Abstract
Alkyltransferase-like proteins (ATLs) share functional motifs with the cancer chemotherapy target O6-alkylguanine DNA-alkyltransferase (AGT) and paradoxically protect cells from the biological effects of DNA alkylation damage, despite lacking the AGT reactive cysteine and alkyltransferase activity. Here we determine S. pombe ATL structures without and with damaged DNA containing endogenous lesion O6-methylguanine or cigarette smoke-derived O6-4-(3-pyridyl)-4-oxobutylguanine. These results reveal non-enzymatic DNA nucleotide flipping plus increased DNA distortion and binding pocket size compared to AGT. Our analysis of lesion-binding site conservation identifies new ATLs in sea anemone and ancestral archaea, indicating ATL interactions are ancestral to present-day repair pathways in all domains of life. Genetic connections to XPG and ERCC1 in S. pombe homologs Rad13 and Swi10 and biochemical interactions with UvrA and UvrC combined with structural results reveal that ATLs sculpt alkylated DNA to create a genetic and structural intersection of base damage processing with nucleotide excision repair.
DNA O6-alkylguanine lesions are mutagenic and cytotoxic: they mis-pair during replication with thymine, resulting in G:C to A:T transition mutations1–4. Human O6-alkylguanine DNA lesions are repaired by O6-alkylguanine-DNA alkyltransferase (AGT), also known as O6-methylguanine-DNA methyltransferase (MGMT), which transfers guanine O6-alkyl adducts to its reactive cysteine reversing damage1. This prevents mutations but resists alkylating chemotherapies2,5. Active site -PCHRV- motif Cys1456,7 plus Arg128 and Tyr114 nucleotide rotating residues are conserved from bacterial to human AGTs1,2,8. Human AGT (hAGT) structures alone9,10 and with small molecule9 or DNA11,12 substrates showed how AGT promotes resistance to anticancer therapies by directly reversing DNA guanine alkylation damage.2
Recently bacterial and yeast proteins with sequence similarity to the AGT DNA-binding domain were identified with the Cys alkyl acceptor replaced by tryptophan, alanine, or another residue,13 and hence named alkyltransferase-like proteins (ATLs). ATLs from S. pombe (Atl1) and E. coli (eAtl) inhibit O6-methylguanine (O6-mG) repair by hAGT14,15. eAtl also binds abasic site-containing dsDNA,16 and Atl1 binds ssDNA containing O6-methyl-, O6-benzyl-, O6-(4-bromothenyl)- or O6-hydroxyethyl-guanine. Yet, ATLs do not cleave the alkyl group, base, or oligonucleotide near the lesion14,15, and eAtl Trp to Cys mutation does not restore alkyltransferase activity14. As S. pombe and T. thermophilus lack AGT, and inactivation of their ATL genes, atl1 and TTHA1564, respectively15,17, reduces their alkylation damage resistance, ATLs protect against biological effects of DNA alkylation damage by an undefined mechanism.
Tight binding affinities16,17 for and inability to repair14,15 O6-alkyl lesions implied ATLs are damage sensors or act in nucleotide excision repair (NER)13,15, which excises bulky, DNA-distorting lesions. However, lack of structures, persuasive evidence, or specific mechanism has obscured how ATL ameliorates DNA damage effects. To clarify this protection, we combined structural, biochemical, and genetic experiments on Atl1 from the fission yeast S. pombe. Our results reveal ATL binding generates a stable complex that sculpts alkylated DNA base damage for NER pathway entry.
Atl1 structure and lesion binding
To characterize Atl1-DNA damage interactions, we crystallized and solved structures to 2.0, 2.7, and 2.8 Å resolution, respectively, for Atl1 alone (Fig. 1a and Supplementary Table 1) and in complex with oligonucleotides containing either O6-mG (Fig. 1b, d and Supplementary Table 1) or O6-4-(3-pyridyl)-4-oxobutylguanine (O6-pobG) (Fig. 1c and Supplementary Table 1), a bulky and toxicologically-relevant adduct18. Atl1 shares the hAGT catalytic domain fold (superposition root mean square difference = 1.6 Å) (Fig. 1a), including residues required for AGT activity, DNA-binding, and nucleotide flipping. Yet, Atl1 specifically lacks AGT’s active site Cys and Asn hinge that couples helix-turn-helix (HTH) DNA binding and active site motifs (Fig. 1a).
Figure 1. Atl1 structure and lesion-binding site.
a, Overlay of Atl1 (yellow) and AGT (cyan; pdb 1EH6) models and comparison of key functional residues. 2Fo-Fc electron density (blue) for Atl1 with the binding site Trp56 side chain omitted. b, Atl1 (magenta) bound to DNA containing O6-mG (orange). 2Fo-Fc simulated annealing composite omit map (blue) shown for DNA. c and d, Atl1 lesion-binding site close-up with O6-pobG (c) or O6-mG (d). Amino acid side chains (ball-and-stick) and hydrogen bonds to the damaged guanine (green dashes) show the damage binding.
Atl1 flips both O6-mG (Fig. 1b, d) and O6-pobG (Fig. 1c) into a pocket containing -PWHRV- motif Trp56, consistent with fluorescence measured flipping for a base opposite an AP site in eAtl16 and for O6-mG in TTHA156417. Atl1 displays no AGT activity,15 suggesting nucleotide flipping is a switch for pathway activation, not catalysis. To our knowledge, Atl1 (Fig. 1b–d), eAtl16, and TTHA156417 are among the first reported non-enzymatic DNA-binding proteins that flip nucleotides. Our Atl1 structures show ATL rotates nucleotides into a specificity pocket. Arg39 intercalates the DNA base stack (Fig. 1d) and hydrogen bonds the orphaned cytosine, thereby stabilizing the extra-helical alkylguanine. Trp56, rather than AGT Cys, is evident in electron density omit maps (Fig. 1a), acting in hydrophobic packing with the alkyl group (Fig. 1c, d). Arg69 guanidinium stacks against the alkylguanine base in a cation-π interaction (Fig. 1d).
Alkylguanine base side and main chain hydrogen bonds are conserved from Atl1 to AGT, but the Atl1 lesion-binding pocket is ~three times larger (Supplementary Table 2). Loop residues 65–73 define one wall of the alkyl-binding pocket, adopting a conformation further from the protein core than in AGT, thereby enlarging the pocket (Fig. 1a and Fig. 2a). Also, lesion-binding pocket Lys45-Pro55 cap is ~5.3 Å further out than the comparable AGT Pro140 (Cα to Cα distance) that interacts with larger alkyl groups9,11,12. Moreover, ATL Ile71 replaces AGT Tyr158, which would clash with the Atl1 Trp56 side chain in its DNA-bound, closed position.
Figure 2. Atl1 DNA binding and damage sculpting.
a, Overlay of DNA-free (yellow) and DNA-bound (magenta) Atl1 with the hAGT C-terminal domain (cyan). The rotated O6-mG (center, spheres) is shown with the binding site loop that determines the open or closed conformation of Atl1. b, Atl1 molecular surface revealing an “open” state. c, DNA-bound Atl1 molecular surface showing the protein “closed” state. d, Atl1-DNA interaction schematic.
Atl1’s larger cavity explains its broad lesion range that includes O6-benzyl-, O6-(4-bromothenyl)- or O6-hydroxyethyl-guanine. In the O6-pobG-DNA complex structure, the pob group is wedged between Pro50 and Trp56, making only these hydrophobic protein interactions (Fig. 1c). No major changes in lesion-binding site, or DNA conformation occur between O6-mG- and O6-pobG-bound Atl1. Pob would push against the active (or binding) site loop in the smaller AGT active site, explaining why AGT repairs pob lesions at a decreased rate compared to O6-mG19. Pob adopts a conformation incompatible with smaller E. coli AGT (Ada-C and Ogt) active site pockets, consistent with its poor repair by Ada-C and Ogt20 and need for ATLs for bulky adducts in organisms like E. coli.
Atl1 DNA-binding
Like AGT, Atl1 uses an HTH motif to bind the DNA minor groove (Fig. 1b and Fig. 2a). All damaged-strand contacts are to alkylguanine and two 3’-adjacent nucleotide phosphate groups (Fig. 2d). DNA binding site loop (Ser67 and Lys70) and loop (Thr92 and Ser93) residues form DNA contacts not found in AGT. Atl1 DNA-binding buries ~1050 Å2 versus 788 Å2 of AGT buried surface area, consistent with tighter DNA binding16,17.
Atl1 bends DNA by ~45° (Fig. 2a), whereas AGT only bends DNA ~30°11. Atl1 achieves greater DNA bending through synergistic N-terminus and binding site loop actions. The Atl1 N-terminal helix extends outward more than the corresponding AGT helix, which follows a loop leading toward the N-terminal domain (Fig. 1a and Fig. 2a). This N-terminal extension pushes against the phosphate backbone of the complementary strand opposite the flipped nucleotide. Moreover, the binding site loop acts as a gate that switches between “open” (Fig. 2b) and “closed” (Fig. 2c) conformations of free and DNA-bound Atl1, respectively, with flanking glycines suggesting flexibility. This “gating” action was proposed in AGT computational simulations21, but not seen in crystal structures11,12. The Atl1 binding site loop open-to-closed conformational switch appears suitable to play an active role in signalling by shifting covering Arg and Ile side chains to expose the C-terminal loop for possible intermolecular interactions.
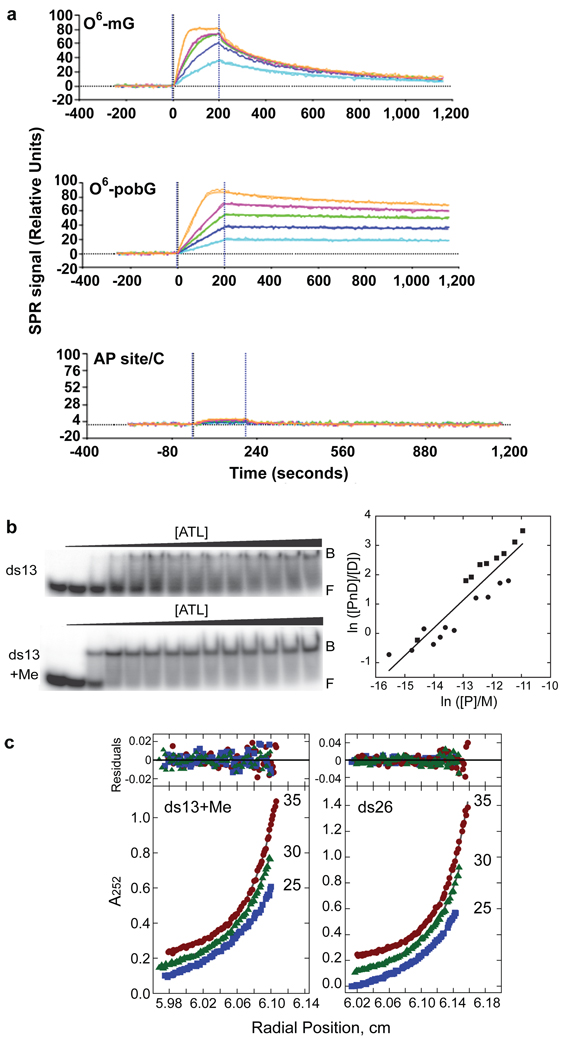
If the closed, bent ATL-DNA complex is a platform for repair protein recognition, then we expect the complex to be stable; yet, KD was estimated by gel-shift as only 0.41 µM for TTHA1564 with O6-mG17. To test binding affinity, we measured Atl1 binding and dissociation to and from oligonucleotides containing O6-mG, O6-pobG, or abasic site by surface plasmon resonance (Fig. 3a). Atl1 binding to oligonucleotides containing alkylG was 0.02–0.3 nM, but binding to abasic site dsDNA was low and/or transient (Fig. 3a). Langmuir fit with mass transfer limitation indicates kass = 1.21×10−7±0.20×10−7 M−1s−1; kdiss = 0.004 ± 0.0006 s−1 and KD = 0.35 ± 0.04 nM for O6-mG and kass = 2.20×10−7± 0.56×10−7 M−1s−1; kdiss = 0.0003 ± 0.00002 s−1 and KD = 0.016 ± 0.004 nM for O6-pobG. Thus, while “on” rates were similar for both lesions, the “off” rate for pob oligonucleotide was slower: higher affinity (KD) for the pob oligonucleotide shows larger O6-alkyl groups are accommodated stably.
Figure 3. Atl1 DNA lesion binding affinity and stoichiometry.
a, Atl1 binding and dissociation for oligonucleotides containing O6-mG (top), O6-pobG (center), or abasic site (bottom). b, Gel-shift assays for Atl1 binding normal and O6-mG 13mer dsDNA (left) and associated O6-mG DNA binding isotherm analysis (right) showing two independent experiments (●,■). c, Sedimentation equilibrium data for O6-mG 13mer dsDNA complexes (left) and normal 26mer dsDNA (right). Small, randomly distributed residuals (top panels) indicate models in which free protein, DNA, and one protein-DNA complex equilibrate in solution. Calculated stoichiometries are 1.15 ± 0.08 for O6-mG ds13mer and 3.03 ± 0.20 for normal ds26mer.
We also measured Atl1 binding to methylated double-strand oligonucleotides by gel-shift (Fig. 3b and Supplementary Fig. 1 and Supplementary Table 3) and verified saturated complex stoichiometries by sedimentation equilibrium analysis (Fig. 3c and Supplementary Table 3). Dominant complexes for 13-mer oligonucleotides in solution have 1:1 stoichiometry (Fig. 3b, c and Supplementary Table 3), consistent with our crystal structures, whereas 16-mers form 2:1 limiting complexes (Supplementary Fig. 1a and Supplementary Table 3) and 26-mers form 3:1 limiting complexes (Supplementary Fig. 1b and Supplementary Table 3). Saturated Atl1-nonmethylated DNA complex is formed without intermediate accumulation, suggesting cooperative binding to nonmethylated DNA. DNAs containing O6-mG form 1:1 complexes before proceeding to saturation in an additional concerted step, suggesting specific binding to O6-mG sites precedes build-up of cooperative assembly, consistent with the open-to-closed switch and a binding site size of ~8 bp. This differs from AGT’s binding site size of 4 bp/protein22, possibly due to AGT's added N-terminal domain and the open-to-closed switch (not seen in AGT) that exposes the C-terminal loop.
Atl1 connections to NER
ATLs tightly bind oligonucleotides containing O6-alkylguanine and switch conformation to expose the C-terminal loop, suggesting ATL-DNA complex binding partners are possible, in vivo. In fact, NER protein UvrA interacts with TTHA156417. Similarly, far-western analysis reveals eAtl interacts with E. coli NER proteins UvrA and UvrC in vitro (Fig. 4a). E. coli DNA repair helicase IV (HelD) is also a potential eAtl binding partner16. Interestingly, S. pombe Atl1 interacts with E. coli UvrA in vitro (Fig. 4b), suggesting ATLs conserve features across species for NER recognition.
Figure 4. Biochemical and genetic connection of Atl1 to NER.
a–b, Coomassie-stained gel (left) and far-western blot (right) probed with FLAG-eAtl (a) or FLAG-UvrA (b). c, MNNG-induced (top) and spontaneous (bottom) mutations of wild-type, Δatl1, Δrad13 and Δatl1 Δrad13 S. pombe strains. d, Atl1 and Rad13 are epistatic for MNNG toxicity. Serial dilutions of wild-type, Δatl1, Δrad13 and Δatl1 Δrad13 S. pombe cells spotted on yeast extract (YE) plates or YE plates containing 0.08 µg/ml MNNG. Results shown are mean ± s.d.; n ≥ 3. e–h, Clonogenic assay, revealing Atl1 is epistatic for MNNG toxicity with Rad13 (e) and Swi10 (f), but not Rhp14 (g) or Rad2 (h). Results shown are mean ± s.e.m; n ≥ 3.
To test for Atl1 functional genetic interactions with the NER pathway in fission yeast, we analyzed S. pombe atl1 and rad13 (NER pathway XPG endonuclease human homolog which cuts 3' of DNA lesions23) single and double deletants. We measured Atl1’s ability to protect cells from MNNG-induced and spontaneous mutations (Fig. 4c–e). MNNG sensitivity of Δatl1 cells was complemented by a plasmid harbouring atl1 (Supplementary Fig. 2), indicating observed cellular phenotypes are due to atl1 deletion. Atl1 inactivation causes ~9-fold increased reversion rate of the ade6-485 mutation, similar to the Δrad13 mutant (Fig. 4c top). The MNNG-induced mutation rate is not further increased in the Δatl1 Δrad13 double mutant, revealing an epistatic relationship between Atl1 and Rad13 (Fig. 4c top). These results are supported by spot tests (Fig. 4d) and clonogenic assays (Fig. 4e) indicating Atl1 and Rad13 are also epistatic for MNNG toxicity.
Strikingly, increased spontaneous mutation rate of Δrad13 cells is suppressed to wild-type levels by additional Atl1 inactivation (Fig. 4c bottom). This effect is not due to decreased cell survival, as all mutants tested here are viable (Supplementary Fig. 3). Clonogenic assays also revealed Atl1 is epistatic with S. pombe Swi10 (Fig. 4f), but not Rhp14 (Fig. 4g) or Rad2 (Fig. 4h) (homologs of human ERCC1, XPA and Fen-1, respectively), for MNNG toxicity. The non-epistatic relationship between Atl1 and Rhp14 suggests Rhp14 has an NER-independent function in response to MNNG, consistent with Rhp14 responses to other DNA damaging agents (R.K. and O.F., unpublished data). As Rad2 plays a role in long-patch base excision repair (BER)24 and the alternative UV excision repair25, lack of epistasis between atl1 and rad2 implies the two proteins work in different pathways. Rad13 and swi10 mutant phenotypes may reflect build-up of stable hard-to-repair ATL-complex intermediates in the absence of these NER proteins, suggesting ATL-DNA complexes may block alternative repair. Similarly, Atl1 protects E. coli cells against MNNG-induced alkylation damage (Supplementary Table 4). Thus, both microbial and eukaryotic genetic evidence suggests ATL bridges DNA-alkylation base damage responses to NER.
Identification of novel ATLs
To see if our structures may characterize other ATLs, we mapped sequence conservation of 197 ATL sequences based upon the Atl1 structure (Supplementary Fig. 4 and Supplementary Fig. 5). For all ATLs, the most conserved residues line the lesion-binding pocket or act in DNA binding in our structures, suggesting our Atl1 structures are paradigmatic for ATLs.
Significantly, our structure-based sequence analyses helped us identify here the first ATL from any multicellular organism, the recently sequenced starlet sea anemone Nematostella vectensis26 plus two archaeal ATLs from Candidatus Korarchaeum cryptofilum27 and Nanoarchaeum equitans28, ancestral to the two established phyla of archaea (Genbank accession numbers XM_001618690, YP_001736655, and NP_963633, respectively; Supplementary Fig. 4 and Supplementary Fig. 6). We verified N. vectensis ATL blocks alkyltransferase activity of hAGT (Supplementary Fig. 7), confirming it is an ATL. The N. vectensis genome, which aided in genome characterization of the long-extinct last common ancestor of all eumetazoans, is surprisingly more similar to vertebrates than fruit flies or nematodes26. Therefore, existence of ATL in this multi-cellular eukaryote, plus yeast, bacteria, and ancestral archaea shows ATL is present in all three domains of life and argues ATL was common to evolutionary branches before complex eukaryotes. This discovery suggests higher eukaryotes and mammals will either have an ATL or have lost or replaced it with an analogous protein.
Discussion
Alkylated DNA base damage is classically repaired by direct damage reversal proteins or by lesion-specific DNA glycosylases, which excise modified bases to create abasic sites and initiate the BER pathway29. These base repair processes differ from the versatile NER removal of bulky, unrelated, helix distorting lesions by excising a lesion-containing DNA patch30. Our combined results reveal a general mechanism for ATL to bind weakly distorting O6-alkylguanine lesions and recruit NER proteins (Fig. 5). We propose ATL binding sculpts alkylguanine into a bulky lesion that is channeled into the NER pathway, explaining NER-mediated repair of O6-alkylguanine lesions31–33.
Figure 5. Alkyl-G lesion recognition allows NER repair of relatively non-distorting base lesions.
The distorted, stable ATL-DNA complex creates a platform to recruit NER enzymes to O6alkyl-G lesions. This general model is based upon our combined structural, biochemical and genetic results.
ATL may be an unrecognized NER element, with analogues in many organisms, that targets endogenous alkylation damage to NER nucleases. In higher eukaryotes, the NER transcription-coupled repair (TCR) sub-pathway engages downstream damage recognition components of global genome repair (GGR), to effect lesion removal from the transcribed strand34. The NER GGR sub-pathway is initiated by XPC recognition of bulky lesions and, like TCR, results in damage removal by incision on either side of the lesion35. As the O6-mG lesion is insufficient to block transcription36, ATL binding may stall RNA polymerase to initiate TCR and/or promote lesion processing analogously to DDB2 of mammalian GGR. Atl1-DNA contacts are with the damaged strand, similar to DDB237, consistent with possible undamaged strand binding by fission yeast XPC homologues Rhp41 or Rhp42, as shown for S. cerevisiae XPC orthologue, Rad438.
ATLs are not alkyltransferases14,15,17 or glycosylases14,15, but inhibit AGT14,15 (Supplementary Fig. 7). Lack of epistasis for MNNG-induced cell killing between atl1 and rad2 shows ATL is not a long-patch BER or alternative UV excision repair protein. Yet, ATL damage recognition resembles AGT and BER glycosylases rather than NER proteins: positive channel for lesion-binding and 180° nucleotide flipping, which allow protein handoffs without release of toxic and mutagenic DNA intermediates, a hallmark of BER and recombination repair pathways39–43.
ATL binding targets base damage to NER, showing how proteins that bind damage, but do not repair it, may redirect lesion processing.44 First, ATL binds base damage analogously to AGT and BER glycosylases2,45, but presents damage to NER similarly to DDB2. Second, in some organisms ATL can block AGT O6-alkylguanine damage recognition and redirect base repair to NER, constituting a crosstalk pathway connection,44 as proposed for AGT33,46, and recently for eATL20. Third, ATL redirects endogenous damage from other repair pathways to NER, as Δrad13 mutator is rescued in Δatl1 Δrad13. Fourth, ancestral archaeal ATL’s are ATL-Endo V fusions, suggesting ATL and Endo V act together in a coordinated pathway47 with BER nuclease Endo V serving a possible XPG-like function in these organisms, as AGT-Endo V fusion proteins retain both activities48. By the Rosetta Stone evolution hypothesis for protein interactions47, ATL-Endo V fusions imply ATL provides a primordial connection joining BER and NER. Indeed, recent structures of EndoV49 and NER complex DDB1-DDB237 support such an ancient BER-NER connection by revealing a mutual, wedge-based binding mechanism50. Thus, non-enzymatic nucleotide flipping emerges as a surprisingly general mechanism to channel specific base damage into the general damage NER pathway by handoff from a non-enzymatic complex.
Methods Summary
Atl1 purification, crystallization, X-ray diffraction data collection, and structural refinement
C-terminally 6x-His tagged Atl1 was expressed in JM109 cells and purified over Ni-NTA agarose and Superdex 75 columns. Atl1:O6-mG- and Atl1:O6-pobG-DNA complexes were prepared at a 1.5:1 DNA:protein molar ratio. Crystals were grown by hanging drop vapor diffusion. Diffraction data were collected at ALS beamline 12.3.1 for Atl1 and at SSRL beamline 11-1 for Atl1:O6-mG and Atl1:O6-pobG DNA complexes, and were processed with HKL2000. Structures were solved by molecular replacement with Phaser, using a modified wild-type Ada-C (PDB code 1SFE) as a search model for Atl1, and the refined Atl1 structure as a search model for Atl1:DNA complexes. Crystallographic refinement was done with Crystallography & NMR System (CNS), and Xfit was used for manual model building.
DNA binding by Atl
Oligonucleotide-Atl1 interactions were analyzed by electrophoretic mobility shift assay using standard methods and by surface plasmon resonance with biotinylated O6-mG-, O6-pobG-, or AP-site-containing or control oligonucleotides immobilized on a streptavidin-coated surface of a Biacore SA chip and serial dilutions of Atl1 applied to the cell. DNA complex stoichiometries formed under protein saturation conditions were established by sedimentation equilibrium analysis.
Other biochemical assays
AGT inhibition assays performed as described previously. Far western analyses performed by standard methods.
Atl1 expression in S. pombe
S. pombe strains originated from GM4 (h− atl1::ura4 ura4-D18 leu1-32 his7-366 ade6-M210), RO131 (h+ rad13::kanMX ura4-D18 his3-D1). MNNG sensitivity determined by agar plate and clonogenic assays. Mutation rates determined as reversions of ade6-485 to Ade+.
Atl1 expression in E. coli
pQE-30 empty vector was expressed in E. coli GWR109 ada− ogt− and pQE-30 or pQE-Atl1 in E. coli GWR109 ada− ogt− atl−. Mutation frequencies determined as number of MNNG-induced Rifampicin resistant mutants (RifR) per 108 surviving cells.
Full Methods and any associated references are available in the Supplementary Information of the online version of the paper at www.nature.com/nature.
Supplementary Material
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Acknowledgements
We thank C. C. Vu and J. Gong for aiding in the synthesis of O6-pobG oligomers, M. N. Boddy, J. Prudden, and A. Sarker for performing genetics and biochemical experiments, G. Guenther, S. Pebernard, R. S. Williams, J. J. Perry, B. R. Chapados, M. Bjorås, D. S. Shin, K. Hitomi, C. Hitomi, G. Williams, S. Tsutakawa, and P. K. Cooper for helpful suggestions, and the staffs at The Advanced Light Source (ALS) and the Stanford Synchrotron Radiation Laboratory (SSRL). Operations at SSRL and ALS are supported by the U.S. Department of Energy and NIH. This work was supported by National Institutes of Health grants CA097209 (JAT, AEP), GM070662 (MGF), and CA59887 (LAP), North West Cancer Research Fund grant CR675 (OF), Cancer Research-UK (GPM) and CHEMORES (GPM).
References
- 1.Pegg AE. Repair of O6-alkylguanine by alkyltransferases. Mutat. Res. 2000;462:83–100. doi: 10.1016/s1383-5742(00)00017-x. [DOI] [PubMed] [Google Scholar]
- 2.Tubbs JL, Pegg AE, Tainer JA. DNA binding, nucleotide flipping, and the helix-turn-helix motif in base repair by O6-alkylguanine-DNA alkyltransferase and its implications for cancer chemotherapy. DNA Repair. 2007;6:1100–1115. doi: 10.1016/j.dnarep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loechler EL, Green CL, Essigmann JM. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc. Natl. Acad. Sci. U. S. A. 1984;81:6271–6275. doi: 10.1073/pnas.81.20.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pauly GT, Hughes SH, Moschel RC. Comparison of mutagenesis by O6-methyl-and O6-ethylguanine and O4-methylthymine in Escherichia coli using double-stranded and gapped plasmids. Carcinogenesis. 1998;19:457–461. doi: 10.1093/carcin/19.3.457. [DOI] [PubMed] [Google Scholar]
- 5.Margison GP, Santibáñez-Koref MF. O6-Alkylguanine-DNA alkyltransferase: role in carcinogenesis and chemotherapy. Bioessays. 2002;24:255–266. doi: 10.1002/bies.10063. [DOI] [PubMed] [Google Scholar]
- 6.Mitra S, Kaina B. Regulation of repair of alkylation damage in mammalian genomes. Progr. Nucleic Acid Res. Mol. Biol. 1993;44:109–142. doi: 10.1016/s0079-6603(08)60218-4. [DOI] [PubMed] [Google Scholar]
- 7.Pegg AE, Dolan ME, Moschel RC. Structure, function and inhibition of O6-alkylguanine-DNA alkyltransferase. Progr. Nucleic Acid Res. Mol. Biol. 1995;51:167–223. doi: 10.1016/s0079-6603(08)60879-x. [DOI] [PubMed] [Google Scholar]
- 8.Daniels DS, Tainer JA. Conserved structural motifs governing the stoichiometric repair of alkylated DNA by O6-alkylguanine-DNA alkyltransferase. Mutat. Res. 2000;460:151–163. doi: 10.1016/s0921-8777(00)00024-0. [DOI] [PubMed] [Google Scholar]
- 9.Daniels DS, et al. Active and alkylated human AGT structures: a novel zinc site, inhibitor and extrahelical base binding. EMBO J. 2000;19:1719–1730. doi: 10.1093/emboj/19.7.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wibley JEA, Pegg AE, Moody PCE. Crystal structure of the human O6-alkylguanine-DNA alkyltransferase. Nucleic Acids Res. 2000;28:393–401. doi: 10.1093/nar/28.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniels DS, et al. DNA binding and nucleotide flipping by the human DNA repair protein AGT. Nat. Struct. Mol. Biol. 2004;11:714–720. doi: 10.1038/nsmb791. [DOI] [PubMed] [Google Scholar]
- 12.Duguid EM, Rice PA, He C. The structure of the human AGT protein bound to DNA and its implications for damage detection. J. Mol. Biol. 2005;350:657–666. doi: 10.1016/j.jmb.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 13.Margison GP, et al. Alkyltransferase-like proteins. DNA Repair. 2007;6:1222–1228. doi: 10.1016/j.dnarep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Pearson SJ, Ferguson J, Santibanez-Koref M, Margison GP. Inhibition of O6-methylguanine-DNA methyltransferase by an alkyltransferase-like protein from Escherichia coli. Nucleic Acids Res. 2005;33:3837–3844. doi: 10.1093/nar/gki696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson SJ, et al. A novel DNA damage recognition protein in Schizosaccharomyces pombe. Nucleic Acids Res. 2006;34:2347–2354. doi: 10.1093/nar/gkl270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CS, et al. A proteome chip approach reveals new DNA damage recognition activities in Escherichia coli. Nat. Methods. 2008;5:69–74. doi: 10.1038/NMETH1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morita R, Nakagawa N, Kuramitsu S, Masui R. An O6-methylguanine-DNA methyltransferase-like protein from Thermus thermophilus interacts with a nucleotide excision repair protein. J. Biochem. (Tokyo) 2008;144:267–277. doi: 10.1093/jb/mvn065. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, et al. Pyridyloxobutyl adduct O6-[4-oxo-4-(3-pyridyl)butyl]guanine is present in 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone-treated DNA and is a substrate for O6-alkylguanine-DNA alkyltransferase. Chem. Res. Toxicol. 1997;10:562–567. doi: 10.1021/tx9602067. [DOI] [PubMed] [Google Scholar]
- 19.Mijal RS, et al. The repair of the tobacco specific nitrosamine derived adduct O6-[4-Oxo-4-(3-pyridyl)butyl]guanine by O6-alkylguanine-DNA alkyltransferase variants. Chem. Res. Toxicol. 2004;17:424–434. doi: 10.1021/tx0342417. [DOI] [PubMed] [Google Scholar]
- 20.Mazon G, et al. The alkyltransferase-like ybaZ gene product enhances nucleotide excision repair of O6-alkylguanine adducts in E. coli. DNA Repair. In Press doi: 10.1016/j.dnarep.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Hu J, Ma A, Dinner AR. A two-step nucleotide-flipping mechanism enables kinetic discrimination of DNA lesions by AGT. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4615–4620. doi: 10.1073/pnas.0708058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasimas JJ, Pegg AE, Fried MG. DNA-binding mechanism of O6-alkylguanine-DNA alkyltransferase. Effects of protein and DNA alkylation on complex stability. J. Biol. Chem. 2003;278:7973–7980. doi: 10.1074/jbc.M211854200. [DOI] [PubMed] [Google Scholar]
- 23.O'Donovan A, et al. XPG endonuclease makes the 3' incision in human DNA nucleotide excision-repair. Nature. 1994;371:432–435. doi: 10.1038/371432a0. [DOI] [PubMed] [Google Scholar]
- 24.Klungland A, Lindahl T. Second pathway for completion of human DNA base excision-repair: Reconstitution with purified proteins and requirement for DNase IV (FEN1) EMBO J. 1997;16:3341–3348. doi: 10.1093/emboj/16.11.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yonemasu R, et al. Characterization of the alternative excision repair pathway of UV-damaged DNA in Schizosaccharomyces pombe. Nucleic Acids Res. 1997;25:1553–1558. doi: 10.1093/nar/25.8.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putnam NH, et al. Sea Anemone Genome Reveals Ancestral Eumetazoan Gene Repertoire and Genomic Organization. Science. 2007;317:86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- 27.Elkins JG, et al. A korarchaeal genome reveals insights into the evolution of the Archaea. Proc. Natl. Acad. Sci. U. S. A. 2008;105:8102–8107. doi: 10.1073/pnas.0801980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waters E, et al. The genome of Nanoarchaeum equitans: Insights into early archaeal evolution and derived parasitism. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12984–12988. doi: 10.1073/pnas.1735403100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sedgwick B. Repairing DNA-methylation damage. Nat. Rev. Mol. Cell Biol. 2004;5:148–157. doi: 10.1038/nrm1312. [DOI] [PubMed] [Google Scholar]
- 30.Hanawalt PC, Spivak G. Transcription-coupled DNA repair: two decades of progress and surprises. Nat. Rev. Mol. Cell Biol. 2008;9:958–970. doi: 10.1038/nrm2549. [DOI] [PubMed] [Google Scholar]
- 31.Samson L, Thomale J, Rajewsky MF. Alternative pathways for the in vivo repair of O6-alkylguanine and O4-alkylthymine in Escherichia coli: the adaptive response and nucleotide excision repair. EMBO J. 1988;7:2261–2267. doi: 10.1002/j.1460-2075.1988.tb03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voigt JM, Van Houten B, Sancar A, Topal MD. Repair of O6-methylguanine by ABC excinuclease of Escherichia coli in Vitro. J. Biol. Chem. 1989;264:5172–5176. [PubMed] [Google Scholar]
- 33.Edara S, Kanugula S, Pegg AE. Expression of the inactive C145A mutant human O6-alkylguanine-DNA alkyltransferase in E. coli increases cell killing and mutations by N-methyl-N'-nitro-N-nitrosoguanidine. Carcinogenesis. 1999;20:103–108. doi: 10.1093/carcin/20.1.103. [DOI] [PubMed] [Google Scholar]
- 34.Mellon I. Transcription-coupled repair: a complex affair. Mutat. Res. 2005;577:155–161. doi: 10.1016/j.mrfmmm.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Branum ME, Reardon JT, Sancar A. DNA repair excision nuclease attacks undamaged DNA. A potential source of spontaneous mutations. J. Biol. Chem. 2001;276:25421–25426. doi: 10.1074/jbc.M101032200. [DOI] [PubMed] [Google Scholar]
- 36.Viswanathan A, Doetsch PW. Effects of nonbulky DNA base damages on Escherichia coli RNA polymerase-mediated elongation and promoter clearance. J. Biol. Chem. 1998;273:21276–21281. doi: 10.1074/jbc.273.33.21276. [DOI] [PubMed] [Google Scholar]
- 37.Scrima A, et al. Structural Basis of UV DNA-Damage Recognition by the DDB1-DDB2 Complex. Cell. 2008;135:1213–1223. doi: 10.1016/j.cell.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Min JH, Pavletich NP. Recognition of DNA damage by the Rad4 nucleotide excision repair protein. Nature. 2007;449:570–575. doi: 10.1038/nature06155. [DOI] [PubMed] [Google Scholar]
- 39.Mol CD, Izumi T, Mitra S, Tainer JA. DNA-bound structures and mutants reveal abasic DNA binding by APE1 and DNA repair coordination. Nature. 2000;403:451–456. doi: 10.1038/35000249. [DOI] [PubMed] [Google Scholar]
- 40.Chapados BR, et al. Structural basis for FEN-1 substrate specificity and PCNA-mediated activation in DNA replication and repair. Cell. 2004;116:39–50. doi: 10.1016/s0092-8674(03)01036-5. [DOI] [PubMed] [Google Scholar]
- 41.Parikh SS, et al. Uracil-DNA glycosylase-DNA substrate and product structures: Conformational strain promotes catalytic efficiency by coupled stereoelectronic effects. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5083–5088. doi: 10.1073/pnas.97.10.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcin ED, et al. DNA apurinic-apyrimidinic site binding and excision by endonuclease IV. Nat. Struct. Mol. Biol. 2008;15:515–522. doi: 10.1038/nsmb.1414. [DOI] [PubMed] [Google Scholar]
- 43.Williams RS, et al. Mre11 dimers coordinate DNA end bridging and nuclease processing in double-strand-break repair. Cell. 2008;135:97–109. doi: 10.1016/j.cell.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cline SD, Hanawalt PC. Who's on first in the cellular response to DNA damage? Nat. Rev. Mol. Cell Biol. 2003;4:361–372. doi: 10.1038/nrm1101. [DOI] [PubMed] [Google Scholar]
- 45.Hitomi K, Iwai S, Tainer JA. The intricate structural chemistry of base excision repair machinery: Implications for DNA damage recognition, removal, and repair. DNA Repair. 2007;6:410–428. doi: 10.1016/j.dnarep.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Hickman MJ, Samson LD. Role of DNA mismatch repair and p53 in signaling induction of apoptosis by alkylating agents. Proc. Natl. Acad. Sci. U. S. A. 1999;96:10764–10769. doi: 10.1073/pnas.96.19.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marcotte EM, et al. Detecting protein function and protein-protein interactions from genome sequences. Science. 1999;285:751–753. doi: 10.1126/science.285.5428.751. [DOI] [PubMed] [Google Scholar]
- 48.Kanugula S, Pauly GT, Moschel RC, Pegg AE. A bifunctional DNA repair protein from Ferroplasma acidarmanus exhibits O6-alkylguanine-DNA alkyltransferase and endonuclease V activities. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3617–3622. doi: 10.1073/pnas.0408719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalhus B, et al. Structures of endonuclease V with DNA reveal initiation of deaminated adenine repair. Nat. Struct. Mol. Biol. 2009;16:138–143. doi: 10.1038/nsmb.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scharer OD, Campbell AJ. Wedging out DNA damage. Nat. Struct. Mol. Biol. 2009;16:102–104. doi: 10.1038/nsmb0209-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.