Abstract
Spontaneous bacterial peritonitis (SBP) is an ascitic fluid infection as a complication of end stage liver disease. The outcome is related to the severity of hepatorenal function, gastrointestinal bleeding, and many others; however it is not well known whether the infection acquisition sites have an effect on the prognosis of SBP. In order to identify the prognostic significance of the acquisition sites, we studied 106 patients who were diagnosed as culture positive SBP between October 1998 and August 2003. Thirty-two episodes were nosocomial and 74 were community acquired. Gram-negative bacilli such as Escherichia coli were dominant in both of the nosocomial and community-acquired SBPs. Despite significantly higher resistance to cefotaxime in nosocomial isolates compared to community-acquired isolates (77.8% vs. 13.6%, p=0.001), no difference was found regarding short or long term prognosis. Infection acquisition sites were not related to short or long term prognosis either. Shock, gastrointestinal bleeding and renal dysfunction were related to short term prognosis. Only Child-Pugh class C was identified as an independent prognostic factor of long-term survival.
Keywords: Liver Cirrhosis, Peritonitis, Cross Infection, Community-Acquired Infections
INTRODUCTION
Spontaneous bacterial peritonitis (SBP), the infection of the ascitic fluid in the absence of primary source of infection, is a serious complication of end-stage liver disease. The prevalence of SBP in cirrhotic patients with ascites has been reported to range between 10% and 25% (1-3). More than 60% of such infections are due to enteric Gram-negative bacteria, mainly Enterobacteriaceae (4). Some of recent studies, however, showed an increased incidence of Gram-positive bacterial infections. Moreover, Staphylococcus aureus is increasingly recognized as an important pathogen in hospitalized cirrhotic patients (5,6).
For the past 20 yr, the prognosis of cirrhotic patients with SBP has improved greatly because of early recognition and effective antibiotics treatment, particularly with third generation cephalosporins. SBP, however, is still the most common cause of death along with variceal bleeding, with a mortality rate between 20% and 40% (3,6). Some factors were known to be associated with the prognosis of SBP such as the severity of underlying liver disease, residual renal function, variceal bleeding, and hepatic encephalopathy (1,7-12). It is still uncertain whether the infection acquisition sites have an effect on the prognosis of SBP or not.
This retrospective study was undertaken to analyze the prognostic significance of infection acquisition sites (nosocomial vs. community-acquired) in cirrhotic patients with SBP. In addition, we intended to compare the clinical and microbiological features between nosocomial and community-acquired SBP.
MATERIALS AND METHODS
Patients identification
From 1 October 1998 through 31 August 2003, all patients with liver cirrhosis and SBP who were admitted to Guro Hospital, Korea University, were included in the study. We reviewed the medical records retrospectively. A community-acquired SBP was considered in any case to be diagnosed during the first 72 hr of hospitalization and nosocomial SBP when the diagnosis was established after this period.
Diagnostic criteria and antimicrobial susceptibility testing
Paracentesis was performed for all patients at the admission. The diagnosis of SBP was based on the combination of positive ascitic fluid culture and and polymorphonuclear leukocyte (PMNL) count of >250 cells/ µL in the absence of clinical, radiological, or laboratory data suggesting secondary peritonitis (4). For the organisms' identification, ascitic fluid specimens were placed in blood culture bottles, incubated at 37℃ for 7 days. Cultures positive specimens were subcultured on specific agar plates. Antibiotic susceptibility was determined by disk diffusion method following the National Committee for Clinical Laboratory standard (NCCLS) guideline (13). Extended-spectrum β-lactamase (ESBL) producing organisms were detected by disc diffusion method using cefotaxime (30 µg) and cefotaxime/clavulanic acid (30 µg/10 µg), recommended by NCCLS (13).
Data collection
Comparative analysis was performed by data from both of nosocomial and community-acquired SBP patients. Data for the clinical manifestations, laboratory findings, etiology of cirrhosis, co-morbidities, empirical antibiotics, and the following parameters were collected; complications, mortality of 10 days (short-term prognosis), mortality of 6 months (long-term prognosis). The severity of liver disease was assessed by the Child-Pugh scoring system, in which 5-15 points are assigned and a higher score indicates greater severity. Patients in Child-Pugh class A have preserved liver function (5-6 points), patients in class B have mild liver dysfunction (7-9 points), and patients in class C have severe liver dysfunction (10-15 points) (14).
Statistical analysis
Data were analyzed with SPSS 10.0. A p-value of <0.05 was considered stastically significant. The results were reported as mean±SD. The Student t-test was used to compare quantitative data, and the chi-square test was used to compare qualitative variables. With the significant prognostic variables obtained from the univariated analysis, multivariated analysis was carried out using a stepwise logistic regression model.
RESULTS
Patient characteristics
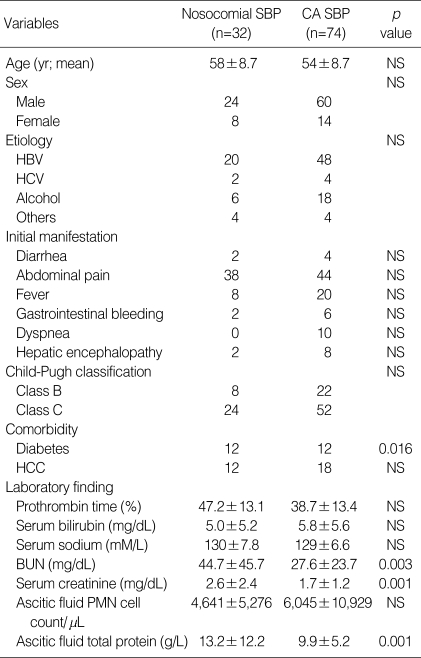
The demographic, clinical and laboratory data at the time of diagnosis of SBP are presented in Table 1. The study included 106 cirrhotic patients (84 male subjects and 22 female subjects) with mean age of 55.3 yr. Thirty-two episodes (30.2%) were nosocomial and 74 (69.8%) were community-acquired. There was no significant difference in age and sex between patients with community-acquired SBP and those with nosocomial SBP. In nosocomial SBP, 8 (25.0%) subjects were in Child-Pugh class B, and 24 (75.0%) subjects were in class C. Likewise, 22 (29.7%) class B and 52 (70.3%) class C subjects were found in community-acquired SBP. Generally, class C was dominant and no subject was in class A. Hepatitis B was a dominant cause of cirrhosis in both groups of patients. Patients complained of abdominal pain, febrile sense, gastrointestinal bleeding, diarrhea, dyspnea, and others. Diabetes was a more common comorbidity in patients with nosocomial SBP than in those with community-acquired SBP (37.5% vs. 16.2%, p=0.016). Serum creatinine, BUN, and ascitic fluid total protein levels were higher in nosocomial SBP comparing to community-acquired SBP. Otherwise, there was no significant difference between nosocomial and community-acquired episodes regarding clinical and laboratory data.
Table 1.
Demographic, clinical and laboratory data of spontaneous bacterial peritonitis (SBP) patients at the time of diagnosis
CA, community-acquired; HBV, Hepatitis B; HCV, Hepatitis C; HCC, hepatocellular carcinoma.
Causative microorganisms and antimicrobial resistance
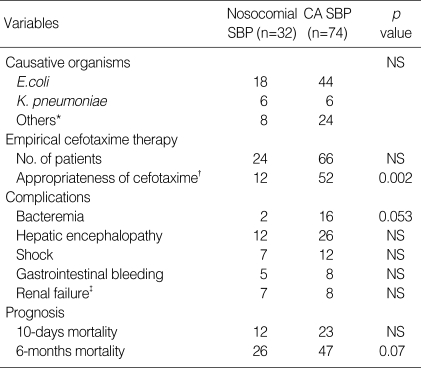
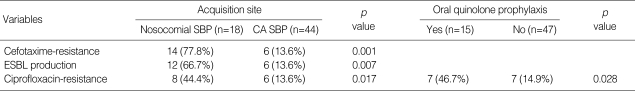
Organisms responsible for SBP, isolated from ascitic fluid, are shown in Table 2. Among the nosocomial SBP, 18 (56.3%) isolates were E. coli, 6 (18.8%) were Klebsiella pneumoniae, and 8 (24.9%) were the rest. The causative organisms were Enterobacteriaceae in 50 (67.6%) episodes of community-acquired SBP (44 cases of E. coli, 6 cases of K. pneumoniae). E. coli was predominant in both types of SBP (>50% of total). However, S. aureus was not isolated in both nosocomial and community-acquired SBPs. E. coli was isolated predominantly as described above, therefore we studied cefotaxime resistance and ESBL producing rate of E. coli (Table 3). As for the nosocomial SBP, the cefotaxime resistance rate was higher comparing to community-acquired SBP (77.8% vs. 13.6%, p=0.001), and ESBL producing rate was also noted higher (66.7% vs. 13.6%, p=0.007). Among cefotaxime resistant E. coli, 2 nosocomial isolates were ESBL negative by disc diffusion method.
Table 2.
Comparison of causative organisms, appropriateness of empirical cefotaxime therapy, complications and prognosis between nosocomial and community-acquired (CA) spontaneous bacterial peritonitis (SBP)
*Others included Streptococcus pneumoniae (8), other streptococci (8), enterococci (6), Pseudomonas aeruginosa (2), Acinetobacter baumannii (6) and Aeromonas hydrophila (2); †Appropriateness was determined by antibiotic susceptibility test; ‡Serum creatinine level increased 3.0 mg/dL more than baseline value.
Table 3.
Antibiotic resistance (%) in E. coli isolates from spontaneous bacterial peritonitis (SBP) patients according to acquisition sites and previous quinolone prophylaxis *
CA, community-acquired; ESBL, extended spectrum β-lactamase.
*Oral quinolone use within recent 1 month.
Cefotaxime was given to most of the patients (75% of nosocomial SBP, 89.2% of community-acquired SBP) as an empirical therapy. We assessed the appropriateness of empirical treatment, depending on the result of antibiotic susceptibility test. The result showed that cefotaxime was rather effective in community-acquired SBP (52, 72.2%), but it was the opposite for nosocomial SBP (12, 37.5%); cefotaxime was not appropriate for empirical treatment of nosocomial SBP (p=0.05, Table 2).
Ciprofloxacin resistance is considered to be increasing because of oral quinolone use in cirrhotic patients for prevention of recurrent SBP. We compared the ciprofloxacin resistance of E. coli isolates from nosocomial and community-acquired SBP patients (Table 3). Ciprofloxacin resistance was noted in 8 (44.4%) cases of nosocomial SBP, and 6 (13.6%) of community-acquired SBP. Ciprofloxacin resistance rate tended to be higher in cases of nosocomial SBP (p=0.017). When it came to quinolone prophylaxis (Table 3), ciprofloxacin resistance rate was also higher in patients with previous oral quinolone prophylaxis within a month (46.7% vs. 14.9%, p=0.028). Accordingly, quinolone resistance could be considered in the treatment of nosocomial SBP and patients who had received oral quinolone prophylaxis within a previous month.
Prognosis and complications of SBP
We tried to identify the influence on complications and prognosis of SBP according to the acquisition sites. We compared the complications of SBP (concomitant bacteremia, hepatic encephalopathy, shock, gastrointestinal bleeding, renal dysfunction, and hepatic failure), 10 days mortality (short-term prognosis), and 6 months mortality (long-term prognosis) between nosocomial and community-acquired SBPs. As for the complications, no significant difference was noted, and neither 10 days nor 6 months mortality showed any statistically significant difference (Table 2). Six months mortality though, was relatively higher in nosocomial SBP (p=0.07).
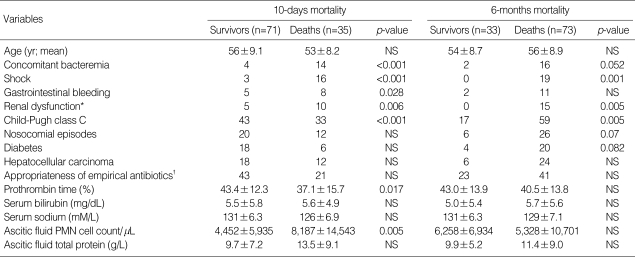
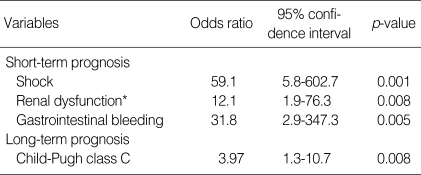
Acquisition site (p=0.519) or appropriateness of empirical antibiotic treatment (p=0.956) did not have an effect on 10 days mortality, which was rather related to some factors, reflecting severity of underlying liver disease or SBP, such as concomitant bacteremia (p=<0.001), shock (p=<0.001), gastrointestinal bleeding (p=0.028), renal dysfunction (p=0.006) and Child-Pugh class C (p=<0.001) as shown in Table 4. In the multivariate analysis, 3 of them were independently correlated with 10 days mortality: gastrointestinal bleeding (odds ratio=31.8), shock (odds ratio=59.1), and renal dysfunction (odds ratio=12.1) (Table 5). Although Child-Pugh class C (p=0.005) as well as concomitant bacteremia (p=0.052), shock (p=0.001) and renal dysfunction (p=0.005) was closely associated with 6 months mortality on univariate analysis (Table 4), only Child-Pugh class C was identified as an independent prognostic factor of long-term survival (odds ratio=3.97) (Table 5). Neither inappropriate initial, empirical antibiotic therapy nor nosocomial episodes were found to affect long term prognosis of SBP.
Table 4.
Variables investigated as possible prognostic factors of spontaneous bacterial peritonitis (SBP)
*Serum creatinine level increased 3.0 mg/dL more than baseline value; †Appropriateness was determined by antibiotic susceptibility test to cefotaxime.
Table 5.
Poor prognostic factors independently related to 10 days/6 months mortality of spontaneous bacterial peritonitis (SBP)
*Serum creatinine level increased 3.0 mg/dL more than baseline value.
DISCUSSION
SBP is a serious complication of cirrhosis in patients who already have the complication of ascites. Even with early diagnosis and prompt therapy, the mortality of an episode of SBP is still approximately 30%, comparable to the mortality of an episode of variceal bleeding. In this study, early SBP-induced mortality rate (10 days mortality) was about the same, with the rate of 32.1% (37.5% of nosocomial episodes vs. 29.7% of community-acquired episodes), and 67.5% of the patients died within 6 months time.
On admission, BUN and creatinine levels were higher and diabetes was a more common comorbidity in patients with nosocomial SBP than in those with community-acquired SBP. In comparison, ascitic fluid protein level was significantly lower in community-acquired SBP than in nosocomial SBP in our study (p=0.001). There was a chance that low protein level might contribute to the SBP occurrence in community stay, in which infection risk was relatively lower than in hospital stay. In the present study, 69.8% of SBP episodes were community-acquired infection. The results rated a bit higher than prior reports (6,15). Our study shows that infection acquisition sites do not have an effect on the outcome of SBP unlike previous studies. Eleven years ago, Toledo et al. reported that community-acquired vs. hospital-acquired peritonitis independently correlate with survival (12). However, selective intestinal decontamination was not popular, and fluoroquinolone use was strictly restricted at the time. Furthermore, their study population was confined to patients treated with cefotaxime, so there was a chance that prognostic significance might have changed. Recently, Fernandez et al. prospectively studied the epidemiological changes of bacterial infections in cirrhotic patients after invasive procedures and norfloxacin prophylaxis; they reported that nosocomial infections had higher hospital mortality with marked increase of Gram positive infections, which was in comparison with the results of this study (16). However, only one fourth of included subjects were SBP patients in that study. Likewise, Campillo et al. also observed that nosocomial and staphylococcal infections were associated with a higher mortality rate than community-acquired, non-staphylococcal infections (17). They intended to evaluate the prognostic significance of isolate type (infecting organisms and acquisition sites) but a few limitations were noted to identify prognostic significance of acquisition sites in SBP; most of the episodes were nosocomial (93.3%), and bacteremic episodes without SBP were included. Evans et al. observed that asymptomatic outpatients had better prognosis than the hospitalized ones (18). Most of outpatients however, had culture-negative neutrocytic ascites (CNNA). Bert et al. also compared the characteristics of nosocomial and community acquired episodes of ascitic fluid infection, but more than half of the episodes were CNNA and non-neutrocytic bacterascites (15). We confined the diagnostic criteria as culture positive neutrocytic ascites. This is the first comparative study mainly designed to assess the prognostic significance of infection acquisition sites in culture positive SBP.
On the other hand, residual renal function, gastrointestinal hemorrhage, ascitic fluid PMNL count, serum sodium level, bilirubin, and prothrombin time were known as prognostic factors, previously (1,8,10,12,19). In the present study, shock, gastrointestinal bleeding, and renal dysfunction showed a significant correlation with short-term prognosis (early mortality in hospital). The most important prognostic factor was the hemodynamic derangement including shock and renal impairment. Ruiz-del-Arbol et al. had also reported that hemodynamic impairment is associated with an extremely poor prognosis despite rapid resolution of infection (20). Underlying liver function was the only independent prognostic factor of long-term survival in SBP.
Another important finding of this study was that empirical cefotaxime treatment did not affect the prognosis notably in spite of high resistance rate in causative organisms of nosocomial SBP. Traditionally, Enterobacteriaceae are known as predominant organisms causing SBP. Some studies however, reported that streptococci were most common in community-acquired episodes, and others showed that S. aureus infection is increasing in nosocomial episodes (3,5,15). Contrarily, we found out that Enterobacteriaceae including E. coli and K. pneumoniae were still predominant in both community-acquired and nosocomial episodes. E. coli was isolated at more than half of episodes, so we studied cefotaxime resistance and ESBL producing rate of E. coli. Both cefotaxime resistance and ESBL producing rate were significantly higher in nosocomial SBP cases. The remarkable point is that ESBL producing rate of E. coli was 66.7% of nosocomial SBP vs. 13.6% of community-acquired SBP. The ESBL producing rate among E. coli in Korea increased significantly from 1% in the 1980s to 30% in the 1990s, and nowadays it is reaching 35% (3). Depending on above data, uniform cefotaxime therapy was not warranted, with the need of secondary antibiotics such as carbapenem. Inappropriate empirical antibiotic therapy though, was not correlated with short-term prognosis of SBP and some reported previously that cefotaxime cured SBP infection despite in vitro resistance; the efficient penetration and greater concentration of cefotaxime in ascitic fluid may account for this finding (12). Therefore, further study is required on the appropriateness of empirical cefotaxime therapy in SBP. On the other hand, ciprofloxacin resistance rate was also remarkably high in nosocomial SBP and patients who had received oral quinolone prophylaxis within 1 month. Because of remarkable resistance rate (>20%), empirical ciprofloxacin is unrecommendable to those patients.
The main limitation of the present study is that it was performed retrospectively. It was observational and not randomized therefore further study is expected in the future.
In conclusion, infection acquisition sites do not have a prognostic significance in SBP. Prognosis is also irrelevant whether empirical antibiotics are appropriately used or not. Though there is not statistical significance, yet careful consideration is required if cefotaxime can be used as an effective empirical agent in nosocomial SBP. Further study is warranted on the appropriateness of empirical antibiotics in SBP.
Footnotes
This work was supported by a grant No (HMP-00-CH-09-0008) from Korea Health Industry Development Institute (KHIDI).
References
- 1.Llovet JM, Planas R, Morillas R, Quer JC, Cabre E, Boix J, Humbert P, Guilera M, Domenech E, Bertran X. Short-term prognosis of cirrhotics with spontaneous bacterial peritonitis: multivariate study. Am J Gastroenterol. 1993;88:388–392. [PubMed] [Google Scholar]
- 2.Mesquita MA, Balbino EP, Albuquerque RS, Carmona CA, Okubo BT, Lorena SL, Montes CG, Soares EC. Ceftriaxone in the treatment of spontaneous bacterial peritonitis: ascitic fluid polymorphonuclear count response and short-term prognosis. Hepatogastroenterology. 1997;44:1276–1280. [PubMed] [Google Scholar]
- 3.Song HG, Lee HC, Joo YH, Jung S, Park YH, Ryu SH, Shin JW, Lee YJ, Chung YH, Lee YS, Suh DJ. Clinical and microbiological characteristics of spontaneous bacterial peritonitis (SBP) in a recent five year period. Taehan Kan Hakhoe Chi. 2002;8:61–70. [PubMed] [Google Scholar]
- 4.Such J, Runyon BA. Spontaneous bacterial peritonitis. Clin Infect Dis. 1998;27:669–674. doi: 10.1086/514940. [DOI] [PubMed] [Google Scholar]
- 5.Campillo B, Dupeyron C, Richardet JP. Epidemiology of hospital-acquired infections in cirrhotic patients: effect of carriage of methicillin-resistant Staphylococcus aureus and influence of previous antibiotic therapy and norfloxacin prophylaxis. Epidemiol Infect. 2001;127:443–450. doi: 10.1017/s0950268801006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guarner C, Sola R, Soriano G, Andreu M, Novella MT, Vila MC, Sabat M, Coll S, Ortiz J, Gomez C, Balanzo J. Risk of a first community-acquired spontaneous bacterial peritonitis in cirrhotics with low ascitic fluid protein levels. Gastroenterology. 1999;117:414–419. doi: 10.1053/gast.1999.0029900414. [DOI] [PubMed] [Google Scholar]
- 7.Follo A, Llovet JM, Navasa M, Planas R, Forns X, Francitorra A, Rimola A, Gassull MA, Arroyo V, Rodes J. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: incidence, clinical course, predictive factors and prognosis. Hepatology. 1994;20:1495–1501. doi: 10.1002/hep.1840200619. [DOI] [PubMed] [Google Scholar]
- 8.Franca AV, De Souza JB, Silva CM, Soares EC. Long-term prognosis of cirrhosis after spontaneous bacterial peritonitis treated with ceftriaxone. J Clin Gastroenterol. 2001;33:295–298. doi: 10.1097/00004836-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Jepsen P, Vilstrup H, Moller JK, Sorensen HT. Prognosis of patients with liver cirrhosis and spontaneous bacterial peritonitis. Hepatogastroenterology. 2003;50:2133–2136. [PubMed] [Google Scholar]
- 10.Ljubicic N, Spajic D, Vrkljan MM, Altabas V, Doko M, Zovak M, Gacina P, Mihatov S. The value of ascitic fluid polymorphonuclear cell count determination during therapy of spontaneous bacterial peritonitis in patients with liver cirrhosis. Hepatogastroenterology. 2000;47:1360–1363. [PubMed] [Google Scholar]
- 11.Thuluvath PJ, Morss S, Thompson R. Spontaneous bacterial peritonitis in-hospital mortality, predictors of survival, and health care costs from 1988 to 1998. Am J Gastroenterol. 2001;96:1232–1236. doi: 10.1111/j.1572-0241.2001.03708.x. [DOI] [PubMed] [Google Scholar]
- 12.Toledo C, Salmeron JM, Rimola A, Navasa M, Arroyo V, Llach J, Gines A, Gines P, Rodes J. Spontaneous bacterial peritonitis in cirrhosis: predictive factors of infection resolution and survival in patients treated with cefotaxime. Hepatology. 1993;17:251–257. [PubMed] [Google Scholar]
- 13.NCCLS. Performance standards for antimicrobial susceptibility testing. 13th international supplement NCCLS document. 2003. p. M100-S13 (M7). [Google Scholar]
- 14.Pugh RN. Pugh's grading in the classification of liver decompensation. Gut. 1992;33:1583. doi: 10.1136/gut.33.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bert F, Andreu M, Durand F, Degos F, Galdbart JO, Moreau R, Branger C, Lambert-Zechovsky N, Valla D. Nosocomial and community-acquired spontaneous bacterial peritonitis: comparative microbiology and therapeutic implications. Eur J Clin Microbiol Infect Dis. 2003;22:10–15. doi: 10.1007/s10096-002-0840-z. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez J, Navasa M, Gomez J, Colmenero J, Vila J, Arroyo V, Rodes J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 17.Campillo B, Richardet JP, Kheo T, Dupeyron C. Nosocomial spontaneous bacterial peritonitis and bacteremia in cirrhotic patients: impact of isolate type on prognosis and characteristics of infection. Clin Infect Dis. 2002;35:1–10. doi: 10.1086/340617. [DOI] [PubMed] [Google Scholar]
- 18.Evans LT, Kim WR, Poterucha JJ, Kamath PS. Spontaneous bacterial peritonitis in asymptomatic outpatients with cirrhotic ascites. Hepatology. 2003;37:897–901. doi: 10.1053/jhep.2003.50119. [DOI] [PubMed] [Google Scholar]
- 19.Perdomo Coral G, Alves de Mattos A. Renal impairment after spontaneous bacterial peritonitis: incidence and prognosis. Can J Gastroenterol. 2003;17:187–190. doi: 10.1155/2003/370257. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-del-Arbol L, Urman J, Fernandez J, Gonzalez M, Navasa M, Monescillo A, Albillos A, Jimenez W, Arroyo V. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38:1210–1218. doi: 10.1053/jhep.2003.50447. [DOI] [PubMed] [Google Scholar]