Abstract
The aim of this study was to analyze long-term survivals in patients with stage IB to IIA cervical cancer treated by neoadjuvant chemotherapy setting. Between February 1989 and January 1998, 94 women with previously untreated stage IB to IIA carcinoma of the uterine cervix who received cisplatin based neoadjuvant chemotherapy were enrolled in this study. All of patients with chemoresponse (complete response, n=15; partial response, n=47) and 16 patients with chemoresistance received radical surgery (RS group). The other 16 patients with chemoresistance received radiotherapy for definite treatment (RT group). In the RS group, the 10 yr survival estimation in patients with bulky tumors (diameter ≥4 cm, n=26) was similar to that with non-bulky tumors (83.3% vs. 89.3%, p=NS). In selected patients with chemoresistance, those treated by radiotherapy (n=16) showed significantly poorer survivals than those treated by radical surgery (n=16) [10 yr survival rates of RT (25%) vs. RS (76.4%), p=0.0111]. Our results support that a possible therapeutic benefit of neoadjuvant chemotherapy plus radical surgery is only in patients with bulky stage IB to IIA cervical cancer. In cases of chemoresistance, radical surgery might be a better definite treatment option.
Keywords: Neoadjuvant Therapy; Hysterectomy, Radiotherapy; Uterine Cervical Neoplasms
INTRODUCTION
In 1999, a National Cancer Institute Alert recommended that concurrent chemoradiation should be considered instead of radiotherapy alone in women with cervical cancer based on the results of five randomized trials (1-5). A subsequent systematic review and meta-analysis of data presented in the previous literature have suggested a large benefit of concurrent chemoradiation for survival, as well as local and distant control rates (6). Thus, concurrent chemoradiotherapy has become a new 'standard of care' for a locally advanced cervical cancer. However, clinical dilemma might be faced with in young patients with bulky stage IB to IIA diseases. This is due to that these patients might lose their sexual functions by either concurrent chemoradiotherapy or post-operative radiotherapy.
Most investigators agree that cervical tumor size is a significant negative prognostic factor, since bulky tumors are associated with a high incidence in lymph node metastasis as well as in recurrence, as compared to smaller sized tumors (7,8). Until now, the best treatment option for bulky stage IB, IIA cervical cancer is uncertain. As one of treatment strategies in bulky stage IB, IIA cervical cancer, neoadjuvant chemotherapy is an attractive option as chemotherapy given prior to surgery can reduce tumor size leading to improvement of overall survival. Several randomized controlled studies have shown survival benefits of neoadjuvant chemotherapy followed by radical surgery in locally advanced cervical cancer (9-12). 'The neoadjuvant chemotherapy for cervical cancer meta-analysis collaboration study group' conducted a meta-analysis from 21 randomized trials of neoadjuvant chemotherapy settings (13), in which little survival benefit was observed in the 18 randomized trials of neoadjuvant chemotherapy followed by radiotherapy. However, 5 randomized controlled studies of neoadjuvant chemotherapy followed by radical surgery showed survival benefits. However, this is yet to be confirmed as the disease stages are heterogeneous and in one trial intra-arterial chemotherapy is used. Furthermore, in two trials post-operative radiotherapy was used in almost all patients.
If neoadjuvant chemotherapy followed by radical surgery offers survival benefits in cervical cancer patients, this might be of great benefit especially to young patients with bulky cervical carcinoma stage IB to IIA. This is because the reduction of tumor size is associated with a decrease in the frequency of post-operative adjuvant radiotherapy, which causes the loss of sexual functions. Since 1989, our group has started neoadjuvant chemotherapy to treat patients with locally advanced cervical cancer. In patients with stage IB to IIA cervical cancer showing poor chemoresponse, either radical surgery or radiotherapy after neoadjuvant chemotherapy has been used without any definite criteria under the assumption that either one could offer similar outcomes. In this retrospective study, we evaluated the impact on survival of neoadjuvant chemotherapy followed by radical surgery. We analyzed the survival difference between radical surgery and radiotherapy after chemotherapy in selected patients with poor chemoresponse.
MATERIALS AND METHODS
Patients
From February 1989 through January 1998, 94 patients with International Federation of Gynecology and Obstetrics (FIGO) stage IB or IIA cervical carcinoma (squamous, adeno- or adenosquamous) treated with neoadjuvant chemotherapy followed by radical surgery or radiotherapy were enrolled into this retrospective study. Eligible criteria were 1) previously untreated, 2) FIGO stage IB to IIA, 3) received 2 or 3 cycles of cisplatin based neoadjuvant chemotherapy, 4) non small cell carcinoma, 5) treated by radical hysterectomy with bilateral pelvic lymphadenectomy or radiotherapy following neoadjuvant chemotherapy.
Treatment
All eligible patients received cisplatin intravenous infusion (100 mg/m2) on day 1, and 5-fluorouracil intravenous infusion (1,000 mg/m2/day) for 4 days from day 2. Two or 3 cycles of chemotherapy were done at 21 days intervals, according to chemoresponse or performance status. Three to four weeks after the last chemotherapy, patients underwent a type III radical hysterectomy with bilateral pelvic lymphadenectomy or radiotherapy. Postoperative radiotherapy was usually prescribed when parametrial extension or positive surgical margins, deep stromal invasion, and lymph node metastasis were detected. Postoperative adjuvant radiotherapy consisted of external radiation by 10 MeV to the whole pelvis of 45 Gy (1.8 Gy/fraction) with a parametrial boost up to 50 Gy with a 4 cm wide midline block. Para-aortic extended field radiation of 45 Gy was administrated if there were multiple, bilateral pelvic, or common iliac node metastases. Patients with poor chemoresponse (stable disease or progressive disease) also received radical surgery as described above, or received radiotherapy for a definite therapy without specific criteria. Radiotherapy for definite therapy was constituted external beam radiation followed by brachytherapy. The pelvis was treated by external beam radiotherapy with a linear accelerator using photon beam energy of 10/25 MeV within a standard four-field box technique, followed by high dose rate brachytherapy. Patients received a dose of 4,500-5,040 centigrays (cGy) as external beam in 25-28 fractions over 5.0-5.5 weeks. This was followed by six courses of intracavitary brachytherapy, after external beam radiotherapy was completed. A dose of 3,000 cGy in 6 fractions was given over 3 weeks.
Evaluation of tumor response
Tumor size was assessed by pelvic examination immediately before each chemotherapy cycle and also at surgery. The clinical response to chemotherapy was evaluated according to the World Health Organization (WHO) criteria (14). Response was measured as the product of the two largest perpendicular diameters of the cervical mass lesion. Patients were evaluated for response using the following criteria. A complete response (CR) was defined as the complete disappearance of all clinically detectable disease. A 50% or more decrease in tumor size constituted a partial response (PR). Stable disease (SD) was defined as no significant change in tumor size, and progressive disease (PD) was defined as a increase of ≥25% in tumor size or the appearance of new lesions.
Three weeks after the last chemotherapy, the final clinical response to chemotherapy was evaluated according to the tumor size assessed by pelvic examination and imaging study. Some of patients underwent radical surgery after 2 cycles of chemotherapy due to good chemoresponse (CR or nearly CR).
Definition of endpoints and follow up
Overall survival, as the primary endpoint, was defined as the time from date of the start of neoadjuvant chemotherapy until death (from cervical cancer related). Surviving patients were censored on the date of last follow-up. After treatment, we followed up patients at 3 month intervals for the first 2 yr, and then at 4-6 month intervals for additional 3 yr, thereafter once a year. For a long term survival analysis, we investigated death date of all enrolled patients with helps from Namgu Ward Office, Daegu, Korea, and confirmed the cause of death by telephone or medical record review.
Statistical analysis
Survival curves were plotted according to the estimate of Kaplan and Meier. The log-rank test was used to determine the significance of differences in survival distribution. Odds ratio and 95% confidence intervals of mortality from cancer were calculated by Cox regression hazard model to determine confounding effects of prognostic factors. The chi-square test, independent t-test, ANOVA (analysis of variance), Mann-Whitney U test, and Fisher's exact test were used to compare covariates. All comparisons were two-sided.
RESULTS
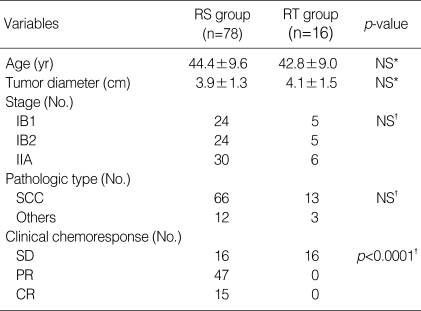
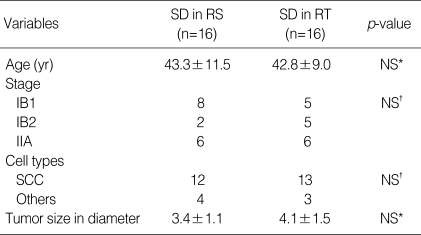
A total of 94 patients were enrolled in this retrospective study. Mean age was 44 yr (26-64 yr), and mean tumor size at presentation was 3.95±1.3 (2.5-7.2) cm in whole series. Seventy eight patients of the radical surgery (RS) group (complete response, n=15; partial response, n=47; stable disease, n=16) received type III radical hysterectomy and pelvic lymphadenectomy with or without postoperative radiotherapy. The other 16 patients of the radiotherapy (RT) group (stable disease, n=16) received radiotherapy following neoadjuvant chemotherapy (Fig. 1). The number of patients was 29, 29 and 36 for stage IB1, IB2 and IIA, respectively. Median follow-up was 85.7 months (3.7-167.7). There was no statistical difference in age, tumor size, stage, pathologic type between the RS and RT groups except for chemoresponse. Characteristics of patients in both groups are summarized in Table 1.
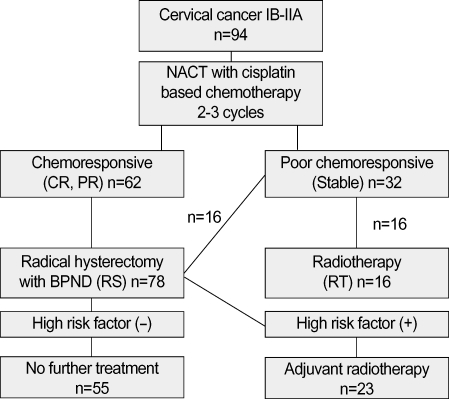
Fig. 1.
Treatment and patient allocation in this study.
NACT, neoadjuvant chemotherapy; CR, complete response; PR, partial response; RS, radical surgery group treated by neoadjuvant chemotherapy plus radical surgery; RT, radiotherapy group treated by neoadjuvant chemotherapy plus radiotherapy; BPND, bilateral pelvic node dissection.
Table 1.
Characteristics of patients
RS group, neoadjuvant chemotherapy followed by radical surgery group; RT group, neoadjuvant chemotherapy followed by radiotherapy group; NS, not statistically significant difference; SCC, squamous cell carcinoma; SD, stable disease; PR, partial response; CR, complete response.
*, t-test; †, chi-square test.
In the RS group, 23 patients (29.5%) received post-operative adjuvant radiotherapy, and in the RT group, 2 patients received adjuvant hysterectomy following radiotherapy. Of 32 patients who showed stable disease after neoadjuvant chemotherapy, 16 patients received radical surgery with or without post-operative radiotherapy, and the other 16 patients received radiotherapy alone for definite therapy (Fig. 1). We did not have any definite criteria of this arrangement of treatment.
Response to neoadjuvant chemotherapy
After neoadjuvant chemotherapy, overall clinical response rate obtained was 66.0% (CR in 15 patients [16.0%], PR in 47 [50.0%], SD in 32 [34.0%]). In the RS group, clinical response rate obtained was 79.5% (CR in 19.2%, PR in 60.3%, SD in 20.5%). All of patients in the RT group had stable disease.
Survival
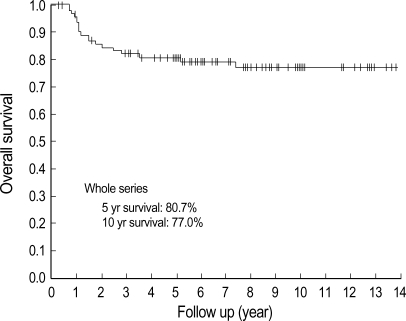
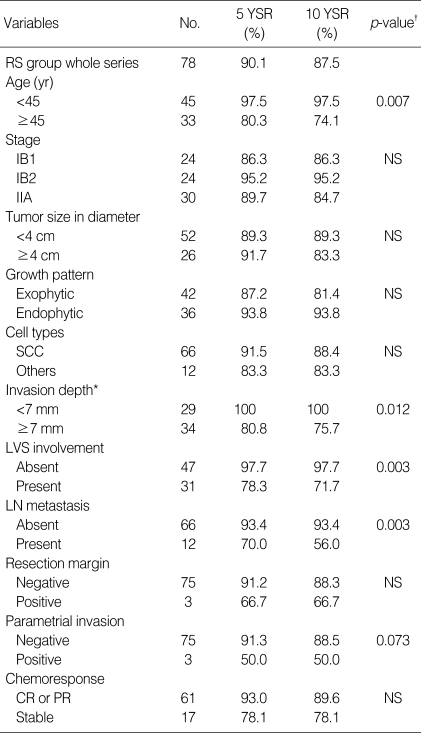
The median duration of follow-up was 85.7 (3.7±167.7) months. Up to the time of this analysis, 19 patients died from cervical cancer, whereas 1 patient died from primary lung cancer (this patient was considered as censored data at death date). In the RS group, 8 of 78 patients died, whereas in the RT group 11 of 16 patients died from cervical cancer. The overall survival of whole series, 5 yr and 10 yr survival rate were 80.7% and 77.0%, respectively (Fig. 2). The 5 yr and 10 yr survival rates of the RS group were 90.1% and 87.5%, respectively. In the RS group, the 10 yr survival estimation in patients with bulky tumors (diameter ≥4 cm, n=26) was similar to that with non-bulky tumors (83.3% vs. 89.3%, p=NS, Table 2).
Fig. 2.
Survival estimation by Kaplan Meier's method of whole series. Five and 10 yr survival rates were 80.7% and 77.0%, respectively.
Table 2.
Kaplan Meier's survival estimation of neoadjuvant chemotherapy followed by radical surgery according to prognostic factors
YSR, year survival rate; RS, radical surgery; NS, not statistically significant difference; SCC, squamous cell carcinoma; LVS, lymphovascular space; LN, lymph node; CR, complete response; PR, partial response.
*, Total number of patients was not 78, because there were some missing data in pathologic report; †, Log rank test.
Survival of patients with chemoresistance
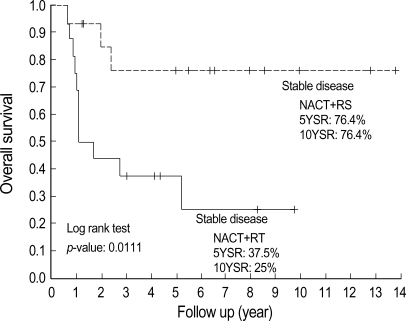
The survival rates of the RT group were very poor considering their stage (5 yr survival rate, 37.5%; 10 yr survival rate, 25.0%). This result might be due to that all of patients in RT group were selected patients showing chemoresistance. The most striking finding in this study was that there was a significant survival difference between the RS group with stable disease and the RT group with stable disease even though they showed similar clinical characteristics (Table 3). Prognostic factors such as lymph node metastasis, lymphovascular space involvement, and invasion depth could not be compared in two groups (RS vs. RT), since surgical specimen was not available in the patients of RT group. The 10 yr survival rates of the RS and RT groups with stable disease were 76.4% and 25.0%, respectively (p=0.0111, Fig. 3).
Table 3.
Comparisons of clinical variables in patients showing stable disease to neoadjuvant chemotherapy
SD, stable disease; RS, radical surgery group treated by neoadjuvant chemotherapy plus radical surgery; RT, radiotherapy group treated by neoadjuvant chemotherapy plus radiotherapy; SCC, squamous cell carcinoma; NS, not statistically significant difference.
*, Mann-Whitney U test; †, Fisher's exact test.
Fig. 3.
Survival estimation by Kaplan Meier's method according to definite therapy following neoadjuvant chemotherapy in patients who showed stable disease after neoadjuvant chemotherapy. Five year survival estimation of stable disease in RS and RT group was 76.4% vs. 37.5%, and 10 yr survival estimation was 76.4% vs. 25%. This difference was statistically significant (p=0.0111) by Log rank test.
NACT, neoadjuvant chemotherapy; RS, radical surgery group treated by neoadjuvant chemotherapy plus radical surgery; RT, radiotherapy group treated by neoadjuvant chemotherapy plus radiotherapy.
Prognostic factors
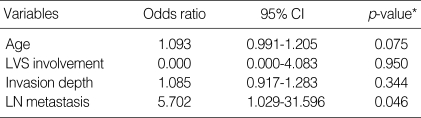
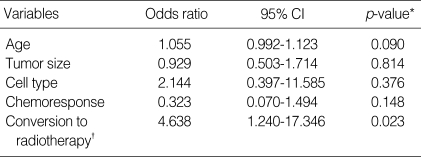
In the RS group, 11 variables (age, stage, tumor size, tumor growth pattern, histologic type, invasion depth, lymphovascular invasion, lymph node metastasis, surgical margin, parametrial extension, chemoresponse) were evaluated for their correlation with survival. Among these prognostic factors, age (≥45 yr), lymphovascular invasion, invasion depth ≥7 mm, lymph node metastasis were significantly associated with overall survival (Table 2). In multivariate analyses using Cox regression hazard model, lymph node metastasis was only an independent prognostic variable associated with overall survival in RS group (Table 4). In whole series, conversion to radiotherapy due to stable disease after neoadjuvant chemotherapy was the most significant prognostic variable (Table 5).
Table 4.
Multivariate analysis of prognostic factors in radical surgery following neoadjuvant chemotherapy
CI, confidence interval; LVS, lymphvascular space; LN, lymph node.
*, Cox regression hazard model.
Table 5.
Multivariate analysis of prognostic factors in whole series
CI, confidence interval.
*, Cox regression hazard model; †, conversion to radiotherapy for definite therapy due to stable disease after neoadjuvant chemotherapy.
DISCUSSION
Concurrent chemoradiotherapy has probably become a 'standard of care' for women with locally advanced disease. This is supported by a National Cancer Institute Alert based on the results of five randomized trials, stating ''strong consideration should be given to the incorporation of chemotherapy into radiotherapy in women who require radiotherapy for the treatment of cervical cancer''. However, we still have clinical dilemma especially in young patients with bulky stage IB, IIA cervical cancer. Under the option of concurrent chemoradiotherapy, the patients will lose their sexual functions and experience menopause. However, if primary radical surgery is chosen, the patients also receive post-operative radiotherapy in the majority of cases. For these reasons, neoadjuvant chemotherapy followed by radical surgery might be still considered to be an attractive treatment option.
Tumor size of cervical carcinoma is an important prognostic factor. The larger tumors are less likely to respond to radiotherapy because their large hypoxic tumor cell population reduces radiosensitivity. In an attempt to further delineate the wide spectrum of behavior of Stage IB cervical cancer, FIGO staging of cervical cancer was modified in August 1995. FIGO established the classifications of stage IB1 (<4 cm) and IB2 (≥4 cm). Traditional therapeutic strategies for the treatment of stage IB2 cervical carcinoma have yielded disappointing results. Although the 5 yr survival rate exceeds 90% for patients with stage IB1 disease, 5 yr survival is only 60 to 70% in patients with stage IB2 disease (7,15). For these reasons, the most effective therapeutic strategies for bulky locally advanced cervical carcinoma, especially stage IB to IIA remain uncertain. Previously, neoadjuvant chemotherapy followed by radical surgery showed survival benefits in many retrospective as well as in several randomized controlled trials (9-12,16-23). Despite these randomized trials and retrospective studies, it is still unclear whether neoadjuvant chemotherapy is effective for the treatment of locally advanced cervical cancer. This is due to that most of retrospective studies included only the patient groups treated by radical surgery following neoadjuvant chemotherapy, which likely has the possibility of selection bias by excluding cases treated by radiotherapy after neoadjuvant chemotherapy due to poor chemoresponse. Furthermore, some of these randomized controlled trials include too wide range of disease stages, even advanced stages (IIB, IIIA, IIIB) which cannot be treated by radical surgery if there is no sufficient chemoresponse.
The probability of survival benefits of neoadjuvant chemotherapy depends on high chemoresponsiveness. The response rate to neoadjuvant chemotherapy was found to be 42 to 85% in randomized controlled trials of neoadjuvant chemotherapy settings (9-13,24). Our study showed 66% of overall clinical response rates including 16% complete response, which are compatible with previous reports. Because 15-40% of patients treated by neoadjuvant chemotherapy result in either stable disease or progressive disease, optimal treatment of these groups of patients is considered of importance.
When our group adopted a neoadjuvant chemotherapybased therapeutic strategy, we thought that neoadjuvant chemotherapy followed by radiotherapy might also have similar survival benefits even in chemoresistant cases. For this reason, we chose either radiotherapy or radical surgery in operation-available patients with poor chemoresponse without specific criteria. In this retrospective study, however, there detected a significant survival difference in 10 yr survival rates between the RS group (76.4%) with stable disease and the RT group (25%) with stable disease (Table 4, Fig. 4). These patients showed similar clinical characteristics in comparison. Because there is no information about lymph node metastasis, lymphovascular invasion, and invasion depth of the RT group, the prognostic factors could not be compared in two groups (RS vs. RT).
Previous two retrospective studies showed similar poor outcomes in patients treated by radiotherapy after neoadjuvant chemotherapy due to poor chemoresponse. In one report, 59 patients with bulky (≥4 cm) stage IB or IIA cervical carcinoma were treated by neoadjuvant chemotherapy, among whom 51 underwent radical surgery and the remaining 8 were treated by definitive radiotherapy. The 5 yr survival rate of these patients who received hysterectomy was 80.3% while only 1 of the 8 patients treated by radiotherapy without hysterectomy survived (survival rate, 12.5%). Of the 7 patients receiving hysterectomy after chemotherapy despite clinical poor response, only 3 patients died (survival rate, 43%) (25). In the other report, 42 patients with FIGO stage IB-IIA bulky (≥4 cm), IIB-IIIB cervical adenocarcinoma were treated by neoadjuvant chemotherapy. The 33 chemoresponders (79%) received laparotomy, among them 29 patients were feasible for radical surgery. The remaining 9 nonresponders received radiotherapy, but all of them were dead, as compared to chemoresponder groups (5 yr survival rate, 84%) (26).
Although chemotherapeutic agents can act as a radiosensitizer in concurrent chemoradiotherapy, pre-exposure to chemotherapeutic agents prior to radiation might render tumor cells to alter their biological systems. Cervical tumors are rapidly proliferating with a median potential doubling time of only 4-4.5 days and a relatively high growth fraction (27). After an effective chemotherapy, tumors can shrink, but in a short time grow again at a more accelerated speed. After tumor cell divisions, tumor volume could be restored and tumor cells can obtain resistance to chemotherapeutic drugs and even to conventionally fractionated radiotherapy, which possibly results from altered tumor cell growth kinetics (13). If tumor cells are cross-resistant to chemotherapy and radiotherapy, the factors, such as the duration of chemotherapy and the delay to radiotherapy for the overall treatment time might have an impact on prognosis (13).
Benedetti-Panici et al. reported that neoadjuvant chemotherapy is of benefit only to Stage IB2, or IIB, not to Stage III (9). Sardi et al. also showed survival benefit only in Stage IB2 cervical cancer, not in Stage IB1 (10,11). Similarly, one Japanese group demonstrated that radiotherapy is not beneficial for stages IIB, IIIA, IIIB, but somewhat helpful only for patients with good chemoresponse treated by radical surgery (12). In our study, patients with bulky stage IB to IIA cervical cancer (≥4 cm) showed a good long term survival (5 yr survival rate, 91.7%; 10 yr survival rate, 83.3%). This appears to be better than survival of previous conventional treatment (7,15). However, the long term survival of patients with non-bulky tumor (<4 cm) looked not improved. These are compatible with previous randomized controlled studies (9-11). It is likely that neoadjuvant chemotherapy strategy might be limited to bulky stage IB to IIA and perhaps IIB cervical cancer.
In summary, survival benefits of neoadjuvant chemotherapy followed by radical surgery are observed only in bulky (≥4 cm) stage IB to IIA cervical cancer, but not in non-bulky stage IB to IIA. In chemoresistant cases, patients undergoing radiotherapy after chemotherapy showed poorer survival rates than patients with radical surgery. Our retrospective study shows that radiotherapy after chemotherapy makes survival outcome deteriorated in chemoresistant patients. Thus, radical surgery might be better treatment option even though patients with stage IB to IIA cervical carcinoma show chemoresistance to neoadjuvant chemotherapy.
References
- 1.Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, 3rd, Walker JL, Gersell D. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 2.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, Rotman M, Gershenson DM, Mutch DG. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 3.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, Stock RJ, Monk BJ, Berek JS, Souhami L, Grigsby P, Gordon W, Jr, Alberts DS. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 4.Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, Clarke-Pearson DL, Insalaco S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 5.Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC, Jr, Clarke-Pearson DL, Liao SY. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–1348. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 6.Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, Williams CJ. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet. 2001;358:781–786. doi: 10.1016/S0140-6736(01)05965-7. [DOI] [PubMed] [Google Scholar]
- 7.Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990;38:352–357. doi: 10.1016/0090-8258(90)90072-s. [DOI] [PubMed] [Google Scholar]
- 8.Piver MS, Chung WS. Prognostic significance of cervical lesion size and pelvic node metastases in cervical carcinoma. Obstet Gynecol. 1975;46:507–510. [PubMed] [Google Scholar]
- 9.Benedetti-Panici P, Greggi S, Colombo A, Amoroso M, Smaniotto D, Giannarelli D, Amunni G, Raspagliesi F, Zola P, Mangioni C, Landoni F. Neoadjuvant chemotherapy and radical surgery versus exclusive radiotherapy in locally advanced squamous cell cervical cancer: results from the Italian multicenter randomized study. J Clin Oncol. 2002;20:179–188. doi: 10.1200/JCO.2002.20.1.179. [DOI] [PubMed] [Google Scholar]
- 10.Sardi J, Sananes C, Giaroli A, Bayo J, Rueda NG, Vighi S, Guardado N, Paniceres G, Snaidas L, Vico C, di Paola G. Results of a prospective randomized trial with neoadjuvant chemotherapy in stage IB, bulky, squamous carcinoma of the cervix. Gynecol Oncol. 1993;49:156–165. doi: 10.1006/gyno.1993.1100. [DOI] [PubMed] [Google Scholar]
- 11.Sardi JE, Giaroli A, Sananes C, Ferreira M, Soderini A, Bermudez A, Snaidas L, Vighi S, Gomez Rueda N, di Paola G. Long-term follow-up of the first randomized trial using neoadjuvant chemotherapy in stage Ib squamous carcinoma of the cervix: the final results. Gynecol Oncol. 1997;67:61–69. doi: 10.1006/gyno.1997.4812. [DOI] [PubMed] [Google Scholar]
- 12.Kigawa J, Minagawa Y, Ishihara H, Itamochi H, Kanamori Y, Terakawa N. The role of neoadjuvant intraarterial infusion chemotherapy with cisplatin and bleomycin for locally advanced cervical cancer. Am J Clin Oncol. 1996;19:255–259. doi: 10.1097/00000421-199606000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy for locally advanced cervical cancer. A systematic review and meta-analysis of individual patient data from 21 randomised trials. Eur J Cancer. 2003;39:2470–2486. doi: 10.1016/s0959-8049(03)00425-8. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. WHO handbook for reporting results of cancer treatment. Vol. 48. Geneva: World Health Organization; 1979. pp. 22–27. [Google Scholar]
- 15.Perez CA, Grigsby PW, Nene SM, Camel HM, Galakatos A, Kao MS, Lockett MA. Effect of tumor size on the prognosis of carcinoma of the uterine cervix treated with irradiation alone. Cancer. 1992;69:2796–2806. doi: 10.1002/1097-0142(19920601)69:11<2796::aid-cncr2820691127>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 16.Aoki Y, Tomita M, Sato T, Watanabe M, Kase H, Fujita K, Kurata H, Tanaka K. Neoadjuvant chemotherapy for patients younger than 50 years with high-risk squamous cell carcinoma of the cervix. Gynecol Oncol. 2001;83:263–267. doi: 10.1006/gyno.2001.6371. [DOI] [PubMed] [Google Scholar]
- 17.Benedetti-Panici P, Greggi S, Scambia G, Amoroso M, Salerno MG, Maneschi F, Cutillo G, Paratore MP, Scorpiglione N, Mancuso S. Long-term survival following neoadjuvant chemotherapy and radical surgery in locally advanced cervical cancer. Eur J Cancer. 1998;34:341–346. doi: 10.1016/s0959-8049(97)10029-6. [DOI] [PubMed] [Google Scholar]
- 18.Eddy GL, Manetta A, Alvarez RD, Williams L, Creasman WT. Neoadjuvant chemotherapy with vincristine and cisplatin followed by radical hysterectomy and pelvic lymphadenectomy for FIGO stage IB bulky cervical cancer: a Gynecologic Oncology Group pilot study. Gynecol Oncol. 1995;57:412–416. doi: 10.1006/gyno.1995.1164. [DOI] [PubMed] [Google Scholar]
- 19.Etcheverry MG, Marantz A, Saine M, Litovska S, Lewi D, Cecchin G, De Pierro AN. Neoadjuvant chemotherapy with cisplatin, ifosfamide and 5-fluorouracil in the treatment of locally advanced cervical cancer. Int J Gynecol Cancer. 2000;10:53–58. doi: 10.1046/j.1525-1438.2000.99077.x. [DOI] [PubMed] [Google Scholar]
- 20.Huang HJ, Chang TC, Hong JH, Tseng CJ, Chou HH, Huang KG, Lai CH. Prognostic value of age and histologic type in neoadjuvant chemotherapy plus radical surgery for bulky (>/=4 cm) stage IB and IIA cervical carcinoma. Int J Gynecol Cancer. 2003;13:204–211. doi: 10.1046/j.1525-1438.2003.13004.x. [DOI] [PubMed] [Google Scholar]
- 21.Hwang YY, Moon H, Cho SH, Kim KT, Moon YJ, Kim SR, Kim DS. Ten-year survival of patients with locally advanced, stage ib-iib cervical cancer after neoadjuvant chemotherapy and radical hysterectomy. Gynecol Oncol. 2001;82:88–93. doi: 10.1006/gyno.2001.6204. [DOI] [PubMed] [Google Scholar]
- 22.Napolitano U, Imperato F, Mossa B, Framarino ML, Marziani R, Marzetti L. The role of neoadjuvant chemotherapy for squamous cell cervical cancer (Ib-IIIb): a long-term randomized trial. Eur J Gynaecol Oncol. 2003;24:51–59. [PubMed] [Google Scholar]
- 23.Paladini D, Raspagliesi F, Fontanelli R, Ntousias V. Radical surgery after induction chemotherapy in locally advanced cervical cancer. A feasibility study. Int J Gynecol Cancer. 1995;5:296–300. doi: 10.1046/j.1525-1438.1995.05040296.x. [DOI] [PubMed] [Google Scholar]
- 24.Chang TC, Lai CH, Hong JH, Hsueh S, Huang KG, Chou HH, Tseng CJ, Tsai CS, Chang JT, Lin CT, Chang HH, Chao PJ, Ng KK, Tang SG, Soong YK. Randomized trial of neoadjuvant cisplatin, vincristine, bleomycin, and radical hysterectomy versus radiation therapy for bulky stage IB and IIA cervical cancer. J Clin Oncol. 2000;18:1740–1747. doi: 10.1200/JCO.2000.18.8.1740. [DOI] [PubMed] [Google Scholar]
- 25.Lai CH, Hsueh S, Chang TC, Tseng CJ, Huang KG, Chou HH, Chen SM, Chang MF, Shum HC. Prognostic factors in patients with bulky stage IB or IIA cervical carcinoma undergoing neoadjuvant chemotherapy and radical hysterectomy. Gynecol Oncol. 1997;64:456–462. doi: 10.1006/gyno.1996.4603. [DOI] [PubMed] [Google Scholar]
- 26.Benedetti-Panici P, Greggi S, Scambia G, Salerno MG, Amoroso M, Maneschi F, Cutillo G, Caruso A, Capelli A, Mancuso S. Locally advanced cervical adenocarcinoma: is there a place for chemo-surgical treatment? Gynecol Oncol. 1996;61:44–49. doi: 10.1006/gyno.1996.0094. [DOI] [PubMed] [Google Scholar]
- 27.Bolger BS, Symonds RP, Stanton PD, MacLean AB, Burnett R, Kelly P, Cooke TG. Prediction of radiotherapy response of cervical carcinoma through measurement of proliferation rate. Br J Cancer. 1996;74:1223–1226. doi: 10.1038/bjc.1996.520. [DOI] [PMC free article] [PubMed] [Google Scholar]