Abstract
Neonatal alloimmune neutropenia (NAN) is a disease that can cause severe and prolonged neutropenia in neonates. However, no report is available on the incidence of granulocyte antibody in neonates, the target antigen of this antibody, and the estimated incidence of NAN in Korea. Among a total of 856 neonates admitted to a neonatal intensive care unit (NICU) over a five year period, a total of 105 neonates with neutropenia were enrolled in this study. Positive reactions were observed in the sera of six neonates (5.7%, 6/105) by mixed passive hemagglutination assay (MPHA). To confirm the presence of NAN, MPHA and granulocyte antigen typing (HNA-1a, -1b, -2a, -4a, and -5a) were performed on neonatal and maternal blood. To differentiate granulocyte antibody and HLA antibody, MPHA was also performed using HLA antibody adsorbed serum. We confirmed three cases (2.9%, 3/105) of NAN among neonates with neutropenia in which granulocyte antibody specificities (two anti-HNA-1b and one anti-HNA-1a) and fetomaternal granulocyte antigen mismatches were identified. In this study, the estimated incidence of NAN was 0.35% (3/856) among neonates admitted to NICUs in Korea.
Keywords: Neutropenia, Neonatal Alloimmune Neutropenia, Granulocyte Antibodies
INTRODUCTION
Neutropenia is frequently observed in neonates. It has been reported that neutropenia occurs in as many as 8% of all patients admitted to neonatal intensive care units (NICUs) (1,2). In many such cases, neutropenia is usually transient and conveys no survival disadvantage. However, in other cases it is prolonged and severe, and neonates are at high risk of developing infections (2). Neonatal alloimmune neutropenia (NAN) is a disease that causes severe and prolonged neutropenia in neonates. NAN occurs when a mother becomes sensitized to a foreign antigen of paternal origin that is present on fetal granulocytes (1-4). These fetal granulocyte antigens sensitize the mother and provoke antibody production. Moreover, maternal immunoglobulin G (IgG) antibody readily crosses the placenta and destroys fetal granulocytes (3). Neutropenia is typically self-limiting and lasts for several weeks. A wide variety of antigenic targets have been identified in NAN (3-10), but target antigens remain unidentified in about a half of cases (8-11). To confirm NAN, granulocyte antibody test and granulocyte antigen typing should be performed on both neonatal and maternal blood. However, granulocyte antibody test is technically complicated and difficult to maintain (11,12). Therefore, in Korea, no report has been issued on the incidence of granulocyte antibody in neonates with neutropenia, the target antigens of this antibody, and the estimated incidence of NAN.
In this study, we detected granulocyte antibodies in a group of neonates with neutropenia, identified the antibody specificities, and confirmed a few cases of NAN. We also estimated the incidence of NAN in Korea.
MATERIALS AND METHODS
Samples and study group
All neonates admitted to the Neonatal Intensive Care Unit of Sanggye Paik Hospital from April 2000 to March 2005 were analyzed (n=856). Complete blood counts and differential cell counts were evaluated. If neonates showed at least one neutropenic WBC during the first 28 days and providing their parents had given formal consent, they were enrolled in this study (n=105). Neutropenia was defined using the reference range established by Manroe et al. (13) and Mouzinho et al. (14). The median age of these neonates at first neutropenia was 11 days (range from 0 to 28 days [mean±SD, 11±5.1 days]). Their male to female ratio was 1.05 to 1 (54 male, 51 female). 112 sera and 105 EDTA sample were collected from the 105 nenoates. In addition, six pairs of serum and EDTA samples were collected from mothers whose babies' sera showed positive reactions by granulocyte antibody testing. Serum samples were stored immediately at -70℃ until required. To detect granulocyte antibody in neonate sera, we used the mixed passive hemagglutination assay (MPHA) (12), using extracted granulocyte antigens coated onto microplates. These were obtained from six voluntary donors whose granulocyte antigen types had been identified. When an antibody was detected, MPHA was re-performed with HLA antibody adsorbed serum (12,15) to differentiate granulocyte antibody and HLA antibody. MPHA was then performed using the mother's serum and granulocyte antigen typing (HNA-1a, HNA-1b, HNA-4a, and HNA-5a genotyping and HNA-2a serotyping) was performed on both neonatal and maternal blood to support alloimmunization due to fetomaternal granulocyte antigen mismatches.
Extracted granulocyte antigen-coated microplates
Granulocytes were isolated and an extracted granulocyte antigen-coated microplate was prepared as a solid phase, according to the protocol described by Araki et al. (12). Granulocytes were isolated from EDTA blood by utilizing a density gradient medium (PMN isolation medium, Robbins Scientific, Sunnyvale, CA, U.S.A.). Isolated granulocytes were suspended in normal saline containing 3% sucrose at a cell density of 3,000/µL and then allowed to stand for three days at 4℃. 25 µL of each supernatant was placed in a well of a U-bottomed microplate (Maxisorp Lockwellmodule, Nunc, Roskide, Denmark) and allowed to stand overnight to allow the granulocyte antigen-coating to form. Microplates were stored at -70℃ before required for MPHA testing.
Granulocyte donors
Six donors were selected from 32 voluntary donors to prepare extracted granulocyte antigen-coated microplates. The six donors' granulocyte antigen types were as follows:
donor 1, HNA-1a+, HNA-1b+, HNA-2a+, HNA-4a+, HNA-5a+; donor 2, HNA-1a+, HNA-1b+, HNA-2a-, HNA-4a+, HNA-5a+; donor 3, HNA-1a+, HNA-1b-, HNA-2a+, HNA-4a+, HNA-5a+; donor 4, HNA-1a-, HNA-1b+, HNA-2a+, HNA-4a+, HNA-5a+; donor 5, HNA-1a+, HNA-1b-, HNA-2a+, HNA-4a+, HNA-5a+; donor 6, HNA-1a-, HNA-1b+, HNA-2a-, HNA-4a+, HNA-5a+. More than two HNA-1a, HNA-1b, and HNA-2a-positive donors were included, and more than two HNA-1a HNA-1a, HNA-1b, and HNA-2a-negative donors. All of donors were HNA-4a-positive and HNA-5a-positive, but none were HNA-4a-negative and HNA-5a-negative.
Granulocyte antibody test using MPHA
To detect granulocyte antibody, sera from neonates and mothers were tested by MPHA. The extracted granulocyte antigens from the six donors coated onto the U-bottomed microplates, were used as a solid phase. The negative control serum was derived from a healthy male donor with no history history of transfusion. Antisera (anti-HNA-1a, anti-HNA-1b, anti-HNA-2b, and anti-HLA antibody) were used as positive controls and sheep RBCs coated with rabbit F(ab')2 anti-human IgG were used as indicator cells. Tests were performed according to the protocols described by Araki et al. (14). To remove HLA antibody from serum, 0.2 mL of serum was incubated with 5×109 pooled platelets at 37℃ for 30 min. Adsorbed serum was recovered after centrifugation at 10,000 g for 5 min (15).
HNA-1a, HNA-1b, and HNA-4a genotyping by PCR
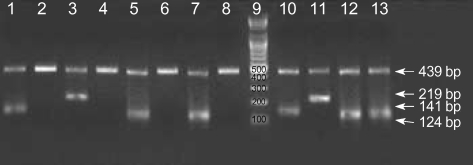
DNA was isolated from the EDTA blood samples of neonates and their mothers using QIAamp DNA Blood Mini kits (Qiagen GmbH, Hilden, Germany). To type HNA-1a, HNA-1b, and HNA-4a, polymerase chain reactions with sequence-specific primers (PCR-SSP) were performed, according to the protocols described by Bux et al. (16) and Clague et al. (17). NA1 (5'-CAGTGGTTTCACAATGAA-3') was used as a sense primer specific for HNA-1a allele (FCGR3B*1) and NA2 (5'-CAATGGTACAGCGTGCTT-3') for HNA-1b allele (FCGR3B*2). NA reverse (5'-ATGGACTTCTAG CTGCAC-3') was used as an antisense primer common to HNA-1a and HNA-1b alleles. Pos-R (5'-AGTGACTCACCCTGCATGC-3') was used as an antisense primer specific for HNA-4a-positive allele and Neg-R (5'-AGTGACTCA CCCTGCATGT-3'for HNA-4a-negative allele. HNA-4a-F (5'-CTCCCCACAGGGTGGTG-3') was used as a sense primer common to HNA-4a-positive and HNA-4a-negative allele. For internal control purposes, two primers (HGH I and HGH II) amplifying a 439 bp fragment of the human growth hormone gene (HGH) were used. Amplification was performed in a 20µL reaction mixture containing the following: 0.2µM of each primer; 200µM dATP, dCTP, dTTP, and dGTP; 10 mM Tris-HCl (pH 9.0), 1.5 mM MgCl2, 40 mM KCl; 1 unit of Taq polymerase (Bioneer, Daejeon, Korea); and 1µL of DNA sample. Amplification was preformed in a DNA thermal cycler (iCycler Thermal Cycler, Bio-Rad Laboratories, Hercules, CA, U.S.A.). Each cycle consisted of the following: predenaturation at 95℃ for 3 min and 30 amplification cycles of (denaturation at 95℃ for 1 min, primer annealing at 58℃ for 1 min, and extension at 72℃ for 1 min). The sizes of the amplified DNA fragments were 141 bp, 219 bp, and 124 bp for the HNA-1a, HNA-1b, and HNA-4a genes, respectively (Fig. 1).
Fig. 1.
HNA-1a, HNA-1b, HNA-4a genotyping by PCR-SSP. Lane 9 shows a DNA ladder marker (Bioneer, Daejeon, Korea). The amplification products (439 bp) of the internal control (HGH gene) are present in each lane. Lanes 1, 3, 5, and 7 are positive controls for HNA-1a (141 bp), HNA-1b (219 bp), HNA-4a-positive (124 bp), and HNA-4a-negative (124 bp), respectively. Lanes 2, 4, 6, and 8 are negative controls for HNA-1a, HNA-1b, HNA-4a+, and HNA-4a-, respectively. Lanes 10-13 contain amplification products of HNA-1a, HNA-1b, HNA-4a+, and HNA-4a-, respectively from a DNA sample that is a HNA-1-heterozygote (HNA-1a/HNA-1b) and a HNA-4a-heterozygote (HNA-4a+/HNA-4a-).
HNA-5a genotyping by reverse transcription (RT) and PCR allele-specific restriction enzyme analysis (PCRASRA)
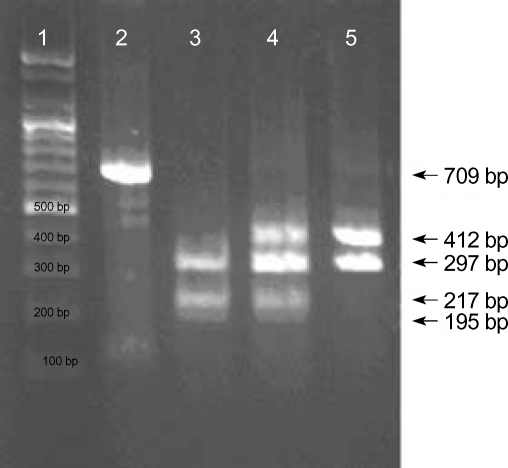
To type HNA-5a, RT and PCR-ASRA were performed according to the protocol described by Simsek et al. (18). RNA was isolated from the EDTA blood samples of neonates and heir mothers using QIAamp RNA Blood Mini kits (Qiagen GmbH, Hilden, Germany). Reverse transcription of 0.5µg of total RNA was performed in a final volume of 20µL containing 5µM random hexamer, 1 mM of each dNTP, 2 units of RNase inhibitor, and 9 units of reverse transcriptase (Bioneer, Daejeon, Korea). After incubation at 42℃ for 60 min, samples were heated for 5 min at 94℃ to terminate reactions. The primers L5 (5'-ATTTCTCTCTTTGGGAGGAGG-3') and L5A (5'-TGGGTATG TTGTGGTCGTGG-3') were used to amplify the coding region of the cDNA. The PCR product (709 bp) was treated with restriction endonuclease Bsp1286I (Takara Biotechnology, Otsu, Japan), size-separated on a 2% agarose gel with ethidium bromide, and visualized with UV light. In HNA-5a-positive homozygote samples, three fragments of 297 bp, 217 bp, and 195 bp were generated; in HNA-5a-negative homozygote samples, two fragments of 412 bp and 297 bp were generated; and in HNA-5a heterozygote samples, four fragments of 412 bp, 297 bp, 217 bp, and 195 bp were generated (Fig. 2).
Fig. 2.
HNA-5a genotyping by Bsp1,286 I allele-specific restriction enzyme analysis (ASRA). Lane 1 shows a DNA ladder marker (Bioneer, Daejeon, Korea); lane 2 shows an undigested 709 bp PCR product of the αL chain of β2integrin cDNA; lane 3 shows an HNA-5a+ homozygote sample (297 bp, 217 bp, and 195 bp); lane 4 shows a HNA-5a heterozygote samples (412 bp, 297 bp, 217 bp, and 195 bp); and lane 5 shows a HNA-5a- homozygote sample (412 bp, and 297 bp).
HNA-2a serotyping using MPHA
To type HNA-2a antigen on neonates' and their mothers' granulocytes, MPHA was performed using the protocol described above. Anti-HNA-2b was used as a typing antiserum and U-bottomed microplates coated with extracted granulocyte antigens from mothers and neonates were used as solid phases.
RESULTS
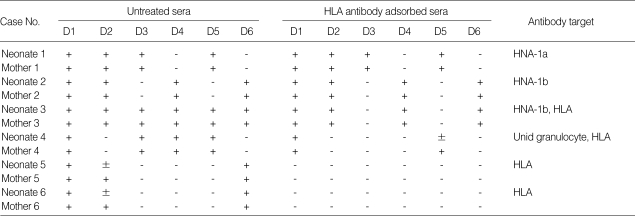
Positive reactions were observed in 13 sera from 6 neonates (5.7%, 6/105) among 105 neonates with neutropenia using MPHA. The positive reactions were as follows: one case of anti-HNA-1a (case 1), one case of anti-HNA-1b (case 2), one case of anti-HNA-1b with HLA antibody (case 3), one case of granulocyte antibody with unknown specificity and HLA antibody (case 4), and two cases of HLA antibody (cases 5, 6) (Table 1). We confirmed three cases (2.9%, 3/105) of NAN (case 1-3), in which granulocyte antibody specificities were identified and fetomaternal granulocyte antigen mismatches were confirmed (Table 1, 2, Fig. 3). In other cases with positive reactions (cases 4-6), maternal sera showed the same reaction patterns as neonatal sera, but there was no fetomaternal granulocyte antigen mismatch (Table 1).
Table 1.
Characteristics of antibodies in sera from neonates with neutropenia and their mothers
D1-D6, donor 1-donor 6; Unid granulocyte, unidentified granulocyte antigen; +, positive; ±, weakly positive; -, negative.
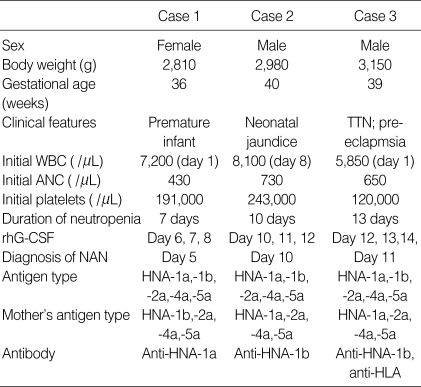
Table 2.
Clinical characteristics of three cases of neonatal alloimmune neutropenia
Days are based on postnatal age.
ANC, absolute neutrophil count; rhG-CSF, recombinant human granulocyte colony-stimulating factor; HNA, human neutrophil antigen; TTN, transient tachypnea of the newborn.
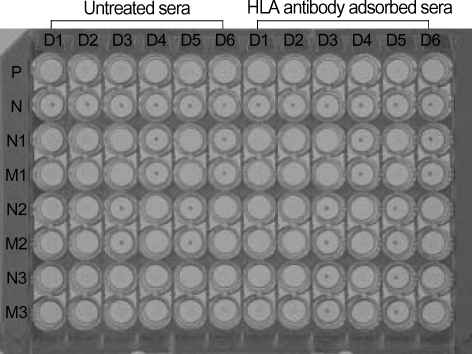
Fig. 3.
Granulocyte antibody test using the mixed passive hemagglutination assay (MPHA). The neonates' and mothers' sera were tested using extracted granulocyte antigen-coated microplates (from six donors) as a solid phase and sheep RBCs coated with rabbit F(ab')2 anti-human IgG as indicator cells. To differentiate granulocyte antibody from HLA antibody, sera (left half) with positive reactions were compared with HLA antibody adsorbed sera (right half). In the sera of neonate 1 (N1) and her mother (M1), anti-HNA-1a; in the sera of neonate 2 (N2) and his mother (M2), anti-HNA-1b; in the sera of neonate 3 (N3) and his mother (M3), anti-HNA-1b and anti-HLA.
P, pasitive control; N, negative control.
The three cases of NAN are summarized in Table 2. All were born by spontaneous vaginal delivery. They were admitted to the NICU because of; prematurity (case 1), neonatal jaundice (case 2), and transient tachypnea of the newborn (case 3). All received prophylactic antibiotics (gentamicin and ampicillin/sublactam) for potential sepsis, although blood, urine, and gastric aspirate cultures proved sterile later. All received subcutaneous injections of recombinant human granulocyte colony-stimulating factor (rhG-CSF) at 10µg/kg/day for three days. Their absolute neutrophil counts increased after treatment and were maintained at more than 1,500/µL until discharge. Maternal investigations were performed and detailed clinical histories were taken to exclude factors related to maternal disease as a cause of neonatal neutropenia. With the exception of case 3 (pre-eclampsia), maternal diseases were not implicated. Findings of maternal complete blood counts were also normal. All were discharged with clinical improvement.
The estimated incidence of neutropenia in neonates admitted to a NICU was about 12.3% (105/856). We also estimated that the incidence of NAN was 0.35% (3/856) among neonates admitted to a NICU.
DISCUSSION
In present study, the estimated incidence of neutropenia in neonates admitted to a NICU was about 12.3% (105/806), which is higher than the incidence (7%) reported in U.S.A. (2). Estimates of the incidence of NAN can vary widely and range from 0.1 to 20% (1,6-8), but such estimates are not easily compared because of differences in study designs, periods, and the test methods used. In the present study, the study population was neonates in a NICU and the estimated incidence of NAN was 0.35% (3/856).
For the NAN development, there should be a fetomaternal granulocyte mismatch and the pregnant woman should be alloimmunized against the granulocyte antigens. Zupanska et al. (5) reported fetomaternal granulocyte antigen mismatches (HNA-1a and -1b only) in 19.6% of mothers, and granulocyte-specific antibodies in 3% of fetomaternal incompatible mothers (alloimmunization in 0.6% of mothers). The authors suggested that NAN related to two antigens (HNA-1a and -1b) occurs in less than 0.1% and severe NAN in 0.06% (5). If HNA-1a and HNA-1b only were involved [HNA-1a and -1b gene frequencies were reported to be 0.52 and 0.48 in Koreans, respectively (20)], fetomaternal granulocyte antigen mismatches would be present in 18% and alloimmunization in 0.5% of mothers in Korea. However, a much higher incidence of antigen mismatches, alloimmunization, and NAN would be expected based on considerations of all possible fetomaternal granulocyte antigen mismatches. In fact the incidence of overall alloimmunization in pregnant women varies widely ranging from 1.1% to 20% (5,7,8). Our unpublished data suggest that the incidence of alloimmunization against granulocyte antigens is 3.5% (6/170) in mothers [HNA-1a and -1b, 2.4% (4/170)] in Korea.
A wide variety of antigens including the human neutrophil antigen (HNA) system and HLA have been identified in NAN (1,3-10), and nearly a half of all cases are mediated by antibodies that bind to HNA-1a, -1b, or -2a (8-10). In the present study, anti-HNA-1b was present in two cases, anti-HNA-1a in one case, and a granulocyte antibody with unknown specificity was present in another. HLA antibodies were present in four cases (two with HLA antibodies only, one with anti-HNA-1b, and one with unidentified granulocyte antibody). HLA antibodies are frequently detected in NAN, but it is generally held that they do not give rise to NAN, because the antibodies are adsorbed by the placenta and by soluble antigens in the fetal circulation (10). However, it has been controversially claimed that HLA antibody can cause NAN in a few cases (8,10). In this study no evidence indicated that HLA antibody is the etiology of neutropenia. In Caucasians HNA-1a is the most common antigen involved in NAN, HNA-1b the second, and HNA-2a the third (8-10). In view of the reported gene frequencies of the HNA system (19), fetomaternal mismatches due to HNA-1b are more common in Asians than in Caucasian, and it is expected that anti-HNA-1b antibody may be more common in Asians than in Caucasians. In the present study, two anti-HNA-1b antibodies and an anti-HNA-1a antibody were identified, which supported the expectation that anti-HNA-1b antibody might be more common than anti-HNA-1a antibody in Korean.
In summary we confirmed three cases of NAN, identified granulocyte antibody, and estimated the incidence of NAN in Korea. We conclude that NAN is not a common disease among neonates, but that it should also be considered as a possible cause of unexplained neutropenia among neonates in Korea.
ACKNOWLEDGMENT
The authors thank Prof. Y. Shibata and Prof. K. Takahashi (University of Tokyo, Japan) for antisera and indicator cells.
Footnotes
This work was supported by an Inje University research grant (2004).
References
- 1.Maheshwari A, Christensen RD, Calhoun DA. Immune-mediated neutropenia in the neonate. Acta Paediatr Suppl. 2002;91:98–103. doi: 10.1111/j.1651-2227.2002.tb02912.x. [DOI] [PubMed] [Google Scholar]
- 2.Christensen RD, Calhoun DA, Rimsza LM. A practical approach evaluating and treating neutropenia in the neonatal intensive care unit. Clin Perinatol. 2000;27:577–601. doi: 10.1016/s0095-5108(05)70040-3. [DOI] [PubMed] [Google Scholar]
- 3.Maheshwari A, Christensen RD, Calhoun DA. Resistance to recombinant human granulocyte colony-stimulating factor in neonatal alloimmune neutropenia associated with anti-human neutrophil antigen-2a (NB1) antibodies. Pediatrics. 2002;109:E64. doi: 10.1542/peds.109.4.e64. [DOI] [PubMed] [Google Scholar]
- 4.Gilmore MM, Stroncek DF, Korones DN. Treatment of alloimmune neonatal neutropenia with granulocyte colony-stimulating factor. J Pediatr. 1994;125:948–951. doi: 10.1016/s0022-3476(05)82014-1. [DOI] [PubMed] [Google Scholar]
- 5.Zupanska B, Uhrynowska M, Guz K, Maslanka K, Brojer E, Czestynska M, Radomska I. The risk of antibody formation against HNA1a and HNA1b granulocyte antigens during pregnancy and its relation to neonatal neutropenia. Transfus Med. 2001;11:377–382. doi: 10.1046/j.1365-3148.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 6.Levine DH, Madyastha PR. Isoimmune neonatal neutropenia. Am Perinatol. 1986;3:231–233. doi: 10.1055/s-2007-999873. [DOI] [PubMed] [Google Scholar]
- 7.Skacel PO, Stacey TE, Tidmarsh CE, Contreras M. Maternal alloimmunization to HLA, platelet and granulocyte-specific antigens during pregnancy: its influence on cord blood granulocyte and platelet counts. Br J Haematol. 1989;71:119–123. doi: 10.1111/j.1365-2141.1989.tb06284.x. [DOI] [PubMed] [Google Scholar]
- 8.Bux J, Jung KD, Kauth T, Mueller-Eckhardt C. Serological and clinical aspects of granulocyte antibodies leading to alloimmune neonatal neutropenia. Transfus Med. 1992;2:143–149. doi: 10.1111/j.1365-3148.1992.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 9.Bux J, Chapman J. Report on the second international granulocyte serology workshop. Transfusion. 1997;37:977–983. doi: 10.1046/j.1537-2995.1997.37997454028.x. [DOI] [PubMed] [Google Scholar]
- 10.Hagimoto R, Koike K, Sakashita K, Ishida T, Nakazawa Y, Kurokawa Y, Kamijo T, Saito S, Hiraoka A, Kobayashi M, Komiyama A. A possible role for maternal HLA antibody in a case of alloimmune neonatal neutropenia. Transfusion. 2001;41:615–620. doi: 10.1046/j.1537-2995.2001.41050615.x. [DOI] [PubMed] [Google Scholar]
- 11.Stroncek D. Granulocyte antigens and antibody detection. Vox Sang. 2004;87:91–94. doi: 10.1111/j.1741-6892.2004.00439.x. [DOI] [PubMed] [Google Scholar]
- 12.Araki N, Nose Y, Kohsaki M, Mito H, Ito K. Anti-granulocyte antibody screening with extracted granulocyte antigens by a micro-mixed passive hemagglutination method. Vox Sang. 1999;77:44–51. doi: 10.1159/000031073. [DOI] [PubMed] [Google Scholar]
- 13.Manroe BL, Weinberg AG, Rosenfeld CR, Browne R. The neonatal blood count in health and disease. I. Reference values for neutrophilic cells. J Pediatr. 1979;95:89–98. doi: 10.1016/s0022-3476(79)80096-7. [DOI] [PubMed] [Google Scholar]
- 14.Mouzinho A, Rosenfeld CR, Sanchez PJ, Risser R. Revised reference ranges for circulating neutrophils in very-low-birth-weight neonates. Pediatrics. 1994;94:76–82. [PubMed] [Google Scholar]
- 15.Helmerhorst FM, van Oss CJ, Bruynes EC, Engelfriet CP, von dem Borne AE. Elution of granulocyte and platelet antibodies. Vox Sang. 1982;43:196–204. doi: 10.1111/j.1423-0410.1982.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 16.Bux J, Stein EL, Santoso S, Mueller-Eckhardt C. NA gene frequencies in the German population, determined by polymerase chain reaction with sequence-specific primers. Transfusion. 1995;35:54–57. doi: 10.1046/j.1537-2995.1995.35195090663.x. [DOI] [PubMed] [Google Scholar]
- 17.Clague HD, Fung YL, Minchinton RM. Human neutrophil antigen-4a gene frequencies in an Australian population, determined by new polymerase chain reaction method using sequence-specific primers. Transfus Med. 2003;13:149–152. doi: 10.1046/j.1365-3148.2003.00435.x. [DOI] [PubMed] [Google Scholar]
- 18.Simsek S, van der Schoot CE, Daams M, Huiskes E, Clay M, McCullough J, van Dalen C, Stroncek D, von dem Borne AE. Molecular characterization of antigenic polymorphisms (Onds(a) and Mart(a)) of the beta 2 family recognized by human leukocyte alloantisera. Blood. 1996;88:1350–1358. [PubMed] [Google Scholar]
- 19.Lucas GF, Metcalfe P. Platelet and granulocyte glycoprotein polymorphisms. Transfus Med. 2000;10:157–174. doi: 10.1046/j.1365-3148.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- 20.Han KS, Um TH. Frequency of neutrophil-specific antigens among Koreans using the granulocyte indirect immunofluorescence test (GIFT) Immunohematology. 1997;13:15–16. [PubMed] [Google Scholar]