Abstract
We investigated the effect of low dose radiation on diabetes induced suppression of neurogenesis in the hippocampal dentate gyrus of rat. After 0.01 Gy, 0.1 Gy, 1 Gy and 10 Gy radiation was delivered, the dentate gyrus of hippocampus of streptozotocin (STZ)-induced diabetic rats were evaluated using immunohistochemistry for 5-bromo-2-deoxyuridine (BrdU), caspase-3, and terminal deoxynucleotidyl transferase-mediated nick end-labeling (TUNEL) staining. The number of BrdU positive cells in the non-diabetic rats, diabetic rats without radiation, diabetic rats with 0.01 Gy radiation, diabetic rats with 0.1 Gy radiation, diabetic rats with 1 Gy radiation and diabetic rats with 10 Gy radiation were 55.4±8.5/mm2, 33.3±6.4/mm2, 67.7±10.5/mm2, 66.6±10.0/mm2, 23.5±6.3/mm2and 14.3±7.2/mm2, respectively. The number of caspase-3 positive cells was 132.6±37.4/mm2, 378.6±99.1/mm2, 15.0±2.8/mm2, 57.1±16.9/mm2, 191.8±44.8/mm2and 450.4±58.3/mm2, respectively. The number of TUNEL-positive cells was 24.5±2.0/mm2, 21.7±4.0/mm2, 20.4±2.0/mm2, 18.96±2.1/mm2, 58.3±7.9/mm2, and 106.0±9.8/mm2, respectively. These results suggest low doses of radiation paradoxically improved diabetes induced neuronal cell suppression in the hippocampal dentate gyrus of rat.
Keywords: Radiation, Hippocampus, Diabetes Mellitus
INTRODUCTION
Diabetes mellitus causes learning and memory deficits (1,2) and cognitive impairment (3), resulting in dementia in severe cases (4). The impact of diabetes mellitus on brain pathology is increasingly being recognized. A recent study showed that streptozotocin-induced diabetes produced a dramatic decrease in cell proliferation in the rat dentate gyrus as compared to controls (5). The dentate gyrus in the hippocampus has been implicated in learning ability and memory capacity. The results from this study suggest a potential role for alterations in neurogenesis in the cognitive decline observed in diabetes mellitus. Neurogenesis occurs in the hippocampal dentate gyrus in a variety of mammals, including humans (6). The factors related to cell proliferation in the dentate gyrus are serotonin (7), enriched environment (8), estrogen (9), and physical exercise (10). Conversely, adrenal steroid hormones (11), stress (12), and aging (13) have been identified as neurogenesis-inhibiting factors.
Radiation is also known to cause neuronal cell death in the developing brain (14), reduced neurogenesis (15-17) and cognitive impairment (18). However, low dose radiation shows an adaptive protection effect in most mammalian cells (19). If cells that had been exposed to a very low dose (1 cGy) of radiography were subsequently exposed to a relatively high dose (1 Gy), approximately half as many chromosome breaks were induced (20). Feinendegen et al. reported that except for apoptosis and terminal cell differentiation, all protective responses to single exposures tend to be expressed maximally after about 0.1 Gy and very little after more than about 0.5 Gy X- or gamma-radiation (21). Because of this protective response, we investigated the effect of low dose radiation for the diabetes-induced neuronal cell damage.
MATERIALS AND METHODS
Animals and treatments
Male Sprague-Dawley rats weighing 250±10 g at 7 weeks of age were used in this experiment. All experimental procedures were performed in accordance with the animal care guidelines established by the National Institute of Health (NIH) and the Korean Academy of Medical Sciences. The animals were housed under laboratory conditions at a controlled temperature (20±2℃) and maintained under light-dark cycles, each cycle consisting of 12 hr of light and 12 hr of darkness (lighting from 7 AM to 7 PM) with food and water available ad libitum.
In order to induce diabetes in the experimental animals, a single intraperitoneal injection of 50 mg/kg streptozotocin (STZ) (Sigma Chemical Co., St. Louis, MO, U.S.A.) was given to each animal. Animals in the control group received injections of an equivalent amount of normal saline. Blood glucose levels were determined 2 days after the STZ injections using a blood glucose tester (Arkray, Kyoto, Japan). Only animals exhibiting blood glucose levels of 300 mg/dL or higher were used in this study.
Radiation
Whole body radiation was delivered by a linear accelerator Clinac 2100 C, Varian, U.S.A.) using 6 MV of radiography. For the determination of the dose-dependence of radiation on cell proliferation in the hippocampal dentate gyrus, rats were divided into three groups: the control group, the 0.1 Gy radiation group, and the 1 Gy radiation group (n=5 in each group).
For the determination of the effect of radiation on cell proliferation and cell death in the hippocampal dentate gyrus of STZ-induced diabetic rats, the animals were divided into six groups: the control group, the STZ-induced diabetes group, the STZ-induced diabetes and 0.01 Gy radiation group, the STZ-induced diabetes and 0.1 Gy radiation group, the STZ-induced diabetes and 1 Gy radiation group, and the STZ-induced diabetes and 10 Gy radiation group (n=5 in each group). Ten min before radiation exposure, the animals were injected intraperitoneally with 50 mg/kg BrdU (Sigma Chemical Co., St. Louis, MO, U.S.A.) and were sacrificed 2 hr after the injection.
Tissue preparation
The animals were first fully anesthetized with Zoletil 50®(10 mg/kg, i.p.; Vibac Laboratories, Carros, France), transcardially perfused with 50 mM phosphate-buffered saline (PBS), and then fixed with a freshly prepared solution consisting of 4% paraformaldehyde (PFA) in 100 mM phosphate buffer (PB, pH 7.4). The brains were then removed, postfixed in the same fixative overnight, and transferred into a 30% sucrose solution for cryoprotection. Coronal sections of 40 µm thickness were produced using a freezing microtome (Leica, Nussloch, Germany).
BrdU immunohistochemical staining
BrdU immunohistochemical staining was used for the detection of newly generated cells in the dentate gyrus according to a previously described method (22). An average of 10 sections within the hippocampal region spanning from Bregma -3.30 mm to Bregma -4.16 mm, of which about 80 sections in average were available, were selected from each brain. The sections were first permeabilized by incubation in a solution of 0.5% Triton X-100 in PBS for 20 min. The sections were then incubated in 50% formamide-2x standard saline citrate at 65℃ for 2 hr, denatured in 2 N HCl at 37℃ for 30 min, rinsed twice in 100 mM sodium borate (pH 8.5), and incubated overnight at 4℃ with a BrdU-specific mouse monoclonal antibody (1:600; Boehringer Mannheim, Mannheim, Germany). The sections were then washed three times with PBS and incubated for 1 hr with a biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA. U.S.A.), followed by another 1 hr incubation with VECTASTAIN®(Elite ABC Kit, 1:100; Vector Laboratories). For staining, the sections were incubated for 5 min in a reaction mixture consisting of 0.02% 3,3'-diaminobenzidine containing nickel chloride (40 mg/mL) (nickel-DAB) and 0.03% H2O2 in 50 mM Tris-HCl (pH 7.6). The sections were then washed three times with PBS and mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and coverslips were mounted using Permount®(Fisher Scientific, Fair Lawn, NJ, U.S.A.).
Caspase-3 immunohistochemical staining
In order to detect caspase-3 expression, caspase-3 immunohistochemical staining was performed. Sections were drawn from each brain and incubated overnight with mouse anticaspase-3 antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) followed by a 1 hr incubation with a biotinylated mouse secondary antibody. The bound secondary antibody was then amplified using a Vector Elite ABC kit®(Vector Laboratories). The antibody-biotin-avidin-peroxidase complexes were visualized using 0.02% DAB and the sections were mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and coverslips were mounted using Permount®(Fisher Scientific).
TUNEL assay
In order to detect apoptotic cell death, a TUNEL assay was performed using the In Situ Cell Death Detection Kit (Roche, Mannheim, Germany) according to a previously described method (22). Briefly, the sections were mounted onto gelatin-coated slides and air-dried overnight at room temperature. The sections were post-fixed in ethanol-acetic acid (2:1) and rinsed. The sections were then incubated with proteinase K (100 µg/mL), rinsed, incubated in 3% H2O2, permeabilized with 0.5% Triton X-100, rinsed again, and incubated in the TUNEL reaction mixture. The sections were rinsed and visualized using a converter-POD with nickel-DAB. Mayer's hematoxylin (Dako, Glostrup, Denmark) was used for counterstaining. The slides were air-dried overnight at room temperature, and coverslips were mounted using Permount. (Fisher Scientific).
Data analysis
The area of the dentate granular layer in the selected hippocampus region was measured using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, U.S.A.). The numbers of BrdU-positive, caspase-3-positive, and TUNEL-positive cells in the dentate gyrus were counted hemilaterally, and are expressed as the number of cells per mm2 of the granular layer. Statistical analysis was performed using a oneway analysis of variance (ANOVA) followed by the Duncan post-hoc test. The results are presented as the mean±standard error mean (SEM). Differences were considered significant at p<0.05.
RESULTS
Effect of radiation on the neuronal cell proliferation in the normal rats
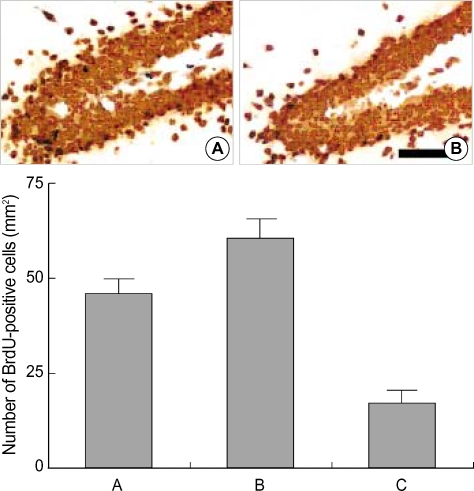
The number of BrdU-positive cells in the dentate gyrus was 45.9±3.4/mm2 in the control group, 59.3±5.2/mm2 in the 0.1 Gy radiation group, and 19.2±2.9/mm2 in the 1 Gy radiation group (Fig. 1). These results suggest that cell proliferation in the dentate gyrus was suppressed by high-dose radiation (1 Gy), in contrast to the low-dose radiation (0.1 Gy) which enhanced cell proliferation.
Fig. 1.
The effect of radiation on the number of 5-bromo-2'-deoxyuridine (BrdU)-positive cells in the dentate gyrus of normal rats. Upper: Photomicrographs of BrdU-positive cells in the dentate gyrus. (A) Control group, (B) 1 Gy radiation group. A scale bar represents 50 µm. Lower: The number of BrdU-positive cells in the dentate gyrus in each group. (A) Control group, (B) 0.1 Gy radiation group, (C) 1 Gy radiation group.
Effect of radiation on the neuronal cell proliferation in the diabetic rats
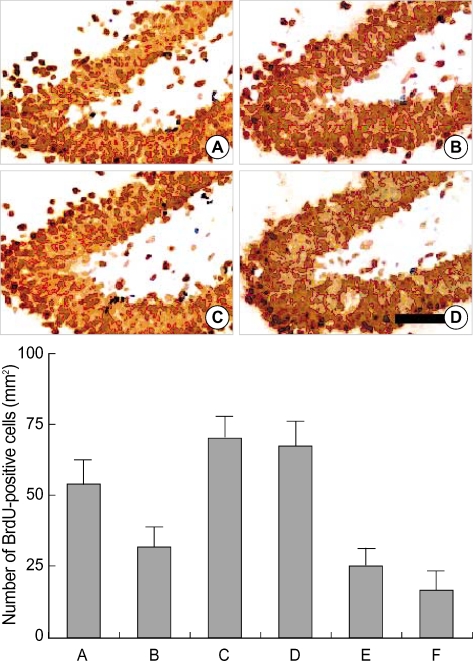
The number of BrdU-positive cells in the dentate gyrus was 55.4±8.5/mm2 in the control group, 33.3±6.4/mm2 in the STZ-induced diabetic rats, 67.7±10.5/mm2 in the STZ-induced diabetic rats with 0.01 Gy radiation, 66.6±10.0/mm2 in the STZ-induced diabetic rats with 0.1 Gy radiation, 23.5±6.3/mm2 in the STZ-induced diabetic rats with 1 Gy radiation, and 14.3±7.2/mm2 in the STZ-induced diabetic rats with 10 Gy radiation (Fig. 2).
Fig. 2.
The effect of radiation on the number of 5-bromo-2'-deoxyuridine (BrdU)-positive cells in the dentate gyrus of streptozotocin (STZ)-induced diabetic rats. Upper: Photomicrographs of BrdU-positive cells in the dentate gyrus. (A) Control group, (B) streptozotocin (STZ)-induced diabetes group, (C) STZ-induced diabetes and 0.01 Gy radiation group, (D) STZ-induced diabetes and 10 Gy radiation group. A scale bar represents 50 µm. Lower: The number of BrdU-positive cells in the dentate gyrus in each group. (A) Control group, (B) STZ-induced diabetes group, (C) STZ-induced diabetes and 0.01 Gy radiation group, (D) STZ-induced diabetes and 0.1 Gy radiation group, (E) STZ-induced diabetes and 1 Gy radiation group, (F) STZ-induced diabetes and 10 Gy radiation group.
These results suggest that the suppression of neuronal cell proliferation in the STZ-induced diabetic rats was aggravated by high doses of radiation (1 Gy and 10 Gy). In contrast, low doses of radiation (0.01 Gy and 0.1 Gy) enhanced cell proliferation in the dentate gyrus of STZ-induced diabetic rats.
Effect of radiation on the caspase-3 expression in the diabetic rats
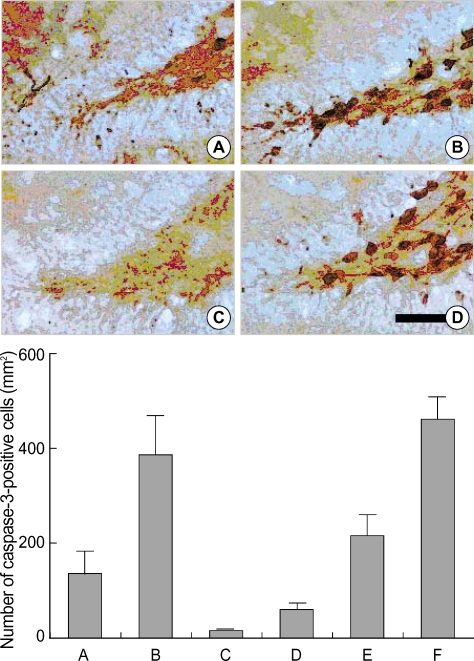
The number of caspase-3-positive cells in the hippocampal dentate gyrus was 132.6±37.4/mm2 in the control group, 378.6±99.1/mm2 in the STZ-induced diabetic rats, 15.0±2.8/mm2 in the STZ-induced diabetic rats with 0.01 Gy radiation, 57.1±16.9/mm2 in the STZ-induced diabetic rats with 0.1 Gy radiation, 191.8±44.8/mm2 in the STZ-induced diabetic rats with 1 Gy radiation, and 450.4±58.3/mm2 in the STZ-induced diabetic rats with 10 Gy radiation (Fig. 3). The caspase-3 expression in the dentate gyrus was increased in the STZ-induced diabetic rats. Radiation therapy suppressed caspase-3 expression in the dentate gyrus of STZ-induced diabetic rats in a dose-reversible manner, except for the 10 Gy radiation group.
Fig. 3.
The effect of radiation on the number of 5-bromo-2'-deoxyuridine (BrdU)-positive cells in the dentate gyrus of streptozotocin (STZ)-induced diabetic rats. Upper: Photomicrographs of BrdU-positive cells in the dentate gyrus. (A) Control group, (B) streptozotocin (STZ)-induced diabetes group, (C) STZ-induced diabetes and 0.01 Gy radiation group, (D) STZ-induced diabetes and 10 Gy radiation group. A scale bar represents 50 µm. Lower: The number of BrdU-positive cells in the dentate gyrus in each group. (A) Control group, (B) STZ-induced diabetes group, (C) STZ-induced diabetes and 0.01 Gy radiation group, (D) STZ-induced diabetes and 0.1 Gy radiation group, (E) STZ-induced diabetes and 1 Gy radiation group, (F) STZ-induced diabetes and 10 Gy radiation group.
Effect of radiation on the apoptosis in the diabetic rats
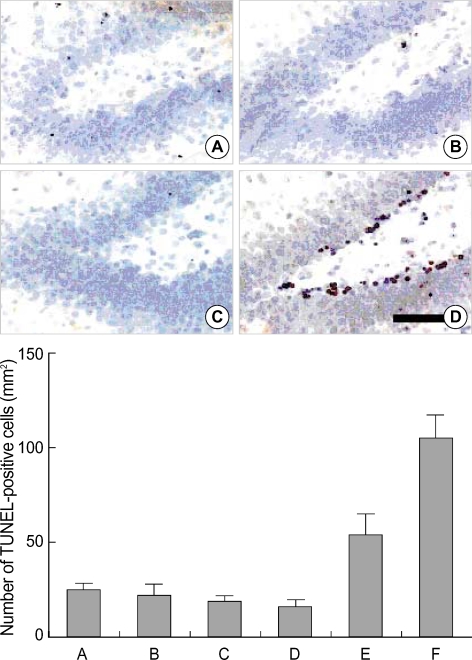
The number of TUNEL-positive cells in the hippocampal dentate gyrus was 24.5±2.0/mm2 in the control group, 21.7±4.0/mm2 in the STZ-induced diabetic rats, 20.4±2.0/mm2 in the STZ-induced diabetic rats with 0.01 Gy radiation, 18.9±2.1/mm2 in the STZ-induced diabetic rats with 0.1 Gy radiation, 58.3±7.9/mm2 in the STZ-induced diabetic rats with 1 Gy radiation, and 106.0±9.8/mm2 in the STZ-induced diabetic rats with 10 Gy radiation (Fig. 4). Low doses of radiation (0.01 Gy and 0.1 Gy) did not have a significant effect on the number of TUNEL-positive cells. However, high doses of radiation (1 Gy and 10 Gy) increased the number of TUNEL-positive cells in the dentate gyrus of the STZ-induced diabetic rats.
Fig. 4.
The effect of radiation on the number of terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL)-positive cells in the dentate gyrus of streptozotocin (STZ)-induced diabetic rats. Upper: Photomicrographs of TUNEL-positive cells in the dentate gyrus. (A) Control group, (B) streptozotocin (STZ)-induced diabetes group, (C) STZ-induced diabetes and 0.01 Gy radiation group, (D) STZ-induced diabetes and 10 Gy radiation group. A scale bar represents 50 µm. Lower: The number of TUNEL-positive cells in the dentate gyrus in each group. (A) Control group, (B) STZ-induced diabetes group, (C) STZ-induced diabetes and 0.01 Gy radiation group, (D) STZ-induced diabetes and 0.1 Gy radiation group, (E) STZ-induced diabetes and 1 Gy radiation group, (F) STZ-induced diabetes and 10 Gy radiation group.
DISCUSSION
The hippocampal dentate gyrus is a region of active proliferation and neurogenesis within the adult brain. The influence of diabetes mellitus on these processes in the dentate gyrus is increasingly becoming recognized. For instance, it has been reported that neuronal cell proliferation in the dentate gyrus is decreased in STZ-induced diabetic rats (23). According to this study, STZ-treated type 1 diabetes rats showed marked astrogliosis and neurogenesis deficit in the hippocampus and increased expression of hypothalamic neuropeptides. Our results showed that the number of BrdU-positive cellsan indicator of the level of cell proliferation-in the STZ-induced diabetic rats was decreased to about 60% that of normal rats. In addition, the suppression of neuronal cell proliferation in the STZ-induced diabetic rats was aggravated by high doses of radiation (1 Gy and 10 Gy). In contrast, low doses of radiation (0.01 Gy and 0.1 Gy) enhanced cell proliferation in the dentate gyrus of STZ-induced diabetic rats. The dose 0.1 Gy was determined to be the threshold point. This was consistent with other studies that reported that normal tissue showed a protective response to a single exposure of radiation around the 0.1 Gy dose. It has been demonstrated that except for apoptosis and terminal cell differentiation, all protective responses to single exposures tend to be expressed maximally after about 0.1 Gy and very little after more than about 0.5 Gy radiation (21,24,25). Feinendegen et al. reported that depending on type of adaptive protection in a given cell system, in most mammalian cells so far examined, the expression of adaptive protection had a maximum above 0.05 Gy and below about 0.2 Gy (19,21).
The present results show that caspase-3 expression increased in the dentate gyrus of STZ-induced diabetic rats with high dose radiation. However, the number of TUNEL-positive cells did not change significantly in the dentate gyrus of the STZ-induced diabetic rats as compared to normal rats. These findings are similar to other reports (26,27). Our present results demonstrate that high doses of radiation resulted in an increase in the number of TUNEL-positive cells in the dentate gyrus of the STZ-induced diabetic rats. With low dose radiation, 0.01 Gy and 0.1 Gy, the diabetes-induced increase in caspase-3 expression in the dentate gyrus was suppressed. The number of TUNEL-positive cells did not change as a result of low doses of radiation in the STZ-induced diabetic rats.
In this study, we have demonstrated that low doses of radiation enhance cell proliferation in the dentate gyrus in both normal and STZ-induced diabetes rats, with no concomitant alterations in the rate of apoptotic neuronal cell death. These results suggest that low doses of radiation may improve diabetes induced neuronal cell suppression in the hippocampal dentate gyrus.
References
- 1.Biessels GJ, Kappelle AC, Kappelle AC, Bravenboer B, Erkelens DW, Gispen WH. Cerebral function in diabetes mellitus. Diabetologia. 1994;37:643–650. doi: 10.1007/BF00417687. [DOI] [PubMed] [Google Scholar]
- 2.Biessels GJ, Kamal A, Ramakers GM, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH. Place learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic rats. Diabetes. 1996;45:1259–1266. doi: 10.2337/diab.45.9.1259. [DOI] [PubMed] [Google Scholar]
- 3.Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J Clin Exp Neuropsychol. 2004;26:1044–1080. doi: 10.1080/13803390490514875. [DOI] [PubMed] [Google Scholar]
- 4.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 5.Jackson-Guilford J, Leander JD, Nisenbaum LK. The effect of streptozotocin-induced diabetes on cell proliferation in the rat dentate gyrus. Neurosci Lett. 2000;293:91–94. doi: 10.1016/s0304-3940(00)01502-0. [DOI] [PubMed] [Google Scholar]
- 6.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 1999;21:46S–51S. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- 8.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 9.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YP, Kim HB, Jang MH, Lim BV, Kim YJ, Kim H, Kim SS, Kim EH, Kim CJ. Magnitude- and time-dependence of the effect of treadmill exercise on cell proliferation in the dentate gyrus of rats. Int J Sports Med. 2003;24:114–117. doi: 10.1055/s-2003-38202. [DOI] [PubMed] [Google Scholar]
- 11.Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- 12.Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrer I, Borras D. Effects of X-irradiation on glial cells in the developing rat brain. Int J Radiat Biol. 1994;66:181–187. doi: 10.1080/09553009414551081. [DOI] [PubMed] [Google Scholar]
- 15.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- 16.Tada E, Parent JM, Lowenstein DH, Fike JR. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience. 2000;99:33–41. doi: 10.1016/s0306-4522(00)00151-2. [DOI] [PubMed] [Google Scholar]
- 17.Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16:129–134. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- 18.Shukitt-Hale B, Casadesus G, McEwen JJ, Rabin BM, Joseph JA. Spatial learning and memory deficits induced by exposure to iron-56-particle radiation. Radiat Res. 2000;154:28–33. doi: 10.1667/0033-7587(2000)154[0028:slamdi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Feinendegen LE. Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol. 2005;78:3–7. doi: 10.1259/bjr/63353075. [DOI] [PubMed] [Google Scholar]
- 20.Wolff S. The adaptive response in radiobiology: evolving insights and implications. Environ Health Perspect. 1998;106(Suppl 1):277–283. doi: 10.1289/ehp.98106s1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feinendegen LE, Loken MK, Booz J, Muhlensiepen H, Sondhaus CA, Bond VP. Cellular mechanisms of protection and repair induced by radiation exposure and their consequences for cell system responses. Stem Cells. 1995;13(Suppl 1):7–20. [PubMed] [Google Scholar]
- 22.Lee KS, Lim BV, Jang MH, Shin MC, Lee TH, Kim YP, Shin HS, Cho SY, Kim H, Shin MS, Kim EH, Kim CJ. Hypothermia inhibits cell proliferation and nitric oxide synthase expression in rats. Neurosci Lett. 2002;329:53–56. doi: 10.1016/s0304-3940(02)00591-8. [DOI] [PubMed] [Google Scholar]
- 23.Revsin Y, Saravia F, Roig P, Lima A, de Kloet ER, Homo-Delarche F, De Nicola AF. Neuronal and astroglial alterations in the hippocampus of a mouse model for type 1 diabetes. Brain Res. 2005;1038:22–31. doi: 10.1016/j.brainres.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 24.Shadley JD, Wiencke JK. Induction of the adaptive response by X-rays is dependent on radiation intensity. Int J Radiat Biol. 1989;56:107–118. doi: 10.1080/09553008914551231. [DOI] [PubMed] [Google Scholar]
- 25.Feinendegen LE, Bond VP, Sondhaus CA, Muehlensiepen H. Radiation effects induced by low doses in complex tissue and their relation to cellular adaptive responses. Mutat Res. 1996;358:199–205. doi: 10.1016/s0027-5107(96)00121-2. [DOI] [PubMed] [Google Scholar]
- 26.Piotrowski P, Wierzbicka K, Smialek M. Neuronal death in the rat hippocampus in experimental diabetes and cerebral ischaemia treated with antioxidants. Folia Neuropathol. 2001;39:147–154. [PubMed] [Google Scholar]
- 27.Li ZG, Zhang W, Grunberger G, Sima AA. Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res. 2002;946:221–231. doi: 10.1016/s0006-8993(02)02887-1. [DOI] [PubMed] [Google Scholar]