Abstract
Purpose
To evaluate the predictive value of basal serum anti-müllerian hormone level and small antral follicle count for high ovarian response to controlled ovarian hyperstimulation.
Methods
A total of 159 patients were prospectively included. Basal serum anti-müllerian hormone and small antral follicle count (2–6 mm) were measured.
Results
Small antral follicle count and anti-müllerian hormone have similar predictive accuracy for high ovarian response with area under curve of 0.961 and 0.922, respectively. The sensitivity and specificity for prediction of high ovarian response were 89% and 92% for small antral follicle count and 93% and 78% for anti-müllerian hormone at the cutoff values of ≥ 16 and ≥ 34.5 pmol/l, respectively.
Conclusions
Small antral follicle count and anti-müllerian hormone are equally accurate predictors of high ovarian response and facilitate determination of the optimal strategy for controlled ovarian hyperstimulation.
Keywords: Anti-müllerian hormone, Embryo quality, High ovarian response, Ovarian hyperstimulation syndrome, Small antral follicle count
Introduction
Prediction of high ovarian response is still a great challenge in assisted reproduction technology (ART). There is a trend toward individualized treatment to decrease complication, patients discomfort, and cost in modern ART. Ovarian hyperstimulation syndrome (OHSS) is a serious and life-threatening iatrogenic complication of controlled ovarian hyperstimulation (COH). It is necessary to identify the patients who are at risk to OHSS and use modified strategies for stimulation, such as GnRH-antagonist regimens and mild stimulation protocols.
A variety of endocrine and ultrasound markers have been assessed for predicting ovarian response and in vitro fertilization (IVF) outcome.
Anti-müllerian hormone (AMH), a member of transforming growth factor β family, is produced in the granulosa cells of preantral and small antral follicles [1]. It has been reported that the highest level of expression of AMH in human is in the antral follicles up 4 mm in diameter and that levels decline as antral follicles increase in size [2]. AMH has an inhibitory effect on follicles recruitment and decreases the sensitivity of follicles to FSH [3, 4], therefore, it may have a regulatory role in follicular development. Furthermore, serum AMH levels have been shown strongly correlate with the number of antral follicles [5, 6]. Serum level of AMH is relatively stable throughout the menstrual cycle [7–10]. It has been demonstrated that AMH is an accurate predictor of ovarian response to COH in ART cycles [11–13]. Clinically, AMH-based approach to COH is a recent strategy and may result in optimized treatment burden and minimization of the risk of OHSS and increased cost-effectiveness [14].
Ultrasound evaluation of the ovaries, early in the menstrual cycle is one of the best diagnostic tools for prediction of ovarian responsiveness. Sonographic parameters such as antral follicle count (AFC) and ovarian volume are important markers of ovarian response [15], and we can predict the ovarian response of patients undergoing IVF/intracytoplasmic sperm injection (ICSI) with the use of this simple procedure. For clinical purpose, some investigators counted the total number of antral follicles with a diameter of 2–10 mm [16], and the others determined the number of antral follicles with a diameter of 2–5 mm [17]. Small antral follicles produce AMH, therefore, they may be better predictors of ovarian reserve and ovarian response than total AFC [18]. Based on these evidence, we preferred to determine the number of small antral follicles with a diameter of 2–6 mm for prediction of high ovarian response in our study.
We designed this prospective cohort study to compare the value of basal serum AMH and small AFC measurements in the prediction of high ovarian response to COH in ART cycles. In addition, the ability of small AFC and AMH for prediction of day two embryo quality was evaluated in this study.
Materials & methods
Patients
The study was performed at our university-based assisted reproduction center between January 1, and December 31, 2008.
A total of 159 patients undergoing their first IVF cycle, were prospectively recruited for the study. To be included in this study, the women had to be <38 years old, have both ovaries and day 3 FSH < 10, and no history of ovarian surgery, chemotherapy, pelvic radiation, and current hormonal therapy. On the day of 2–3 of the spontaneous menstrual cycle before initiating treatment with a long GnRH agonist protocol in the mid-luteal phase of the same cycle, venipuncture for assay of AMH, FSH, estradiol (E2), and transvaginal ultrasound scan were performed. The exclusion criteria was ovarian cyst > 10 mm on basal ultrasound scans. Patients who had poor ovarian response to COH in current study were also excluded. Poor ovarian response was considered when three or fewer follicles with a mean diameter of 16 mm were achieved and/or serum E2 level measured on the day of hCG administration was ≤500 pg/ml, and/or three or fewer oocyted were retrieved.
This study was approved by ethics committee of Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Science. All patients were required to sign a written informed consent before initiation of the treatment cycles.
Assays
All Transvaginal ultrasonographic evaluations were performed by a single investigator, blinded to results of any hormonal assays, using a conventional two-dimensional ultrasound (HS-4000, Honda, Japan) equipped with a 7.5-MHz vaginal transducer. The total number of 2–6 mm antral follicles in both ovaries was used for calculation. Intraanalysis coefficient of variation for follicular diameter measurements was < 5%, and the lower limit of detection was 2 mm.
Blood samples were collected from each patients and immediately centrifuged to separate the serum. FSH and E2 assays were performed and the other part of serum samples were frozen at−20°C and stored until sufficient samples were available for AMH assays. FSH concentrations were measured by competitive immunoassay (IDCS, Korbach, Germany), intra-assay and inter-assay coefficients of variation were 6% and 6.8% respectively. E2 concentrations were measured using an enzyme-immunoassay kit (DRG, Marburg, Germany), intra-assay and inter-assay coefficients of variation were 6.3% and 6.4% respectively. Measurement of serum AMH levels was performed using AMH/MIS enzyme-linked immunosorbent assay kit (Beckman Coulter Immunotech Com., Fullerton, CA). The lowest detection rate limit and intra-assay and inter-assay coefficients of variation were 1 pmol/l or 0.14 ng/mL, 12.3%, and 14.2%, respectively.
Treatment protocol
All patients in the initial cohort were treated with a long protocol for ovarian stimulation. For pituitary suppression, patients were treated with daily administration of 0.5 mg buserelin (suprefact, Aventis, Frankfurt, Germany), started in the luteal phase of menstrual cycle. When ovaries were quiescent on ultrasound, buserelin was reduced to 0.25 mg and continued until the day of hCG administration. The COH was initiated with recombinant FSH (Gonal F, Serono, Aubnne, Switzerland) 150 IU/day on the day 2 of menstrual cycle. Based on clinic-specific protocol, patients >35 years old received recombinant FSH 225 IU/day. Ovarian response was monitored by serial ultrasound examinations and evaluation of serum E2 levels, and then gonadotropin dose adjustments were done as required. Human chorionic gonadotropin (pregnyl, Organon, Oss, the Netherlands) 10,000 IU was administered when at least two follicles reached a mean diameter of 18 mm. Cycle cancellation was considered when fewer than two follicles with normal growth pattern were noted.
Oocyte retrieval was performed 34–36 h after hCG administration and conventional insemination or ICSI was performed as clinically appropriate. Embryos were transferred on day 2 or 3 under ultrasound guidance, with a C.C.D. embryo transfer catheter (Laboratory C.C.D., Paris, France). According to local criteria, in patients who were high risk for OHSS, all embryos were cryopreserved. Luteal support with progesterone in oil (Progesterone, Aburaihan Co., Tehran, Iran) 100 mg daily IM was started on the day of oocyte retrieval.
Serum β-hCG level was measured 14 days after embryo transfer and a transvaginal ultrasonography was performed 3 weeks later for documentation of gestational sacs and fetal viability.
Outcome measures
For statistical analysis, we assessed two outcome measures. The first one was high ovarian response, according to local criteria, defined as the presence of ≥ 15 follicles with a mean diameter ≥ 12 mm per each ovary at the end of the follicular phase of COH, and/or E2 levels on the day of hCG administration >3,000 pg/mL, and/or > 15 oocytes retrieved and/or cycle cancellation on the day of hCG, and/or cryopreservation of all embryos because of high risk of OHSS.
The second outcome measure was embryo quality, based on embryo morphology on day 2. Two embryologists evaluated the embryo morphology, embryos with 4–6 evenly sized blastomers on day 2 with ≤ 20% fragmentation and no multinucleation were classified as top-quality embryos. Embryos with 2–6 even or uneven blastomers with ≤ 20% fragmentation and no multinucleation were classified as good-quality embryos.
Statistical analysis
The parameters relevant to demographic and COH characteristics were presented as mean ± SD and range. The Statistical Package for Social Science (SPSS, version 15.0 for windows; SPSS Inc., Chicago. IL) was used for data analysis. Normality was evaluated with the Kolmogorov-Smirnov test. Student’s t-test and Chi-square test were used for analysis as appropriate. Logistic regression was performed to determine the independent effect of age, body mass index (BMI), basal FSH, E2, AMH, and AFC on the ability to predict high ovarian response and embryo quality. Receiver operating characteristics (ROC) curve analysis was used to estimate the predictive accuracy of the variables. The ability of any predictors was compared by calculating the areas under the ROC curves (ROCAUC) and their 95% confidence intervals (CIs). Areas under the ROC curves were compared using the MEDCALC software package (version 9.20; MedCalc Software, Mariakerke, Belgium). The sensitivity, specificity, positive, and negative predictive values were calculated for selected cutoff levels.
A P value of less than 0.05 was considered statistically significant.
Results
Patients were divided into three groups, based on their ovarian response to COH. Of the 159 participants, 16 were defined as poor responders and excluded, 98 as normal responders, and 45 as high responders.
The basal patients demographic and IVF cycle characteristics are shown in Table 1. There was no significant difference among the normal responders and high responders regarding to woman’s age (28.6 ± 4 vs. 27.5 ± 3.6 years), BMI (25.1 ± 2.3 vs. 24.5 ± 2.8 Kg/m²), basal FSH (5.3 ± 1.4 vs. 5 ± 0.7 mIU/mL), and E2 (46.8 ± 11.1 vs. 45.9 ± 8.6 pg/mL). Infertility etiology distribution did not differ between groups (data not shown). Basal AMH (25.1 ± 9.7 vs. 54.7 ± 24.8 pmol/l) levels, small AFC (13.1 ± 2.9 vs. 21.2 ± 5.7 2–6 mm AFC/both ovaries), the E2 levels on the day of hCG administration(1,557.3 ± 651.2 vs. 3,451.7 ± 728.1 ), and the number of retrieved oocytes (8.1 ± 2.9 vs. 17.7 ± 3.3) were significantly higher in high responders. There was no statistically significant difference among the groups regarding the percentage of patients who had top- and good-quality embryos (60.2% vs. 73%).
Table 1.
Patients and IVF cycles characteristics
| Variable | Normal responders (n = 98) | High responders (n = 45) | P value |
|---|---|---|---|
| Age(years) | 28.6 ± 4(21–37) | 27.5 ± 3.6(18–34) | 0.96 |
| BMI (Kg/m²) | 25.1 ± 2.3(19.9–31.1) | 24.5 ± 2.8(19–31) | 0.184 |
| Basal FSH (mIU/mL) | 5.36 ± 1.4(3–8.9) | 5.05 ± 0.7(3.5–6.8) | 0.163 |
| Basal E2 (pg/mL) | 46.8 ± 11.1(27–67) | 45.9 ± 8.6(29–65) | 0.624 |
| Basal AMH (pmol/l) | 25.1 ± 9.7 (10–54) | 54.7 ± 24.8(22.14–121.5) | 0.000 |
| Small AFC (n) | 13.1 ± 2.9 (6–20) | 21.2 ± 5.7(16–36) | 0.000 |
| Oocyte retrieved (n) | 8.1 ± 2.9(4–14) | 17.7 ± 3.3(11–25) | 0.000 |
| E2on day hCG (pg/mL) | 1557.3 ± 651.2(630–2850) | 3451.7 ± 728.1(2,180–5,100) | 0.000 |
| Patients with top- & good-quality embryos (%) | 60.2% | 73% | 0.138 |
Values are presented as mean ± SD (range).
Multiple logistic regression analysis of age, BMI, basal FSH, E2, AMH, and small AFC demonstrated that small AFC and AMH, both were significant predictors of high ovarian response. Table 2 shows the correlation between variables and high ovarian response by multiple logistic regression analysis.
Table 2.
Predictive value of variables for high ovarian response by multiple logistic regression analysis
| Variable | Coefficient | Odds ratio | 95% confidence interval | P |
|---|---|---|---|---|
| Age | 0.178 | 1.194 | 0.911–1.565 | 0.198 |
| BMI | 0.003 | 1.003 | 0.721–1.395 | 0.986 |
| Basal FSH | 0.440 | 1.553 | 0.551–4.375 | 0.405 |
| Basal E2 | −0.019 | 0.981 | 0.891–1.081 | 0.702 |
| AMH | −0.178 | 0.837 | 0.755–0.928 | 0.001 |
| Small AFC | −1.125 | 0.325 | 0.178–0.591 | 0.000 |
The ROC curve analysis for the predicting factors for high ovarian response were performed and the results are presented in Table 3.
Table 3.
Comparison of performance of variables for high ovarian response by ROC curve analysis
| Variable | AUC | Cutoff value | Sensitivity(%) | Specificity(%) | PPV | NPP |
|---|---|---|---|---|---|---|
| Age(y) | 0.409(0.312–0.506) | 26.5 | 58 | 30 | 0.39 | 0.72 |
| BMI(Kg/m²) | 0.468(0.362–0.574) | 24.1 | 67 | 42 | 0.25 | 0.64 |
| Basal FSH(mIU/mL) | 0.385(0.294–0.475) | 5.05 | 51 | 36 | 0.37 | 0.72 |
| Basal E2(pg/mL) | 0.474(0.377–0.572) | 43.5 | 69 | 33 | 0.31 | 0.68 |
| AMH(pmol/l) | 0.922(0.876–0.968) | 34.5 | 93 | 78 | 0.65 | 0.96 |
| Small AFC(n) | 0.961(0.933–0.989) | 16 | 89 | 92 | 0.83 | 0.94 |
PPV positive predictive value, NPP negative predictive value
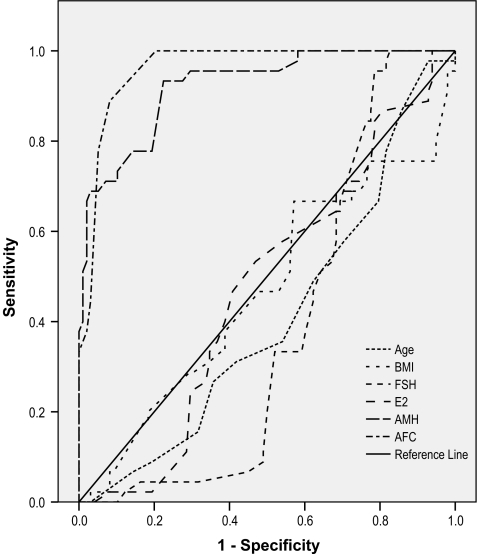
Figure 1 shows the ROC curve for the predicting factors for high ovarian response. The ROC curve analysis showed that small AFC and AMH were equally predictive of high ovarian response, as demonstrated by a similar area under the curve (AUC) of 0.961 and 0.922, respectively.
Fig. 1.
Comparison of predictive values for ovarian high response using the ROC curve analysis
The sensitivity, specificity, positive and negative predictive values for prediction of high ovarian response at optimum cutoff levels of variables are shown in Table 3. The sensitivity and specificity for prediction of high ovarian response were 89 % and 92 % for small AFC and 93% and 78% for AMH at the cutoff values of ≥ 16 and ≥ 34.5 pmol/l, respectively.
Multiple logistic regression analysis of all variables demonstrated that AMH, small AFC and FSH were significant predictors of day 2 embryo quality (Table 4).
Table 4.
Predictive value of variables for embryo quality by multiple logistic regression analysis
| Variable | Coefficient | Odds ratio | 95% confidence interval | P |
|---|---|---|---|---|
| Age | 0.005 | 1.005 | 0.903–1.118 | 0.927 |
| BMI | 0.064 | 1.066 | 0.906–1.254 | 0.440 |
| Basal FSH | 0.495 | 1.641 | 1.160–2.320 | 0.005 |
| Basal E2 | 0.030 | 1.030 | 0.991–1.071 | 0.129 |
| AMH | −0.070 | 0.932 | 0.897–0.969 | 0.000 |
| Small AFC | 0.090 | 1.094 | 1.006–1.191 | 0.036 |
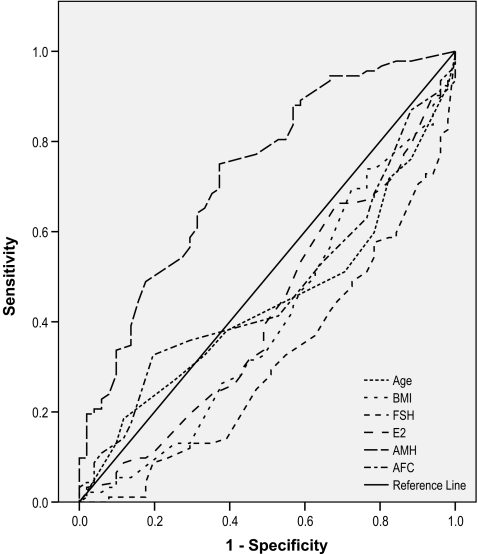
Figure 2 shows the ROC curve for the predicting factors for day 2 embryo quality. AMH was the most significant predictor of day 2 embryo quality, and had the largest area under curve (AUC= 0.728), although, its predictive accuracy was low.
Fig. 2.
Comparison of predictive values for embryo quality using the ROC curve analysis
The ROC curve analysis for the predicting factors for day 2 embryo quality, sensitivity, specificity, positive and negative predictive values for prediction of day2 embryo quality at optimum cutoff levels of variables are shown in Table 5.
Table 5.
Comparison of performance variables for embryo quality by ROC curve analysis
| Variable | AUC | Cutoff value | Sensitivity(%) | Specificity(%) | PPV | NPP |
|---|---|---|---|---|---|---|
| Age(y) | 0.439(0.344–0.533) | 28.5 | 45 | 45 | 0.68 | 0.40 |
| BMI(Kg/m²) | 0.405(0.307–0.503) | 24.1 | 58 | 33 | 0.69 | 0.39 |
| Basal FSH(mIU/mL) | 0.313(0.224–0.401) | 5.05 | 51 | 25 | 0.78 | 0.45 |
| Basal E2(pg/mL) | 0.422(0.325–0.520) | 44.5 | 66 | 31 | 0.65 | 0.36 |
| AMH(pmol/l) | 0.728(0.642–0.814) | 21 | 83 | 43 | 0.72 | 0.61 |
| Small AFC(n) | 0.473(0.377–0.569) | 14 | 53 | 35 | 0.58 | 0.29 |
PPV positive predictive value, NPP negative predictive value
Discussion
In this study, we investigated the value of small AFC and AMH as predictors for high ovarian response in young women < 38 years, with normal day 3 FSH. Prediction of high ovarian response to COH and individualization of treatment strategies for patients undergoing IVF reduce the incidence of OHSS, cancellation rate, and cost and also increase chance of pregnancy and clinical safety. Our results demonstrated that we could identify with reasonable accuracy high responders, using either small AFC or AMH measurement prior to COH (AUC= 0.961 and AUC= 0.925, respectively). Our results, along with other previous publications, validate small AFC and AMH as highly predictive of high ovarian response [19–22].
In current study, there was no significant difference between the accuracy of small AFC and AMH in prediction of high ovarian response (AUC= 0.961 and AUC= 0.922, respectively).
Assessment of AFC by use of ultrasound is easy, inexpensive, and feasible. It has been reported that AFC is the best single predictor of response to COH [23–27]. The number of antral follicles depends on the size of primordial follicle pool from which they are recruited. There are some evidence in the literature that the number of small antral follicles represents the functional ovarian reserve better [18], therefore, small AFC is superior to total AFC in predicting ovarian response. Small AFC is operator-dependent and has intercycle, inter-observer, and intraobserver variability [28, 29]. However, it was reported that intercycle variability of AFC appeared to be more significant in young ovulatory women than in infertile ones [30]. In some studies follicle counts were made using 3D ultrasound, which has been shown to provide more reliable and valid measurements [29, 31]. Several investigators reported that AFC was only a valid measurement of ovarian reserve and a good predictor of ovarian response, therefore, it could not predict oocyte/embryo quality or IVF outcome [32]. On the contrary, recently an investigation demonstrated that AFC was a significant predictor of live birth in IVF cycles and could be used as a prognostic factor for the outcome of ART cycles. [33]. In agreement with the former studies, our results showed that small AFC was not a significant predictor of embryo quality (AUC= 0.473) and did not have the value of prediction of IVF outcome.
In recent years, AMH has emerged as a useful, reliable, accurate, and reproducible predictor of response to COH and IVF outcome [19–22]. It has been suggested that AMH has the potential to replace FSH as the widely used test of ovarian reserve, with significantly better predictive accuracy [31]. The AMH results are not influenced significantly by menstrual cycle day, E2, contraceptives, or gonadotropin-releasing hormone agonists [34], but there are some reports that show AMH has limited intercycle variability [12]. AMH levels decreases after ovulation. Streuli et al. reported that the changes in AMH after ovulation were slight, yet statistically significant. The fluctuations observed were smaller than intercycle variability and, therefore, were not clinically relevant as far as AMH measurement for clinical purposes were concerned. They suggested that in daily practice, AMH could be measured any time during the menstrual cycle [35].
There are two AMH ELISA assays, DSL and Beckman Coulter. We used Beckman Coulter kit. It has been reported that AMH levels were nearly 4.6 fold lower with the DSL assay than with the Beckman Coulter [36]. The cutoff value for prediction of high ovarian response in our study was 34.5 pmol/l, that was higher than previous studies [19, 22]. This difference may reflect different AMH ELISA assays and the need for standardization of the assays in ART. Furthermore, it is necessary to define reliable cutoff values for prediction of ovarian response.
The main aim of our study was to assess the predictive value of small AFC and AMH for high ovarian response. Moreover, we evaluated the predictive value of small AFC and AMH for day 2 embryo quality as a prognostic factor for the outcome of IVF/ICSI cycles.
Our results showed that AMH was the most significant predictor of embryo quality (AUC =0.728). Determination of the probability of achieving good quality embryos is important because of its prognostic value. The AMH is a reliable marker of ovarian activity and follicular growth dynamics and may be the association between AMH and oocyte quality [37]. AFC is believed to represent only the quantitative aspect of ovarian response [38], in contrast, AMH may show some qualitative aspect of ovarian response.
Both AFC and AMH have some disadvantages. The AFC necessitates skilled ultrasound operators who carefully identify, measure, and count ovarian follicles. There is a moderate intercycle and interobsrever variability in AFC [28, 29]. On the other hand, in some country AMH assay is not routinely available. The availability of the AMH assay may present some problems but surely this test system will soon become part of one of the large automated platforms [21].
In conclusion, small AFC and AMH are equally accurate predictors of high ovarian response to COH and allow us to identify the patients who are at increased risk of OHSS prior the commencement of stimulation, and help to determine the appropriate treatment protocols. In modern ART, this approach should be a routine practice to attain safe and effective clinical outcomes. Further work remains to be done in standardizing these tests in ART cycles.
Acknowledgements
The authors are grateful to the nursing and laboratory staff of the Research and Clinical Center for Infertility for their assistance.
Footnotes
Capsule Small antral follicle count and anti-müllerian hormone are the most significant predictors of high ovarian response to stimulation with high degree of predictive ability.
Contributor Information
Abbas Aflatoonian, Phone: +98-351-8247085, FAX: +98-351-8247087, Email: abbas_aflatoonian@yahoo.com.
Homa Oskouian, Phone: +98-351-8247085, FAX: +98-351-8247087, Email: homaoskouian@gmail.com.
Shahnaz Ahmadi, Phone: +98-351-8247085, FAX: +98-351-8247087, Email: AHMADISHAHNAZ2005@yahoo.com.
Leila Oskouian, Phone: +98-351-8247085, FAX: +98-351-8247087, Email: leilaoskouian0695@gmail.com.
References
- 1.Lee MM, Donahoe PK, Hasegawa T, Silverman B, Crist GB, Best S, et al. Mullerian inhibiting substance in humans: normal levels from infancy to adulthood. J Clin Endocrinol Metab. 1996;81:571–6. doi:10.1210/jc.81.2.571. [DOI] [PubMed]
- 2.Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-mullerian hormone expression pattern in the human ovary: potential implication for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi:10.1093/molehr/gah015. [DOI] [PubMed]
- 3.Durlinger ALL, Gruijters MJG, Kramer P, Karles B, Kumer TR, Matzuk MM, et al. Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891–9. doi:10.1210/en.142.11.4891. [DOI] [PubMed]
- 4.Durlinger ALL, Gruijters MJG, Kramer P, Karles B, Ingraham HA, Nachtigal MW, et al. Anti-mullerian hormone inhibits initiation of primordial follicle growth in mouse ovary. Endocrinology. 2002;143:1076–84. doi:10.1210/en.143.3.1076. [DOI] [PubMed]
- 5.Gruijters MJG, Visser JA, Durlinger ALL, Themmen AP. Anti-mullerian hormone and its role in ovarian function. Mol Cell Endocrinol. 2003;211:85–90. doi:10.1016/j.mce.2003.09.024. [DOI] [PubMed]
- 6.van Rooji IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, Jong FH, et al. Serum anti-mullerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–71. doi:10.1093/humrep/17.12.3065. [DOI] [PubMed]
- 7.Hehenkamp WJ, Looman CW, Thememen AP, de Jong FH, te Velde ER, Broekmans FJ. Anti-mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057–63. doi:10.1210/jc.2006-0331. [DOI] [PubMed]
- 8.La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-mullerian hormone throughout the human menstrual cycle. Hum Reprod. 2006;21:3103–7. doi:10.1093/humrep/del291. [DOI] [PubMed]
- 9.Tsepelidis S, Devreker F, Demeestere I, Flahaut A, Gervy C, Englert Y. Stable serum levels of anti-mullerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22:1837–40. doi:10.1093/humrep/dem101. [DOI] [PubMed]
- 10.La Marca A, Giulini S, Tirelli A, Bertucci E, Marsella T, Xella S, et al. Anti-mullerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum Reprod. 2007;22:766–71. doi:10.1093/humrep/del421. [DOI] [PubMed]
- 11.Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum mullerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77:468–71. doi:10.1016/S0015-0282(01)03201-0. [DOI] [PubMed]
- 12.Fanchin R, Taieb J, Mendez Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005;20:923–7. doi:10.1093/humrep/deh688. [DOI] [PubMed]
- 13.Fleming R, Deshpande N, Traynor I, Yates RW. Dynamics of FSH-induced follicular growth in subfertile women: a relationship with age, insulin resistance, oocyte yield and anti-mullerian hormone. Hum Reprod. 2006;21:1436–41. doi:10.1093/humrep/dei499. [DOI] [PubMed]
- 14.Nelson SM, Yates RW, Lyall H, Jamieson M, Traynor I, Gaudoin M, et al. Anti-Mullerian hormone-based approach to controlled ovarian stimulation for assisted conception. Hum Reprod. 2009;24(4):867–75. [DOI] [PubMed]
- 15.Kline J, Kinney A, Kelly A, Reuss ML, Levin B. Predictors of antral follicle count during the reproductive years. Hum Reprod. 2005;20:2179–89. doi:10.1093/humrep/dei048. [DOI] [PubMed]
- 16.Frattarelli JL, Levi AJ, Miller BT, Segars JH. A prospective assessment of the predictive value of basal antral follicles in in vitro fertilization cycles. Fertil Steril. 2003;80:350–5. doi:10.1016/S0015-0282(03)00664-2. [DOI] [PubMed]
- 17.Chang MY, Chiang CH, Hsieh TT, Soong YK, Hsu KH. Use of antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril. 1998;69:505–10. doi:10.1016/S0015-0282(97)00557-8. [DOI] [PubMed]
- 18.Haadsma ML, Bukman A, Groen H, Roeloffzen EMA, Groenewoud ER, Heineman MJ, et al. The number of small antral follicles (2–6 mm) determines the outcome of endocrine ovarian reserve tests in a subfertile population. Hum Reprod. 2007;22:1925–31. doi:10.1093/humrep/dem081. [DOI] [PubMed]
- 19.Lee T, Liu C, Huang C, Wu Y, Shih Y, Ho H, et al. Serum anti-mullerian hormone and esteradiol levels as predictors of ovarian hyperstimulation syndrome in assisted reproduction technology cycles. Hum Reprod. 2008;23:160–7. doi:10.1093/humrep/dem254. [DOI] [PubMed]
- 20.Riggs RM, Duran EH, Baker MW, Kimble TD, Hobeika E, Yin L, et al. Assessment of ovarian reserve with anti-Mullerian hormone: a comparison of the predictive value of anti-Mullerian hormone, follicle-stimulating hormone, inhibin B, and age. Am J Obstet Gynecol. 2008;199:202.e1–202e8. [DOI] [PubMed]
- 21.Broer SL, Mol BWJ, Hendriks D, Broekmans FJM. The role of anti-mullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril. 2009;91:705–14. doi:10.1016/j.fertnstert.2007.12.013. [DOI] [PubMed]
- 22.Nardo L, Gelbaya T, Wilkinson H, Roberts S, Yates A, Pemberton P, et al. Circulating basal anti-Mullerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2008. doi:10.1016/j.fertnstert.2008.08.127. [DOI] [PubMed]
- 23.Tomas C, Nuojua-Huttunen S, Martikainen H. Pretreatment transvaginal ultrasound examination predicts ovarian responsiveness to gonadotropins in in-vitro fertilization. Hum Reprod. 1997;12:220–3. doi:10.1093/humrep/12.2.220. [DOI] [PubMed]
- 24.Scheffer GJ, Broekmans FJ, Dorland M, Habbema JD, Looman CW, te Velde ER. Antral follicle counts by Transvaginal ultrasonography are related to age in woman with proven natural fertility. Fertil Steril. 1999;72:854–51. doi:10.1016/S0015-0282(99)00396-9. [DOI] [PubMed]
- 25.Ng EH, Yeung WS, Ho PC. The significance of antral follicle count in controlled ovarian stimulation and intrauterine insemination. J Assist Reprod Genet. 2000;17:323–8. doi:10.1023/A:1009453011321. [DOI] [PubMed]
- 26.Pohl M, Hohlagschwandtner M, Obruca A, Poschalko G, Weigert M, Feichtinger W. Number and size of antral follicles as predictive factors in vitro fertilization and embryo transfer. J Assist Reprod Genet. 2000;17:315–8. doi:10.1023/A:1009448810413. [DOI] [PMC free article] [PubMed]
- 27.Nahum R, Shifren JL, Chang Y, Leykin L, Isaacson K, Toth TL. Antral follicle assessment as a tool for predicting outcome in IVF— is it a better predictor than age and FSH? J Assist Reprod Genet. 2001;18:151–5. doi:10.1023/A:1009424407082. [DOI] [PMC free article] [PubMed]
- 28.Hansen KR, Morris JL, Theyer AC, Soules MR. Reproductive aging and variability in the ovarian antral follicle count: application in the clinical setting. Fertil Steril. 2003;80:577–83. doi:10.1016/S0015-0282(03)00741-6. [DOI] [PubMed]
- 29.Jayaprakasan K, Campbell BK, Clewes JS, Johnson IR, Raine-Fenning NJ. Three-dimensional ultrasound improves the interobserver reliability of antral follicle counts and facilitates increased clinical work flow. Ultrasound Obstet Gynecol. 2008;31:439–44. doi:10.1002/uog.5301. [DOI] [PubMed]
- 30.Bancsi LFJMM, Broekmans FJM, Looman CWN, Habbema JDF, Velde ER. Impact of repeated antral follicle counts on the prediction of poor ovarian response in woman undergoing in vitro fertilization. Fertil Steril. 2004;81:35–41. doi:10.1016/j.fertnstert.2003.06.011. [DOI] [PubMed]
- 31.Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2008. doi:10.1016/j.fertnstert.208.10.042. [DOI] [PubMed]
- 32.Melo MAB, Garrido N, Alvarez C, Bellver J, Meseguer M, Pellicer A, et al. Antral follicle count (AFC) can be used in the prediction of ovarian response but can not predict the oocyte/embryo quality or the in vitro fertilization outcome in an egg donation program. Fertil Steril. 2009;91:148–56. doi:10.1016/j.fertnstert.2007.11.042. [DOI] [PubMed]
- 33.Maseelall PB, Hernandez-Rey AE, Oh C, Maagdenberg T, McCulloh DH, McGovern PG. Antral follicle count is a significant predictor of livebirth in in vitro fertilization cycles. Fertil Steril. 2009;91:1595-7. doi:10.1016/j.fertnstert.2008.11.001. [DOI] [PubMed]
- 34.Seifer DB, Maclaughlin DT. Mullerian inhibiting substance is an ovarian growth factor of emerging clinical significance. Fertil Steril. 2007;88:539–46. doi:10.1016/j.fertnstert.2007.02.014. [DOI] [PubMed]
- 35.Streuli I, Fraisse T, Chapron C, Biiaoui G, Bischof P, Ziegler D. Clinical uses of anti-Mullerian hormone assays: pitfalls and promises. Fertil Steril. 2009;91:226–30. doi:10.1016/j.fertnstert.2007.10.067. [DOI] [PubMed]
- 36.Freour T, Mirallie S, Bach-Ngohou K, Denis M, Barriere P, Masson D. Measurement of serum anti-Mullerian hormone by Beckman Coulter ELISA and DSL ELISA: comparison and relevance in assisted reproduction technology (ART). Clin Chim Acta. 2007;375:162–4. doi:10.1016/j.cca.2006.06.013. [DOI] [PubMed]
- 37.Ebner T, Sommergruber M, Moser M, Shebel O, Schreier-Lechner E, Tews G. Basal level of anti-Mullerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022–6. doi:10.1093/humrep/del127. [DOI] [PubMed]
- 38.Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685–718. doi:10.1093/humupd/dml034. [DOI] [PubMed]