Abstract
Objective
To elucidate the relationship between embryo grade and ART outcomes and determine how to decrease multiple pregnancy rates by assigning patients to single embryo transfer (SET) instead of dual embryo transfer (DET) according to embryo grade and/or availability.
Design
Retrospective medical record review.
Setting
IVF fertility center.
Patients
247 women undergoing day 5 DET after in vitro fertilization or intracytoplasmic sperm injection treatment.
Methods
We retrospectively investigated embryo grade and outcomes on day 5 DET and calculated theoretical multiple pregnancy rates by assigning patients to SET instead of DET according to a combination of embryo grades and availability.
Main Outcomes Measures
Implantation, pregnancy, multiple pregnancy, expected pregnancy and expected multiple pregnancy rates.
Results
Embryo grade affects implantation rates so that embryo transfer of at least one embryo with grade ≥ 3BB resulted in high multiple pregnancy rates as well as high pregnancy rates. By performing DET, the clinical pregnancy rate was 39.9% with a multiple pregnancy rate of 33.3%; however, had SET been performed with an embryo graded 3BB or better, theoretical calculated pregnancy rates would have dropped to 35.8% but with a multiple pregnancy rate of 7.2%.
Conclusions
Our model showed that cases having at least one embryo with grade ≥ 3BB might serve as suitable candidates for SET.
Keywords: Double embryos transfer (DET), Single embryos transfer (SET), Multiple pregnancies, Assisted reproductive technologies (ART), Blastocyst grade
Introduction
When embryos are cultured to cleavage-stage, more than one embryo has to be transferred to compensate for the low implantation rate of each embryo in order to achieve satisfactory pregnancy results. After the advent of the extended embryo culture technique, higher implantation rates have been reported because of better embryo selection compared to earlier developmental stages and because of better synchronization between embryo developmental stage and uterine environment. However, increases in implantation rates have also been accompanied by significant increases in multiple pregnancy rates. Multiple pregnancies pose unwanted morbidity risks to the mother and unborn child as well as creating additional financial costs related to prenatal and postpartum health care [1, 2]. If we could reduce multiple pregnancy rates while maintaining acceptable pregnancy rates on blastocyst transfer, we would be then able to reduce the overall multiple pregnancy rate. Multiple pregnancies are mainly a result of the transfer of more than one embryo, when the quality of the embryos is high. Gardner et al showed that dual blastocyst transfers do not significantly increase pregnancy rates but increased multiple pregnancies compared to single blastocyst transfers in selected cases [3].
Thus, single embryo transfer (SET) has been the strategy used recently to curtail multiple pregnancies in several countries, especially in Europe where the cost of in vitro fertilization (IVF) treatments are covered or offset by government health agencies [4-6]. Although SET is an effective means to prevent multiple pregnancies, pregnancy rates from non-selective SET remain disappointing.
It is the aim of this study is to investigate whether embryo grade affects implantation rates, pregnancy rates and multiple pregnancy rates and to identify those embryos with high-implantation potential using DET and determine which grade embryo should then be considered as candidate for SET. Once we identify the embryos with high-implantation potential, we can then select and use them in SET procedures. Furthermore, embryos that fail to meet the selection criteria for SET can then still be used on DET cases. The second aim would then be to use this information to predict how we could decrease multiple pregnancy rates by assigning patients to either SET or DET according to a combination of embryo grades and availability.
Methods
Patients
This study was retrospectively conducted from routine clinical data compiled between January 2004 and December 2005 at the Advanced Fertility Center of Fuchu Nozomi, Osaka, Japan, from a total of 316 completed treatment cycles from 247 patients. As a matter of principle, patients who had more than five oocytes during the oocytes retrieval procedure were invited to have the embryos cultured to blastocyst stage for transfer. Patients were counseled at least on one occasion and they could eventually select cleavage or blastocyst-stage embryo transfer.
Ovarian stimulation and embryo culture
Patients were down regulated with luteinzing hormone-releasing hormone agonist and ovarian stimulation was started with human menopausal gonadotrophin (HMG) and urinary follicle stimulating hormone (uFSH) or recombinant follicle stimulating hormone (rFSH). Follicle growth was monitored by ultrasound and 6,000–10,000 IU of human chorionic gonadotropin (HCG) was administered when at least two follicles were ≥ 18 mm in diameter. Oocyte retrieval was performed 35–37 h after HCG administration under ultrasound guidance. For the standard IVF fertilization, oocytes in groups of 3 to 4 were inseminated between 4 h and 6 h after retrieval with 1 × 105 motile sperm/ml in G-FERT media or IVF-30 (Vitrolife, Gothenburg, Sweden). For the intracytoplasmic sperm injection (ICSI) procedure, oocytes were injected with a single sperm between 4 h and 6 h after the retrieval. Mature oocytes were identified by the presence of the first polar body in the ICSI cycles after removal of the corona cells following exposure to hyaluronidase. These oocytes were then cultured in 1 ml G1.3 media for 1–3 oocytes (Vitrolife, Gothenburg, Sweden), after ICSI. The fertilization check was performed between 16 h and 18 h after insemination or ICSI. These zygotes were cultured individually in 25 μl droplets of G1.3 media (Vitrolife, Gothenburg, Sweden) overlaid with 4 ml of oil in Falcon 3001 culture dishes (Becton Dickinson Labware, Franklin Lakes, NJ). All cultures were incubated at 37℃ in a 5% CO2 atmosphere. On the morning of day 3, groups of 3 to 5 embryos were transferred into 60 μl droplets of G2.3 media (Vitrolife, Gothenburg, Sweden).
Embryo grading, dual embryo transfer
On the morning of day 5, blastocysts were assigned a score using the grading system established by Gardner and Schoolcraft as described in Table 1 prior to DET [7]. By using this scoring system, two embryos were selected for the transfer. Embryos were then transferred into G2.3 media early in the afternoon. When an embryo developed to more than morula, an embryo transfer was performed. When only one embryo was available for transfer, compulsory SET was performed. When no embryo was available, embryo transfer was then cancelled.
Table 1.
Blastocyst scoring criteria
| Size | Degree of expansion and hatching status |
|---|---|
| 1 | An early blastocyst with a blastocoel that is less than half of the volume of the embryo. |
| 2 | A blastocyst with a blastocoel that is half of or greater than half of the volume of the embryo. |
| 3 | A full blastocyst with a blastocoel completely filling the embryo. |
| 4 | An expanded blastocyst with a blastocoel volume larger than that of the early embryo, with a thinning zona. |
| 5 | A hatching blastocyst with the trophectoderm starting to herniated through the zona. |
| 6 | A hatched blastocyst, in which the blastocyst has completely escaped from zona. |
| Grade | Inner cell mass |
| A | Tightly packed, many cells. |
| B | Loosely grouped, several cells. |
| C | Very few cells. |
| Grade | Tropoderm |
| A | Many cells forming a cohesive epithelium. |
| B | Few cells forming a loose epithelium. |
| C | Very few large cells. |
(Gardner et al, 1999)
Criteria assignment
We examined the relationship between embryo grade versus implantation rates, pregnancy rates and multiple pregnancy rates in DET cases. Cases were grouped according to embryo grade (criteria I and II) and number of high-grade embryos available (group A, B and C) as summarized in Table 2. Criteria I included those cases with embryos graded 3AA or better. These were further subdivided into three groups as follows: group A, having two blastocysts of ≥ 3AA; group B, having one blastocyst of ≥ 3AA; and group C, having no blastocysyt ≥ 3AA. Criteria II consisted of cases with embryos graded 3BB or better. Similarly, cases were subdivided into: group A, having two blastocysts of ≥ 3BB; group B, having one blastocyst of ≥ 3BB; and group C, having no blastocysyt of ≥ 3BB.
Table 2.
Selection criteria for high grade embryos
| Pregnancy and multiple pregnancy rates were analyzed using 2 different criteria: |
|---|
| Criteria I; high grade blastocytes are defined as being greater than 3AA |
| Group A: two blastocysts of ≥ grade 3AA (24) |
| Group B: one blastocyst of ≥ grade 3AA (63) |
| Group C: no blastocyst of ≥ grade 3AA (229) |
| Criteria II; high grade blastocytes are defined as being greater than 3BB |
| Group A: two blastocysts of ≥ grade 3BB (101) |
| Group B: one blastocyst of ≥ grade 3BB (106) |
| Group C: no blastocyst of ≥ grade 3BB (109) |
Outcome measures
Implantation rates were defined as the number of gestational sacs observed on ultrasound scanning divided by the number of embryos transferred. Pregnancy was determined by the detection of gestational sacs in the uterus. When multiple fetal heartbeats were observed within a single gestational sac, we defined it as monozygotic pregnancies.
Projected pregnancy rates and multiple pregnancy rates
A theoretical mathematical model based on retrospective clinical data was developed to compare overall pregnancy rates and multiple pregnancy rates if SET was performed. Based on a subset of patient data for SET cases performed at our clinic at about the same time period (2004–2006), we calculated projected pregnancy rates to demonstrate decreases in multiple pregnancy rates had we hypothetically performed SET instead of DET in these cases. Projected values were extrapolated by multiplying the actual pregnancy or multiple pregnancy rates from SET cases to the corresponding totals of DET cases. In order to compensate for multiple pregnancies from SET, which are monozygotic, we used the rate of monozygotic multiple pregnancy rate from this study.
Statistical analysis
Data were statistically analyzed using the χ2 test and Student’s t-test as appropriate and differences were considered to be significant at P < 0.05. Power analysis was performed with a nominal alpha of 0.50 and power of %80 was considered acceptable. All statistical analysis was carried out using SigmaStat 3.5 (Systat Software, Inc., San Jose, CA).
Results
There was a total of 316 treatment cycles (247 patients) with DET on day 5. Patient’s demographic and clinical characteristics are presented in Table 3. Indications, method of fertilization, treatment cycle number and patient age were similar between each group in both criteria.
Table 3.
Patient background and outcomes
| DET on DAY5 316 cycles | Criteria I | Criteria II | |||||
|---|---|---|---|---|---|---|---|
| Total (n = 316) | Group A (n = 24) | Group B (n = 63) | Group C (n = 229) | Group A (n = 101) | Group B (n = 106) | Group C (n = 109) | |
| Oocyte number (mean ± SD) | 11.0 ± 4.38 | 12.3 ± 4.311, 2 | 11.8 ± 5.311 | 10.7 ± 4.072 | 12.6 ± 4.00 | 10.3 ± 4.80 | 10.2 ± 3.91 |
| Previous failure cycle | 2.2. ± 1.94 | 2.3 ± 1.37 | 2.0 ± 1.38 | 2.3 ± 2.12 | 2.1 ± 1.48 | 2.3 ± 2.11 | 2.3 ± 2.14 |
| Age (mean ± SD) | 33.9 ± 1.00 | 32.4 ± 4.193 | 33.3 ± 3.78 | 34.2 ± 4.003 | 33.0 ± 3.374 | 34.0 ± 4.02 | 34.7 ± 4.084 |
| IVF/ICSI cycle | 133/183 | 8/16 | 21/42 | 104/125 | 46.5%(47/101) | 37.7% (40/106) | 42.2% (46/109) |
| Clinical Pregnancy ratea | 39.9% (126/316) | 58.3% (14/24) 5 | 58.7%(37/63) 6 | 32.8% (75/229) 5, 6 | 55.4% (56/101) 7, 8 | 41.5% (44/106) 7, 9 | 23.9% (26/109) 8, 9 |
| Implantation rateb | 26.3% (166/632) | 52.1% (25/48) 10 | 40.5% (51/126) 11 | 19.7% (90/458) 10, 11 | 39.6% (80/202) 12, 13 | 25.9% (55/212) 12, 14 | 14.2% (31/218) 13, 14 |
| Monozygotic multiple pregnancy ratec | 2.38% (3/126) | 7.1% (1/14) | 2.7% (1/37) | 1.3% (1/75) | 3.6% (2/56) | 0.0% (0/44) | 3.8% (1/26) |
| Multiple pregnancy rated | 33.3% (42/126) | 78.6% (11/14) 15, 16 | 40.5% (15/37) 15, 17 | 21.3% (16/75) 16, 17 | 44.6% (25/56) 18 | 25.0% (11/44) 18 | 23.1% (6/26) |
aclinical pregnancy rate was defined by detection of gestational sac with ultrasound
bimplantation rate was calculated by the number of gestational sac divided by embryo number of transfer
cmonozygotic multiple pregnancy was defined as being more than two fetus in one gestational sac which included one case with two gestational and three fetus
dmultiple pregnancy rate was defined as either detection of two gestational sac or fetus which included one case with two gestational and three fetus
Corresponding superscript number pairs indicate significant differences between corresponding groups.
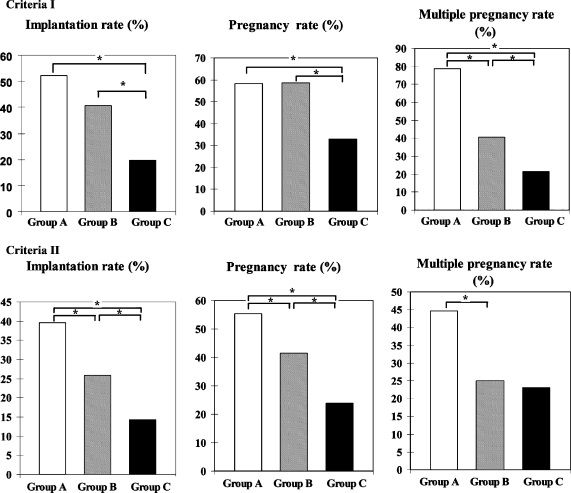
As shown in Fig. 1, criteria I, implantation rates, pregnancy rates and multiple pregnancy rates for groups A, B and C, were 52.1%(25/48), 40.5%(51/126) and 19.7% (90/458); 58.3% (14/24), 58.7% (37/63) and 32.8% (75/229); 78.6% (11/14), 40.5% (15/37) and 21.3% (16/75), respectively. There was no significant difference in implantation rates and pregnancy rates between groups A and B. However, significant differences were noted in implantation rates and pregnancy rates between groups A and C and groups B and C. Significant differences were also noted in multiple pregnancy rate among three groups.
Fig. 1.
Implantation rates, pregnancy rates and multiple pregnancy rates of DET for selection criteria I and II. As described in the text, criteria I consists of cases classifying embryos scoring greater than 3AA as high-grade, whereas criteria II includes embryos greater than 3BB as high-grade embryos. Groups A, B and C had either two, one and no high-grade embryos, respectively. Statistically significant differences at * p < 0.05
In criteria II, implantation rates, pregnancy rates and multiple pregnancy rates for groups A, B and C, were 39.6% (80/202), 25.9% (55/212) and 14.2% (31/218); 55.4% (56/101), 41.5 (44/106) and 23.9 (26/109); 44.6% (25/56), 25% (11/44) and 23.1% (6/26), respectively (Fig. 1 and Table 3). Significant differences were found between groups A and B, group A and C and groups B and C in implantation rates. Moreover, significant differences were found between groups A and B, groups A and C and groups B and C in pregnancy rates. Significant differences were also found between groups A and B in multiple pregnancy rates, Additionally, significant difference in multiple pregnancy rates between groups B and C were not observed.
Projected pregnancy rates were extrapolated from a representative and separate subset of SET cases with a grade ≥ 3AA (45 patients) and grade ≥ 3BB (86 patients) embryos on day 5 to project what the comparable pregnancy rate would be in the DET group had they had SET instead. The overall pregnancy rate was 39.9% for DET cases, and was compared to projected values as shown in Tables 4 and 5. There was no significant difference in the overall projected pregnancy rate that would have resulted had SETs been performed between all groups containing at least one embryo graded 3BB or better (Table 5).
Table 4.
Projected pregnancy and multiple pregnancy rates
| Projected pregnancy rate | Projected multiple pregnancy rate | ||||
|---|---|---|---|---|---|
| SET for criteria I group A and DET for others | SET for criteria I group A and DET for others | ||||
| Criteria I | Actual pregnancy rate | Projected pregnancy rate | Criteria I | Actual multiple pregnancy rate | Projected pregnancy rate |
| Group A | 58.3% (14/24) | 46.7% (11.2/24) | Group A | 78.6% (11/14) | 2.38% (0.27/11.2) |
| Group B | 58.7% (37/63) | 58.7% (37/63) | Group B | 40.5% (15/37) | 40.5% (15/37) |
| Group C | 32.8% (75/229) | 32.8% (75/229) | Group C | 21.3% (16/75) | 21.3% (16/75) |
| Total | 39.9% (126/316) | 39% (123.2/316) | Total | 33.3% (42/126) | 25.4% (31.3/123.2) |
| SET for criteria I groups A and B and DET for others | SET for criteria I groups A and B and DET for others | ||||
| Criteria I | Actual pregnancy rate | Projected pregnancy rate | Criteria I | Actual multiple pregnancy rate | Projected pregnancy rate |
| Group A | 58.3% (14/24) | 46.7% (11.2/24) | Group A | 78.6% (11/14) | 2.38% (0.27/11.2) |
| Group B | 58.7% (37/63) | 46.7% (29.4/63) | Group B | 40.5% (15/37) | 2.38% (0.7/29.4) |
| Group C | 32.8% (75/229) | 32.8% (75/229) | Group C | 21.3% (16/75) | 21.3% (16/75) |
| Total | 39.9% (126/316) | 36.6% (116/316) | Total | 33.3% (42/126) | 14.7% (17/116) |
| SET for criteria II group A and DET for others | SET for criteria II group A and DET for others | ||||
| Criteria II | Actual pregnancy rate | Projected pregnancy rate | Criteria II | Actual multiple pregnancy rate | Projected pregnancy rate |
| Group A | 55.4% (56/101) | 41.9% (42.3/101) | Group A | 44.6% (25/56) | 2.38% (1.01/42.3) |
| Group B | 41.5% (44/106) | 41.5% (44/106) | Group B | 25% (11/44) | 25% (11/44) |
| Group C | 23.9% (26/109) | 23.9% (26/109) | Group C | 23.1% (6/26) | 23.1% (6/26) |
| Total | 39.9% (126/316) | 35.5% (112.3/316) | Total | 33.3% (42/126) | 16.1% (18/112) |
| SET for criteria II groups A and B and DET for others | SET for criteria II groups A and B and DET for others | ||||
| Criteria II | Actual pregnancy rate | Projected pregnancy rate | Criteria II | Actual multiple pregnancy rate | Projected pregnancy rate |
| Group A | 55.4% (56/101) | 41.9% (42.3/101) | Group A | 44.6% (25/56) | 2.38% (1.01/42.3) |
| Group B | 41.5% (44/106) | 41.9% (44.4/106) | Group B | 25% (11/44) | 2.38% (1.06/44.4) |
| Group C | 23.9% (26/109) | 23.9% (26/109) | Group C | 23.1% (6/26) | 23.1% (6/26) |
| Total | 39.9% (126/316) | 35.8% (113/316) | Total | 33.3% (42/126) | 7.2% (8.07/113) |
#1 Pregnancy rates were adjusted according the pregnancy rates of SET in our clinic at about same period:
SET pregnancy rate (grade ≥ 3AA); 46.7% (selective SET 88%, compulsory SET 12%)
SET pregnancy rate (grade ≥ 3BB); 41.9% (selective SET 75%, compulsory SET 25%)
#2 Multiple pregnancy rates were adjusted according the monozygotic twin rate of this study (2.38%)
Table 5.
Projected pregnancy and multiple pregnancy rates
| DET for all cases | Actual pregnancy rate (%) | Actual multiple pregnancy rate (%) | ||||
|---|---|---|---|---|---|---|
| 39.9 % (126/316) | 33.3 % (42/126) | |||||
| Projected pregnancy rate (%) | Power(exact)a | P-valueb | Projected multiple pregnancy rate (%) | Power (exact)a | P-valueb | |
| SET for criteria I group A and DET for others | 39.0 % (123/316) | 0.035 | 0.805 | 25.4 % (17/116) | 0.216 | 0.159 |
| SET for criteria I groups A, B and DET for others | 36.6 % (116/316) | 0.117 | 0.253 | 14.7 % (17/116) | 0.904 | <0.001 |
| SET for criteria II group A and DET for others | 35.5 % (112/316) | 0.184 | 0.141 | 16.0 % (18/112) | 0.835 | 0.002 |
| SET for criteria II groups A, B and DET for others | 35.8 % (113/316) | 0.163 | 0.165 | 7.2 % (8.1/113) | 0.999 | <0.001 |
aPower analysis of n for projected pregnancy and multiple pregnancy rates; alpha, 0.050
bCompared with actual pregnancy and multiple pregnancy rates from DET cases
We used the monozygotic multiple pregnancy rate of 2.38% from 126 pregnancy cases from DET to predict the multiple pregnancy rate from SET. The same extrapolations were performed to predict multiple pregnancy rates as described in the previous paragraph (Table 4). The actual multiple pregnancy rate was 33.3% for all DET cases. Interestingly, projected multiple pregnancy rates decreased significantly in groups A (25.4%), and A and B (14.7%) in criteria I; and groups A (16.1%), and A and B (7.2%) in criteria II (Table 5).
Discussion
In efforts to reduce complications from multiple pregnancies, ASRM (American Society for Reproductive Medicine) and ESHRE (European Society of Human Reproduction and Embryology) guidelines recommend the number of embryos transferred to be no more than 2 for good-prognosis IVF patients [8-10]. As a result, many countries, especially in Europe, have restricted the number of embryos transferred to reduce complications from multiple pregnancies. For instance, United Kingdom has restricted the number of embryos transferred to two and a law introduced in Italy in 2004 forbids the fertilization of more than three oocytes in one fresh cycle for assisted reproduction [11, 12]. After Sweden started to regulate the number of embryos transferred for women younger than 35, delivery rates were maintained around 26% while the multiple birth rate decreased dramatically, from 35% to around 5% which would be acceptable for countries where governments cover their expense. In exchange for restricting the number of embryos transferred, the Belgian government introduced a new law that reimburses laboratory expenses for up to six IVF cycles for women up to the age of 42 years and although the SET rate increased from 14% to 49% and the over all pregnancy rate remained at 32.5%, multiple pregnancies decreased from 25.9% to 8.0% [4, 13]. To decrease the multiple pregnancy rate from ART, in 1996, the Japanese Society of Obstetrics and Gynecology began regulating the number of embryos transferred in each cycle to three; however, the multiple pregnancy rate from ART was still reported high (17.2%) in 2003.
With SETs being established as the primary course of action to reduce multiple pregnancies, the question of embryo selection becomes eminent in order to establish the highest pregnancy rates achievable. There have been numerous attempts to select high implantation potential embryos based on morphology at different stages. Scott et al reported that pronuclear embryo morphology was related to implantation and pronuclear embryos could be successfully selected for embryo transfer [14]. There certainly were relationships between pronuclear embryo morphology and implantation rates; however, implantation rates of top-morphology-pronuclear embryos was still 28%, which is lower than in other stage-selection, so in countries where extended culture is allowed, pronuclear embryo parameters are not usually used for embryo selection of SETs.
Blastocyst transfer has been reported to have a higher success rate than cleavage embryo transfer; the rates of delivery of single blastocyst—stage transfer and single cleavage-stage embryo are 32% and 21.6%, respectively [15]. The implantation rate from a blastocyst (50%) was reported higher when compared to a cleavage stage embryo transfer (30.1%) [16]. Mainly two reasons have been considered; first, the human cleavage stage embryo normally resides in the oviduct and does not enter the uterus until after compaction, so there is the hypothesis that for this reason, the transfer of cleavage stage contributes to lower pregnancy rate than blastocysts [17]. Secondly, more viable embryos can be naturally selected by extended culture. Based on our clinic data, DET on blastocyst stage resulted in higher pregnancy rates (39.9%) and higher multiple pregnancy rates (33.3%) compared to DET based on cleavage stage pregnancy rates (24.8%) and higher multiple pregnancy rates (20%) (data not shown) as similarly reported by others [15]. Gardner reported that the transfer of a single blastocyst in good prognosis cases resulted in an ongoing pregnancy rate of 60.9% with no twins. In contrast, the transfer of two blastocysts resulted in an implantation rate of 56%, an ongoing pregnancy rate of 76% with a 47.4% incidence of twins [3].
Grade ≥ 3AA embryos are considered as the best candidates for SET; however, cases with at least one embryo with grade ≥ 3AA are not frequent [18]. In fact, in our study, 27% of DET cases had an embryo graded greater than 3AA and only 7.6% had two embryos graded 3AA or higher. Therefore, it is impossible to reduce the overall multiple pregnancy rate for SETs without increasing the selection of candidate embryos. We confirmed that grade ≥ 3AA embryos provide the highest clinical pregnancy rates; we also demonstrated that grade ≥ 3BB embryos could provide adequate clinical pregnancy results as well in cases when higher-grade embryos are not available. Thus, we can assume that if we use at least grade a 3BB embryo in SETs, we can significantly contribute to a lower multiple pregnancy rate.
Because this is a retrospective study, we calculated theoretical values from this study group based on data gathered from an actual subset of SET cases. By performing DET, the clinical pregnancy rate was 39.9% with a multiple pregnancy rate of 33.3%. However, if SET had been performed with at least one 3BB grade embryo, the pregnancy rate would have dropped to 35.8% but with a pregnancy rate of 7.2%
We extrapolated our pregnancy rate values from SET of embryos with grade ≥ 3AA (46.6%) in which 40 cases were elective SET with extra embryo to transfer and 5 cases that were compulsory with no extra embryo to transfer. Additionally, we use our pregnancy rate for SET from embryos with grade ≥ 3BB (41.9%) in which 80 cases were elective SET with extra embryo to transfer and 15 compulsory cases with no extra embryo to transfer.
An increased incidence of monozygotic twinning has been reported in pregnancies conceived after ovarian induction, IVF/ICSI procedure, especially in blastocyst transfer(s) [19-23]. We projected multiple pregnancy rates from the results of blastocyst stage transfers in our clinic from this study that included 3 cases of monozygotic twinning in 126 pregnancies (2.38%). In comparison, incidence of monozygotic twinning from blastocyst-stage embryos has been reported from 1–5%; “1.6% Wright et al., 2004,” “5% Behr et al., 2000” and “5.6% Milki et al., 2003.”
Other predictive factors leading to multiple pregnancies may exist and need further investigation, for instance, the number of attempts, other stage morphology and female age. In general, young females with or without failed previous IVF/ICSI treatment are considered as good candidates for SET. Thus, selective single blastocyst transfer is preferred for females < 38 years of age [24]. However, Veleva et al. reported successful elective SET in older women (aged 36–39 years) [25]. Furthermore Schmit et al reported that quality and number of embryos to transfer but not number of previous failed cycles were important in determining outcome in cases of women aged 35 to 37 [26]. Due to a growing population of older women in ART and the high maternal risk associated, SETs for these older women, including those over 40 years of age, have to be investigated further. In addition, we can assume that multiple parameters on different stages embryo development influence in some degree the implantation of the embryo transfer and subsequent pregnancy success. At present, we consider that blastocyst-stage selection is the most practical approach for the selection of high-implantation embryos for SET. In fact, in 2004 and 2005, the SET rate in our clinic was only 28.6% (213/744 cases). However, after this study, we began to perform SETs to cases with only one blastocyst as well as cases with an extra embryo to transfer and the SET rate has increased to 56.1% (288/406 cases) in 2007.
Conclusion
In this study, we have observed that blastocyst grade affects implantation, pregnancy and multiple pregnancy rates in day 5 DET. Furthermore, we confirmed that transferring at least one high-grade embryo leads to extremely high multiple pregnancy rates as well as high pregnancy rates in DET. More importantly, our model suggests that if we use embryos graded 3BB or better for SET, we may significantly reduce multiple pregnancy rates without significantly decreasing pregnancy rates. The data is based on a retrospective study using a theoretical model; therefore, prospective analysis needs to be carried out further to implement these findings.
Conflict of interest statement
The authors have no conflict of interest to disclose.
References
- 1.Kjellberg AT, Carlsson P, Bergh C. Randomized single versus double embryo transfer: obstetric and paediatric outcome and a cost-effectiveness analysis. Hum Reprod. 2006;21:210–6. doi:10.1093/humrep/dei298. [DOI] [PubMed]
- 2.Fiddelers AA, Severens JL, Dirksen CD, Dumoulin JC, Land JA, Evers JL. Economic evaluations of single- versus double-embryo transfer in IVF. Hum Reprod. 2007;13:5–13. [DOI] [PubMed]
- 3.Gardner DK, Surrey E, Minjarez D, Leitz A, Stevens J, Schoolcraft WB. Single blastocyst transfer: a prospective randomized trial. Fertil Steril. 2004;81:551–5. doi:10.1016/j.fertnstert.2003.07.023. [DOI] [PubMed]
- 4.Debrock S, Spiessens C, Meuleman C, Segal L, De Loecker P, Meeuwis L, et al. TM New Belgian legislation regarding the limitation of transferable embryos in in vitro fertilization cycles does not significantly influence the pregnancy rate but reduces the multiple pregnancy rate in a threefold way in the Leuven University Fertility Center. Fertil Steril. 2005;83:1572–4. doi:10.1016/j.fertnstert.2005.01.087. [DOI] [PubMed]
- 5.Karlström PO, Bergh C. Reducing the number of embryos transferred in Sweden-impact on delivery and multiple birth rates. Hum Reprod. 2007;22:2202–7. doi:10.1093/humrep/dem120. [DOI] [PubMed]
- 6.Gerris J, De Neubourg D, Mangelschots K, Van Royen E, Vercruyssen M, Barudy-Vasquez J, et al. Elective single day 3 embryo transfer halves the twinning rate without decrease in the ongoing pregnancy rate of an IVF/ICSI programme. Hum Reprod. 2002;17:2626–31. doi:10.1093/humrep/17.10.2626. [DOI] [PubMed]
- 7.Gardner DK, Schoolcraft WB, Jansen R, Mortimer D. In vitro culture of human blastocyst: Towards reproductive certainty: infertility and genetics beyond 1999. Carnforth: Parthenon; 1999. p. 378–8.
- 8.Gianaroli L, Plachot M, van Kooij R, Al-Hasani S, Dawson K, Devos A, et al. ESHRE guidelines for good practice in IVF laboratories. Committee of the Special Interest Group on Embryology of the European Society of Human Reproduction and Embryology. Hum Reprod. 2000;15:2241–6. doi:10.1093/humrep/15.10.2241. [DOI] [PubMed]
- 9.Practice Committee. Society for Assisted Reproductive Technology and American Society for Reproductive Medicine Guidelines on the number of embryos transferred. Fertil Steril. 2004;82:773–4. doi:10.1016/j.fertnstert.2004.06.031. [DOI] [PubMed]
- 10.Stern JE, Cedars MI, Jain T, Klein NA, Beaird CM, Grainger DA, et al. Society for Assisted Reproductive Technology Writing Group Assisted reproductive technology practice patterns and the impact of embryo transfer guidelines in the United States. Fertil Steril. 2007;88:275–82. doi:10.1016/j.fertnstert.2006.09.016. [DOI] [PubMed]
- 11.Mayor S. UK authority sets limit on number of embryos transferred. BMJ. 2004;328:7431. [DOI] [PMC free article] [PubMed]
- 12.Levi Setti PE, Albani E, Novara P, Cesana A, Negri L. Results of in vitro fertilization in Italy after the introduction of a new law. Fertil Steril. 2007; Epub ahead of print. [DOI] [PubMed]
- 13.Gordts S, Campo R, Puttemans P, Brosens I, Valkenburg M, Norre J, et al. Belgian legislation and the effect of elective single embryo transfer on IVF outcome. Reprod Biomed Online. 2005;10:436–41. [DOI] [PubMed]
- 14.Scott LA, Smith S. The successful use of pronuclear embryo transfers the day following oocyte retrieval. Hum Reprod. 1998;13:1003–13. doi:10.1093/humrep/13.4.1003. [DOI] [PubMed]
- 15.Papanikolaou EG, Camus M, Kolibianakis EM, Van Landuyt L, Van Steirteghem A, Devroey P. In vitro fertilization with single blastocyst-stage versus single cleavage-stage embryos. N Engl J Med. 2005;354:1139–46. doi:10.1056/NEJMoa053524. [DOI] [PubMed]
- 16.Gardner DK, Schoolcraft WB, Wagley L, Schlenker T, Stevens J, Hesla J. A prospective randomized trial of blastocyst culture and transfer in in-vitro fertilization. Hum Reprod. 1998;13:3434–40. doi:10.1093/humrep/13.12.3434. [DOI] [PubMed]
- 17.Croxatto HB, Ortiz ME, Díaz S, Hess R, Balmaceda J, Croxatto HD. Studies on the duration of egg transport by the human oviduct. II. Ovum location at various intervals following luteinizing hormone peak. Am J Obstet Gynecol. 1978;132:629–34. [DOI] [PubMed]
- 18.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–8. doi:10.1016/S0015-0282(00)00518-5. [DOI] [PubMed]
- 19.Wright V, Schieve LA, Vahratian A, Reynold MA. Monozygotic twinning associated with day 5 embryo transfer in pregnancies conceived after IVF. Hum Reprod. 2004;19:1831–6. doi:10.1093/humrep/deh338. [DOI] [PubMed]
- 20.Schieve LA, Meikle SF, Peterson HB, Jeng G, Burnett NM. Wilcox LS Does assisted hatching pose a risk for monozygotic twinning in pregnancies conceived through in vitro fertilization? Fertil Steril. 2000;74:288–94. doi:10.1016/S0015-0282(00)00602-6. [DOI] [PubMed]
- 21.Schachter M, Raziel A, Friedler S, Strassburger D, Bern O, Ron-El R. Monozygotic twinning after assisted reproductive techniques: a phenomenon independent of micromanipulation. Hum Reprod. 2001;16:1264–9. doi:10.1093/humrep/16.6.1264. [DOI] [PubMed]
- 22.Behr B, Fisch JD, Racowsky C, Miller K, Pool TB, Milki AA. Blastocyst-ET and monozygotic twinning. J Assist Reprod Genet. 2000;17:349–51. doi:10.1023/A:1009461213139. [DOI] [PMC free article] [PubMed]
- 23.Milki AA, Jun SH, Hinckley MD, Behr B, Giudice LC, Westphal LM. Incidence of monozygotic twinning with blastocyst transfer compared to cleavage-stage transfer. Fertil Steril. 2003;79:503–6. doi:10.1016/S0015-0282(02)04754-4. [DOI] [PubMed]
- 24.Henman M, Catt JW, Wood T, Bowman MC, de Boer KA, Jansen RP. Elective transfer of single fresh blastocysts and later transfer of cryostored blastocysts reduces the twin pregnancy rate and can improve the in vitro fertilization live birth rate in younger women. Fertil Steril. 2005;84:1620–7. doi:10.1016/j.fertnstert.2005.05.064. [DOI] [PubMed]
- 25.Veleva Z, Vilska S, Hydén-Granskog C, Tiitinen A, Tapanainen JS, Martikainen H. Elective single embryo transfer in women aged 36–39 years. Hum Reprod. 2006;21:2098–102. doi:10.1093/humrep/del137. [DOI] [PubMed]
- 26.Schmidt DW, Engmann LL, Siano LJ, Benadiva CA, Nulsen JC, Maier DB. Influence of embryo quality and number of previous cycles on pregnancy and multiple pregnancy rates in women aged 35 to 37 years who received two or three embryos. Fertil Steril. 2005;84:1748–51. doi:10.1016/j.fertnstert.2005.04.069. [DOI] [PubMed]