Abstract
Purpose
To determine the most optimal stage for antioxidant supplementation of culture medium to improve developmental competence, cryotolerance and DNA-fragmentation of bovine embryos.
Methods
Presumptive zygotes were first cultured in presence or absence of β-mercaptoethanol (β-ME), for 8 days. Subsequently, half of the expanded blastocysts developed in both groups were vitrified, warmed within 30 min and post-warming embryos along with their corresponding non-vitrified embryos were cultured for two further days in presence or absence of (100 µM) βME.
Results
For vitrified and non-vitrified embryos, the best effect was found when βME was added from day 1 of in vitro culture in continuation with post-warming culture period. Day 1–8 supplementation significantly increased the rates of cleavage, day 7 and day 8 blastocyst production. For non-vitrified embryos, βME addition during day 1–8 and/or 9–10 of embryo culture improved both hatching rate and quality of hatched embryos. For vitrified embryos, however, the percentage of DNA-fragmentation (18.5%) was significantly higher (p ≤ 0.05) than that of embryos developed in absence of βME but supplemented with βME during post-warming period (13.5%).
Conclusions
Exogenous antioxidant increases the chance of embryos, even those of fair-quality, to develop to blastocyst. However, antioxidant inclusion during in vitro embryo development is not sufficient to maintain the redox state of these embryos during the critical period of post-warming embryo culture, and therefore, there should be a surplus source of exogenous antioxidant during post-warming embryo culture.
Keywords: β-mercaptoethanol (βME), Bovine embryo, Developmental competence, Cryosurvival
Introduction
Since the first successful cryopreservation of mammalian embryos [1], numerous agricultural and biomedical opportunities have been proposed based on this technology [2, 3]. Accordingly, the ability to cryopreserve embryos without critical loss of viability has a profound effect on the success of assisted reproductive techniques (ART) [4]. However, the survival of cryopreserved in vitro produced (IVP) embryos, as measured either by post-warming survival in culture or by established pregnancies after embryo transfer, has lagged behind that of in vivo-derived embryos [5–8]. Although the background of this difference is not completely understood, it seems that the culture medium in which embryos are grown has dramatic effect(s) on developmental competence and cryo-withstand of the resultant embryos [6]. In this regard, a number of different embryo culture protocols have been proposed in order to maximize post-warming survival of vitrified embryos. Most of these studies have been mainly focused on the possible effects of serum, glucose, NADPH (nicotinamide adenosine dinucleotide phosphate), BSA (bovine serum albumin) and co-culturing on the freezability of IVP embryos [2, 3, 9, 10]. However, little attention has been paid to the contributing effects of antioxidants on cryosurvival of the vitrified/warmed embryos.
Oxidative stress has been implicated in many different types of cell injuries, including membrane lipids peroxidation, oxidation of amino acids and nucleic acids, apoptosis and necrosis which may subsequently decrease the viability of IVP embryos [11–13]. To overcome this, exogenous antioxidants such as β-mercaptoethanol (βME), a low molecular weight thiol compound, have been frequently used to increase antioxidant capacity of embryos via increasing intracellular levels of reactive oxygen species (ROS) scavengers such as glutathione (GSH) [14–18]. Following cryopreservation, embryos become more amenable to the deleterious effects of ROS [2, 4], and as a consequence, the importance of ROS detoxification seems to be greater than the normal sate of in vitro embryo development. In these circumstances, however, it is not clear if antioxidant supplementing of culture medium during in vitro embryo development is sufficient for ROS detoxification during the critical period of post-warming embryo culture, or, there should be a surplus source of exogenous antioxidant following cryopreservation. The answer of this question may also help human and also animal ART specialists to know if there is a place for in vitro culture of vitrified-warmed embryos before being transferred into recipients or efficient supplementing of embryos before vitrification but not after warming is sufficient for direct transfer into recipients. The present study, therefore, was undertaken to systematically investigate whether the embryo culture stage in which βME is supplemented can have an effect on developmental competence, hatching rate, DNA fragmentation and also cryosurvival of bovine IVP embryos.
Materials and methods
Chemicals and media
Unless otherwise stated, all chemicals and media were obtained from Sigma Chemical Co. (St. Louis, MO, USA) and Gibco (Grand Island, NY, USA), respectively.
In vitro embryo production
The culture procedure used for production of preimplantation bovine embryos was as described previously [19]. Briefly, bovine ovaries were obtained from a local abattoir and cumulus-oocyte complexes (COCs) were aspirated from antral follicles (2–8 mm). COCs were then washed with Hepes-buffered TCM199 (HTCM199) medium and then TCM199 before being cultured upon the established monolayers of vero cells (approximately 1 × 105 cells/ml) in 100 µl droplets of maturation medium (ten oocytes per droplet), covered with mineral oil for 24 h at 38.5°C, 5% CO2 and humidified air (Labotect C200, Germany). The medium used for maturation was TCM199, supplemented with 10% fetal calf serum (FCS), 10 mg/ml ovine FSH, 1 mg/ml ovine LH, 1 mg/ml oestradiol, and 100 mM cysteamine. Frozen-thawed and washed sperm from a single Holstein sire of proven in vitro fertility were used for fertilization after capacitation by the swim-up procedure [20]. Spermatozoa (1 × 106 sperm/ml) and matured COCs (40–45 COCs/200 µl) were co-incubated in modified fert-TALP medium containing 0.01 mM heparin, 0.2 mM penicillamine, and 0.1 mM hypotaurine for 18–24 h at 38.5°C under 5% CO2 in humidified air [20]. The presumptive zygotes were then vortexed for 90 s in Hepes-TALP to remove the cumulus cells, washed once in H-TCM199 and twice in the culture medium (B2 medium; CCD, Paris, France) and then each five zygotes were placed in drops of 50 µl B2 medium supplemented with 10% FCS, seeded with vero cells and overlaid with mineral oil at 38.5°C, 5% CO2, 5% O2 and humidified air. Those embryos which had cleaved beyond the 2-cell stage were refreshed in new culture dishes every 2 days [21].
Vitrification of IVP embryos
The vitrification protocol used in this study was adopted from Martínez et al. [3] with some modifications. Briefly, embryos were pre-equilibrated in a solution of 7.5% ethylene glycol (EG) + 7.5% dimethyl sulfoxide (DMSO) in Dulbecco Modified Eagles’ Medium (DMEM) supplemented with 20% FCS until the expanded blastocysts endured a period of shrinkage and returning (up to 8 min, at 38.5°C). Equilibrated embryos were then exposed to vitrification solution consisting of 15% EG + 15% DMSO in holding medium at room temperature for one min, and then were loaded into the tips of the cryotops (Cryologic; CVM™, Fibreplug & Sleeve, Australia) with the minimum amount of vitrification solution and immediately plunged into the liquid nitrogen (LN2). For warming, embryos were removed from LN2 and quickly exposed into DMEM + 20% FCS supplemented with 1 M sucrose for 1 min on a warm plate (38.5°C). Then, embryos were transferred to DMEM + 20%FCS containing 0.25 M sucrose for 5 min and finally washed thoroughly in DMEM + 20%FCS. Embryos were then co-cultured over vero cells in B2 medium + 10% FCS. The percentages of re-expansion, hatching and degeneration were determined at 12–48 h post-warming and hatched blastocysts were used for assessment of TCN, viability, and DNA fragmentation.
Experimental design
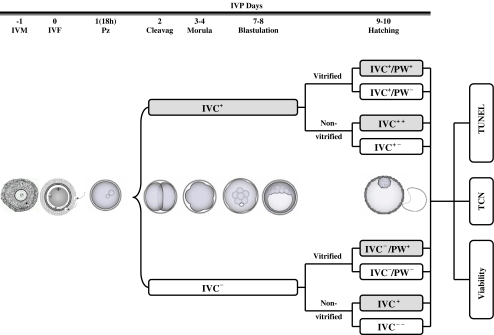
As depicted in Fig. 1, presumptive zygotes were first cultured in presence (IVC+) or absence (IVC−) of βME (100 µM) for up to 8 days, and the rates of cleavage, morula, and day 7–8 blastocyst formation were determined. Subsequently, expanded blastocysts in both IVC+ and IVC− groups were selected, counted, and half of them were randomly divided and cultured for two further days in presence (IVC+ +, IVC− +) or absence (IVC+ −, IVC− −) of βME. Meanwhile, the other half of the expanded blastocysts in IVC+ and IVC− groups were vitrified, warmed within 30 min and then post-warmed (PW) embryos were randomly divided and cultured in presence (IVC+/ PW+, IVC−/ PW+) or absence (IVC+/ PW−, IVC−/PW−) of βME. The percentages of hatched/degenerated non-vitrified embryos, along with the proportions of immediate/total degenerated, re-expanded and hatched vitrified embryos developed under different culture conditions (Fig. 1) were determined. Finally, half of the hatched embryos developed in each group were used for determining total cell number (TCN) and viability and the other half used for TUNEL assessment of DNA-fragmentation.
Fig 1.
Experimental design: Immature bovine oocytes were matured/fertilized in vitro and then presumptive zygotes were cultured in presence (IVC+) or absence (IVC−) of βME for up to 8 days. On day 8 post fertilization, expanded blastocysts developed in each group were selected, counted and then half of these embryos were randomly divided and further cultured for 2 other days in presence (IVC+ +, IVC− +) or absence (IVC+ −, IVC− −) of βME. Meanwhile, the other half of the expanded blastocysts in IVC+ and IVC− groups were vitrified, thawed within 30 min and then post-warming (PT) embryos were randomly divided and cultured in presence (IVC+/PW+, IVC−/PW+) or absence (IVC+/PW −, IVC−/PW −) of βME. Gray boxes indicated the presence of βME
Differential staining to detect viable vs. dead blastomeres
To this end, hatched blastocysts in each group were first incubated for 30 min in pre-equilibrated culture medium containing propidium iodide (PI; 300 µg/ml) and Hoechst 33342 (H33342; 5 µg/ml) fluorescent dyes. Then residual dyes removed from embryos by thoroughly washing in warm calcium and magnesium free phosphate buffer saline (PBS−), fixed in 2.5% glutharaldehyde and washed again in PBS−. Stained embryos were then mounted in a drop of glycerol over a microscopic slide and visualized by an epifluorescent microscope (Olympus BX51) using the same excitation wavelength [330–385 nm] and barrier filter [400 nm] to distinguish and record viability (viable cells/ TCN) as they appeared in red and blue, respectively [21].
TUNEL measurement of DNA-fragmentation
Detection of embryonic apoptosis was performed using terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-digoxigenin nick end labeling (TUNEL) with an in-situ cell death detection kit (Promega Diagnostic Corporation, USA) according to the manufacturer’s recommendation and Kitagawaa et al. [13]. Briefly, hatched blastocysts in each group were fixed overnight at 4°C in 3.7% (w/v) paraformaldehyde diluted in PBS−. Fixed embryos were then washed thrice in PBS− containing 3% (w/v) polyvinyl alcohol (PVA) and permeabilized in 0.5% (v/v) Triton X-100 for 60 min, and subsequently incubated in a blocking solution (PBS− containing 10 mg/ml BSA) overnight at 4°C. As a positive control, one or two embryos per TUNEL analysis were incubated in 1,000 U/ml deoxyribonuclease I (DNase I; Sigma) for 20 min. After washing in PBS−-PVA, the positive controls and all experimental embryos were incubated in TUNEL reaction cocktail at 37°C for 1 h in the dark. The embryos were then counterstained with 50 μg/ml DAPI (DAPI) for 20 min to label all nuclei. Embryos were washed extensively and mounted with slight coverslip compression and examined under epiflouresence microscope (Olympus, BX 51) [13]. In the present study, the ratio of TUNEL-labeled nuclei to total cell number was defined as DNA-fragmented index (DFI).
Statistical analysis
During this study, all the experimental design was repeated 3 times including 3 independent runs of each group. Hatched blastocysts in each group were then divided and used for viability and TUNEL assays. The differences recorded were evaluated by analysis of variance (ANOVA) using the GLM procedures of SAS (SAS Institute Inc., Cary, NC, USA). The mean of the treatments were compared using Duncan’s multiple range test (DMRT) and a confidence level of P < 0.05 was considered to be significant.
Results
Effect of βME supplementation on in vitro embryo production
Two thousand seven hundred ninety-three bovine oocytes were subjected to IVM/F and 2,575 presumptive zygotes were then subsequently cultured in presence (IVC+) or absence (IVC−) of βME for 8 days. Developed blastocysts were then further cultured in presence (IVC+ + or IVC− +) or absence (IVC+ − or IVC− −) of βME. As shown in Table 1, day 1–8 βME supplementation of culture medium improved developmental competence of IVC+ vs. IVC− embryos, and this improvement was significant at three stages; 8–16 cell (87.0 ± 2.3% vs. 66.0 ± 3.5%), day 7 (28.3 ± 2.2% vs. 17.1 ± 1.4%), and day 8 (40.1 ± 1.1% vs. 33.3 ± 2.3%) blastocyst. By addition of βME during day 9 and 10 of embryo culture, hatching rate of IVC+ + embryos (83.3 ± 3.6%) was non-significantly greater than IVC+ − (75.0 ± 4.0%) and IVC− + (61.1 ± 4.5%), but significantly greater than IVC− − (38.9 ± 3.3%) embryos. Analysis of quality of hatched blastocysts indicated overall improved quality of IVP embryos when cultured in presence of βME. Accordingly, blastocyst cell number of IVC+ + embryos was 248.0 ± 4.5 which was non-significantly greater than IVC+ − (170.6 ± 5.5) and IVC− + (168.4 ± 3.8) but significantly greater than IVC− − (163.7 ± 5.8) embryos. Furthermore, DFI index indicated that the mean percentages of fragmented cells in bovine embryos developed in IVC+ + condition (6.1 ± 0.0), was significantly lower than embryos cultured in IVC− − (15.1 ± 0.9%) conditions.
Table 1.
Effect of βME supplementation on developmental ability of bovine in vitro produced (IVP) embryos
| IVP culture design | Presumptive zygotes (n) | Proportion of embryos developed to1 | Quality analysis of blastocysts | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cleavage | 8–16 cell | Morula | D7 Bls | D8 Bls | D9–10 Hatching | TCN (n) | Viability2(%) | DNA-fragmented3 (%) | ||
| IVC + + | 1,250 | 92.0 ± 3.1 | 87.0 ± 2.3a | 75 ± 3.1 | 28.3 ± 2.2a | 40.1 ± 1.1a | 83.3 ± 3.6a | 248.0 ± 4.5a | 96.3 ± 0.3 | 6.1 ± 0.0a |
| IVC+ − | 75.0 ± 4.0ab | 170.6 ± 5.5ab | 94.0 ± 1.0 | 9.2 ± 0.3ab | ||||||
| IVC− + | 1,325 | 75.1 ± 2.5 | 66.0 ± 3.5b | 68 ± 1.5 | 17.1 ± 1.4b | 33.3 ± 2.3b | 61.1 ± 4.5ab | 168.4 ± 3.8ab | 93.5 ± 0.0 | 10.4 ± 0.2ab |
| IVC− − | 38.9 ± 3.3b | 163.7 ± 5.8b | 90.8 ± 1.0 | 15.1 ± 0.9b | ||||||
1: Ratios are expressed based on the number of presumptive zygotes.
2: Percentage of live cells/TCN
3: Percentage of TUNEL-positive/TCN
Different letters within a column are significantly different between treatments (P < 0.05)
Within βME supplemented groups, the best effect was found when βME was added throughout the culture period. Day 1–8 supplementing significantly increased the rate of blastocyst production. However, the quality of IVC+ − embryos was not significantly different with that of embryos developed in IVC− + condition (Table 1).
Effect of βME on post-warming survival of vitrified blastocysts
As shown in Table 2, βME supplementation of culture medium during embryo development and/or after vitrification-warming improved overall survival of frozen-thawed embryos as assessed by the ratios of re-expansion at 12 h post-warming, hatching rate, and post hatching quality analysis. In this regard, within the βME supplemented groups, the proportion of morphological survival and also total hatching of vitrified-warmed embryos were 15.7 ± 1.5 % and 74.3 ± 2.0% (IVC+/PW+), 16.7 ± 1.0% and 50.0 ± 2.4% (IVC+/PW−), and 15.0 ± 1.1% and 57.8 ± 1.2% (IVC−/ PW+) which were non-significantly different with each other but were significantly different with the related values of IVC−/PW− culture group (35.9 ± 2.4% and 31.4 ± 3.2%). Analysis of quality of cryopreserved embryos indicated no significant difference of TCN and the ratio of viable cells/TCN between the groups. However, TUNEL assessment of the cryopreserved embryos revealed that embryos developed in IVC+/PW+ culture condition contained the lowest rate of DNA-fragmentation (10.0 ± 1.0%), which was significantly different with IVC+/PW− (18.5 ± 1.5%) and IVC−/PW− (20.6 ± 0.5%) groups.
Table 2.
Effect of BME supplementation of culture medium (IVC+vs. IVC¯) and/or post-warming medium (PW+vs. PW¯) on cryosurvival of bovine IVP blastocysts
| Comparisons | Survived embryos (%) | Post-warming re-expansion after 12 h (%) | Total hatching (%) | Total degeneration (%) | Quality assay of blastocysts | ||
|---|---|---|---|---|---|---|---|
| TCN (n) | Viability 1 (%) | DNA fragmented 2 (%) | |||||
| IVC+/PW+ | 15.7 ± 1.5a | 84.3 ± 1.6a | 74.3 ± 2.0a | 25.7 ± 3.0a | 170.4 ± 4.2a | 95.2 ± 1.0a | 10.0 ± 1.0a |
| IVC+/PW − | 16.7 ± 1.0a | 83.3 ± 1.0a | 50.0 ± 2.4a | 50.0 ± 2.5b | 160.4 ± 4.5a | 93.4 ± 0.0a | 18.5 ± 1.5b |
| IVC−/PW+ | 15.0 ± 1.1a | 85.0 ± 1.1a | 57.8 ± 1.2a | 42.2 ± 2.2b | 168.0 ± 5.0a | 94.5 ± 1.0a | 13.5 ± 0.2a |
| IVC−/PW − | 35.9 ± 2.4b | 64.2 ± 1.1a | 48.1 ± 3.2b | 51.9 ± 2.3b | 158.0 ± 3.8a | 88.2 ± 2.0a | 20.6 ± 0.5b |
1 : Percentage of Live cells/TCN
2 : Percentage of TUNEL-positive/TCN cells nuclei
Different letters within a column are significantly different between treatments (P < 0.05)
Within βME supplemented groups, IVC+/PW+ induced the best cryoprotection protection. However, there was a trend for better survival, re-expansion, and also quality of embryos cultured in IVC−/PW+ vs. IVC+/PW− culture conditions. Accordingly, the percentage of DFI within IVC−/PW+ embryos (13.5 ± 0.2%) was significantly lower than IVC+/PW− embryos (18.5 ± 1.5%).
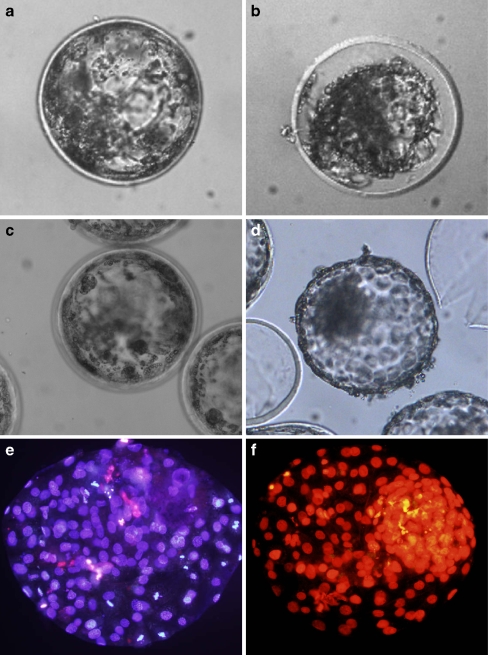
Figure 2 represent blastocyst morphology form a fully expanded blastocysts before vitrification (A), during vitrification process (B), after warming and recovery (C) and completely recovered and hatched after culture for 12 h (D), respectively. Figure 2 also represents pictures from frozen-thawed viable stained (E) and TUNEL assessed fragmented DNA (F) embryos.
Fig. 2.
Bovine blastocyst morphology before and after vitrification and viability assay of embryonic cells in fully recovered blastocysts. a Day 8 bovine blastocysts produced in vitro. b Collapsed blastocyst during vitrification procedure. c Vitrified/warmed blastocysts after 12 h (c) of culture. d A fully recovered hatched blastocyst. e Differential viable staining of the survived hatched blastocysts with viable (blue) and dead (red) cells. e Tunnel assessment of the hatched blastocyst indicating apoptotic (yellow color) within the total cells (red) of the blastocyst
Effect of βME on developmental competence of vitrified vs. non-vitrified embryos
In Table 3 the results of quality assessment of non-vitrified (Table 1) vs. vitrified (Table 2) blastocysts which have been cultured under the same conditions were paired and compared. The levels of difference (LD) between the values of each comparison were also compared (based on statistical analysis of the replicates data). As shown, vitrified-warmed IVP embryos had lower developmental competence compared to their corresponding non-vitrified embryos at all comparisons. However, these differences were not significant except for TCN, percentages of DFI and hatching of ICV+/PW+ vs. IVC+ +, IVC+/PW − vs. IVC+ − and IVC+/PW − vs. IVC+ −, respectively. Moreover, the levels of difference (LD) of different comparisons were not significantly different, except for LD-values of hatching and DNA-fragmentation IVC+/PW− vs. IVC+ − comparison (25.0 ± 0.6% and 9.3 ± 0.2%) and TCN LD-value of IVC+/PW+ vs. IVC+ + (77.6 ± 0.3) which were significantly greater than other comparisons.
Table 3.
Comparison on different cryosurvival parameters of vitrified vs. non vitrified embryos cultured at the same conditions of βME supplementation
| Parameters | Comparisons | |||||||
|---|---|---|---|---|---|---|---|---|
| IVC+/PW+vs. IVC+ + | IVC+/PW −vs. IVC+ − | IVC−/PW+vs. IVC− + | IVC−/PW −vs. IVC− − | |||||
| Values | LD# | Values | LD | Values | LD | Values | LD | |
| Hatching (%) | 74.3 ± 2.0 vs. 83.3 ± 3.6 | 9.0 ± 1.6 a | *50.0 ± 3.4 vs. 75.0 ± 4.0 | 25.0 ± 0.6 b | 57.8 ± 4.2 vs. 61.1 ± 4.5 | 3.3 ± 0.3 a | 31.4 ± 3.3 vs. 38.9 ± 3.3 | 7.5 ± 0.0 a |
| TCN (n) | *170.4 ± 4.2 vs. 248.0 ± 4.5 | 77.6 ± 0.3 a | 160.4 ± 4.5 vs. 170.6 ± 5.5 | 10.0 ± 1.0 a | 168.0 ± 5.0 vs. 168.4 ± 3.8 | 0.4 ± 1.2 a | 158.0 ± 3.8 vs. 163.7 ± 5.8 | 5.7 ± 2.0 a |
| Viability (%) | 95.2 ± 1.0 vs. 96.3 ± 0.3 | 0.1 ± 0.3 a | 93.4 ± 0.0 vs. 94.0 ± 1.0 | 0.6 ± 1.0 a | 94.5 ± 1.0 vs. 93.5 ± 0.0 | 1.0 ± 1.0 a | 88 ± 2.0 vs. 90.8 ± 1.0 | 2.8 ± 1.0 a |
| DNA-fragmentation(%) | 10.0 ± 1.0 vs. 6.1 ± 0.0 | 5.9 ± 1.0 a | *18.5 ± 0.5 vs. 9.2 ± 0.3 | 9.3 ± 0.2 b | 13.5 ± 0.2 vs. 10.4 ± 0.2 | 3.1 ± 0.0 a | 20.6 ± 0.5 vs.15.1 ± 0.9 | 5.5 ± 0.4 a |
# Level of difference between the two values compared
*Indicates significant difference between non-vitrified vs. Vitrified values of a given comparison (P < 0.05)
In each row, comparisons with different letters are significantly different (P < 0.05)
Discussion
Pathological levels of ROS have been implicated in impaired development of in vitro mammalian embryos [13, 22]. Following cryopreservation, oocytes and embryos become especially more sensitive to the oxidative stress, resulting in lipid peroxidation, membrane injury and structural destruction [3, 23, 24]. Low molecular-weight thiol compounds, such as βME, maintain the redox state of cells and protect them against the harmful effects of oxidative injuries and a number of studies have indicated the promoting effects of βME during in vitro embryo production [14–18]. However, data on the effect of βME are conflicting or inconsistent regarding favorable concentration of βME used in culture medium, and regarding the stage of IVP in which βME supplementation better supports embryo development. For example, Feugang et al [23] demonstrated no protective effect of βME on embryo development when added at 50 µM. In contrast, Geshi et al. [16] showed that 10 µM βME in a co-culture system improves embryo development and Cammano et al. [15] did not observed any difference between a low (10 µM) or high (100 µM) concentration of βME in development of resulting embryos. In addition, Geshi et al. [16], Caamano et al. [14] and Feuagang et al. [23] reported better promoting effect of βME when added at 4–8 cell, 8–16 cell and beyond the morula stages, respectively. Therefore, it seems that the apparent differences among studies could be attributed to the culture conditions or developmental stage in which βME has been added.
In this study, 100 µM βME was chosen because it was demonstrated to be the best concentration to promote pre-implantation embryo development [15, 24]. Furthermore, due to increased inherent structural fragility of embryos after cryopreservation, the highest recommended concentration of βME (100 µM) was used.
The results of this study indicated that βME supplementation of IVC medium, either during days 1–8 and/or 9–10 of embryo culture, promotes overall developmental competence and quality of bovine IVP embryos as measured by the proportion of blastocyst formation, hatching, TCN, cell viability, and apoptosis. However, these effects were more evident and became significant when IVC+ + and IVC− − derived embryos were compared (p < 0.05) (Table 1). Moreover, cryosurvival analysis indicated that supplementing culture medium with βME, either during embryo development and/or after vitrification-warming, not only enhances overall ability of the blastocysts to survive cryopreservation, but also significantly decreases the ratios of survival, hatching and apoptosis of the embryos cultured in IVC+/ PW+vs. IVC−/PW − culture condition (p < 0.05) (Table 2).
Within non-vitrified embryos, the best supplementing effect was found when βME was added throughout the culture period. However, hatching rate and quality of IVC+ − and IVC− + embryos were not significantly different. Therefore, it seems that although early inclusion of βME significantly increases the chance of embryos to develop to blastocyst, final quality of blastocysts is influenced by the stage of βME addition (p < 0.05) (Table 1).
Within vitrified embryos, the best cryoprotective effect was also found when βME was added throughout the culture period. Cryosurvival and the overall trend of embryo development in embryos supplemented with βME following vitrification-warming was better than embryos supplemented before cryopreservation. However, this difference became significant only regarding to DNA-fragmentation index of IVC−/PW+vs. IVC+/PW − embryos (p < 0.05) (Table 2). Therefore, it seems that while developmental competence and quality of embryos may not mainly differ regarding the stage of antioxidant supplementing, cryo-withstand of these embryos would be more in favor if βME was added during the critical period of post-warming embryo culture. Furthermore, by looking at Tables 1 and 2, it can be stated that although exogenous antioxidant increase the resistance of embryos to reactive oxygen species (ROS) and may increase the chance of embryos to develop to blastocyst, antioxidant inclusion during in vitro embryo development is not sufficient for preventing DNA-fragmentation during the critical period of post-warming embryo culture, and hence, there appears to be a need to surplus source of exogenous antioxidant during post-warming embryo culture.
The mechanism of action through which βME exerts its effect on embryos is not yet completely understood. Literatures reveal that although βME can directly interacts with some oxidized radicals and can chelate metallic ions, its main action is to protect oxidation of cysteine, a precursor of GSH, into cystine and increase its entry into the cell, which is known to trigger GSH synthesis [12, 14, 15]. GSH is a tripeptide (gamma-glutamylcysteinylglycine) and acts as a major antioxidant in the elimination of ROS and maintaining the intracellular redox state [25]. It is noteworthy that in agreement with the suggestion of Hamano et al. [26] that 2-cell bovine embryos respond poorly to the addition of βME compared with 8- and 16-cell embryos, Caamano et al. [15] observed that 8- to 16-cell bovine embryos are more capable of utilizing this source of cysteine to synthesize GSH than other less-developed embryos. It is of interest to note that during the present study, the beneficial effects of βME was observed at 8–16 cell stage and not at the cleavage stage (Table 1).
Apoptosis is a type of programmed cell death characterized phenotypically by cell shrinkage, chromatin compaction, plasma-membrane blebbing, cytoplasmic vacuolization, and collapse of the cell into small intact fragments that are removed by phagocytes [13, 17, 23]. The results indicate that addition of βME reduced DNA-fragmentation especially when βME was added throughout the culture period (Table 1). These results are also consistent with the suggestion of Feugang et al. [23] that addition of exogenous antioxidant such as βME plays a critical role in increasing the resistance of vitrified embryos to oxidative stress, which in turn could reduce DNA-fragmentation. However, quality assay of vitrified and non-vitrified blastocysts in this study (Tables 1 and 2) implicates that although the quality of non-vitrified embryos is not critically dependent on the stage of antioxidant supplementing, addition of βME after freezing/ warming have had a critical role in increasing the resistance of embryos to oxidative stress, which in turn have reduced the number of DNA-fragmented cells (Table 2). Therefore, it seems that the right time of antioxidant supplementation may differ for non-vitrified and vitrified embryos and presence of antioxidant at the same time of ROS production may work better than pre-enrichment of the embryo redox state. Furthermore, regarding to the great potential of co-culture systems to support embryos against different toxic factors [19], it seems that establishment of vero cells co-culture system in the present study has had a role in decreasing deleterious effects of ROS which developed during vitrification procedure. However, to better discriminate the suggested protective value of βME from those reported for vero cells, these observations needs to be repeated in cell free culture media such as synthetic oviductal medium (SOF).
Comparison between the results of viability and apoptosis of IVC+ +vs. IVC− − (Table 1) and IVC+/ PW+vs. IVC−/PW − (Table 2) revealed that while the number of viable/dead confirms no significant difference between these groups, the number of apoptotic cells are significantly different in the same comparison (p < 0.05). This suggests that the assessment of viability on its own, is not a very suitable parameter for assessment of embryo development and cryosurvivability.
Comparison between different quality parameters of vitrified and non-vitrified blastocysts developed under the same culture conditions (Table 3) suggests that the vitrification procedure used in this study had no such a dramatic influence on the viability of embryos. However, there seen a significant drop in term of hatching and apoptosis rates of IVC+/PW −vs. IVC+ − blastocysts compared to all the other culture conditions (p < 0.05). Due to well-known correlation between the culture condition and the quality of the embryos following cryopreservation [6], it seems that addition of βME antioxidant during early stage of in vitro embryo development but not after freezing/warming has increased the chance of more embryos, even those of fair-quality, to develop to the blastocyst stage. However, following stressful process of freezing, some of these fair-quality embryos did not survive and fail to hatch and hence degenerate.
Currently, direct embryo transfer of vitrified-warmed embryos, without further in vitro culture, has become the method of choice, due to its simplicity and cost effectiveness, for not only human ART specialists [27, 28] but also for commercial livestock industry [29]. However, the viability of these vitrified-warmed embryos cannot be completely assessed prior to embryo transfer because the limited time between warming and embryo transfer procedures. The results of this study indicates that although pre-enrichment of embryo culture medium with antioxidant may increase the overall chance of embryos to survive cryopreservation, post-warming culture period is the critical stage for a) supporting survived embryos against harmful effects of cryopreservation and b) selection of the best survived embryos to be used for embryo transfer.
In conclusion, this study indicates that supplementation of exogenous antioxidants such as βME plays a critical role in increasing the resistance of embryos to reactive oxygen species (ROS) and their effect would be maximized if added throughout the culture period. However, antioxidant inclusion during in vitro embryo development is not sufficient for ROS detoxification during the critical period of post-warming embryo culture and, hence; there appears to be a need for a surplus source of exogenous antioxidant during post- warming embryo culture. From these results, further studies are needed to investigate the exact mechanism(s) by which βME and other antioxidants protect the cells and also to investigate the post implantation embryo developmental competence of these IVP vitrified or non-vitrified embryos.
Acknowledgments
This study was funded by the grant of Royan Institute of I.R.I. The authors would like to thank Dr. H. Gurabi and Dr. A. Vosough for their full support.
References
- 1.Whittingham DG, Leibo SP, Mazur P. Survival of mouse embryos frozen to −196 and −269°C. Science. 1972;178:411–4. doi:10.1126/science.178.4059.411. [DOI] [PubMed]
- 2.Donnay I, Auquier P, Kaidi S, Carolan C, Lonergan P, Mermillod P, et al. Vitrification of in vitro produced bovine blastocysts: methodological studies and developmental capacity. Anim Reprod Sci. 1998;21:93–104. doi:10.1016/S0378-4320(98)00098-0. [DOI] [PubMed]
- 3.Martínez AG, Valcárcel A, de las Heras MA, de Matos DG, Furnus C, Brogliatti G. Vitrification of in vitro produced bovine embryos: in vitro and in vivo evaluations. Anim Reprod Sci. 2002;16:11–21. doi:10.1016/S0378-4320(02)00121-5. [DOI] [PubMed]
- 4.Lane M, Maybach JM, Gardner DK. Addition of ascorbate during cryopreservation stimulates subsequent embryo development. Hum Reprod. 2002;17:2686–93. doi:10.1093/humrep/17.10.2686. [DOI] [PubMed]
- 5.Massip A, Mermillod P, Van Langendonckt A, Touze JL, Dessy F. Survival and viability of fresh and frozen-thawed in vitro bovine blastocysts. Reprod Nutr Dev. 1995;35(1):3–10. [DOI] [PubMed]
- 6.Kaidi S, Donnay I, Van Langendonckt A, Dessy F, Massip A. Comparison of two co-culture systems to assess the survival of in vitro produced bovine blastocysts after vitrification. Anim Reprod Sci. 1998;30:39–50. doi:10.1016/S0378-4320(98)00089-X. [DOI] [PubMed]
- 7.Lonergan P, Rizos D, Kanka J, Nemcova L, Mbaye AM, Kingston M, et al. Temporal sensitivity of bovine embryos to culture environment after fertilization and the implications for blastocyst quality. Reproduction. 2003;126:337–46. doi:10.1530/rep. 0.1260337. [DOI] [PubMed]
- 8.Gomez E, Muñoz M, Rodríguez A, Caamaño JN, Facal N, Díez C. Vitrification of bovine blastocysts produced in vitro inflicts selective damage to the inner cell mass. Reprod Domest Anim 2008 [Epub ahead of print] [DOI] [PubMed]
- 9.Vajta G, Rindom N, Peura TT, Holm P, Greve T, Callesen H. The effect of media, serum and temperature on in vitro survival of bovine blastocysts after Open Pulled Straw (OPS) vitrification. Theriogenology. 1999;52:939–48. doi:10.1016/S0093-691X(99)00184-3. [DOI] [PubMed]
- 10.Rizos D, Ward F, Boland MP, Lonergan P. Effect of culture system on the yield and quality of bovine blastocysts as assessed by survival after vitrification. Theriogenology. 2001;56:1–16. doi:10.1016/S0093-691X(01)00538-6. [DOI] [PubMed]
- 11.Johnson MH, Nasr-Esfahani MH. Radical solutions and cultural problems: could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays. 1994;16:31–8. doi:10.1002/bies.950160105. [DOI] [PubMed]
- 12.Ali AA, Bilodeau JF, Sirard MA. Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology. 2003;59:939–49. doi:10.1016/S0093-691X(02)01125-1. [DOI] [PubMed]
- 13.Kitagawaa Y, Suzukib K, Yonedaa A, Watanabea T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology. 2004;62:1186–97. doi:10.1016/j.theriogenology.2004.01.011. [DOI] [PubMed]
- 14.Caamaño JN, Ryoo ZY, Thomas JA, Youngs CR. Beta-mercaptoethanol enhances blastocyst formation rate of bovine in vitro-matured/in vitro-fertilized embryos. Biol Reprod. 1996;55:1179–84. doi:10.1095/biolreprod55.5.1179. [DOI] [PubMed]
- 15.Caamaño JN, Ryoo ZY, Youngs CR. Promotion of development of bovine embryos produced in vitro by addition of cysteine and beta-mercaptoethanol to a chemically defined culture system. J Dairy Sci. 1998;81:369–74. [DOI] [PubMed]
- 16.Geshi M, Yonai M, Sakaguchi M, Nagai T. Improvement of in vitro co-culture systems for bovine embryos using a low concentration of carbon dioxide and medium supplemented with beta-mercaptoethanol. Theriogenology. 1999;51:551–8. doi:10.1016/S0093-691X(99)00009-6. [DOI] [PubMed]
- 17.Mori M, Otoi T, Wongsrikeao P, Agung B, Nagai T. Effects of beta-mercaptoethanol and cycloheximide on survival and DNA damage of bovine embryos stored at 4 degrees C for 72 h. Theriogenology. 2006;5:1322–32. doi:10.1016/j.theriogenology.2005.07.018. [DOI] [PubMed]
- 18.Takahashi M, Nagai T, Okamura N, Takahashi H, Okano A. Promoting effect of beta-mercaptoethanol on in vitro development under oxidative stress and cystine uptake of bovine embryos. Biol Reprod. 2002;66:562–267. doi:10.1095/biolreprod66.3.562. [DOI] [PubMed]
- 19.Moulavi F, Hosseini SM, Ashtiani SK, Shahverdi A, Nasr-Esfahani MH. Can Vero cell co-culture improve in-vitro maturation of bovine oocytes? Reprod Biomed Online. 2006;13:404–11. [DOI] [PubMed]
- 20.Parrish JJ, Susko-Parrish JL, Leibfried-Rutledge ML, Critser ES, Eyestone WH, First NL. Bovine in vitro fertilization with frozen-thawed semen. Theriogenology. 1986;25:591–600. doi:10.1016/0093-691X(86)90143-3. [DOI] [PubMed]
- 21.Gómez E, Rodríguez A, Muñoz M, Caamaño J, Hidalgo C, Morán E, et al. Serum free embryo culture medium improves in vitro survival of bovine blastocysts to vitrification. Theriogenology. 2003;69:1013–21. doi:10.1016/j.theriogenology.2007.12.015. [DOI] [PubMed]
- 22.Nasr-Esfahani MH, Aitken JR, Johnson MH. Hydrogen peroxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo. Development. 1990;109:501–7. [DOI] [PubMed]
- 23.Feugang JM, de Roover R, Moen A, Léonard S, Dessy F, Donnay I. Addition of beta-mercaptoethanol or Trolox at the morula/blastocyst stage improves the quality of bovine blastocysts and prevents induction of apoptosis and degeneration by prooxidant agents. Theriogenology. 2004;61:71–90. doi:10.1016/S0093-691X(03)00191-2. [DOI] [PubMed]
- 24.Nedambale TL, Du F, Yang X, Tian XC. Higher survival rate of vitrified and thawed in vitro produced bovine blastocysts following culture in defined medium supplemented with beta-mercaptoethanol. Anim Reprod Sci. 2006;93:61–75. doi:10.1016/j.anireprosci.2005.06.027. [DOI] [PubMed]
- 25.de Matos DG, Furnus CC, Moses DF, Martinez AG, Matkovic M. Stimulation of glutathione synthesis of in vitro matured bovine oocytes and its effect on embryo development and freezability. Mol Reprod Dev. 1996;45:451–7. doi:10.1002/(SICI)1098-2795(199612) 45:4<451::AID-MRD7>3.0.CO;2-Q. [DOI] [PubMed]
- 26.Hamano SM, Kuwayama M, Takahashi N, Okamura A, Okano Nagai T. Effect of β-mercaptoethanol on the preimplantation development of bovine embryos fertilized in vitro. J Reprod Dev. 1994;40:355–9.
- 27.Saito H, Ishida GM, Kaneko T, Kawachiya S, Ohta N, Takahashi T, et al. Application of vitrification to human embryo freezing. Gynecol Obstet Invest. 2000;49:145–9. doi:10.1159/000010236. [DOI] [PubMed]
- 28.Lai AC, Lin BP, Chang CC, Tsai HD, Hwang VW, Lo HY. Pregnancies after transfer of ultrarapidly frozen human embryos. J Assist Reprod Genet. 1996;13:625–8. doi:10.1007/BF02069640. [DOI] [PubMed]
- 29.Dochi O, Yamamoto Y, Saga H, Yoshiba N, Kano N, Maeda J, et al. Direct transfer of bovine embryos frozen-thawed in the presence of propylene glycol or ethylene glycol under on-farm conditions in an integrated embryo transfer program. Theriogenology. 1998;49:1051–8. doi:10.1016/S0093-691X(98)00053-3. [DOI] [PubMed]