Abstract
Accurate and timely molecular test results play an important role in patient management; consequently, there is a customer expectation of short testing turnaround times. Baseline data analysis revealed that the greatest challenge to timely result generation occurred in the preanalytic phase of specimen collection and transport. Here, we describe our efforts to improve molecular testing turnaround times by focusing primarily on redesign of preanalytic processes using the principles of LEAN production. Our goal was to complete greater than 90% of the molecular tests in less than 3 days. The project required cooperation from different laboratory disciplines as well as individuals outside of the laboratory. The redesigned processes involved defining and standardizing the protocols and approaching blood and tissue specimens as analytes for molecular testing. The LEAN process resulted in fewer steps, approaching the ideal of a one-piece flow for specimens through collection/retrieval, transport, and different aspects of the testing process. The outcome of introducing the LEAN process has been a 44% reduction in molecular test turnaround time for tissue specimens, from an average of 2.7 to 1.5 days. In addition, extending LEAN work principles to the clinician suppliers has resulted in a markedly increased number of properly collected and shipped blood specimens (from 50 to 87%). These continuous quality improvements were accomplished by empowered workers in a blame-free environment and are now being sustained with minimal management involvement.
Molecular diagnostic laboratories, just as for other areas of pathology, face challenges associated with increasing testing volumes, decreasing reimbursement, and maintaining and improving quality levels. Diagnostic accuracy is crucial in pathology; nucleic acid-based diagnostic test results are often important for subsequent therapeutic decision making. Accurate and timely molecular testing can add a great deal of value to total patient management. Specimen types such as peripheral blood, bone marrow aspirates, and formalin-fixed, paraffin-embedded (FFPE) tissue, are routinely evaluated using molecular techniques. For tissue-based nucleic acid assays to enter a clinical setting, nucleic acids must be obtainable through current practices of diagnostic pathology. This might involve dealing with individuals who are based at off-site locations, have different priorities, and often have very little understanding of molecular testing requirements. Finally, the isolation of nucleic acids from FFPE tissue, which makes it possible to bring molecular testing to surgical pathology, requires close collaboration between molecular and histology personnel. For accurate and reliable test results, FFPE tissue must be handled in a standardized fashion, similar to how blood and other body fluids are used in routine clinical assays. Furthermore, it is important for individuals doing molecular testing on blood samples collected at different locations to understand the factors outside of their laboratories or sphere of influence. All of these factors might require molecular laboratory personnel to collaborate and become intimately involved with the education of different customer and supplier groups involved in the preanalytic and sometimes postanalytic phases of the testing cycle. This way, roles and boundaries of responsibility pertaining to each group become well defined and the expertise of each group can be used in the most efficient way.
Issues with the preanalytic phase of the testing cycle in particular are not unique to molecular laboratories. Other studies have shown that many laboratory errors occur during the preanalytic phase. These usually consist of all activities leading up to actual analysis of the specimen.1,2,3 In 2006 Plebani4 reported that defects in specimen adequacy occurred most often, with more than 60% of preanalytic errors involving inadequate quantity or unacceptable quality of specimen. Causes of unacceptable quality included collection in the wrong container, improper collection procedure, and improper storage and transportation techniques. Preanalytic factors during collection, processing, and storage of blood specimens may affect DNA and RNA quality and their subsequent use as biomarkers.5,6,7 In terms of FFPE tissues, factors such as fixation and storage can also affect quality of specimens,8 as can preanalytic tissue processing.9
To streamline overall laboratory services at our institution a continuous quality improvement initiative was implemented in early 2006 as the Henry Ford Production System (HFPS).10 This approach to quality improvement was initially adopted in the various sections of the surgical pathology laboratory at Henry Ford Hospital but now is practiced as LEAN management by more than 500 anatomical and clinical pathology employees at Henry Ford Health System. The encompassing goal is to streamline work processes of the pathology department so they are analogous to manufacturing processes (in the creation of value in its work product). Therefore, our pathology laboratory benchmarked the continuous process improvement disciplines of the very successful Toyota LEAN Production System11 as well as those of Henry Ford.12 Our laboratory-based quality effort melded a cultural transformation of management's role and the employees' work approach to go beyond simple approaches of leaning out operations, with the aim of reducing commonly encountered defects and waste.13,14 The chief focus of LEAN is a continuous effort to eliminate process defects and waste while improving practice efficiency.
Baseline data analysis in our laboratory revealed that the greatest challenge for timely molecular test result generation was defects that occurred during the preanalytic phase of specimen collection and transport. We have defined defects to measure waste and reworked imperfections in product requirements including discrepancies in flow or deficiencies in specimen collection and processing resulting in a bottleneck. These defects caused work to be delayed, stopped, or returned to the sender, but once defects and waste were so defined, processes were modified and standardized in accordance with our own HFPS principles. Two types of specimens are currently used in the molecular laboratory: formalin-fixed, paraffin-embedded tissue, which is processed and archived in histology, and blood and bone marrow aspirate specimens, which are collected at Henry Ford Hospital, associated hospitals, and regional medical centers.
In this study we share our experience with the participation of the molecular pathology laboratory in the Henry Ford Production System as a framework for implementation of LEAN process redesign. Through a blameless work environment and contributions from all workers, we undertook the process of eliminating non-value-added waste and standardizing the process of electronic test ordering, of reporting, and of specimen collection, triaging, and transport, all of which contributed to an increase in overall efficiency and greatly reduced testing turnaround times (TATs).
Materials and Methods
Identifying Sources of Defects
Formalin-Fixed, Paraffin-Embedded Tissue Specimens
Before implementation of HFPS, different divisions and sections of pathology operated as independent units. No formal channels of communication were in place such as electronic test ordering or team meetings between departments. Any communication that did occur between groups tended to focus on fault finding, blame, and denial, rather than on using a team effort for reaching common goals successfully. Work performed in different sections lacked a method of standardization, which added to overall testing delays and waste. Our molecular laboratory is centrally located and is in close proximity to the histology laboratory. Despite being a part of the pathology department, the molecular laboratory remained isolated from the rest of the anatomical pathology laboratory because of its focus on nucleic acid-based testing rather than total tissue-based testing. Working in the molecular laboratory requires a highly specialized set of skills. Frequently, individuals who have mastered molecular biology techniques have little familiarity with important histology-based tissue processing techniques such as tissue fixation, paraffin embedding, sectioning on a microtome, and staining.
To incorporate FFPE specimens into the molecular work flow, the choice was either to train molecular personnel in basic tissue processing or to rely on the expertise of histology personnel to prepare tissue sections. The histology laboratory is a large and very busy laboratory that already handles multiple requests for tissue processing. Having to take on the additional burden of training molecular laboratory personnel was seen as just creating more inefficiency, so the histology personnel reluctantly chose to take on the responsibility of providing tissue sections for molecular testing. At baseline, molecular testing was perceived by histology as “research” and was assigned a very low priority in regard to other forms of testing. The process for molecular testing went as follows. A pathologist would be sitting in his or her office (in a section approximately 500 feet from the molecular laboratory) reviewing and signing out cases. If gene rearrangement testing was needed, for example, the pathologist would have to stop his or her work and carry a written request to the molecular laboratory. A busy pathologist might have preferred to wait until the end of the day or the following morning to submit that request. Someone from the laboratory would then have to walk over to the histology area (approximately 100 feet away) and manually enter the request in histology's “recut log.” If the pathologist forgot to specify which tissue block was needed for testing, the molecular technologists then had to contact the pathologist to clarify the order, thus causing an additional delay. Once the correct request was entered in the recut log in histology, it took several days until someone “got around” to it. In addition, the tissue sections submitted by histology were not always appropriate for molecular testing, necessitating more walking back and forth. The testing process itself took 1 to 2 business days to complete, yet the total testing cycle, from request to results being available for signing out, might have taken as many as 6 business days.
Blood and Bone Marrow Specimens
Blood and bone marrow specimens are routinely submitted to the molecular laboratory by clinician customers, primarily for hematology-based tests. A lack of standardization in this process was unveiled as well, as delays in specimen delivery to the laboratory occurred. At Henry Ford Hospital patients are routinely seen at the hospital's hematology/oncology clinic and at oncology clinics at several off-site locations. Phlebotomy is performed by nursing personnel, who at the baseline were uneducated in molecular testing. Most specimens would either be collected and shipped incorrectly or directed to wrong laboratory areas. Frequently blood was collected into a wrong collection tube or was transported and stored at a wrong temperature. Storage temperature has been shown to affect quality of extracted nucleic acids, especially RNA.15,16 DNA is more stable, but storage at room temperature for more than 3 days affects specimen quality (unpublished data). Prolonged storage at room temperature occurred when specimens were directed to wrong laboratory sections. It generally took several days for them to be re-routed to the molecular laboratory. Suboptimal specimen quality necessitated repeating the tests or, when the quality was unacceptable, calling patients back to the clinic for recollection.
Having to ask a patient to return to the clinic for re-collection because a blood specimen was rejected by the laboratory caused considerable frustration, cost, and inconvenience. The patient, who might have been in poor health to begin with, had to arrange for transportation, travel a distance of up to 100 miles round trip, endure another sample extraction, and spend time waiting at the clinic. Delayed test results further affected patient care either because of delayed diagnosis or delayed treatment.
Results
Bringing LEAN into Pathology
Customer-supplier meetings were introduced by HFPS team members as an effort to “hear the voice of the customer.” These meetings proved to be instrumental as collaborative intra- and interdepartmental efforts to assist in defining problems, increasing accountability, determining root causes, brainstorming solutions, and eventually implementing our efforts. Weekly customer-supplier meetings were undertaken with the mission of congregating workers to discuss their expectations and customer requirements as product was produced and passed from one work cell to another.10 The goal was to create highly specified requirements to aid in direct handoffs between customers and suppliers so that the main types of waste in processes could be eliminated. Molecular pathology personnel were educated in HFPS principles in May 2006. The group also participated in a Saturday 5S exercise to reorganize the physical aspects of the laboratory.10 This consisted of applying the 5S concepts of Sort, Stabilize, Shine, Standardize, and Sustain to clean, eliminate non-value added equipment and supplies, and organize and label what remained so that the changes could be sustained. This weekend event was the first opportunity for the individuals in the molecular laboratory to interact informally with personnel from other areas of pathology. The camaraderie generated during this informal exercise was subsequently carried over into the customer-supplier meetings and different phases of the LEAN process improvement, making the change less challenging. Although a minority of individuals viewed the process at first as “a lot of extra work” and change that distracted them away from their main goals (sample processing and testing), a gradual acceptance of the necessity of continuous improvement developed, as increases in efficiency became the most strategic, forward thinking plan.
Redesign of Tissue-Based Testing Process
Histology-Molecular Laboratory Communication
Focused communication between the histology and molecular groups brought to light reasons for delayed tissue sectioning. Histology suppliers explained that tissue sectioning for molecular testing (cutting tissue ribbons and cutting thicker sections on charged or uncharged slides) was different from the routine protocols of histology. Only a few histotechnologists felt comfortable doing these tasks, and each approached the process a bit differently. And because molecular requests were seen as “low priority,” there never seemed to be enough time for these highly sought after individuals to do “additional work.” Once the LEAN process had been implemented in histology, the entire team began thinking more creatively. Histology personnel became educated about molecular testing and its importance in patient care. That resulted in increased effort to process molecular section requests in a timely manner. It is well known that fixation and sectioning of the tissue block can impair the recovery of nucleic acids.8,17,18 Furthermore, contamination of specimens with a different tissue source could occur and has been reported.19 Education of histology personnel was undertaken to prevent misunderstandings and ensure correct processing of molecular requests. On a suggestion from the leaders of the histology group, the molecular laboratory director provided an informal lecture and a clear set of instructions on how FFPE tissue blocks needed to be handled to avoid contamination and maintain nuclease-free conditions. These efforts were so well received that over a period of 1 week a “Molecular Tissue Processing” procedure was implemented in histology and everyone in the laboratory was trained.
For training purposes (and as a quick reference) the molecular laboratory also provided a chart listing all of the tests performed, names that would show up on the electronic log, and types of sections required for each test (Table 1). This chart is now posted in the general histology cutting area and can quickly be consulted as a visual aid for histology personnel when they are unsure about the electronic section requests (eg, 10 μm sections, 4 μm sections on uncharged slides, one set of sections for two tests, and others).
Table 1.
Histology Protocol: Tissue Sectioning Requirements for Molecular Testing
| AP Molecular specimen cutting |
| Test-curls → 5–6 curls at 10 μm into tubes provided |
| Test-slides → 5 blanks on uncharged slides (no oven) |
| Bcell If both ordered, cut once only |
| Tcell |
| MGMT If both ordered, cut once only |
| LOH |
| EGFRvIII |
| KRAS |
| MLH1 |
| EGFR |
| MSI |
| ID |
| Finished specimens go to Molecular Lab or into Molecular box in Histology Lab |
Other process improvements were being implemented simultaneously throughout pathology,13,14 which had a “ripple” effect on the molecular testing process. A very significant improvement was made possible when the pathology informatics group developed a system for electronic test requests and result reporting. With some modifications, this system was expanded to cover the molecular laboratory. The newly created electronic pathways eliminated the need for the pathologists to walk to the molecular laboratory with a test request and for molecular personnel to walk to histology to manually log in the tissue sectioning request. Orders for sectioning of the blocks could be entered electronically by the pathologist while he or she was in the process of reviewing a case and thus the right block could be selected from the start. Implementation of the LEAN processes and standardization of tissue processing in the histology laboratory resulted in greater efficiency in that area, a dramatic reduction in tissue sectioning delays, and a better leveling of workload for all types of tissue specimens. As a result, “one piece” work flow was made possible, which resulted in molecular tissue sections being cut through the day. This meant that the molecular laboratory was now able to start nucleic acid extractions from tissue sections shortly after the request for testing was submitted or at the latest the following morning (if requests were received late in the day). The combination of these streamlined processes in the preanalytic phase significantly reduced the waiting, extra steps, and needs for recuts, which in the past might have accounted for 2 to 3 days of wasted time in the total testing cycle.
Developing Standardized Molecular Tissue Processing Protocol for Histology
Routine tissue sectioning.
The histology protocol for how FFPE tissue blocks need to be handled for molecular testing to achieve optimal DNA or RNA recovery is now very specific. For isolation of nucleic acids, fresh sections are cut from blocks with a new microtome blade after general cleaning of the microtome with sodium hypochlorite solution and 100% ethanol to avoid any potential issues with contamination. During tissue sectioning, the first section of the block is discarded and disposable microtome blades are replaced when different blocks are sectioned. When microdissection is not needed because the block contains mostly tumor tissue, paraffin sections are cut as ribbons and are placed directly into a microcentrifuge tube. When some form of microdissection is required because of focal involvement, the sections are applied to a solid support, such as a glass slide, typically by floating the tissue in a water bath to obtain an unwrinkled section. To avoid contamination, water baths must be emptied and cleaned between processing of samples from different individuals.
Tissue section storage.
Storage of unstained microscope slides can be problematic as precut sections can degrade over a relatively short period of time.20,21 When microdissection is to be performed, sections are cut just before testing and immediately delivered to the molecular laboratory. As soon as the sections are received the molecular laboratory initiates isolation of DNA or RNA.
Suboptimal specimens.
Some tissue blocks are considered to be suboptimal from the start because of their very small size or extensive necrosis. Histotechnologists are the experts in tissue processing and can quickly judge how to approach cutting sections from very small specimens, such as skin biopsies, or from necrotic tissues. Occasionally tissue blocks from very small specimens need to be re-embedded in paraffin before sectioning, which is accomplished quickly and efficiently by a histotechnologist.
Small biopsy specimens.
When immunostains are ordered on the same block, it is a general practice to cut those sections first. More tissue is needed for most molecular tests (five to six sections at 10-μm thickness compared with 4-μm thickness for immunostains). When a small biopsy specimen is involved, the tissue block will be quickly depleted and the area of tumor involvement may even be lost. Yet there are instances for which molecular testing is deemed more valuable than immunostaining. When one is dealing with very small biopsy specimens, it is always a good idea to consult the case pathologist about establishing the cutting order.
Measuring Effectiveness of Process Improvements
Total test times and individual steps were compared during three time periods (Figure 1). We listed individual steps and measured average processing times needed to complete the entire testing cycle (test ordering to result sign out) for each phase.
Figure 1.
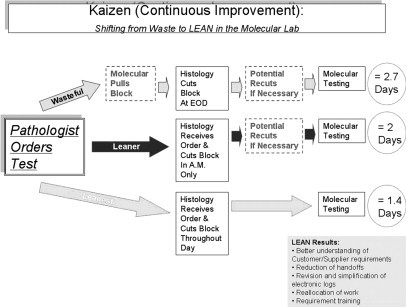
Process map for tissue-based molecular testing: phase I (wasteful phase)—baseline measurements were defined and establishing customer- supplier relationships were established; phase II (leaner phase)—LEAN process improvements were implemented across different disciplines; phase III (leanest phase)—several “Rapid Process Improvement” modifications were added to the initial LEAN work flow to further increase efficiency. LEAN process improvements in histology and molecular pathology resulted in creation of a unidirectional work flow, greatly reduced testing times, and elimination of extra steps.
Phase I (wasteful phase): Baseline measurements were defined and customer-supplier relationships were established. The time period was January to December 2005, and 158 specimens were included in the study (61 B-cell gene rearrangement and 98 T-cell gene rearrangement).
Phase II (leaner phase): LEAN process improvements were implemented across different disciplines. Measurement of efficacy of the process was done for 3 months, June to August 2006, and 116 specimens were included in the study (40 B-cell gene rearrangement, 19 T-cell gene rearrangement, and 57 MGMT promoter methylation).
Phase III (leanest phase): Several “Rapid Process Improvement” modifications were added to the initial LEAN work flow to further increase efficiency. Measurement of efficacy of the process was done from January to December 2007, and 338 specimens were included in the study (92 B-cell gene rearrangement, 145 T-cell gene rearrangement, and 101 MGMT promoter methylation).
The total testing cycle at the baseline (time the pathologist realizes the testing is needed to the time the test results are generated in the molecular laboratory) took 33.5 to 145 hours (1.4 to 6.0 days), average 64.8 hours (2.7 days). Although the analytical phase itself remained consistent at 24 to 48 hours (1 to 2 days), the rest of the time was wasted on hand-offs between different sections of the laboratory (Figure 1, top row). In phase II electronic test ordering and reporting became available, and LEAN process improvement was implemented in histology (Figure 1, middle row). We estimate that just the use of an electronic ordering and reporting system alone cut hours and sometimes even 1 to 2 days from the total testing cycle. In phase III additional posted visual aids were created for histology personnel to reduce misunderstanding of test requests and reduce the need for recuts. This resulted in additional time saving, eliminating the need for recuts, and TATs of 24.2 to 49 hours (1 to 2 days), average 36.5 hours (1.5 days) (Figure 1, bottom row).
Standardizing Blood Collection and Transport
Observation: “Go and See”—the Voice of the Customer
The quality improvement project of standardizing specimen collection and transport was initiated in February 2008 as an effort to improve service to clinician customers. The molecular laboratory enlisted help from the departmental quality improvement data analysis coordinator who accompanied the laboratory director on visits to clinics and laboratories at off-site locations. At each site a customer-supplier meeting was implemented to understand issues and gain input toward process improvements. Individuals from different disciplines such as hematology/oncology clinics, regional laboratories, and triaging areas were invited to participate. Clinicians, nurses, site management, laboratory personnel, and, when possible, specimen transport and triaging personnel were included in customer-supplier meetings. Several issues were identified at the very first meeting.
Clinic personnel had limited knowledge of nucleic acid-based testing and little understanding of how this highly specialized type of testing contributed to patient care.
Rejected specimens created a great deal of frustration for clinicians, clinic personnel, and especially for the patients. The clinics were notified by the molecular laboratory about specimen inadequacy several days after the patient had been seen at the clinic. This necessitated calling the patient at home and asking for a return visit to the clinic for recollection. In cases of therapy monitoring, such as BCR/ABL1 quantitative reverse transcription-PCR testing for minimal residual disease detection, vital treatment might have been delayed owing to a lack of reliable test results.
There were also misconceptions in how specimens needed to be collected and shipped to the molecular laboratory, where the laboratory was located, the testing menu, and whom to call for questions and information.
To answer the question of how the services could be improved, the molecular laboratory was asked to provide the following: 1) clearly specified contact information (individuals to contact, phone numbers, location of the laboratory, and business hours); 2) a list of tests offered; 3) a flow chart of the process for specimen collection and shipment; 4) a protocol for dealing with after hours and weekend specimens; 5) special instructions for collecting blood for DNA-based versus RNA-based tests, and 6) provision of a molecular test requisition that was unique in some way (for oncologists) and that made the blood sample easy to spot during the triaging process (for the Laboratory Support Services section).
Responding to Customer Needs: Redesigning Specimen Collection and Transport Protocol
To put a process in place that would ensure timely delivery of blood and bone marrow specimens, cooperation was needed from the clinics, regional laboratories, and transport/triaging personnel. Leadership at each site also had to be included for continuity of process. Molecular specimens represent only a small fraction of the total sample volume that individuals in these facilities routinely handle, so any instructions provided had to be very specific and brief but also unique in a way that made instant recognition possible. Because the process involved a rare event from the customer-supplier point of view, compared with a “sea” of hematology and chemistry requests, a highly visual system needed to be developed that would make molecular test requests and molecular specimens stand out.
To address the need for a molecular sample to stand out for these busy individuals and to assist specimen triaging personnel in quickly identifying molecular specimens, the molecular laboratory started printing test requisition forms on colored paper. “Hot pink” was selected as the color of choice, because it was easy to see and because none of the other departments or laboratory areas seemed to be using that particular color. To save the clinics time and effort in having to find pink paper for extra copies, the molecular laboratory offered to provide as many copies of the colored test request form as each site needed. This visual aid was well received by all, and was fondly named by the triaging personnel as “the pink form.” To deal with the issues of rapidly outdated pink forms as the new tests are added, the laboratory continues to monitor all paper test requisitions that come back accompanied with a specimen. If it seems that a particular site has an outdated version of the form, that site is immediately contacted and supplied with updated forms.
To preserve specimen integrity during prolonged shipment, the molecular laboratory validated the use of PAXgene blood RNA tubes (PreAnalytiX, Hombrechtikon, Switzerland). The laboratory's standard protocol for RNA-based testing is to accept two types of blood specimens for RNA testing: EDTA samples less than 24 hours old and PAXgene stabilized specimens up to 5 days old when kept cold. The PAXgene tubes were subsequently provided to all outside clinics and regional laboratory sites during educational meetings. Following the process outlined on the pink form, each site was requested to collect blood for DNA-based assays into an evacuated lavender-top tube and blood for RNA-based assays into a PAXgene tube. To avoid damage to specimens during prolonged storage at room temperature, as might occur over weekends and holidays, the laboratory requested that all molecular specimens be kept cold during transport. Specimens received after hours and on weekends were to be stored in a specially designated area in the specimen triaging (Laboratory Support Services section) refrigerator. Bone marrow aspirate specimens were to be collected according to the clinic's routine protocol and were to be shipped cold. For blood specimens collected at the main hospital, where the molecular laboratory is located, no special PAXgene tubes were provided, because specimen pickup and delivery generally took less than 2 hours and no weekend clinic visits take place.
Once received in the molecular laboratory, the specimens were to be either processed immediately or refrigerated. The laboratory implemented an automated DNA extraction method (QuickGene, AutoGen, Holliston, MA), a 10-minute protocol, for blood sample processing. RNA extraction is still performed manually, but RNA stabilization allows timing of specimen processing for optimal efficiency. In either case, the specimens had to be taken to a point where the extracted nucleic acid could be safely stored for possible downstream batch processing.
Measuring Effectiveness of Process Redesign
A baseline study performed in 2007 (Figure 2) showed that of the 28 specimens received from off-site clinics, 14 (50%) had at least one defect: blood in a wrong tube (2 of 28), specimen at an incorrect temperature (4 of 28), or specimen routed to a wrong location (8 of 28). A wrong tube was defined as blood that was drawn into a Vacutainer tube with sodium heparin as anticoagulant. The wrong temperature was defined as blood that was stored at room temperature for longer than 6 hours. For 6 of 28 samples, either extracted DNA or RNA was of marginal quality and the specimen had to be rejected.
Figure 2.
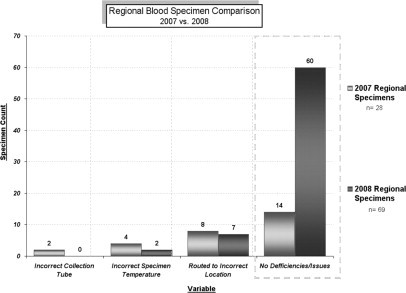
Comparison of regional laboratory specimen volumes. Baseline measurements were performed for 12 months, January to December 2007, and 28 blood specimens were received during that time period. Follow-up measurement was done for 5 months, March to July 2008 (a total of 69 blood specimens were received).
The redesigned process was implemented in February 2008. A follow-up study was done 5 months after implementation (Figure 2). Sixty-nine blood specimens had been received from the off-site locations during that time, compared with a total of 28 specimens during the entire previous year (a 44% increase). The standardized process had been followed for 60 of 69 (87%) specimens. DNA and RNA isolated from these blood specimens was of good quality. Nine of the samples for which the process had not been followed (two at a wrong temperature and seven sent to a wrong laboratory) were collected at smaller clinics that had not yet been visited as part of process implementation. Only three of these specimens were of suboptimal quality and were rejected as inappropriate for testing. The other six were “flagged” by triaging personnel, who by now were familiar with the molecular testing menu and were routed correctly.
Implementing LEAN in the Molecular Laboratory
Pathology informatics played a very important role in the overall increase of efficiency in the molecular laboratory. As part of the HFPS effort, the informatics team was assigned a project of eliminating and revising hundreds of part types in the pathology database.13 That involved creating new part types and pathways for the molecular laboratory, for which none had existed previously. The pathology informatics team members were not familiar with molecular testing and were initially unsure how to approach this project. Formal and informal meetings between pathology informatics team and the molecular laboratory personnel resulted in greater understanding of work requirements and challenges that each team faced. The requirements of the molecular laboratory were to have a simple process in place that would allow electronic test request generation and result sign outs. Once they understood the changes the molecular team was seeking, the pathology informatics team found ways in which they could customize the electronic database to meet the laboratory's needs. Improvements such as molecular-specific part types, electronic result reporting, pathway to the histology recut log for tissue sectioning, and method descriptions in a form of standardized electronic format, became a routine part of the testing process. Molecular test results are now quickly incorporated into the electronic medical records either as stand-alone test reports or as procedure addenda within a composite case report. As such, they are immediately accessible to both clinical and pathology customers. In designing molecular test result report formats, an effort was made to make these short and informative, yet easy to read, with clearly identified result section, interpretation section, specimen type, and testing parameters information.
Increased demand for molecular testing necessitated further effort in streamlining the work flow. As more new tests were added to the testing menu and customers became more aware of those, the laboratory experienced a substantial increase in testing volumes. In 2007 a total of 765 specimens were submitted for molecular testing. That number increased to 952 in 2008, while the staffing remained constant. To keep up with the increase in testing volumes and still satisfy the needs of customers, the molecular laboratory introduced limited batching. Some of the more specialized tests, such as KRAS and EGFR mutation detection, are performed twice a week rather than three times per week as had been done previously. Even with running some assays less frequently, the average testing TATs (from request received in the laboratory to result reporting) has remained below 3 days (Figure 3).
Figure 3.
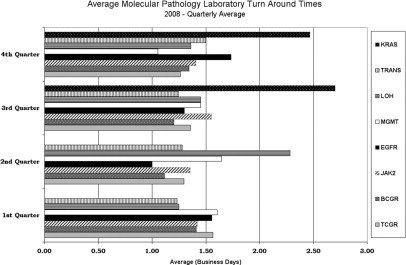
Quarterly average testing turnaround times in the molecular pathology laboratory in 2008. These TATs are estimations of average times (in business days) from the time a testing request was received until results were ready for signing out. KRAS, KRAS mutation detection; TRANS, quantitative RT-PCR assays for detection of t(9;22) and t(15;17) translocations; LOH, 1p/19q loss of heterozygosity; MGMT, MGMT promoter methylation detection; EGFR, epidermal growth factor receptor exon 19 and exon 21 mutation detection; JAK2, JAK2 mutation detection; BCGR, B-cell (IgH) gene rearrangement; TCGR, T-cell receptor γ gene rearrangement.
Educating Customers and Suppliers
Focus on customer and supplier education became an integral part of the effort to remove roadblocks to efficient molecular test utilization. Other than test reports, there were no formal channels in place to reach potential users of the laboratory's services. Even within the department of pathology, not all of the pathologists had molecular training or familiarity with this type of testing. One of the laboratory's goals became a consistent effort to educate internal and external customers (clinicians and pathologists). This education was provided through formal lectures, as part of the customer-supplier meetings, and informally as quick consultation, process recommendation, and specimen requirement information. At all times the laboratory tried to be respectful of customers' time constraints. The laboratory also developed a Website, which was designed to provide a quick overview of laboratory's testing menu, indications for testing, method(s) used, specimen requirements, TATs, and assay parameters.
The second goal was education of the suppliers (histology laboratory, pathology informatics, and triaging and clinic personnel) that the laboratory depended on. Because many of these individuals are either nonlaboratory personnel or they have limited understanding of their role in the total molecular testing cycle, the laboratory initiated a focused supplier education program. For each test, specimen collection instructions included detailed information about the required quantity, proper collection container, and storage and transportation conditions. These were made available as part of the “Lab Users Guide,” a system-wide electronic laboratory testing catalog. A brief overview of how molecular testing affects patient care was also presented at every face-to-face encounter.
Education of customers and suppliers resulted in greatly improved communication and trust between the molecular laboratory and these different groups and their increased willingness to see the laboratory as a source of information as well as timely and reliable test results. Whether talking to a clinician inquiring about a particular test, a nurse calling about collection clarification, or a histotechnologist asking about section preferences, laboratory personnel view each contact as an opportunity to educate, inform, and, most importantly, make a positive impact on patient care.
Discussion
Reasons for delayed test results may vary, and during the preanalytic phase of the testing cycle are often caused by lack of standardized protocols for specimen collection, transport, and preanalytical processing as well as lack of understanding of customer-supplier relationships that are involved in specimen hand-offs between different areas. There are a number of steps involved in molecular testing, and time waste can occur at any one of those steps. In anatomical pathology, a request for molecular testing on a patient's surgical specimen is often driven by a pathologist's or a clinician's uncertainty about the presence or extent of malignancy. Likewise, a blood specimen is submitted for molecular testing when an oncologist suspects the presence of cancer or when monitoring of therapy efficacy is needed. There is often a sense of urgency involved in expecting timely and accurate molecular results. Our laboratory's efforts in improving services were welcomed by both laboratory and clinical customers, and both groups have come to see this LEAN process as a combined project. We did not formally solicit customer feedback; however, our clinical customers now routinely express satisfaction with our services and use molecular tests more frequently, as is evidenced by consistently increasing testing volumes. Clinician customers are now more comfortable with calling the laboratory with questions about methodology, testing indications, and result availability. The use of electronic medical records makes it easier for pathologists to order molecular tests and for both pathologists and clinician customers to receive the test results as soon as they are signed out.
An important factor in the overall success of this project was the focus on communication. Educating the health care community, including pathologists, clinicians, and technical personnel, is essential for bringing timely, accurate molecular test results into the clinical setting. Because a number of different individuals across different disciplines are involved in collection and handling of molecular specimens during the preanalytic phase, the molecular laboratory had little control over how these specimens would be collected or when they would be received.
As a consequence of the focused educational efforts of the laboratory, the users of molecular testing have a better understanding of methods involved and can judge more accurately when addition of molecular tests would be beneficial for patient management. Because the laboratory is creating value from their perspective, the clinic sites are taking an active role in sustaining the processes of standardized specimen collection and transport. Personnel at different locations monitor their supply of PAXgene tubes and customized test request forms, train new personnel as needed, and call the laboratory when supplies are low.
The molecular laboratory process redesign was well received within the department of pathology as well, as different stakeholders in the process began to understand their role in improved patient care. By maintaining mutually supportive customer-supplier relationships between histology, the molecular laboratory, the pathology informatics division, and the pathologists using molecular testing, total molecular diagnostic time for tissue-based molecular assays was reduced from an average of 2.7 days to an average of 1.5 days, a 45% improvement (Figure 1). Because of the short turnaround times, pathologists are now able to rapidly integrate molecular diagnostic test results into case reports and thus ultimately better serve the patients' needs.
The overall concept of analyzing a process to eliminate waste and improve overall manufacturing dates back to Henry Ford in the 1920s,12 but Toyota gave the concept new life as LEAN continuous process improvement.11 Toyota gave the concept structure through actual practice, emphasizing practicality over theoretical analysis.22 The Henry Ford Production System, which is based on these concepts,10 provided us with the framework for implementing LEAN process improvements in molecular pathology testing. As part of this process we examined our own, as well as our customers' and suppliers' processes and procedures, looking for better ways to optimize time, human resources, and assets, while improving the quality level of our products and services.
HFPS LEAN process improvement fosters a collegial, nonadversarial, collaborative team approach to the management of quality in the department, with patient safety being the common goal. The emphasis is on camaraderie and praise during process redesign efforts rather than on fault finding and assignment of blame. As different changes were put in place, these were discussed during monthly “Share the Gain” meetings. Frequently, suggestions for additional improvements were made by group participants, sometimes formally and sometimes during casual conversations in the hallway or in the lounge area. As different process improvements became implemented, even the “slow adapters” learned to appreciate the benefits of more streamlined processes. Integral to HFPS principles, all team members were expected to take turns in presenting at the Share the Gain meetings. Individuals who might have previously felt “ignored” or “unappreciated” now had an opportunity to publicly share their ideas and suggestions, many of which contributed to increase in overall efficiency.
In summary, the aim of this study was to focus on the specimen and the result while eliminating non-value-added waste involved in specimen handoffs between internal and external suppliers and customers. By focusing primarily on the preanalytic phase and by implementing process improvements such as education, electronic test ordering, standardized specimen collection and transport, standardized specimen delivery schedules, and color-coded triaging, we were able to significantly reduce delays and maintain short overall testing TATs (Figure 3). Better defining and standardizing protocols and approaching blood and tissue specimens as analytes for molecular testing allowed us to implement and maintain LEAN process redesign and thus enhance the quality and reproducibility of nucleic acid-based testing in our laboratory.
Acknowledgements
We are grateful to the staff of Henry Ford Surgical Pathology division for their continuous quality improvement efforts. We are also grateful to the staff of the hematology/oncology department and regional laboratory of Henry Ford West Bloomfield Hospital for their assistance in implementation of standardized blood collection and transport protocol and to the Laboratory Support Services (LSS) section at Henry Ford Hospital for their efforts in correctly triaging molecular specimens. Special thanks go to Bernd Barthel, M.D., Hematology/Oncology; Beverly Mahar, histology supervisor; Ralph Benitez, LSS supervisor; Linda Trombley, R.N., Hematology/Oncology; Kathy Schoen and Olivia Seske, West Bloomfield Regional Laboratory; Joanne Beher, Molecular Pathology; and Mark Tuthill, M.D., division head, Pathology Informatics.
Footnotes
This article is partly based on material presented by the authors at the William Beaumont Hospital 17th Annual Symposium on Molecular Pathology: Clinical Applications of Genomic Medicine, which took place on September 10 to 12, 2008 in Troy, MI.
References
- 1.Bonini P, Plebani M, Ceriotti F, Rubboli F. Errors in laboratory medicine. Clin Chem. 2002;48:691–698. [PubMed] [Google Scholar]
- 2.Lippi G, Salvagno GL, Montagnana M, Franchini M, Guidi GS. Phlebotomy issues and quality improvement in results of laboratory testing. Clin Lab. 2006;52:217–230. [PubMed] [Google Scholar]
- 3.Persoon TJ, Zaleski S, Frerichs J. Improving preanalytic processes using the principles of lean production (Toyota Production System) Am J Clin Pathol. 2006;125:16–25. [PubMed] [Google Scholar]
- 4.Plebani M. Errors in clinical laboratories or errors in laboratory medicine? Clin Chem Lab Med. 2006;44:750–759. doi: 10.1515/CCLM.2006.123. [DOI] [PubMed] [Google Scholar]
- 5.Holland NT, Smith MT, Eskenazi B, Bastaki M. Biological sample collection and processing for molecular epidemiological studies. Mutat Res. 2003;543:217–234. doi: 10.1016/s1383-5742(02)00090-x. [DOI] [PubMed] [Google Scholar]
- 6.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Moser K, Ortmann WA, Espe KJ, Balasubramanian S, Hughes KM, Chan JP, Begovich A, Chang S-YP, Gregersen PK, Behrens TW. Expression levels for many genes in human peripheral blood cells are highly sensitive to ex vivo incubation. Genes Immun. 2004;5:347–353. doi: 10.1038/sj.gene.6364098. [DOI] [PubMed] [Google Scholar]
- 7.Pahl A, Brune K. Stabilization of gene expression profiles in blood after phlebotomy. Clin Chem. 2002;48:2251–2253. [PubMed] [Google Scholar]
- 8.Srinivasan M, Sedmak D, Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol. 2002;161:1961–1971. doi: 10.1016/S0002-9440(10)64472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cronin M, Pho M, Dutta D, Stephans JC, Shark S, Kiefer MC, Esteban JM, Baker JB. Measurement of gene expression in archival paraffin-embedded tissues: development and performance of a 92-gene reverse transcriptase-polymerase chain reaction assay. Am J Pathol. 2004;164:35–42. doi: 10.1016/S0002-9440(10)63093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarbo RJ, D'Angelo R. Transforming to a quality culture: the Henry Ford Production System. Am J Clin Pathol. 2006;126(suppl 1):S21–S29. [Google Scholar]
- 11.Ohno T. Toyota Production System: Beyond Large Scale Production. Productivity Press; Portland: 1988. [Google Scholar]
- 12.Ford H. Today and Tomorrow. Doubleday; New York: 1926. [Google Scholar]
- 13.Zarbo RJ, D'Angelo R. The Henry Ford Production System: effective reduction of process defects and waste in surgical pathology. Am J Clin Pathol. 2007;128:1015–1022. doi: 10.1309/RGF6JD1NAP2DU88Q. [DOI] [PubMed] [Google Scholar]
- 14.D'Angelo R, Zarbo RJ. The Henry Ford Production System: measures of process defects and waste in surgical pathology as a basis for quality improvement initiatives. Am J Clin Pathol. 2007;128:423–429. doi: 10.1309/X6N1Y3V2CB9HUL8G. [DOI] [PubMed] [Google Scholar]
- 15.Kim SJ, Dix DJ, Thompson KE, Murrell RN, Schmid JE, Gallagher JE, Rockett JC. Effects of storage. RNA extraction, genechip type, and donor sex on gene expression profiling of human whole blood. Clin Chem. 2007;53:1038–1045. doi: 10.1373/clinchem.2006.078436. [DOI] [PubMed] [Google Scholar]
- 16.Tanner MAS, Berk LS, Felten DL, Blidy AD, Bit SL, Ruff DW. Substantial changes in gene expression level due to the storage temperature and storage duration of human whole blood. Clin Lab Haematol. 2002;24:337–341. doi: 10.1046/j.1365-2257.2002.00474.x. [DOI] [PubMed] [Google Scholar]
- 17.Hewitt SM, Lewis FA, Cao Y, Conrad RC, Cronin M, Danenberg KD, Goralski TJ, Langmore JP, Raja RG, Williams PM, Palma JF, Warrington JA. Tissue handling and specimen preparation in surgical pathology—issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. 2008;132:1929–1935. doi: 10.5858/132.12.1929. [DOI] [PubMed] [Google Scholar]
- 18.Medeiros F, Rigl CT, Anderson GG, Becker SH, Halling KC. Tissue handling for genome-wide expression analysis: a review of the issues, evidence and opportunities. Arch Pathol Lab Med. 2007;131:1805–1816. doi: 10.5858/2007-131-1805-THFGEA. [DOI] [PubMed] [Google Scholar]
- 19.Bernsen MR, Dijkman HB, de Vries E, Figdor CG, Ruiter DJ, Adema GJ, van Muijen GN. Identification of multiple mRNA and DNA sequences from small tissue samples isolated by laser-assisted microdissection. Lab Invest. 1998;78:1267–1273. [PubMed] [Google Scholar]
- 20.Fergenbaum JH, Garcia-Closas M, Hewitt SM, Lissowska J, Sakoda LC, Sherman ME. Loss of antigenicity in stored sections of breast cancer tissue microarrays. Cancer Epidemiol Biomarkers Prev. 2004;13:667–672. [PubMed] [Google Scholar]
- 21.Jacobs TW, Prioleau JE, Stillman IE, Schnitt SJ. Loss of tumor marker immunostaining intensity on stored paraffin slides of breast cancer. J Natl Cancer Inst. 1996;88:1054–1059. doi: 10.1093/jnci/88.15.1054. [DOI] [PubMed] [Google Scholar]
- 22.Liker JK. The Toyota Way: 14 Management Principles from the World's Greatest Manufacturer. McGraw-Hill; New York: 2004. pp. 35–41. [Google Scholar]


