Abstract
When Caenorhabditis elegans encounters an unfavourable stimulus at its anterior, it responds by initiating an avoidance response, namely reversal of locomotion. The amphid neurons, ASHL and ASHR, are polymodal in function, with roles in the avoidance responses to high osmolarity, nose touch, and both volatile and non-volatile repellents. The mechanisms that underlie the ability of the ASH neurons to respond to such a wide range of stimuli are still unclear. We demonstrate that the inositol 1,4,5-trisphosphate receptor (IP3R), encoded by itr-1, functions in the reversal responses to nose touch and benzaldehyde, but not in other known ASH-mediated responses. We show that phospholipase Cβ (EGL-8) and phospholipase Cγ (PLC-3), which catalyse the production of IP3, both function upstream of ITR-1 in the response to nose touch. We use neuron-specific gene rescue and neuron-specific disruption of protein function to show that the site of ITR-1 function is the ASH neurons. By rescuing plc-3 and egl-8 in a neuron-specific manner, we show that both are acting in ASH. Imaging of nose touch–induced Ca2+ transients in ASH confirms these conclusions. In contrast, the response to benzaldehyde is independent of PLC function. Thus, we have identified distinct roles for the IP3R in two specific responses mediated by ASH.
Author Summary
In order to avoid potential hazards, animals detect and discriminate between a wide range of aversive stimuli. To detect some of these stimuli, animals use polymodal sensory neurons, that is neurons of a single type that can detect a range of different stimuli and transmit an appropriate signal to the downstream nervous system. Pain-sensing nociceptors in humans and the ASH neurons in C. elegans are both polymodal. The ASH neurons mediate responses to high osmotic strength, nose touch, high ambient oxygen, and volatile and non-volatile compounds. It remains unclear how these cells detect and discriminate between these different stimuli. We show that signalling through the second messenger inositol 1,4,5-trisphosphate (IP3) and its receptor (IP3R) is required in ASH for animals to respond to nose touch. We also show that IP3Rs are required for the response to the volatile compound benzaldehyde. However, these signalling components are not required for a range of other ASH-mediated responses. Thus, we have identified a signalling mechanism that is specific to a small subset of ASH-mediated responses. These results add to our understanding of how ASH discriminates between a variety of stimuli and thus to our understanding of polymodal neurons in general.
Introduction
Like other animals, C. elegans negotiates its environment by responding to a range of noxious stimuli, by changing its direction of movement to avoid the source of the stimulus and thus avoid imminent injury. Mechanical stimulation is one type of stimulation that exerts such an effect. Depending on the position and strength of the mechanical stimulus, the neuronal circuitry responsible for this response differs. The response to nose touch relies primarily on the ASH pair of sensory neurons [1], which output to the command interneurons, AVA, AVB, AVD, AVE and PVC, which control forwards and backwards movement [2],[3]. These command neurons interact synaptically with one another and ultimately output to the motor neurons that control the body wall contractions necessary for sinusoidal movement. In contrast, the response to light anterior body touch relies on the ALM and AVM sensory neurons, which act upon these same command neurons.
The ASH neurons are particularly interesting in that they are polymodal nociceptive neurons, implicated in avoidance responses to a diverse range of sensory cues, namely, high osmotic strength, nose touch, high ambient oxygen, volatile compounds and non-volatile repellents such as heavy metals, protons and detergents [4]–[8]. ASH is thus analogous to human nociceptors, capable of responding to heat, mechanical stimulation and chemicals such as capsaicin. So understanding the signalling pathways that underlie the polymodal function of ASH is proving important to our understanding of human pain sensation. The molecular mechanisms that enable ASH to sense such a wide range of inputs are still poorly described. Work thus far has identified “general” components that are required for responses to all stimuli, and has also identified “specific” molecules that are required for single, or a small subset of, responses. The transient receptor potential vanilloid (TRPV)-related channel proteins OCR-2 and OSM-9 [9],[10], for example, appear to be required for all ASH-mediated responses, while GPA-3, a G-protein α subunit, is required for only a small subset [11]. Thus ASH utilises specific signalling pathways for individual stimuli, but these may converge on a common pathway.
In the present study, we identify signalling through the inositol 1,4,5-trisphosphate receptor (IP3R) (Figure 1A) as a specific component, required for a small subset of ASH-mediated responses. IP3Rs in Caenorhabditis elegans are encoded by a single gene, itr-1, and are widely expressed throughout the animal, including in the nervous system [12]–[14]. A wide range of functions for itr-1 have been identified. Genetic approaches have identified roles for itr-1 in ovulation and meiotic maturation ([13],[15],[16], defecation [13],[16],[17], male mating [18] and in ventral enclosure [19]. We used a dominant-negative construct (IP3 sponge), as well as loss-of-function mutants and RNA interference, to demonstrate that IP3 signalling and IP3Rs function in the regulation of pharyngeal pumping rate and in multiple stages of embryogenesis [20].
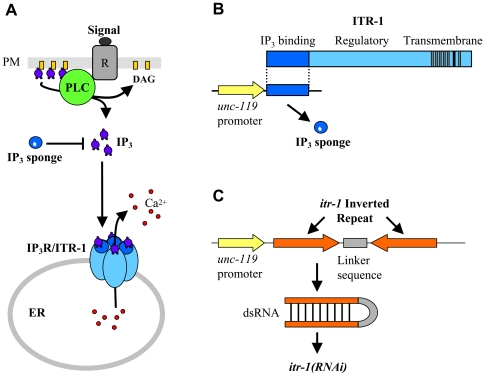
Figure 1. Disruption of IP3 signalling in the nervous system.
(A) Schematic diagram showing the IP3 signalling cassette. Stimulation of a receptor (R) at the cell surface leads to the activation of phopholipase C (PLC), which catalyses the hydrolysis of phosphatidylinositol 4,5-bisphosphate to produce inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 diffuses to the endoplasmic reticulum (ER), where it activates the IP3 receptor (IP3R), resulting in the release of Ca2+ into the cytoplasm. Expression of an IP3 sponge [see (B)] should mop up free IP3, thus interfering with its ability to activate IP3Rs. (B) Strategy used to disrupt IP3 signalling in the nervous system using IP3 sponges. ITR-1, the C. elegans IP3R subunit, consists of 3 functional regions, including an IP3 binding domain. Overexpression of the binding domain allows it to act as an IP3 sponge. Expression under the control of the unc-119 promoter leads to nervous system-wide expression of the IP3 sponge. (C) Strategy used to express dsRNA, and thus disrupt itr-1 expression, in the nervous system. Forward and reverse copies of an itr-1 cDNA fragment are expressed under the control of the unc-119 promoter. A “linker” region allows the complementary RNA regions to form dsRNA.
For some of these functions, we have some insights into the nature of events upstream of itr-1 and in particular into the member(s) of the phospholipase C (PLC) family responsible for IP3 production (Figure 1A). For example, PLC-3 (PLCγ) appears to act upstream of ITR-1 in the regulation of gonadal sheath contraction (with the receptor tyrosine kinases LET-23 and VAB-1 presumably acting further upstream, [16]), and also during the defecation motor program [17]. We also know that PLC-1 (PLCε) acts upstream of ITR-1 to regulate ventral enclosure [21]. Finally, there is evidence that EGL-8 (PLCβ) functions upstream of ITR-1 in the control of sperm transfer [18].
In the present study we have used transgenic approaches to disrupt either IP3 signalling or itr-1 function in the nervous system, and demonstrated a role in the avoidance responses to nose touch and benzaldehyde. Our evidence indicates that, for nose touch, two PLCs, PLC-3 and EGL-8, act as the source of IP3 upstream. We use cell-specific expression of an IP3 sponge and cell-specific rescue to show that itr-1 and both PLCs are acting in the ASH neurons; and demonstrate that all three genes function in the production of nose touch-induced Ca2+ transients in ASH. Thus we have identified signalling components that are specific to two of the group of stimuli sensed by ASH.
Methods
Strains and constructs
The C. elegans strains used in this study are listed in Table S1. Strains containing the plc-3(tm1340) allele were maintained as balanced heterozygous strains, and assayed as homozygotes. itr-1, egl-8 and plc-3 strains carrying the sra-6p::YC2.12 construct were made by crossing the appropriate strain with AQ1444 [22]. Strains carrying itr-1(sy290gf) also carry a closely linked allele of unc-24, unc-24(e138), which results in a locomotion (weak kinker) phenotype, which may interfere with the avoidance response. When using this allele we therefore rescued the unc-24 deficiency by transgenic expression of a genomic fragment containing the wild type unc-24 gene under the control of its own promoter. As a control, we rescued unc-24(e138) animals in the same way [18].
To construct sra-6, glr-1 and unc-119 promoter plasmids, we used 3.8 Kb [23], 5.2 Kb [24] and 1.3 Kb [25], respectively, of upstream DNA. IP3 sponge derivatives were constructed as described previously [20]. RNAi inverted repeat constructs were constructed using pHAB200 [12] by inserting forward and reverse copies of the same region of E. coli lacZ or itr-1 cDNA either side of a “linker” made from gfp or a unique part of lacZ, respectively. plc-3 rescue plasmids were constructed using a full length genomic fragment. itr-1 and egl-8 rescue plasmids were constructed using the Gateway system (Invitrogen), using the full-length cDNA (itr-1) or a “minigene (egl-8, as in [26]). These were introduced, along with the relevant promoter, into the destination vector pHP2 (see Table S1).
Constructs were introduced into C. elegans by injection [27] with a mec-7p::gfp marker plasmid, pPD117.01 (a gift from A. Fire).
RNA-mediated interference (RNAi) of itr-1
RNA-mediated interference (RNAi) of itr-1 was carried out using E. coli HT115 carrying derivatives of the vector pPD129.36 [28], which contains two flanking T7 RNA polymerase promoters. For RNAi of PLC genes we used derivatives of pPD129.36, pHAB301 (egl-8) and pHAB303 (plc-3) [18]. As a control we used a derivative of pPD129.36 with an E. coli chloramphenicol acetyltransferase (CAT) DNA insert. Plasmids were transformed into E. coli HT115 (DE3) and these strains used to perform RNAi feeding experiments [28].
Behavioural assays
The response to nose touch was assayed on food at 20°C using an eyelash, as described by Kaplan and Horvitz [1]. The response to anterior body touch was assayed similarly. Reversal responses (initiated within 3 seconds of the stimulus) were quantified as distance reversed, expressed in worm lengths. Three categories of response were used, >1 worm length, which corresponds to a “good” reversal response, >0.1 worm length and 0 worm lengths, considered “poor” avoidance responses. A minimum of 40 animals were assayed for each genotype or condition shown. The response to repellents was assayed using the “dry drop” test [6], except for octanol, which was assayed using a “smell-on-a-stick” assay [23], as described by Chao et al. [29]. Results were analysed using Chi-squared tests.
In vivo Ca2+ imaging
Optical recordings were performed essentially as described [30],[31] on a Zeiss Axioskop 2 upright compound microscope equipped with a Dual View beam splitter and a Uniblitz Shutter. The following filters and dichroics were used: excitation: 400–440 nm bandpass; excitation dichroic: 455 nm; CFP emission: 465–495 nm bandpass; emission dichroic: 505 nm; YFP emission: 520–550 nm bandpass. Individual adult worms (∼24 h past L4) were glued with Nexaband S/C cyanoacrylate glue to pads composed of 2% agarose in extracellular saline (145 mM NaCl, 5 mM KCl, 1 mM CaCl2, 5 mM MgCl2, 20 mM D-glucose, 10 mM HEPES buffer, pH 7.2, 2 mM serotonin). Fluorescence images were acquired using MetaVue 6.2. Acquisitions were taken at 28 Hz (35 ms exposure time) with 4×4 or 2×2 binning, using a 63× Zeiss Achroplan water immersion objective.
Nose touch stimulation was performed as described [32]. A rounded glass needle was placed perpendicular to the worm's body at a distance of 150 µm from the side of the nose, displaced 8 µm into the side of the worm's nose, held in position for 1 second, and then pulled back to its original position. For each strain, we recorded 2 responses for 10 animals, with 5 minutes between stimuli. Results were compared using a Mann-Whitney rank sum test.
Results
Disruption of itr-1 function in the nervous system
In order to investigate the role of IP3 signalling in the nervous system, we expressed the cDNA encoding the IP3 binding domain of itr-1 (an “IP3 sponge”, [20]) under the control of the promoter of unc-119, which is widely, and exclusively, expressed in the nervous system [25]. Two derivatives of the IP3 sponge were used, as described previously [33]. The “control sponge” (K579Q, R582Q), is deficient in IP3 binding and therefore should not disrupt IP3 signalling, while the “super sponge” (R511C) has increased affinity for IP3. In a second approach, to disrupt IP3R function rather than IP3 signalling, we used the unc-119 promoter to control expression of an itr-1 dsRNAi “snapback” construct [34]. Figure 1 illustrates these approaches.
IP3 signalling and itr-1 function in the aversive response to nose touch
We determined the role of IP3 signalling and itr-1 in the avoidance response to nose touch. Mechanical stimuli were delivered to the nose of moving animals using an eyelash, essentially as described by Kaplan and Horvitz [1]. In order to detect differences in the type of movement response exhibited, we used a scoring system in which the length of reversal was expressed in worm lengths (see Methods). Three categories of response were used, >1 worm length, >0.1 worm length and 0 worm lengths. The first is considered a “good” response, while the latter two correspond to “poor” responses. This scoring method is similar to that used by Kindt et al. [32], with the >0.1 worm length category usually corresponding to a “head withdrawal” response [32],[35].
As Figure 2A shows, when cDNA encoding the IP3 super sponge is expressed under the control of the unc-119 promoter, the reversal response to nose touch is severely disrupted, while expression of the control sponge in the same way has no effect. However, the response to light anterior body touch, which uses a different neuronal circuitry but relies on the same command neurons and muscle groups, remains unaffected, indicating that the defect is specific to nose touch, rather than a general movement defect. The avoidance responses to harsh anterior body touch and both harsh and light posterior body touch are similarly unaffected (DSW and HAB, unpublished). Thus, IP3 signalling functions in the avoidance response to nose touch.
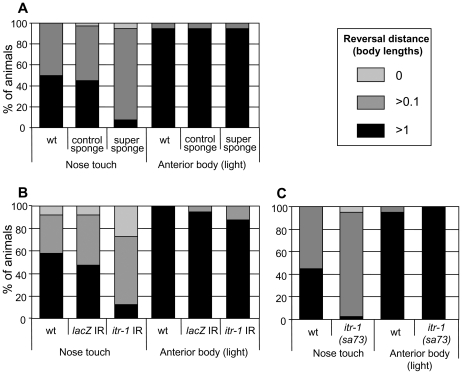
Figure 2. IP3 and itr-1 function in the aversive response to nose touch.
The reversal response (measured in worm lengths) of animals exposed to nose touch or light anterior body touch. (A) Animals expressing IP3 sponge derivatives under the control of the unc-119 promoter. (B) Animals expressing dsRNA under the control of the unc-119 promoter. IR, inverted repeat. (C) Animals carrying the itr-1(sa73) loss-of-function allele. All three methods of disrupting ITR-1 function significantly disrupt the nose touch response in comparison to wt animals (P<0.001, Chi-squared test, in each case). The control sponge (A) and lacZ control IR (B) do not disrupt the response (P>0.05).
As Figure 2B shows, when an itr-1 dsRNAi “snapback” construct is expressed in the nervous system, the response to nose touch is significantly disrupted. A dsRNAi construct for the E. coli lacZ gene, expressed in the same way, has no effect. As Figure 2C shows, itr-1(sa73) (temperature sensitive, loss-of-function) animals also demonstrate a defective response to nose touch at 20°C, a partially restrictive temperature. In both cases, the response to light anterior body touch is unaffected, as were the responses to other types of mechanical stimuli (DSW and HAB, unpublished). Since the vast majority of animals still exhibit a slight movement response (>0.1 worm length), head withdrawal appears to be unaffected. Thus, itr-1 functions specifically in the reversal response to nose touch.
itr-1 functions in the avoidance response to a volatile repellent, benzaldehyde, but not in other ASH-mediated responses
The response to nose touch is largely mediated through the ASHL and ASHR pair of amphid sensory neurons, although minor roles appear to be played by FLP and OLQ neurons [1]. The ASH neurons are polymodal in function, with roles identified not only in the avoidance of nose touch, but also in the avoidance of high osmolarity and both volatile and non-volatile repellents [4],[6],[8]. To determine whether itr-1 has a global role in ASH responses or is specifically required for nose touch, we tested whether it has a similarly important role in other responses known to be mediated by ASH. As Figure 3A–3F shows, expression of the itr-1 dsRNAi construct under the control of the unc-119 promoter does not significantly disrupt the avoidance responses to high osmolarity (fructose), SDS, copper, quinine or glycerol (although our experiments do not exclude more subtle roles). However, the response to the volatile repellent benzaldehyde is disrupted (Figure 3G). Similarly, itr-1(sa73) animals display a defective response to benzaldehyde (Figure 3H). Interestingly, however, the use of an IP3 sponge failed to disrupt the aversive response to benzaldehyde (Figure 3I), suggesting that this response could be independent of IP3. We tested another volatile repellent, octanol, and found that, in the presence of food, the responses to 30% and 100% octanol are unaffected (Figure 3J) whilst wild type, and tph-1 and mod-5 mutants, behave as expected [29]. Thus itr-1 appears to function in a very limited subset of ASH-mediated avoidance responses, to nose touch and benzaldehyde.
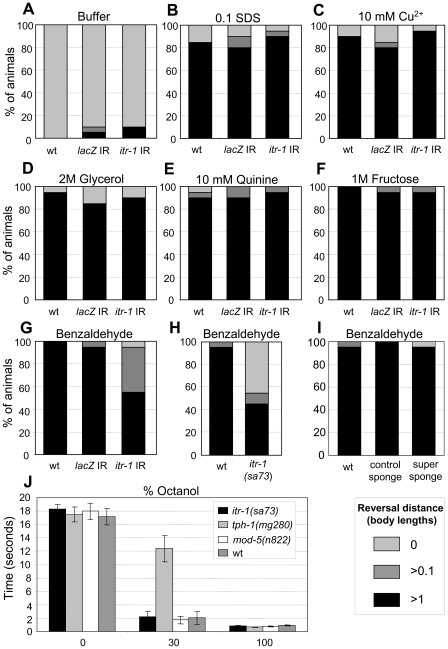
Figure 3. itr-1 functions in the avoidance response to a volatile repellent, benzaldehyde, but not in other ASH-mediated responses.
(A–I) The reversal response of animals (measured in worm lengths; see key, bottom right) exposed to a range of stimuli. (A–F) Reversal responses in animals expressing itr-1 or lacZ dsRNA (IR, inverted repeat) under the control of the unc-119 promoter and treated with: (A) Buffer alone (30 mM Tris [pH 7.5], 100 mM NaCl, 10 mM KCl), (B) SDS, (C) copper, (D) glycerol, (E) quinine, and (F) fructose, at the concentrations indicated, using a “dry drop” assay [6]. (G–I) Reversal response to benzaldehyde (undiluted) of (G) animals expressing dsRNA under the control of the unc-119 promoter (IR, inverted repeat); (H) wild type and itr-1 loss-of-function animals; (I) animals expressing IP3 sponge derivatives under the control of the unc-119 promoter. (J) Response to octanol, measured as the time taken to reverse, following administration of the octanol concentrations indicated, as a “smell-on-a-stick” [29]. Animals in which itr-1 is knocked down in the nervous system, or which carry the itr-1(sa73) mutation are defective in the response to benzaldehyde [P<0.001, (G)] but not to other repellants (P>0.05). However, the IP3 sponge failed to disrupt the response to benzaldehyde [P>0.05, (I)]. All P values are from Chi-squared tests.
PLCβ and PLCγ function in the aversive response to nose touch through the production of an IP3 signal
PLCs catalyse the hydrolysis of PIP2, to produce IP3 (Figure 1) and are therefore good candidates for the source of signal that activates the IP3R. We therefore investigated the role of C. elegans PLCs in the avoidance response to nose touch. In C. elegans five PLC genes and one further PLC-like gene have been identified in the genome [18]. They correspond to vertebrate PLC-β (egl-8 [26],[36],[37]), PLC-δ, PLC-γ (plc-4 and plc-3, respectively [16]), PLC-ε (plc-1 [37]), and an unusual, β-like protein (plc-2 [18]). As Figure 4A shows, both egl-8 and plc-3 loss-of-function mutants exhibit a significant defect in the aversive response to nose touch, while loss-of-function mutants for the other PLC genes remain unaffected. The response to light anterior body touch is unaffected, as were the responses to other types of stimuli (data not shown). Thus, egl-8 and plc-3 function specifically in the aversive response to nose touch.
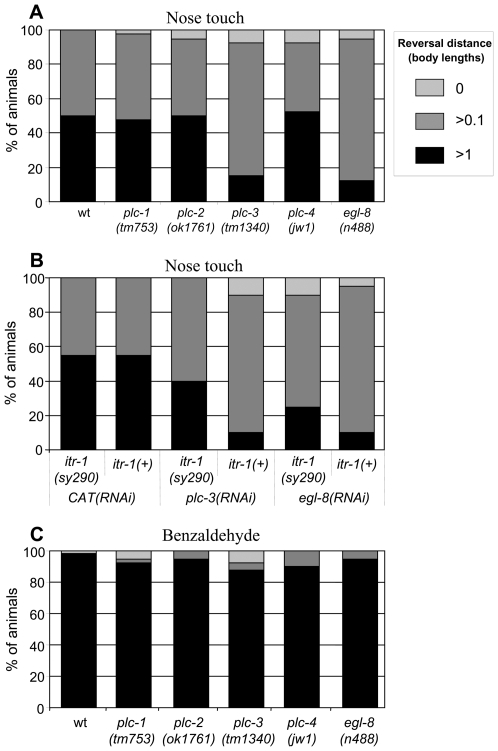
Figure 4. PLCβ and PLCγ function, through the production of an IP3 signal, in the aversive response to nose touch, but not to benzaldehyde.
(A) Reversal response of PLC deficient animals to nose touch. All showed a wild type response (>90% reversing >1 worm length) to anterior body touch (data not shown). (B) Reversal response of itr-1(sy290) gain-of-function animals to nose touch, following depletion of plc-3 or egl-8 by RNAi. CAT, E. coli chloramphenicol acetyltransferase. All showed a wild type response (>90% reversing >1 worm length) to anterior body touch (data not shown). (C) Reversal response of PLC deficient animals to benzaldehyde. All showed a wild type response (<5% reversing >1 worm length) to buffer alone (data not shown). Both plc-3 and egl-8 loss-of-function mutants exhibit defective responses to nose touch (P<0.001) compared to wt animals. RNAi of plc-3 or egl-8 similarly disrupted the response (P<0.001) compared to the CAT(RNAi) control animals. However, RNAi of plc-3 or egl-8 in an itr-1(sy290) background failed to disrupt the response to such an extent (plc-3, P<0.001; egl-8, P<0.05, when compared to RNAi of the same genes in wt animals). All P values are from Chi-squared tests.
Since PLC-β and PLC-γ catalyse the production of two second messengers, IP3 and diacylglycerol (DAG), we wished to demonstrate that it is via the generation of an IP3 signal that they function in the nose touch response. To this end, we investigated whether itr-1(sy290), a gain-of-function allele [38], could rescue the defects in nose touch response that resulted from egl-8 or plc-3 RNAi. itr-1(sy290) has a mutation, R582Q, in the IP3 binding site [38], which results in a two-fold increase in IP3 binding affinity [20]. As Figure 4B shows, RNAi of plc-3 and of egl-8 (in a wild type itr-1 background) is able to reproduce the defect in nose touch response that was observed for loss-of-function mutants. However, RNAi of egl-8 and plc-3 on itr-1(sy290) animals failed, significantly, to disrupt the nose touch response to such an extent. Thus, an itr-1 mutation that increases the receptor's affinity for IP3 partially rescues the defects in nose touch response that result from knockdown of either plc-3 or egl-8, suggesting that IP3 is an important component of the downstream signal from these PLCs.
We investigated the role of PLCs in the response to benzaldehyde. As Figure 4C shows, the response to benzaldehyde remained intact in all of the PLC loss-of-function mutants. As both egl-8 and plc-3 are implicated in the response to nose touch we also attempted to test a plc-3,egl-8, double mutant for responses to benzaldehyde, however the double mutant animals had severe locomotive defects and we were not able to perform the relevant assays. Thus it appears that the response to benzaldehyde, although IP3R-dependent, may be independent of PLC function. Although we cannot rule out that the action of PLCs is redundant in this response, this data is compatible with the suggestion that this response does not depend on IP3 (Figure 3I).
itr-1 functions in ASH
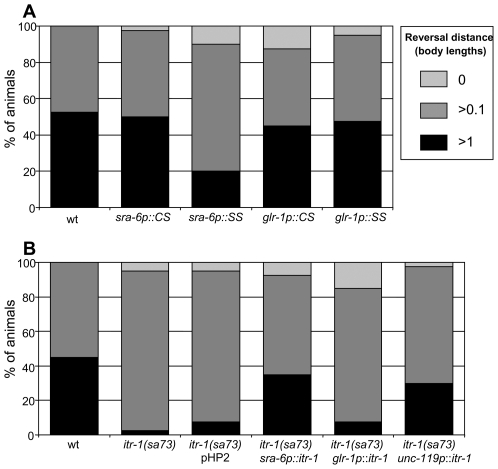
The most likely candidates for the site of action of itr-1 in the nose touch response are the ASH neurons themselves or the downstream command neurons. In order to distinguish between these possibilities (and the alternative, which is that it functions elsewhere to influence the function of these neurons in some way), we exploited the availability of neuron-specific promoters. The promoter of sra-6 directs expression in ASH, and (weakly) in ASI and PVQ [23], while that of glr-1 directs expression in the command neurons AVA, AVB, AVD, AVE and PVC and several others, but not in ASH [35]. We therefore used these promoters to carry out cell-specific rescue and disruption of itr-1 function. As Figure 5A shows, when the IP3 super sponge is expressed under control of the sra-6 promoter, the response to nose touch is disrupted. In contrast, when it is expressed under control of the glr-1 promoter the response is unaffected. Likewise, expression of the control sponge, using either promoter, does not disrupt the response. Thus, disruption of IP3 signalling in ASH, but not the command neurons, interferes with the response to nose touch.
Figure 5. itr-1 functions in ASH.
Reversal response to nose touch. (A) Animals expressing IP3 sponge derivatives under the control of cell-specific promoters. CS, control sponge; SS, super sponge. (B) itr-1(sa73) loss-of-function animals expressing full-length itr-1 cDNA under the control of cell-specific promoters. pHP2 is the empty destination vector used in construction of the other plasmids. All showed a wild type response (>90% reversing >1 worm length) to anterior body touch (data not shown). When the IP3 sponge is expressed under control of the sra-6 promoter, nose touch response is disrupted (P>0.001), while expression under control of the glr-1 promoter has no effect (P>0.05). Expression of the control sponge using either promoter has no effect (P>0.05). All P values are from Chi-squared tests.
We also used the opposite approach, expressing itr-1 cDNA under control of specific promoters and assessing whether this could rescue the defect in nose touch response that is observed in JT73 animals, which carry the itr-1(sa73) loss-of-function mutation. As Figure 5B shows, expression of itr-1 under the control of the sra-6 promoter rescues the defect seen in itr-1(sa73) animals, as does the expression of itr-1 under control of the pan-neuronal unc-119 promoter. In contrast, expression of itr-1 using the glr-1 promoter failed to rescue this defect. Thus, the defect in the reversal response to nose touch observed in itr-1(sa73) animals can be rescued by expression of itr-1 cDNA in ASH, but not in the command neurons.
plc-3 and egl-8 function in ASH
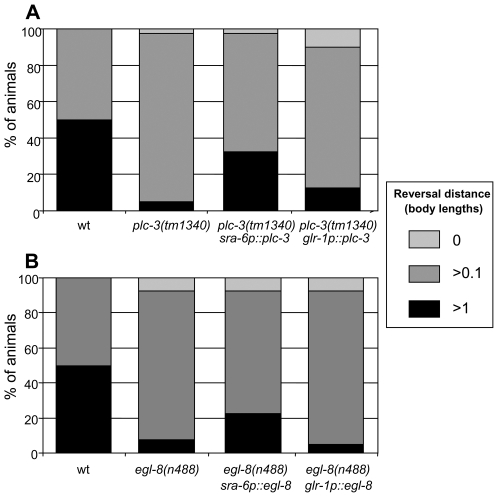
Since plc-3 and egl-8 appear to act upstream of itr-1, we hypothesised that they also effect their role in the nose touch response in the ASH neurons. To test this, we used sra-6 and glr-1 promoters to rescue plc-3 and egl-8 in a neuron-specific manner in loss-of-function mutants. As Figure 6A shows, when plc-3 is expressed under the control of the sra-6 promoter in plc-3(tm1340) homozygotes, the defect in nose touch response is significantly rescued, while expression of plc-3 under the control of the glr-1 promoter fails to rescue. Thus, as predicted, the site of plc-3 function in the response to nose touch also appears to be the ASH neurons.
Figure 6. egl-8 and plc-3 function in ASH.
Reversal response to nose touch. (A) plc-3(tm1340) loss-of-function animals expressing plc-3 genomic DNA under the control of cell-specific promoters. (B) egl-8(n488) loss-of-function animals expressing an egl-8 rescuing “minigene” under the control of cell-specific promoters. All showed a wild type response (>90% reversing >1 worm length) to anterior body touch (data not shown). Expression of plc-3 or egl-8 under control of the sra-6 promoter significantly rescues nose touch response in their respective mutants (P<0.001), while expression using the glr-1 promoter does not (P>0.05). All P values are from Chi-squared tests.
As Figure 6B shows, when egl-8 is expressed under the control of the sra-6 promoter in egl-8(n488) animals, the defect in nose touch response is significantly rescued, while expression of egl-8 under the control of the glr-1 promoter fails to rescue. Thus the site of egl-8 function in the response to nose touch is also the ASH neurons.
itr-1, egl-8, and plc-3 function in nose touch–induced Ca2+ transients in ASH
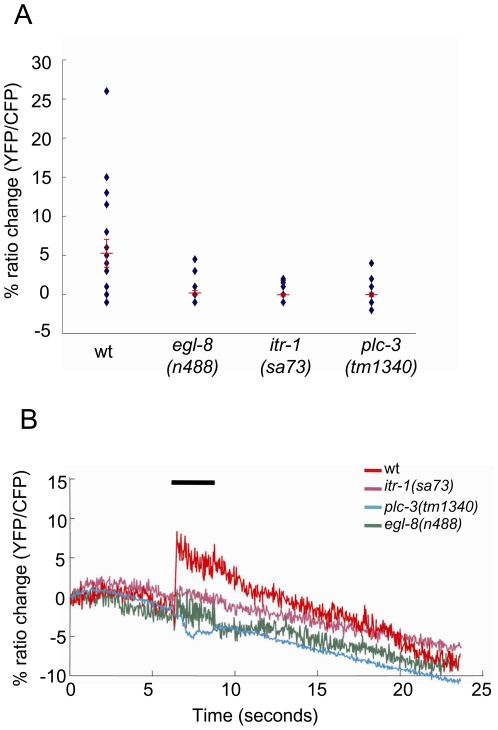
In order to more directly observe how itr-1, egl-8 and plc-3 affect sensory responses in ASH, we used the genetically encoded Ca2+ sensor, cameleon, to examine in vivo calcium transients evoked by nose touch in ASH. As previously observed [22],[32], mechanical stimulation of the nose evoked calcium influx in the ASH neurons of wild-type animals expressing cameleon under control of the sra-6 promoter (Figure 7). However, nose touch-evoked calcium transients were significantly disrupted in itr-1 mutant animals, indicating that the IP3 receptor is required for ASH mechanosensory responses. Likewise, mutants defective in egl-8 or plc-3 showed significantly reduced calcium transients in ASH in response to nose touch. Together, these results indicate that the IP3 pathway is required for nose touch mechanosensation in ASH.
Figure 7. itr-1, egl-8, and plc-3 all function in nose touch–induced Ca2+ transients in ASH.
Ratio changes in cameleon-expressing ASH neurons, following nose touch. (A) Quantification of responses. Diamonds are individual observations; longer red lines are mean; error bars are s.e.m. (n = 20). (B) Representative responses, for wild type animals and the mutants indicated. Black bar indicates duration of stimulation. The CFP/YFP ratio decreases over the course of the recordings because YFP photobleaches faster than CFP; noise is relatively low in some animals, due to higher cameleon expression levels. Nose touch–evoked Ca2+ transients were significantly disrupted in itr-1, egl-8, and plc-3 loss-of-function animals (P<0.05, Mann-Whitney rank sum test).
Discussion
We have shown that signalling through IP3Rs is required for aversive responses to nose touch and benzaldehyde in C. elegans. The response to nose touch requires itr-1 function and the action of plc-3 and egl-8 in the polymodal ASH neurons, where they function in the generation of Ca2+ transients. The ability of the IP3 sponge to disrupt nose touch and the rescue of nose touch defects in plc-3 and egl-8 RNAi animals by an itr-1 gain-of-function allele, both support the conclusion that IP3 is the signalling molecule downstream of PLC and upstream of itr-1 activation. Thus nose touch requires a canonical IP3 signalling pathway in ASH.
ASH neurons display striking polymodality and clearly distinguish functionally between different stimuli. For example, habituation to repeated nose touch has no effect on the response to octanol or high osmotic strength [39]. Likewise, prolonged exposure to copper affected behavioural and neural responses to copper but not to other repellents detected by ASH such as glycerol [22]. The ability of ASH neurons to discriminate between different aversive stimuli and undergo stimulus-specific adaptation indicates that at some level ASH uses different sensory transduction mechanisms for different modalities. At the molecular level, genes which are required for subsets of responses have been identified (see review in [40]). For example, OSM-10 is required to sense osmolarity but not for other sensory responses [39], while GPA-3 specifically affects acute responses to quinine [22]. Our new results identify the IP3 pathway as playing a specific role in the mechanosensory modality of ASH.
Interestingly, itr-1 also affects a second ASH-dependent behaviour - avoidance of high concentrations of benzaldehyde. Thus it would be interesting to determine to what extent these responses show segregation or interact, for example whether habituation to nose touch alters responses to benzaldehyde or vice versa. The molecular overlap between the responses to these two very different stimuli is intriguing. Although both require itr-1 they do show some differences. The response to benzaldehyde is not disrupted by the use of IP3 sponges, and appears not to be dependant on PLC (although we cannot eliminate the possibility that egl-8 and plc-3 act redundantly). This would suggest that, although both responses use the IP3R, the upstream components may be different. One explanation for these results is that the benzaldehyde response is mediated by an IP3-independent mechanism. IP3 independent activation of IP3Rs by proteins ligands such as CaBP (Ca2+ binding protein), CIB1 (Ca2+ and integrin binding 1, also known as calmyrin) and G-protein βγ subunits has been shown in other systems [41], Homologues of CIB1 and Gβγ are both present in worms so such mechanisms could act within ASH. It would be interesting to know whether the site of itr-1 function in the benzaldehyde response is also ASH. However, due to technical limitations, we have been unable to test this. The profound movement defects of egl-30 mutants have also prevented us from testing whether this response is also Gαq-independent.
The TRPV channels OSM-9 and OCR-2 are required for all ASH mediated responses, suggesting that response-specific signalling pathways converge on a common mechanism of activation. However, how the detection of such a wide range of stimuli is coupled to gating of these channels is unclear. The identification of signalling components that are required for detection of single stimuli, or small subsets, is vital to resolving this issue. OSM-10, for example, is only required for detection of osmotic stimuli [39]. Similarly we have shown that itr-1 is only required for two responses, nose touch and benzaldehyde. It remains to be established how itr-1 mediated signals are coupled to the activation of OSM-9 and OCR-2. It is probable that signals downstream of itr-1 are transduced by Ca2+ released from the ER. Many TRP channels are regulated by calmodulin, a key target of intracellular Ca2+ release. Calmodulin can act as both a positive and negative regulator of TRP channels [42]. Interestingly, TRPV4 and 6 are both positively regulated by CaM. The TRPV4 C-terminal CaM binding site which is required for positive regulation [43] shows some conservation with OSM-9 (HAB, unpublished). A range of other signals are known to be involved in regulating TRP channel function. For example polyunsaturated fatty acids (PUFAs) are known to play an important role in regulating the activity of some TRP channels [44] and it has been suggested that PUFAs play a key role in regulating OSM-9/OCR-2 function [45]. Thus itr-1 might also regulate OSM-9/OCR-2 indirectly through other pathways.
Our results place egl-8 (PLC-β) and plc-3 (PLC-γ) as being upstream of itr-1. In each case we observed partial rescue when the genes were expressed in ASH in loss-of-function backgrounds. This partial rescue may be due to inadequate expression from the sra-6 promoter or could reflect a requirement for these genes in other cells, although we do not observe any rescue on expression in command neurons. The identification of a role for two PLC subtypes is at first glance surprising, however there are many examples of multiple PLC subtypes being utilised in physiological processes (see for example [17]). In ASH our results suggest that both act, at least in part, through IP3. The signals upstream of PLC are unknown. PLC-β is usually regulated by members of the Gqα subunits of heterotrimeric G-proteins. We were unable to test the role of EGL-30 (Gqα) in this process as egl-30 mutants have widespread defects in locomotion. In addition, ASH expresses at least 9 G-alpha subunits. Some of these have identified and specific functions whilst some are more general; e.g. odr-3 appears to be required for all known ASH-mediated responses, while gpa-3 is only required for the response to water soluble repellents [11],[46]. Whether any of the other Gα subunits are specifically required in nose touch remains unclear. We tested the effect of mutations in the Gα subunits expressed in ASH, however, we found that most impaired nose touch, to varying degrees (DSW and HAB, unpublished data). It seems likely that their role is complex, involving multiple cells types and perhaps redundancy between subunit types.
How does PLC and IP3 signalling facilitate the response to nose touch? The mechanism by which ASH neurons detect nose touch is unclear. Kindt et al. [32] have shown that the response of two other neurons QLQ and Il1 to nose touch involves the mechanosensitive TRPA channel TRPA-1. However TRPA1 does not appear to be required in ASH [32]. Thus mechanosensation in ASH may use a different mechanism. One possibility is that IP3 signalling in ASH lies downstream of ligand independent activation of GPCRs. Analysis of the “Bayliss Response” in which small resistance arterial blood vessels constrict in response to rises in blood pressure has identified a pathway which is initiated by the activation of GPCRs by membrane stretch [47]. In these vascular smooth muscle cells ligand independent activation of the Angiotensisn II AT1 receptor by membrane stretch regulates a TRP channel, TRPC6, through a mechanism that requires both Gαq and PLC. Other Gαq linked GPCRs also demonstrate mechanosensitive properties [47]. The signal between PLC and TRPC6 has not been identified. As discussed above we have shown that in ASH, nose touch is mediated by PLC-β (egl-8) which is normally downstream of Gαq coupled GPCRs so our data are compatible with such a mechanism operating in these cells. Alternatively, IP3 signalling might not be directly activated by nose touch. Many mechanosensory processes involve ion channels that are directly activated by force; thus, IP3 signalling might regulate the activity of a mechanosensitive channel responsible for sensing nose touch in ASH. In this model, IP3 signalling does not mediate sensory transduction per se, but rather acts downstream of G-protein-mediated neuromodulatory pathways to modify touch sensitivity. Pathways of this sort would be critical for modality-specific adaptation in a polymodal neuron such as ASH.
In summary, we have shown that the IP3 signalling cassette is part of the specific signalling machinery for nose touch in ASH neurons. This adds to our molecular understanding of the molecular mechanisms that enable the segregation of signals in these polymodal sensory neurons and contributes to our understanding of how polymodal neurons, such as human nociceptors, function in general.
Supporting Information
Strains used in this work.
(0.09 MB DOC)
Acknowledgments
We are grateful to Mario de Bono, Andrew Fire, and Helen Peterkin for gifts of plasmids and to the C. elegans Knockout Consortium and Mitani laboratory for the provision of strains. Some nematode strains used in this study were supplied by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources. We are grateful to Sung Ly for technical support and to Marios Chatzigeorgiou for help with Ca2+ imaging and analysis.
Footnotes
The authors have declared that no competing interests exist.
This work was supported by the MRC and the National Institute on Drug Abuse (NIH). RPV-M was supported by the BBSRC and a “Value in People Award”, provided by the Wellcome Trust to the University of Cambridge. HAB was an MRC Senior Fellow. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kaplan JM, Horvitz HR. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1993;90:2227–2231. doi: 10.1073/pnas.90.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, et al. The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci. 1985;5:956–964. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White JG, Southgate E, Thomson JN, Brenner S. The Structure of the Nervous System of the Nematode Caenorhabditis elegans. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 4.Bargmann CI, Thomas JH, Horvitz HR. Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol. 1990;55:529–538. doi: 10.1101/sqb.1990.055.01.051. [DOI] [PubMed] [Google Scholar]
- 5.Culotti JG, Russell RL. Osmotic avoidance defective mutants of the nematode Caenorhabditis elegans. Genetics. 1978;90:243–256. doi: 10.1093/genetics/90.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hilliard MA, Bargmann CI, Bazzicalupo P. C. elegans responds to chemical repellents by integrating sensory inputs from the head and the tail. Curr Biol. 2002;12:730–734. doi: 10.1016/s0960-9822(02)00813-8. [DOI] [PubMed] [Google Scholar]
- 7.Rogers C, Persson A, Cheung B, de Bono M. Behavioral motifs and neural pathways coordinating O2 responses and aggregation in C. elegans. Curr Biol. 2006;16:649–659. doi: 10.1016/j.cub.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Troemel ER, Kimmel BE, Bargmann CI. Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell. 1997;91:161–169. doi: 10.1016/s0092-8674(00)80399-2. [DOI] [PubMed] [Google Scholar]
- 9.Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, et al. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–318. doi: 10.1016/s0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 11.Hilliard MA, Bergamasco C, Arbucci S, Plasterk RH, Bazzicalupo P. Worms taste bitter: ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. Embo J. 2004;23:1101–1111. doi: 10.1038/sj.emboj.7600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baylis HA, Furuichi T, Yoshikawa F, Mikoshiba K, Sattelle DB. Inositol 1,4,5-trisphosphate receptors are strongly expressed in the nervous system, pharynx, intestine, gonad and excretory cell of Caenorhabditis elegans and are encoded by a single gene (itr-1). J Mol Biol. 1999;294:467–476. doi: 10.1006/jmbi.1999.3229. [DOI] [PubMed] [Google Scholar]
- 13.Dal Santo P, Logan MA, Chisholm AD, Jorgensen EM. The inositol trisphosphate receptor regulates a 50-second behavioral rhythm in C. elegans. Cell. 1999;98:757–767. doi: 10.1016/s0092-8674(00)81510-x. [DOI] [PubMed] [Google Scholar]
- 14.Gower NJ, Temple GR, Schein JE, Marra M, Walker DS, et al. Dissection of the promoter region of the inositol 1,4,5-trisphosphate receptor gene, itr-1, in C. elegans: a molecular basis for cell-specific expression of IP3R isoforms. J Mol Biol. 2001;306:145–157. doi: 10.1006/jmbi.2000.4388. [DOI] [PubMed] [Google Scholar]
- 15.Corrigan C, Subramanian R, Miller MA. Eph and NMDA receptors control Ca2+/calmodulin-dependent protein kinase II activation during C. elegans oocyte meiotic maturation. Development. 2005;132:5225–5237. doi: 10.1242/dev.02083. [DOI] [PubMed] [Google Scholar]
- 16.Yin X, Gower NJ, Baylis HA, Strange K. Inositol 1,4,5-trisphosphate signaling regulates rhythmic contractile activity of myoepithelial sheath cells in Caenorhabditis elegans. Mol Biol Cell. 2004;15:3938–3949. doi: 10.1091/mbc.E04-03-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espelt MV, Estevez AY, Yin X, Strange K. Oscillatory Ca2+ signaling in the isolated Caenorhabditis elegans intestine: role of the inositol-1,4,5-trisphosphate receptor and phospholipases C β and γ. J Gen Physiol. 2005;126:379–392. doi: 10.1085/jgp.200509355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gower NJ, Walker DS, Baylis HA. Inositol 1,4,5-trisphosphate signaling regulates mating behavior in Caenorhabditis elegans males. Mol Biol Cell. 2005;16:3978–3986. doi: 10.1091/mbc.E05-02-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas-Virnig CL, Sims PA, Simske JS, Hardin J. The inositol 1,4,5-trisphosphate receptor regulates epidermal cell migration in Caenorhabditis elegans. Curr Biol. 2004;14:1882–1887. doi: 10.1016/j.cub.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Walker DS, Gower NJ, Ly S, Bradley GL, Baylis HA. Regulated disruption of inositol 1,4,5-trisphosphate signaling in Caenorhabditis elegans reveals new functions in feeding and embryogenesis. Mol Biol Cell. 2002;13:1329–1337. doi: 10.1091/mbc.01-08-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vazquez-Manrique RP, Nagy AI, Legg JC, Bales OA, Ly S, et al. Phospholipase C-ε regulates epidermal morphogenesis in Caenorhabditis elegans. PLoS Genet. 2008;4:e1000043. doi: 10.1371/journal.pgen.1000043. doi: 10.1371/journal.pgen.1000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hilliard MA, Apicella AJ, Kerr R, Suzuki H, Bazzicalupo P, et al. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. Embo J. 2005;24:63–72. doi: 10.1038/sj.emboj.7600493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell. 1995;83:207–218. doi: 10.1016/0092-8674(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Y, Brockie PJ, Mellem JE, Madsen DM, Maricq AV. Neuronal control of locomotion in C. elegans is modified by a dominant mutation in the GLR-1 ionotropic glutamate receptor. Neuron. 1999;24:347–361. doi: 10.1016/s0896-6273(00)80849-1. [DOI] [PubMed] [Google Scholar]
- 25.Maduro M, Pilgrim D. Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics. 1995;141:977–988. doi: 10.1093/genetics/141.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lackner MR, Nurrish SJ, Kaplan JM. Facilitation of synaptic transmission by EGL-30 Gqα and EGL-8 PLCβ: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron. 1999;24:335–346. doi: 10.1016/s0896-6273(00)80848-x. [DOI] [PubMed] [Google Scholar]
- 27.Mello C, Fire A. DNA transformation. Methods Cell Biol. 1995;48:451–482. [PubMed] [Google Scholar]
- 28.Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- 29.Chao MY, Komatsu H, Fukuto HS, Dionne HM, Hart AC. Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc Natl Acad Sci U S A. 2004;101:15512–15517. doi: 10.1073/pnas.0403369101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerr R. Imaging the activity of neurons and muscles. WormBook. 2006:1–13. doi: 10.1895/wormbook.1.113.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerr R, Lev-Ram V, Baird G, Vincent P, Tsien RY, et al. Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans. Neuron. 2000;26:583–594. doi: 10.1016/s0896-6273(00)81196-4. [DOI] [PubMed] [Google Scholar]
- 32.Kindt KS, Viswanath V, Macpherson L, Quast K, Hu HZ, et al. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nature Neuroscience. 2007;10:568–577. doi: 10.1038/nn1886. [DOI] [PubMed] [Google Scholar]
- 33.Walker DS, Ly S, Lockwood KC, Baylis HA. A direct interaction between IP3 receptors and myosin II regulates IP3 signaling in C. elegans. Curr Biol. 2002;12:951–956. doi: 10.1016/s0960-9822(02)00868-0. [DOI] [PubMed] [Google Scholar]
- 34.Tavernarakis N, Driscoll M. Caenorhabditis elegans degenerins and vertebrate ENaC ion channels contain an extracellular domain related to venom neurotoxins. J Neurogenet. 2000;13:257–264. doi: 10.3109/01677060009084497. [DOI] [PubMed] [Google Scholar]
- 35.Hart AC, Sims S, Kaplan JM. Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature. 1995;378:82–85. doi: 10.1038/378082a0. [DOI] [PubMed] [Google Scholar]
- 36.Miller KG, Emerson MD, Rand JB. Goα and diacylglycerol kinase negatively regulate the Gqα pathway in C. elegans. Neuron. 1999;24:323–333. doi: 10.1016/s0896-6273(00)80847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibatohge M, Kariya K, Liao Y, Hu CD, Watari Y, et al. Identification of PLC210, a Caenorhabditis elegans phospholipase C, as a putative effector of Ras. J Biol Chem. 1998;273:6218–6222. doi: 10.1074/jbc.273.11.6218. [DOI] [PubMed] [Google Scholar]
- 38.Clandinin TR, DeModena JA, Sternberg PW. Inositol trisphosphate mediates a RAS-independent response to LET-23 receptor tyrosine kinase activation in C. elegans. Cell. 1998;92:523–533. doi: 10.1016/s0092-8674(00)80945-9. [DOI] [PubMed] [Google Scholar]
- 39.Hart AC, Kass J, Shapiro JE, Kaplan JM. Distinct signaling pathways mediate touch and osmosensory responses in a polymodal sensory neuron. J Neurosci. 1999;19:1952–1958. doi: 10.1523/JNEUROSCI.19-06-01952.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Bono M, Maricq AV. Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci. 2005;28:451–501. doi: 10.1146/annurev.neuro.27.070203.144259. [DOI] [PubMed] [Google Scholar]
- 41.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu MX. Multiple roles of calmodulin and other Ca2+-binding proteins in the functional regulation of TRP channels. Pflugers Arch. 2005;451:105–115. doi: 10.1007/s00424-005-1427-1. [DOI] [PubMed] [Google Scholar]
- 43.Strotmann R, Schultz G, Plant TD. Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J Biol Chem. 2003;278:26541–26549. doi: 10.1074/jbc.M302590200. [DOI] [PubMed] [Google Scholar]
- 44.Hardie RC. TRP channels and lipids: from Drosophila to mammalian physiology. Journal of Physiology-London. 2007;578:9–24. doi: 10.1113/jphysiol.2006.118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahn-Kirby AH, Dantzker JL, Apicella AJ, Schafer WR, Browse J, et al. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell. 2004;119:889–900. doi: 10.1016/j.cell.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Jansen G, Thijssen KL, Werner P, van der Horst M, Hazendonk E, et al. The complete family of genes encoding G proteins of Caenorhabditis elegans. Nat Genet. 1999;21:414–419. doi: 10.1038/7753. [DOI] [PubMed] [Google Scholar]
- 47.Schnitzler MMY, Storch U, Meibers S, Nurwakagari P, Breit A, et al. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. Embo Journal. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Strains used in this work.
(0.09 MB DOC)