Abstract
A real-time polymerase chain reaction (PCR) assay for the direct identification of Mycobacterium tuberculosis and M. bovis using molecular beacons was developed. The assay was modified for use in regular thermal cyclers. Molecular beacons that were specific for M. tuberculosis (Tb-B) and M. bovis (Bo-B) were designed. The fluorescence of the target PCR product-molecular beacon probe complex was detected visually using a transilluminator. The results were then compared with those of conventional multiplex PCR (CM-PCR) assays and biochemical identification. The detection limit of Tb-B and Bo-B beacons was 500 fg and 50 fg by the visual format and real-time PCR assay, respectively, compared with 5 pg by CM-PCR assay. Pulmonary and extrapulmonary samples were examined. The agreement between culture and the two assays was very good in sputum samples and fair in extrapulmonary samples. The agreement between clinical diagnoses with the two assays was moderate in extrapulmonary samples. There was very good agreement between CM-PCR and visual format assays for all samples used in the study. Concordance in the identification of isolates by the visual, CM-PCR assay, and biochemical identification was seen. Hence, the use of molecular beacon detection of M. tuberculosis and M. bovis in clinical samples is feasible by setting up two asymmetric PCRs concurrently. The assay is sensitive, specific, simple to interpret, and takes less than 3 hours to complete.
For effective treatment of tuberculosis (TB), rapid and accurate diagnosis is essential. Conventional polymerase chain reaction (PCR)-based assays designed to detect Mycobacterium tuberculosis that involve electrophoresis based analysis of amplicons are relatively fast but are laborious and the potential risk of inadvertent dispersal of amplicons leading to contamination of untested samples exists. To overcome these difficulties a number of probes, including TaqMan probes, molecular beacons, and side-by-side probes, that can report the amplification of the correct amplicon in sealed tubes have been developed.1,2,3 Several different instruments that measure increase in fluorescence of these probes while simultaneously performing amplification have also become available. Although such real-time PCR assays are more quantitative than conventional ones, the instruments used are not commonly available in resource-poor locations. Since such localities are often where tuberculosis is more prevalent, it is important to develop sealed tube assays that can be implemented on conventional PCR machines but have the simplicity and accuracy of probe-based detection.
In the previously developed tests different coding and intergenic regions from the M. tuberculosis genome, such as devR, IS6110, IS986, RNA polymerase gene, and ribosomal RNA gene have been used as targets for amplification using molecular beacons or fluorescent probes.4,5,6,7 One of the limitations of these tests is that they have exclusively focused on M. tuberculosis. However, other closely related bacteria such as M. bovis have been often associated clinically with human and bovine samples in practice.8,9,10 Therefore an ideal test should distinguish between M. tuberculosis and M. bovis. We used the mce3 operon that has a differential organization in the M. tuberculosis and M. bovis genome as a suitable target for the assay.11
To develop a simple sealed tube test that can reliably detect M. tuberculosis in clinical samples using conventional PCR instrument for amplification and a common lamp for transillumination, we used molecular beacons as probes. However, the signal intensity in PCRs performed with molecular beacons is often limited because the two product strands of the amplicon bind to each other excluding the probe from the target strand. We improved the fluorescence intensity of these reactions by performing asymmetric PCR that produced more of the molecular beacon target strand than of the opposite strand. The optimizations were performed using a real-time instrument. We show that our assay is robust, specific, sensitive, and can be completed in less than 3 hours. The presence of mycobacteria in the sample can be conclusively established by visualizing the fluorescent amplicon in a blue light transilluminator by the naked eye. We also compared and correlated our endpoint visual format assay with acid-fast bacilli (AFB) smear microscopy; a gel-based conventional multiplex PCR (CM-PCR) assay, culture, and clinical diagnosis. The simplified visual format assay was found to be an accurate predictor of the presence of M. tuberculosis and M. bovis in clinical samples.
Materials and Methods
Samples
Sputum samples were collected from 97 pulmonary tuberculosis patients attending the outpatient department of LRS Institute of tuberculosis and Respiratory Disease, New Delhi. Seventy-one aseptically aspirated pleural fluid samples were obtained from patients registered in the Department of Respiratory Medicine, Safdarjung Hospital, New Delhi. The study was approved by the institutional ethical committee. The clinical criteria as described by Light12 were adopted for the diagnosis of pleural tuberculosis. The definitive criterion for tuberculous pleural effusion was the demonstration of acid-fast bacilli in sputum and or pleural fluid by microscopy or culture. Suggestive criteria included: 1) patients with clinical history of fever, pleuritic chest pain, cough, breathlessness, and chest radiography with evidence of pleural effusion; 2) cytological examination of the pleural fluid for predominance of lymphocytes and paucity of mesothelial cells; 3) biochemical estimation for protein content (>3 g/dl) in pleural fluid: serum protein ratio (>0.5); and 4) response of patients to antituberculous treatment. Tuberculous pleural effusion was diagnosed if the definitive criterion or all of the suggestive criteria were met. Based on these criteria, 51 patients were classified as patients with tuberculous pleural effusion.
Criteria for including malignant pleural effusion patients (controls) were: clinical history suggestive of rapidly refilling pleural effusion with or without focal malignant lesion elsewhere in the body; pleural fluid being exudative, usually hemorrhagic; and on cytological examination, positive for malignant cells. Based on these criteria 20 patients were classified as nontuberculous pleural effusion patients (non-TB).
The cerebrospinal fluid (CSF) sample was obtained from a clinically suspected case of tuberculous meningitis. The patient was admitted to the pediatric ward at Kalawati Saran Children Hospital, New Delhi.
Processing of Samples
All samples except CSF were processed by the NALC-NaOH method.13 For processing of CSF, the filtration method was used as described earlier.14 The sediments were processed for AFB microscopy, isolation of mycobacteria, and target DNA. A total of 118 samples (97 sputum, 20 pleural fluid, and the CSF sample) were inoculated into MGIT tubes (BD BACTEC MGIT 960 system for mycobacteria testing). The remaining 51 pleural fluid samples were inoculated on Lowenstein-Jensen medium and into 7H9 broth. All cultures were examined up to 8 weeks. Species level identification of isolates was done by standard biochemical tests (niacin production, nitrate reduction, catalase and aryl sulfatase activity, Tween hydrolysis, thiopen-2-carboxylic acid hydrazide sensitivity) as recommended by the Centers for Disease Control and Prevention, Atlanta, GA, with appropriate controls.15
Mycobacterial Strains
M. tuberculosis (M. tuberculosis, H37Rv) DNA was obtained from Tuberculosis Research Material, NIAD, National Institutes of Health. M. bovis (AN5) was obtained from National JALMA Institute for Leprosy and Other Mycobacterial Diseases, Agra, India.
Design and Characterization of the Molecular Beacons
M. tuberculosis beacon (Tb-B) was designed targeting the unique 12.7-kb fragment of the M. tuberculosis genome. The M. bovis beacon (Bo-B) was designed commencing 1 bp 5′ to the deletion site of the 12.7-kb fragment in the M. bovis genome. The positions of the beacons and primers are shown in Figure 1.11
Figure 1.
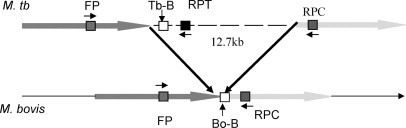
Position of primers and beacons in the mce3 operon11 used for the detection and identification of M. tuberculosis and M. bovis. 12.7-kb insert prevents Taq polymerase-mediated amplification in M. tuberculosis, hence amplification seen only in M. bovis.
Thermal denaturation profile studies were conducted to determine the optimal annealing temperature for the real-time PCR. Real-time PCR was done as follows: molecular beacon buffer alone (negative control); buffer + Tb-B (negative control); buffer + Tb-B + target oligonucleotide of M. tuberculosis (TO-tb, positive control); buffer + Tb-B + target oligonucleotide M. bovis (TO-bo). Identical combination of reagents was used with M. bovis beacon (Bo-B). For the assay the molecular beacon buffer (3.5 mmol/L MgCl2 and 10 mmol/L Tris-HCl), with 0.3 μmol/L each of molecular beacon (Tb-B and Bo-B) and 0.6 μmol/L each of target oligonucleotide (5′-GTACTATGCTGACCCATGCGCCCT-3′ and TO-Bo, 5′-CGGAGAGCGCCGTTGTAGGCC-3′) was used. Target oligonucleotides contained the complementary sequence of the beacon's loop. Each tube was subjected to sequential decrease in temperature (1°C at a time) from 96 to 45°C. Fluorescence was estimated during the 30-second hold period at each temperature in the real-time thermal cycler. The melt curve of the molecular beacons was obtained by plotting a –dF/dT curve.
The signal-to-noise ratio of the molecular beacons was determined by measuring the fluorescence at 491 nm excitation and 515 nm emission in a spectrofluorimeter (Spectra MAX Gemini XS, Molecular Devices). Background fluorescence of the buffer and beacons was assessed and deducted from the fluorescence estimated following target hybridization. The signal-to-noise ratio (STNR) of the molecular beacons was determined as follows: STNR = Fopen – Fbuffer/Fclosed – Fbuffer, where Fbuffer = fluorescence of molecular beacons buffer (containing 1 mmol/L MgCl2 + 20 mmol/L Tris-HCl, pH 8); Fclosed = fluorescence of 0.3 μmol/L molecular beacons in the molecular beacons buffer; Fopen = fluorescence of molecular beacon buffer containing 0.3 μmol/L molecular beacons and 0.6 μmol/L target oligonucleotide.
The readings obtained with M. tuberculosis beacon were as follows: molecular beacon buffer was Fbuffer = 5.4, for M. tuberculosis beacon with buffer Fclosed = 136.2, M. tuberculosis beacon with target oligonucleotide of M. tuberculosis Fopen = 3055.1. Similarly for M. bovis beacon the reading obtained was as follows: buffer alone Fbuffer = 4.4, for M. bovis beacon with buffer Fclosed = 38.1, M. bovis beacon with target oligonucleotide of M. bovis Fopen = 905.6. The signal-to-noise ratio of the respective molecular beacons was 27 and 23 for M. bovis and M. tuberculosis beacons, respectively. These characterizations were performed at the room temperature. The signal to noise ratio for the beacons were determined with twofold increase in target oligonucleotide by the fluorometric and visual format methods.
PCR Assay
The assay was first standardized in a real-time format (FP, forward primer common to both M. tuberculosis and M. bovis; RPT, reverse primer from 12.7-kb fragment specific for M. tuberculosis; RPC, reverse primer from the sequence present in both M. tuberculosis and M. bovis). For M. tuberculosis the primers used were FP (5′-ATGACGCCTTCCTAACCAGAA-3′) and RPT (5′-ATGCATCGGAATAAGATGTCAGGC-3′) with Tb-B (FAM-5′-CCGCGGAGGGCGCATGGGTCAGCATAGTACCGCGG-3′-BHQ2) and for M. bovis the primers used were FP and RPC (5′-ACCGGATATCTTAGCTGGTCAA-3′) with Bo-B (FAM-5′-CCGCGCTGGCCTACAACGGCGCTCTCCGCGCGG-3′–BHQ2). Sequences in italic demarcate the beacon stem. Subsequently the real-time format assay was modified for the visual format. The visual format assay was compared with the CM-PCR assay.
Real-Time PCR Assay
The experiments were performed with an iCycler iQ real-time detection system (Bio-Rad). For the asymmetric PCR assay, two reaction mixtures were set up for detection of M. tuberculosis and M. bovis. The first mixture (50 μl reaction) contained the primer FP (0.1 μmol/L) and RPT (0.5 μmol/L) and the Tb-B (0.6 μmol/L). The second mixture (50 μl reaction) contained the FP and RPC (0.5 μmol/L) and the Bo-b (0.6 μmol/L). The PCR reaction mixture contained 1X AmpliTaq Gold PCR buffer, 4 mmol/L MgCl2, 0.25 mmol/L dNTPs (Eppendorf), and 2.5 U AmpliTaq Gold enzyme (Applied Biosystems). The thermal cycle parameters were 95°C for 10 minutes and 40 cycles of each for 45 seconds at 95°C, 45 seconds at the optimized annealing temperature, 45 seconds at 72°C, and final extension at 72°C for 10 minutes. Annealing temperature for asymmetric PCR assay was determined by the melt curve analysis of the beacons with their target oligonucleotides.
Visual Format Using Molecular Beacons
The asymmetric PCR parameter and conditions in the visual format were identical to the real-time PCR assay with the exception of the Taq polymerase enzyme (MBI Fermentas) in place of AmpliTaq Gold enzyme.
Multiplex PCR
Three primers were used: FP common to M. tuberculosis and M. bovis and two reverse primers, RPT and RPC, specific for M. tuberculosis and M. bovis, respectively. The assay mixture (25 μl reaction) contained FP (0.625 μmol/L), RPT and RPC (0.325 μmol/L each), 1X PCR buffer (100 mmol/L Tris/HCl, pH 8.8, 500 mmol/L KCl, 0.8% Nonidet P-40), 2.5 mmol/L MgCl2, 0.3 mmol/L dNTPs, and 1.25 U TaqDNA polymerase. The thermal cycle parameters were 95°C for 10 minutes and 40 cycles of each, 45 seconds at 95°C, 45 seconds at 58°C, 45 seconds at 72°C, and final extension at 72°C for 10 minutes. The standardized assay was used for detection of M. tuberculosis as well as M. bovis in clinical samples. The amplified product obtained in CM-PCR assay is 162 and 127 bp for M. tuberculosis and M. bovis, respectively.
Detection of PCR-Amplified Product
For the gel-based CM-PCR, amplicon(s) were detected by standard ethidium bromide staining and ultraviolet illumination of 2.2% agarose gels. For the visual format using molecular beacons, tubes were positioned over a dark reader box (Clare Chemical Research). Tubes were illuminated with a source of visible excitation blue light devoid of ultraviolet radiation. An amber screen separated the light source from the viewer. The fluorescence was viewed and assessed in the dark by at least two individuals.
Sensitivity of the Assays
The limit of sensitivity of the PCR assays (real-time PCR, CM-PCR, and molecular beacons using visual format) was determined by serial dilutions of M. tuberculosis and M. bovis DNA.
Statistical Analysis
All statistical analyses were done using STATA software, version 9.2 (StataCorp, College Station, TX). The agreement between the assays used was assessed using the kappa coefficient (κ). The strength of agreement was defined as: poor, κ ≤ 0.2; fair, κ = 0.21–0.4; moderate, κ = 0.41–0.6; good, κ = 0.61–0.8; very good, κ = 0.81–1.16
Results
Rationale for the PCR Target Used in the Study
Sequence analysis of M. tuberculosis and M. bovis has shown that M. bovis lacks the 12.7-kb fragment containing the mce3 operon.11 All M. tuberculosis isolates examined showed the presence of the 12.7-kb fragment, while all of the M. bovis strains lacked this fragment.11 We exploited the differences in the organization of the mce3 operon in the two species.11 Primers were designed as follows: FP was common to both M. tuberculosis and M. bovis; RPT was derived from the 12.7-kb fragment and RPC from the region adjacent to the 12.7-kb fragment (Figure 1).11 RPT primer was specific for M. tuberculosis, while the sequence of the RPC reverse primer was present in both M. tuberculosis and M. bovis. However, amplification occurs in case of M. bovis with the FP and RPC exclusively and not in the case of M. tuberculosis, as the 12.7-kb insert prevents Taq polymerase-mediated amplification.
Characterization of Molecular Beacons
The beacons were characterized by visual and fluorometric techniques. The specificity of molecular beacons was assessed by adding excess of the target oligonucleotides and an aliquot of each molecular beacon. Photographs were taken. Figure 2A shows that M. tuberculosis target oligonucleotide elicited fluorescence with Tb-B and M. bovis target oligonucleotide elicited fluorescence with Bo-B and not vice versa. In absence of the target oligonucleotides, no fluorescence was generated by the molecular beacons in the tubes. The signal-to-noise ratio of the respective molecular beacons was determined as described in Materials and Methods. The signal-to-noise ratio of the respective molecular beacons was 27 and 23 for M. bovis and M. tuberculosis beacons, respectively.
Figure 2.
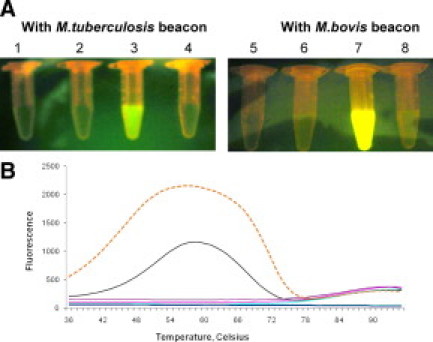
A: Signal-to-noise ratio of molecular beacons. The signal-to-noise ratio of M. tuberculosis and M. bovis beacons was determined by the visual format assay. Tube 1, buffer (negative control); tube 2, buffer + M. tuberculosis beacon (negative control); tube 3, buffer + M. tuberculosis beacon + target oligonucleotide of M. tuberculosis (positive control); tube 4, buffer + M. tuberculosis beacon + target oligonucleotide of M. bovis; tube 5, buffer (negative control); tube 6, buffer + M. bovis beacon (negative control); tube 7, buffer + M. bovis beacons + target oligonucleotide M. bovis (positive control); tube 8, buffer + M. bovis beacon + target oligonucleotide M. tuberculosis. B: Thermo-denaturation profile for molecular beacons. Dotted line denotes M. bovis beacon with its target oligonucleotide melt curve. Continuous line denotes M. tuberculosis beacon with its target oligonucleotide melt curve. All other lines denotes various controls.
Further, the thermal denaturation profile for each molecular beacon was determined by real-time PCR. As shown in Figure 2B, the probe target hybrid for M. tuberculosis beacon melts at 58°C and that of M. bovis melts at 56°C, and the stems of each molecular beacon melt at a range between 74 and 76°C. These features indicate that the detection temperatures can be below 60°C, at which the probe-target hybrids would be allowed to form when the target is produced in the reaction and the stems would remain closed when the target is absent.
Real-Time PCR and Multiplex PCR Sensitivity
As the signal intensities generated by real-time PCR were inadequate, asymmetric PCR was performed to intensify and maximize the fluorescence. To demonstrate the limits of detection of M. tuberculosis and M. bovis, serial dilutions of the DNA ranging from 500 ng to 10 fg were added to the asymmetric PCR reaction. Two separate reactions were set up using Tb-B and Bo-B. The limit of detection by real-time PCR is shown in Figure 3, A and B. The detection limit with Tb-B and Bo-B beacons was 500 fg (50 bacilli) and 50 fg (5 bacilli), respectively. The sensitivity of the visual format using molecular beacons was similar to that detected by real-time PCR (Figure 4A). Gel-based CM-PCR assay showed lower sensitivity of detection of mycobacterial DNA (5 pg, equivalent to 500 bacilli; Figure 4B).
Figure 3.
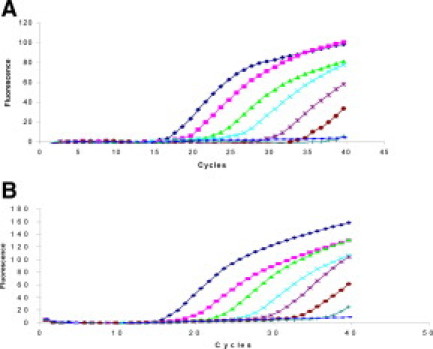
Amplification plots show the detection of the serially diluted DNA of M. tuberculosis (A) and M. bovis (B) by real-time PCR using molecular beacons. Tenfold serial dilution of the mycobacterial DNA, ranging from 5 × 106 to 5 bacilli/reaction (from left to right) and the negative control were amplified in individual tubes. PCR cycles have been plotted against fluorescence intensity.
Figure 4.
Limit of detection of M. tuberculosis and M. bovis DNA by PCR assay using molecular beacons by the visual format (A) and gel-based assays (B). M. tuberculosis and M. bovis DNA were added as follows: tube 1, 50 ng (5 × 106 bacilli); tube 2, 5 ng (5 × 105 bacilli); tube 3, 500 pg (5 × 104 bacilli); tube 4, 50 pg (5 × 103 bacilli); tube 5, 5 pg (5 × 102 bacilli); tube 6, 500 fg (5 × 101 bacilli); tube 7, 50 fg (5 bacilli); tube 8, negative control in visual format; tube 8′, 5 fg (∼1 bacilli) in gel-based; tube 9, negative control.
Comparative Analysis of the Four Methods for Detection of Mycobacteria in 97 Sputum Samples
Four methods were used for detecting mycobacteria in sputum samples: AFB smear microscopy, isolation by culture, visual format using molecular beacons, and CM-PCR assays (Table 1). The results of the detection of AFB by smear microscopy were compared with culture, visual format, and CM-PCR assays. Of the 97 sputum samples processed, in 45 samples (45/97, 46.3%) AFB was detected. All 45 AFB smear positive samples were positive by the three methods used, namely culture, visual format, and CM-PCR assays. Among the 52 AFB negative samples, in 33 samples mycobacteria were isolated by culture. By the visual format assay 30 of the 52 (57.6%) and by the CM-PCR assay 26 of the 52 (50%) samples were positive for M. tuberculosis. On comparing these assay results with culture, in the visual format assay using molecular beacons and CM-PCR assay a very good agreement was seen (κ = 0.91 and 0.80 respectively; Table 1). Between the visual format and CM-PCR assays there was near perfect agreement (κ = 0.89). However, between culture and AFB detection by smear microscopy, the agreement was limited (κ = 0.35).
Table 1.
Comparative Analysis of AFB Smear Microscopy of Sputum Samples with Isolation of Mycobacteria by Culture, Visual Format, and CM-PCR Assay
| AFB |
κ (P value)* |
|||||
|---|---|---|---|---|---|---|
| Assays | P (n = 45) | N (n = 52) | Total (n = 97) | Culture versus assays | Culture versus AFB | Visual versus CM-PCR |
| Culture† | ||||||
| P | 45 | 33 | 78 | 0.35 (<0.001) | 0.89 (<0.001) | |
| N | 0 | 19 | ||||
| Visual format‡ | ||||||
| P | 45 | 30 | 75 | 0.91 (<0.001) | ||
| N | 0 | 22 | ||||
| CM-PCR | ||||||
| P | 45 | 26 | 71 | 0.80 (<0.001) | ||
| N | 0 | 26 | ||||
P, positive; N, negative.
Agreement between the assays calculated using STATA software version 9.2.
Inoculated in MGIT tubes, incubated at 37°C for 6–8 weeks.
Detection of M. tuberculosis and M. bovis using beacons.
Comparative Analysis of the Four Methods for Detection of Mycobacteria in Pleural Fluid Samples
Mycobacteria in pleural fluid samples were detected by the four methods described (Table 2). The results obtained by these methods were compared, with isolation of mycobacteria by culture and clinical diagnosis. The distribution of these 71 samples is detailed in Table 2.
Table 2.
Comparative Analysis of Clinical Diagnosis and Isolation of Mycobacteria from Samples Derived from Pleurisy Patients with AFB Smear Microscopy, Visual Format, and CM-PCR Assay
| Clinical diagnosis* |
κ (P value)† |
|||||||
|---|---|---|---|---|---|---|---|---|
| TB patients (n = 51) Culture‡ |
Non-TB patients (n = 20) Culture |
|||||||
| Assay method | P 13 | N 38 | P 0 | N 20 | Culture versus assays | Clinical diagnosis versus assays | Culture versus clinical diagnosis | Visual versus CM-PCR |
| Visual format§ | ||||||||
| P | 11 | 24 | 0 | 0 | 0.26 (<0.01) | 0.55 (<0.001) | 0.16 (<0.01) | 0.89 (<0.001) |
| N | 2¶ | 14 | 0 | 20 | ||||
| CM-PCR | ||||||||
| P | 11 | 20 | 0 | 0 | 0.33 (<0.001) | 0.47 (<0.001) | ||
| N | 2¶ | 18 | 0 | 20 | ||||
| AFB | ||||||||
| P | 3 | 4 | 0 | 0 | 0.20 (<0.04) | 0.08 (<0.04) | ||
| N | 10 | 34 | 0 | 20 | ||||
P, positive; N, negative.
Clinical diagnosis of TB and non-TB patients based on criteria detailed in Materials and Methods.
Agreement between the assays calculated using STATA software version 9.2.
Culture: sample inoculated into MGIT/LJ/7H9, incubated at 37°C for 6–8 weeks.
Detection of M. tuberculosis and M. bovis using beacons.
MOTT isolates M. fortuitum and M. chelonae.
Of the 51 clinically diagnosed pleural tuberculosis patients, mycobacteria were detected in 17 samples (33.3%) either by AFB microscopy or by culture. No mycobacteria were detected in non-TB patients by any of the assays used. By the visual format and CM-PCR assays M. tuberculosis was detected directly in 11 of the 13 culture positive samples. In two samples negative by both the visual format and CM-PCR assays, the mycobacteria isolated from these samples were identified as M. fortuitum and M. chelonae, respectively. In culture negative samples, 24 (24/38, 63.1%) samples were positive for M. tuberculosis by the visual format assay, compared with 20 (20/38, 52.6%) samples that were positive by the CM-PCR assay (Table 2). There was fair to moderate agreement among the results obtained by the two assays (visual format and CM-PCR assays) compared with culture and clinical diagnoses except with AFB smear microscopy. Fair agreement between culture and visual format and culture and CM-PCR assays was observed (κ = 0.26 for visual format and 0.33 for CM-PCR assay, respectively). Between clinical diagnosis and the assays used, moderate agreement was observed (κ = 0.55 for visual format and 0.47 for CM-PCR assay, respectively). In 35 of the 51 (68.6%) samples mycobacteria were detected by the visual format assay compared with 31 (60.7%) by the gel-based assay, 13 (25.5%) by culture, and 7 (13.7%) by AFB smear microscopy. Poor agreement was seen between AFB detection and culture and AFB detection and clinical diagnosis (κ = 0.20 and 0.08, respectively). Agreement between the visual format and CM-PCR assays was very good (κ = 0.89).
Detection of Mixed Infection in CSF Sample Clinically Suspected of Tuberculous Meningitis
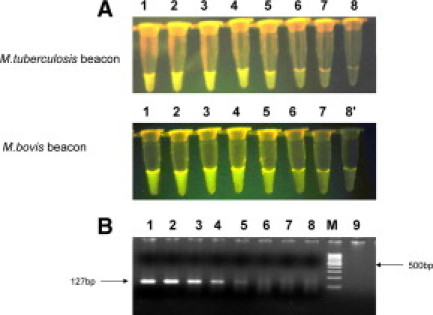
The CSF sample was processed as described previously.14 The CSF sample was filtered, and the residue on the filter (0.45 μm) membrane was directly used for detection of M. tuberculosis and/M. bovis by culture (BACTECMGIT960) as well as by the assays described herein. Using molecular beacons (visual format assay) (Figure 5B) and CM-PCR assay, the sample was positive for M. tuberculosis as well as M. bovis (Figure 5A). The dual PCR-amplified products seen in the sample align to the 162- and 127-bp products obtained in the standard strains of M. tuberculosis and M. bovis (positive controls), respectively. Two weeks later, the MGIT culture of the CSF sample was similarly positive for both pathogens. The assays have been performed three times to confirm the results. Efforts are under way to separate the AFB obtained in MGIT media on 7H11 Dubos agar.
Figure 5.
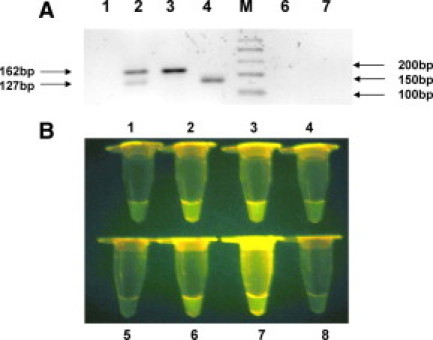
A: CM-PCR for detecting and differentiating M. tuberculosis and M. bovis in the CSF sample, CS-112. The ethidium bromide stained amplification products of M. tuberculosis and M. bovis generated by using FP, RPT, and RPC primers were electrophoresed on 2.2% agarose gel. The 162- and 127-bp products obtained in M. tuberculosis and M. bovis respectively, are indicated. Lane 1, PF-7; lane 2, CSF; lane 3, M. tuberculosis (positive control, H37Rv); lane 4, M. bovis (positive control AN-5); lane M, 50-bp molecular weight marker; lanes 6 and 7, negative control. B: Visual format using molecular beacons for detecting and differentiating M. tuberculosis and M. bovis in the CSF sample, CS-112. Tubes 1–4 showing fluorescence obtained using primer pair FP and RPT with M. tuberculosis beacon and tubes 5–8 showing fluorescence obtained using primer pair FP and RPC with M. bovis beacon. Tubes 1 and 5, PF-7; tubes 2 and 6, CSF; tube 3, M. tuberculosis positive control; tube 7, M. bovis positive control; tubes 4 and 8, negative control.
Agreement between Direct Detection of Mycobacteria in Clinical Samples by Multiplex PCR Assay and the Visual Format Beacon Assay Versus Classical Identification of Isolates
Seventy-eight isolates were obtained from 97 sputum and 13 from 51 pleural tuberculosis patients. All isolates were AFB positive and were identified by biochemical criteria.15 Of the 91 isolates, 89 were identified as M. tuberculosis with the exception of two isolates from pleural tuberculosis patients. These two isolates were identified as M. fortuitum and M. chelonae. The mixed culture obtained from the CSF sample in MGIT media has been subcultured on 7H11 Dubos agar to isolate and characterize the strain(s) by standard biochemical tests. There was complete concordance in the direct identification of mycobacteria present in the clinical sample before isolation by the visual format and CM-PCR assays. M. fortuitum and M. chelonae was isolated from the pleural tuberculosis patients that were negative by both assays. The 20 DNA samples derived from M. bovis isolates (courtesy Dr. Niamh Corbally) were identified as M. bovis by both assays.
Discussion
The molecular beacon-based PCR assay described has the potential for direct detection of specific mycobacterial pathogens, namely M. tuberculosis and M. bovis, that could potentially be present in clinical samples. In the visual format assay, a sample was considered positive if the fluorescence intensity was more than the negative control (without DNA). In comparison with culture, the visually configured assay using molecular beacons described in this study showed the sensitivity of 96.1% compared with earlier reports of 86.7% for devR and 88.3% for IS6110 PCR.4 To the best of our knowledge, this is the first report of molecular beacons used in combination with asymmetric PCR for detection of dual infection due to M. tuberculosis and M. bovis in a sample. This confirms our earlier report using the hupB-based assay17 describing dual detection of M. tuberculosis and M. bovis in clinical samples. Similar reports of mixed mycobacterial infection in individual clinical samples have been described.18,19,20
The classical microbiological methods for diagnosing tuberculosis have poor sensitivity and specificity.21 Traditionally, the diagnosis of tuberculosis depends on demonstration of mycobacteria by AFB smear microscopy and growth on Lowenstein-Jensen medium. AFB microscopy is rapid; the results are obtained within a few minutes compared with the 2- to 6-week time period required by the MGIT-960 culture system.22,23 AFB microscopy is inexpensive, easy to perform, and requires limited training and equipment. The major disadvantage of smear microscopy is its sensitivity, as a smear to be considered positive should have a concentration of 10,000 AFB/ml.24,25 The low sensitivity is often reflected by the high rates of smear-negative TB cases.4 On the other hand, there have been some improvements in the culture-based detection systems, but the requirement of expensive instrumentation (such as BACTEC-460, MGIT-960) has restricted their use to specialized laboratories. Mycobacterial culture is more sensitive compared with smear microscopy, as 10 to 100 bacilli/ml are required for culture-based detection.24,25 Our method is similar in its simplicity compared with smear microscopy but has improved sensitivity and specificity, requiring 5 to 50 bacilli for detection by the visual format assay.
Due to the inherent paucibacillary nature of pleural fluid samples, the isolation of mycobacteria by culture has been inefficient.26,27 The rate of isolation of mycobacteria from pleural fluid has been reported to range from 12% to 33%.26,28,29 Besides pleural fluid, inclusion of sputum samples has been considered appropriate to improve the demonstration of mycobacteria by smear microscopy and its isolation28 (submitted for publication by P Kumar, MK Sen, DS Chauhan, VM Katoch, S Singh, HK Prasad). In the present study in 17 of the 51 (33.3%) samples derived from pleural TB patients, AFB was detected in seven samples and mycobacteria were isolated from 13 (Table 2). These patients meet the definitive criterion for tuberculous pleural effusion. Fifteen of these 17 samples (88.2%) were positive by the visual as well as the CM-PCR assay for M. tuberculosis. Besides the definitive criterion, 34 patients met the suggestive criteria for tuberculous pleural effusion, such as clinical history of fever, chest pain, cough, breathlessness, and chest radiography with evidence of pleural effusion and pleural fluid cytology commensurate with pleural TB and responded to antituberculous treatment. In 70% of these (24/34) patients M. tuberculosis was detected by the visual format assay. PCR-based detection of M. tuberculosis in smear negative samples has been reported.30 Further, improvement of detection and isolation of mycobacteria in pleural effusion patients has been reported with inclusion of pleural biopsies.29,31 However, this approach is disadvantaged owing to its invasiveness, complications of the procedure, and the inability to sample infectious foci of the pleural tissue.
Besides demonstration of mycobacteria in patient-derived samples, the detection of mycobacterial DNA in clinical samples using PCR has been widely adopted as a rapid, sensitive, and specific diagnostic method.32 Use of the nested PCR assay has provided a remarkable increase in sensitivity and specificity of DNA amplification compared with the conventional single-step PCR assay.18,33,34 However, the nested PCR assay is laborious and time-consuming, which carries the risk of contamination in clinical samples. Hence it has yet to be widely used in tuberculosis diagnosis.33,34 To overcome the problems of sample contamination and to reduce detection time, real-time PCR technique has been developed as an alternate rapid and sensitive assay for detection of M. tuberculosis in clinical samples.35,36 Although real-time PCR assay has qualitatively advanced microbial detection assays, owing to their technological sophistication, equipment and reagent costs have restricted its utility in the diagnosis of infectious diseases for specialized laboratories.
The simplicity of the assay and visual interpretation makes it ideally suited for routine use in clinical laboratories with limited resources, such as in developing countries. The assay could be further simplified from the present format by using molecular beacons tagged with different fluorophores in a multiplex assay.37 Such a multiplex assay would reduce the number of tubes, time, labor, and cost required to set up an assay for a clinical sample. However, the clinical samples suspected of dual infection must necessarily be investigated and analyzed using the visual format assay as outlined in this study. Cross-contamination with PCR products does not occur, as assay tubes are not opened after amplification. The study shows that simple end point detection using molecular beacons and a blue light transilluminator has a great potential in the clinical diagnosis of tuberculosis and that of other pathogens.
Acknowledgements
We acknowledge the help of Dr. Jaya S. Tyagi and Sagarika Haldar and the technical assistance of Mr. Shailendra Kumar and Indresh Kumar. We acknowledge Professor Niamh Corbally of the Microbiology Department of Mater Hospital, Dublin, Ireland, for the DNA of mycobacterial strains. The biotechnology information system facility of the department is kindly acknowledged.
Footnotes
Supported by Department of Biotechnology, Government of India, through Indo-US Vaccine Action Program project and a fellowship from the Indian Council of Medical Research (to P.K.). Mycobacteria H37Rv DNA was obtained from TB Research Materials and Vaccine Testing under NIH/NIAID grant AI-75320.
S.T. and Public Health Research Institute receive royalties from the sale of molecular beacons.
References
- 1.Lee LG, Connell CR, Bloch W. Allelic discrimination by nick-translation PCR with fluorogenic probes. Nucleic Acids Res. 1993;21:3761–3766. doi: 10.1093/nar/21.16.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyagi S, Kramer FR. Molecular beacons: probes that fluoresce upon hybridization. Nature Biotechnol. 1996;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- 3.Lay JY, Wittwer CT. Real-time fluorescence genotyping of factor V Leiden during rapid-cycle PCR. Clin Chem. 1997;43:2262–2267. [PubMed] [Google Scholar]
- 4.Halder S, Chakravorty S, Bhalla M, Majumdar SD, Tyagi JS. Simplified detection of Mycobacterium tuberculosis using smear microscopy and PCR with molecular beacons. J Med Microbiol. 2007;56:1356–1362. doi: 10.1099/jmm.0.47265-0. [DOI] [PubMed] [Google Scholar]
- 5.Li QG, Liang JX, Luan GY, Zhang Y, Wang K. Molecular beacons based homogeneous fluorescence PCR assays for diagnosis of infectious disease. Anal Sci. 2000;16:248. 248. [Google Scholar]
- 6.Piatek AS, Tyagi S, Pol AC, Telenti A, Miller LP, Kramer FR, David A. Molecular beacon sequence analysis for detecting drug resistance in Mycobacterium tuberculosis. Nature Biotechnol. 1998;16:359–363. doi: 10.1038/nbt0498-359. [DOI] [PubMed] [Google Scholar]
- 7.Soini H, Musser JM. Molecular diagnosis of mycobacteria. Clin Chem. 2001;47:809–814. [PubMed] [Google Scholar]
- 8.Rodwell TC, Moore M, Moser KS, Brodine SK, Strathdee SA. Tuberculosis from Mycobacterium bovis in binational communities, United States. Emerg Infect Dis. 2008;14:909–916. doi: 10.3201/eid1406.071485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans JT, Smith EG, Banerjee A, Smith RMM, Dale J, Innes JA, Hunt D, Tweddell A, Wood A, Anderson C, Hewinson RG, Smith NH, Hawkey PM, Sonnenberg P. Cluster of human tuberculosis caused by Mycobacterium bovis: evidence for person-to- person transmission in the UK. Lancet. 2007;369:1270–1276. doi: 10.1016/S0140-6736(07)60598-4. [DOI] [PubMed] [Google Scholar]
- 10.Mishra A, Singhal A, Chauhan DS, Katoch VM, Srivastava K, Thakral SS, Bharadwaj SS, Sreenivas V, Prasad HK. Direct detection and identification of Mycobacterium tuberculosis and Mycobacterium bovis in bovine samples by a novel nested PCR assay: correlation with conventional techniques. J Clin Microbiol. 2005;43:5670–5678. doi: 10.1128/JCM.43.11.5670-5678.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zumarraga M, Bigi F, Alito A, Romano MI, Cataldi A. A 12.7-kb fragment of the M. tuberculosis genome is not present in M. bovis. Microbiology. 1999;145:893–897. doi: 10.1099/13500872-145-4-893. [DOI] [PubMed] [Google Scholar]
- 12.Light RW. Pleural Diseases. ed 4. Lippincott Williams & Wilkins; Philadelphia: 2001. Clinical manifestations and useful tests; pp. 42–86. [Google Scholar]
- 13.Kent TP, Kubica GP. Public health mycobacteriology: a guide for the level III laboratory. US Department of Health and Human Services, Centers for Disease Control; Atlanta, GA, USA: 1985. pp. 57–63. [Google Scholar]
- 14.Kumar P, Srivatsava MVP, Singh S, Prasad HK. filtration of cerebrospinal fluid improves isolation of mycobacteria. J Clin Microbiol. 2008;46:2824–2825. doi: 10.1128/JCM.00210-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vestal AL. Procedures of isolation and identification of mycobacteria. Centers for Disease Control; Atlanta: 1977. pp. 65–98. [Google Scholar]
- 16.Kampmann B, Whittaker E, Williams A, Walters S, Gordon A, Martinez-Alier N, Williams B, Crook AM, Hutton AM, Anderson ST. Interferon- gamma release assays do not identify more children with active TB than TST. Eur Respir J. 2009;33:1374–1382. doi: 10.1183/09031936.00153408. [DOI] [PubMed] [Google Scholar]
- 17.Shah NP, Singhal A, Jain A, Kumar P, Uppal SS, Srivatsava MVP, Prasad HK. Occurrence of overlooked zoonotic tuberculosis: detection of Mycobacterium bovis in human cerebrospinal fluid. J Clin Microbiol. 2006;44:1352–1358. doi: 10.1128/JCM.44.4.1352-1358.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suffys P, Rocha AD, Brandao A, Vanderborght B, Mijs W, Jannes G, Mello FCQ, Pedro HSP, Fonseca LS, Oliveira RS, Leao SC, Saad MHF. Detection of mixed infections with Mycobacterium lentiflavum and Mycobacterium avium by molecular genotyping methods. J Med Microbiol. 2006;55:127–131. doi: 10.1099/jmm.0.46218-0. [DOI] [PubMed] [Google Scholar]
- 19.Lévy-Frébault V, Pangon B, Buré A, Katlama C, Marche C, David HL. Mycobacterium simiae and Mycobacterium avium-M. intracellulare mixed infection in acquired immune deficiency syndrome. J Clin Microbiol. 1987;25:154–157. doi: 10.1128/jcm.25.1.154-157.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tortoli E, Simonetti MT, Dionisio D, Meli M, Sterrantino G: Mycobacterium genavense and Mycobacterium avium mixed infection in an AIDS patient. Clin Microbiol Newslett 15: 175–176
- 21.Chain K. Clinical microscopy. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of Clinical Microbiology. ed 6. American Society for Microbiology; Washington, DC: 1995. pp. 33–51. [Google Scholar]
- 22.Small PM, Perkins MD. More rigour needed in trials of new diagnostic agents for tuberculosis. Lancet. 2000;9235:1048–1049. doi: 10.1016/S0140-6736(00)02724-0. [DOI] [PubMed] [Google Scholar]
- 23.Daniel TM. The rapid diagnosis of tuberculosis: a selective review. J Lab Clin Med. 1990;116:277–282. [PubMed] [Google Scholar]
- 24.Mirza S, Restrepo IB, McCormick JB, Fisher-Hoch SP. Diagnosis of tuberculosis lymphadenitis using polymerase reaction on peripheral blood mononuclear cells. Am J Trop Med Hyg. 2003;69:461–465. [PubMed] [Google Scholar]
- 25.Gopi A, Madhavan SM, Sharma SK, Sahn SA. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest. 2007;131:880–889. doi: 10.1378/chest.06-2063. [DOI] [PubMed] [Google Scholar]
- 26.Villegas MV, Labrada LA, Saravia NC. Evaluation of polymerase chain reaction, adenosine deaminase, and interferon-γ in pleural fluid for the differential diagnosis of pleural tuberculosis. Chest. 2000;118:1355–1364. doi: 10.1378/chest.118.5.1355. [DOI] [PubMed] [Google Scholar]
- 27.Trajman A, Pai M, Dheda K, van Zyl Smit R, Zwerling AA, Joshi R, Kalantri S, Daley P, Menzies D. Novel tests for diagnosing tuberculous pleural effusion: what works and what does not? Eur Respir J. 2008;31:1098–1106. doi: 10.1183/09031936.00147507. [DOI] [PubMed] [Google Scholar]
- 28.Conde MB, Loivos AC, Rezende VM, Soares SLM, Mello FCQ, Reingold AL, Daley CL, Kritski AL. Yield of sputum induction in the diagnosis of pleural tuberculosis. Am J Respir Crit Care Med. 2003;167:723–725. doi: 10.1164/rccm.2111019. [DOI] [PubMed] [Google Scholar]
- 29.Hasaneen NA, Zaki ME, Shalaby HM, El-Morsi AS. polymerase chain reaction of pleural biopsy is a rapid and sensitive method for the diagnosis of tuberculous pleural effusion. Chest. 2003;124:2105–2111. doi: 10.1378/chest.124.6.2105. [DOI] [PubMed] [Google Scholar]
- 30.Liu KT, Su WJ, Perng RP. Clinical utility of polymerase chain reaction for diagnosis of smear-negative pleural tuberculosis. J Chin Med Assoc. 2007;70:148–151. doi: 10.1016/S1726-4901(09)70348-X. [DOI] [PubMed] [Google Scholar]
- 31.Epstein DM, Kline LR, Albelda SM, Miller WT. Tuberculous pleural effusions. Chest. 1987;91:106–109. doi: 10.1378/chest.91.1.106. [DOI] [PubMed] [Google Scholar]
- 32.Palomino JC. Nonconventional and new methods in the diagnosis of tuberculosis: feasibility and applicability in the field. Eur Respir J. 2005;26:239–350. doi: 10.1183/09031936.05.00050305. [DOI] [PubMed] [Google Scholar]
- 33.Liu PY, Shi ZY, Lau YZ, Hu BS. Rapid diagnosis of tuberculous meningitis by a simplified nested amplification protocol. Neurology. 1994;44:1161–1164. doi: 10.1212/wnl.44.6.1161. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi T, Nakayama T, Tamura M, Ogawa K, Tsuda H, Morita A. Nested polymerase chain reaction for assessing the clinical course of tuberculous meningitis. Neurology. 2005;64:1789–1793. doi: 10.1212/01.WNL.0000162052.13838.B2. [DOI] [PubMed] [Google Scholar]
- 35.Rindi L, Lari N, Bonanni D, Garzelli C. Detection of Mycobacterium tuberculosis genotypic groups by a duplex real-time PCR targeting the katG and gyrA genes. J Microbiol Methods. 2004;59:283–287. doi: 10.1016/j.mimet.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Aldous WK, Pounder JI, Cloud JL, Woods GL. Comparison of six methods of extracting Mycobacterium tuberculosis DNA from processed sputum for testing by quantitative real-time PCR. J Clin Microbiol. 2005;43:2471–2473. doi: 10.1128/JCM.43.5.2471-2473.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marras SAE, Kramer FR, Tyagi S. Multiplex detection of single-nucleotide variations using molecular beacons: genetic analysis. Biomol Eng. 1999;14:151–156. doi: 10.1016/s1050-3862(98)00018-7. [DOI] [PubMed] [Google Scholar]


