Abstract
Neopterin is a pyrazino-pyrimidine compound, and is known to be a marker associated with cell-mediated immunity in various diseases. We hypothesized that the levels of serum and urine neopterin would be elevated in renal disease, and would correlate with certain clinical parameters. We evaluated serum and urinary neopterin levels in patients with several renal diseases, including nephrotic syndrome (NS, n=19), chronic renal failure (CRF, n=8), end stage renal disease (ESRD, n=64) and controls (n=22). Serum neopterin was elevated in patients with CRF and ESRD, as compared to controls. Urinary neopterin levels were also found to be elevated in patients with CRF and ESRD, as compared to controls. Serum neopterin levels showed significant positive correlation with age, serum BUN and creatinine levels, and inverse correlation with WBC, hemoglobin, hematocrit, serum albumin and total iron binding capacity. Urine neopterin levels exhibited positive correlation with age and serum creatinine levels, and inverse correlation with WBC, hemoglobin, hematocrit, BUN and serum albumin. In conclusion, increased serum and urinary neopterin levels were found in some patients with renal disease and were correlated with certain clinical parameters.
Keywords: Kidney Diseases, Neopterin, Nephrotic Syndrome, Kidney Failure
INTRODUCTION
Neopterin is an intermediate product of biopterin, a nerve transmitter coenzyme. It is a pyrazino-pyrimidine compound, synthesized by monocytes and macrophages as a result of stimulation by endotoxin and interferon γ in activated T cells. Neopterin levels in body fluids are elevated in a variety of diseases in which T cells or macrophages are activated (1,2). Therefore, neopterin levels can be useful for the evaluation of disease activity and prognosis in a variety of conditions (3-7).
Lin reported that increased urinary neopterin levels are related to increases in interlukin-2 and the interferon γ observed in the membranous nephropathy associated with hepatitis B, which is generally accompanied by the nephrotic syndrome or severe proteinuria (3). He noted that neopterin is not specific in this case, but it is clear that neopterin is useful in the monitoring the clinical course, or even as a prognostic marker. Several studies have reported that neopterin levels are increased in serum or urine in the nephrotic syndrome, in chronic and acute glomerulonephritis, and in chronic renal disease (4-7). Fuchs et al. reported that neopterin levels were increased in end-stage renal disease patients who had undergone hemodialysis for more than 1 yr (8). They attributed this to various interactions between blood coagulation molecules and the hemodialysis membrane, as well as to the activated cellular immune response associated with exposure to the dialysis membrane.
The purpose of our study was to determine whether serum and urine neopterin levels were elevated in some renal diseases, including nephrotic syndrome (NS), chronic renal failure (CRF), and end-stage renal disease (ESRD). We also assessed correlations between serum and urinary neopterin levels and some of the clinical parameters seen in these conditions.
MATERIALS AND METHODS
Subjects
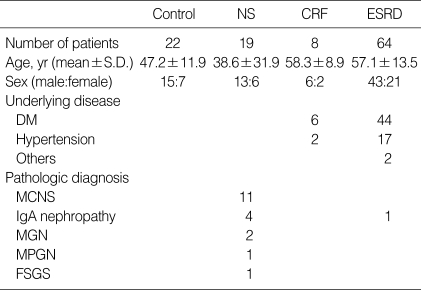
The study group was composed of 91 persons who were outpatients or inpatients at the Kangbuk Samsung Hospital in June 2002. This group included 19 NS patients whose diseases had been proven by biopsy, 8 CRF patients with glomerular filtration rates of 10-60 mL/min/1.73 m2 and 64 ESRD patients who had undergone maintenance hemodialysis for more than 3 months. The pathologic diagnoses of the NS patients included 11 patients with minimal-change nephrotic syndrome, 4 with IgA nephropathy, 2 with membranous glomerulonephritis, 1 with membrano-proliferative glomerulonephritis, and 1 case of focal segmental glomerulosclerosis. The underlying causes of CRF included 6 cases of diabetes mellitus, and 2 patients with severe hypertension. The underlying causes of ESRD included 44 cases of diabetes mellitus, 17 cases of hypertension, 1 with IgA nephropathy, 1 patient with systemic lupus nephritis, and 1 with autosomal dominant polycystic kidney disease. The controls were selected in order to determine a normal range for neopterin. They were 22 patients with GFR values of more than 90 mL/min/1.73 m2. Control subjects had no renal disease, hepatic disease, infections, or autoimmune diseases (Table 1).
Table 1.
Demographic data of the patient population
NS, nephrotic syndrome; CRF, chronic renal failure; ESRD, end-stage renal disease; DM, diabetes mellitus; MCNS, minimal change nephrotic syndrome; MGN, membranous glomerulonephritis; MPGN, membrano-proliferative glomerulonephritis; FSGS, focal segmental glomerulosclerosis.
Methods
Serum neopterin was measured as follows: 3 mL of serum was obtained from a clotted blood sample collected in a shaded tube, as neopterin is sensitive to light. In ESRD patients, blood samples were taken before hemodialysis. The tube was maintained at 4℃. Serum and urinary neopterin levels were measured by radioimmune assay (ICN Biomedicals, Costa Mesa, California, U.S.A.) within 30 min, using a double antibody technique. In order to measure urinary neopterin levels, 100 mL of a first-void morning urine was collected from patients who were able to urinate. The urine was kept in a urine cup which was shaded and maintained at 4℃. The specimens were then centrifuged for 10 min, and the supernatant was separated. Creatinine levels were measured in the supernants, and the remaining urine was diluted with neopterin urine buffer to concentrations of 1:50 and 1:500. The creatinine levels of the remaining urine were then measured. Neopterines are excreted by the kidney, and therefore urine neopterin levels were higher than serum neopterin levels. Urinary neopterin levels are expressed per mole of creatinine so as to standardize differences in renal function vs. neopterin excretion.
Statistical analysis
Statistical analysis of the data was performed with SPSS version 11.0 software, and continuous variables were presented as means±standard deviation. Comparisons between each renal disease group and the control group were carried out using the Mann-Whitney test. The relationship between serum and urinary neopterin levels and clinical parameters was determined using the Spearman correlation analysis and the linear regression method. p values of less than 0.05 were considered to be statistically significant.
RESULTS
Serum neopterin
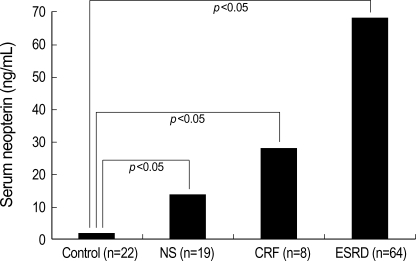
Serum neopterin levels were elevated in patients with NS (14.1±30.9 ng/mL), CRF (28.2±19.4 ng/mL), and ESRD (68.6±25.5 ng/mL) in comparison to control subjects (1.6 ±0.3 ng/mL). Patients with CRF and ESRD, in particular, exhibited statistically significant elevated levels of serum neopterin compared to controls (p<0.05) (Fig. 1).
Fig. 1.
Levels of serum neopterin in patient population.
NS, nephrotic syndrome; CRF, chronic renal failure; ESRD, end-stage renal disease.
Urine neopterin
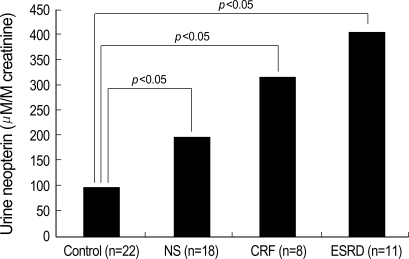
Urinary neopterin levels were elevated in patients with NS (203.2±349.6 µM/M creatinine), CRF (319.2±107.7 µM/M creatinine) and ESRD (407.9±256.9 µM/M creatinine), in comparison to controls (108.9±57.9 µM/M creatinine). Patients with CRF and ESRD, in particular, exhibited statistically significant higher urine neopterin than control (p<0.05) (Fig. 2).
Fig. 2.
Levels of urine neopterin in patient population.
NS, nephrotic syndrome; CRF, chronic renal failure; ESRD; end-stage renal disease.
The relationship between serum neopterin and other clinical parameters
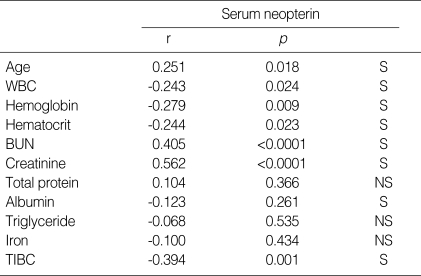
Serum neopterin levels were positively correlated with age, BUN and serum creatinine levels. Serum neopterin levels exhibited inverse correlation with WBC, hemoglobin, hematocrit, serum albumin and total iron binding capacity (Table 2).
Table 2.
Correlation between serum neopterin and clinical parameters
TIBC, total iron binding capacity; r, regression coefficient; S, statistically significant defined by p<0.05; NS, statistically non-significant defined by p≥0.05.
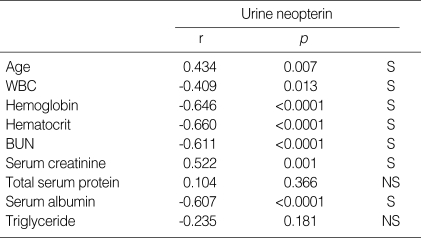
The relationship between urine neopterin and clinical parameters
Urine neopterin levels exhibited a positive correlation with age and serum creatinine levels (p<0.05). Urine neopterin levels showed significant inverse correlation with WBC, hemoglobin, hematocrit, BUN and serum albumin (p<0.05) (Table 3).
Table 3.
Correlation between urine neopterin and clinical parameters
r, regression coefficient; S, statistically significant defined by p<0.05; NS, statistically non-significant defined by p≥0.05.
DISCUSSION
Neopterin, which is a biochemical marker of cellular immune response, belongs to the group of compounds known as pteridines. Pteridines are pyrazino-(2,3-d-) pyrimidine derivative compounds which are synthesized from guanosine triphosphate. Neopterin is 253 daltons in size, twice the size of creatinine (113 daltons). The first pteridines were initially identified in 1889, and in 1979, Wachter et al. reported that urine neopterin levels were elevated in cases of viral infections and tumors (9). Recently, a marked abnormality in pteridine metabolism has been noted in cases of chronic renal disease (10). Neopterin is produced in monocytes and macrophages as a result of stimulation by interferon γ and endotoxin. The mechanism underlying the generation of neopterin involves the accumulation of precursor 7,8-dihydroneopterin triphosphate, due to deficiencies in 6-pyruvoyl tetrahydropterin synthetase. This precursor is then converted to neopterin by phosphatase. Neopterin is also associated with the activity of T-lymphocytes, monocytes and macrophages, and it is closely associated with the ability of these cells to break down hydrogen peroxide (1,2).
In recent studies, increased serum and urinary neopterin levels have been used as diagnostic or prognostic markers in several conditions. These include: various immune-mediated clinical conditions, including transplantation rejection (11); hepatitis and other viral infections, such as human immunodeficiency virus and cytomegalovirus; bacterial infections (3,12, 13); autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis; tumors, such as hepatocellular carcinoma, stomach cancer, or uroepithelial cancer (14,15); congestive heart failure; renal failure; coronary artery disease; and, myocardial infarction. Weiss et al. reported that neopterin is a marker for disease progression and complication in diabetes mellitus, such as glycation end-product pentosidine, and other inflammatory molecules, such as interleukin-6 and C-reactive protein (CRP) are (16). Murr et al. reported that serum neopterin is closely related to the activation of immune mechanisms in anemia, weight loss, or cachexia in malignant tumors or chronic disease, γ and is also associated with interferon γ and tumor necrosis factor-α(17). Therefore, increased neopterin levels are observed primarily in diseases in which monocytes or macrophages have been activated. Thus, they provide valuable information with regard to cellular immunity, acting not only as an initial inflammatory marker, but also as a predictive factor for the progression of disease. In our study, we did not measure CRP or cytokines such as interleukin-6, interferon γ and tumor necrosis factor-α . However, serum neopterin had an inverse correlation with other proteins that decline in the presence of inflammation, such as albumin and total iron binding capacity.
In our study, serum and urine neopterin levels were increased in patients with CRF and ESRD. Serum neopterin levels showed a positive correlation with serum creatinine levels. These results are consistent with the report of Godai et al., in that serum neopterin levels have a positive relationship with creatinine, and a negative relationship with creatinine clearance (4). Pecoits-Filho et al. also reported that neopterin levels were significantly higher in the subgroup of patients with a low glomerular filtration rate, suggesting either impaired renal elimination, increased generation in uremia, or an adverse effect of inflammation on renal function (18). In our study, there was a trend towards increased serum and urine neopterin levels in patients with NS compared to the control group, but it was not statistically significant. The standard deviation of serum and urinary neopterin levels in patients with NS was greater than in patients with other renal diseases. Oda et al. reported that in patients with steroid-responsive nephrotic syndrome, elevated neopterin levels decreased after steroid treatment. However, in a steroid-resistant group, neopterin levels increased significantly. They suggested that steroid therapy may decrease increased neopterin production by normalizing disturbed T-cell function (7).
Serum neopterin varies in response to monocyte and macrophage activation, according to increases in the immune response. Activation of monocytes and macrophages results in iron deficiency, due to the utilization of iron by the blood. Iron deficiency stimulates increased neopterin release, and neopterin aggravates anemia via the indirect inhibition of hematopoiesis. Berdowska et al. reported that serum neopterin levels are inversely related to iron, transferrin, and hemoglobin, and are positively related to levels of ferritin, which functions as an intracellular iron carrier (19). In our study, serum neopterin levels were also inversely related to hemoglobin levels and the total iron binding capacity. Anemia treatment in patients with CRF and ESRD may have some influence on our results.
The present study has several limitations. Our study did not directly relate serum and urinary neopterin levels with observed monocyte or macrophage activation. Also, we were not sure of the validity of urinary neopterin or creatinine in patients with ESRD, since these patients are mostly oliguric.
In conclusion, serum and urinary neopterin levels were elevated in patients with certain renal diseases. In addition, neopterin levels were correlated with some clinical parameters in those patients. We suggest that serum and urinary neopterin levels may be useful marker for predicting disease activity and prognosis in some patients with renal disease. This should be confirmed by a prospective long term study in a larger group of patients.
References
- 1.Huber C, Batchelor JR, Fuchs D, Hausen A, Lang A, Niederwieser D, Reibnegger G, Swetly P, Troppmair J, Wachter H. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med. 1984;160:310–316. doi: 10.1084/jem.160.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoedon G, Troppmair J, Fontana A, Huber C, Curtius HC, Niederwieser A. Biosynthesis and metabolism of pterins in peripheral blood mononuclear cells and leukemia lines of man and mouse. Eur J Biochem. 1987;166:303–310. doi: 10.1111/j.1432-1033.1987.tb13515.x. [DOI] [PubMed] [Google Scholar]
- 3.Lin CY. Urinary neopterin as a new biochemical marker for the monitoring of disease activity and prognosis in membranous nephropathy associated with hepatitis B surface antigenemia. Nephron. 1989;53:115–120. doi: 10.1159/000185722. [DOI] [PubMed] [Google Scholar]
- 4.Godai K, Uemasu J, Kawasaki H. Clinical significance of serum and urinary neopterins in patients wth chronic renal disease. Clin Nephrol. 1991;36:141–146. [PubMed] [Google Scholar]
- 5.Park YH, Moon KH, Park KK, Cho KW, Eum SH, Kim KS, Choi KC, Kang YJ. A study of serum neopterin levels in chronic renal failure patients undergoing hemodialysis. Korean J Intern Med. 1990;39:213–218. [Google Scholar]
- 6.Neale J, Hill J, Cooke R, Dunbar R. Neopterin quantitation indicates participation of cell mediated immunity in human glomerulonephritis. [abstract] Kidney Int. 1990;37:443. [Google Scholar]
- 7.Oda K, Arai T, Nagase M. Increased serum and urinary neopterin in nephrotic syndrome indicate cell-mediated immune dysfunction. Am J Kidney Dis. 1999;34:611–617. doi: 10.1016/S0272-6386(99)70383-5. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs D, Hausen A, Reibnegger G, Werner ER, Dittrich PV, Wachter H. Neopterin levels in long-term hemodialysis. Clin Nephrol. 1988;30:220–224. [PubMed] [Google Scholar]
- 9.Wachter H, Hausen A, Grassmayr K. Increased urinary excretion of neopterin in patients with malignant tumors and with virus diseases. Hoppeseyler's Z Physiol Chem. 1979;360:1957–1960. [PubMed] [Google Scholar]
- 10.Yokoyama K, Tajima M, Yoshida H, Nakayama M, Tokutome G, Sakagami H, Hosoya T. Plasma pteridine concentrations in patients with chronic renal failure. Nephrol Dial Transplant. 2002;17:1032–1036. doi: 10.1093/ndt/17.6.1032. [DOI] [PubMed] [Google Scholar]
- 11.Aulitzky WE, Tilg H, Niederwieser D, Riccabona G, Obendorf L, Margreiter R, Pfaller W, Huber C. Comparison of serum neopterin levels and urinary neopterin excretion in renal allograft recipients. Clin Nephrol. 1988;29:248–252. [PubMed] [Google Scholar]
- 12.Fahey JL, Taylor JM, Detels R, Hofmann B, Melmed R, Nishanian P, Giorgi JV. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322:166–172. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 13.Halota W, Jaruga B, Pawlowska M. Serum neopterin and beta2-microglobulin concentration as prognostic markers of AIDS [abstract] Pol Merkuriusz Lek. 2002;13:126–128. [PubMed] [Google Scholar]
- 14.Aulitzky W, Frick J, Fuchs D, Hausen A, Reibnegger G, Wachter H. Significance of urinary neopterin in patients with malignant tumor of the genitourinary tract. Cancer. 1985;55:1052. doi: 10.1002/1097-0142(19850301)55:5<1052::aid-cncr2820550521>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Bichler A, Fuchs D, Hausen A, Hetzel H, Konig K, Wachter H. Urinary neopterin excretion in patients with genital cancer. Clin Biochem. 1982;15:38–40. doi: 10.1016/s0009-9120(82)90421-0. [DOI] [PubMed] [Google Scholar]
- 16.Weiss MF, Rodby RA, Justice AC, Hricik DE, Collaborative Study Group Free pentosidine and neopterin as markers of progression rate in diabetic nephropathy. Kindey Int. 1998;54:193–202. doi: 10.1038/sj.ki.4495352. [DOI] [PubMed] [Google Scholar]
- 17.Murr G, Neurauter G, Wirleitner B, Fuchs D. Neopterin to predict prognosis of infection and neoplasia. International Eurogin-east conference; Sept; Vilnius. 2001. [Google Scholar]
- 18.Pecoits-Filho R, Heimburger O, Barany P, Suliman M, Fehrman-Ekholm I, Lndholm B, Stenvinkel P. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003;41:1212–1218. doi: 10.1016/s0272-6386(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 19.Berdowska A, Zwirska-Korczala K. Neopterin measurement in clinical diagnosis. J Clin Pharm Ther. 2001;26:319–329. doi: 10.1046/j.1365-2710.2001.00358.x. [DOI] [PubMed] [Google Scholar]