Abstract
The clinical outcome and prognostic factors of patients with synchronous brain metastases from non-small cell lung cancer (NSCLC) who were treated with gamma knife radiosurgery (GKS) were analyzed. A total of 35 patients with NSCLC underwent GKS as an initial treatment for metastatic brain lesions of synchronous onset. The period of survival and various prognostic factors such as age, gender, performance status, multiplicity of the brain lesions, intracranial tumor volume, and extent of the primary tumor were analyzed. The overall median survival time for this series was 12 months (range 0.75 to 43 months) from the diagnosis. Of the 21 patients who were no longer alive at the conclusion of this study, only 7 (33.3%) died of neurological causes. Multivariate analysis of these data revealed that N stage, whole-brain radiotherapy (WBRT), and chemotherapy were significant predictors for survival (p<0.05). Survival of patients with NSCLC and synchronous brain metastases is mainly dependent upon the progression of the systemic disease, provided that the cerebral lesions are treated adequately with local treatment modalities including radiosurgery. Application of radiosurgery as an initial treatment option and aggressive local and systemic modalities to control extracranial disease may improve survival.
Keywords: Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis; Radiosurgery
INTRODUCTION
Lung cancer ranks among the most commonly occurring malignancies and currently is the leading cause of cancer-related deaths worldwide (1). It is also the most common origin of brain metastases, accounting for 40-50% of such cases (2).
Patients with brain metastases who go untreated have a median survival of 1 month and this can be prolonged for about 1 month by treatment with corticosteroids. Radiotherapy prolongs median survival to 3-8 months (3-5). Total excision of tumor combined with radiotherapy resulted in better survival compared with radiotherapy alone (6-8). Recently, stereotactic radiosurgery became a widely used treatment modality, achieving effective local control of brain metastases (9). Radiosurgery is a noninvasive alternative to surgical excision and very useful for multiple or deep-seated lesions which cannot be managed with surgery. The survival of cancer patients with brain metastases who are treated with radiosurgery is mainly influenced by the progression of their primary site tumor or extracranial metastases, rather than by the brain lesions themselves (10). Because clinical pattern of disease progression is different according to type of cancer, it is necessary to investigate the clinical outcome of metastatic brain tumors in each individual type of cancer. Synchronous brain metastases accompany 7.4-10% of newly diagnosed non-small cell lung cancer (NSCLC) (11,12)and the probability of the concurrent brain lesion at initial diagnosis of NSCLC is much higher than other solid organ cancers.
Optimal treatment strategy in this specific situation can be established based on the outcome data also specific in the situation. Although there have been numerous reports demonstrating that stereotactic radiosurgery provides effective local control of metastatic brain lesions, little is known about the overall outcome of NSCLC with synchronous cerebral metastases treated with radiosurgery. Therefore, we carried out a retrospective study to determine the survival time and identify the prognostic factors for synchronous brain metastases from NSCLC in which gamma knife radiosurgery (GKS) was performed as an initial treatment.
MATERIALS AND METHODS
In the period between May 2001 and October 2004, 35 patients with synchronous metastatic cerebral lesions from NSCLC underwent GKS as a part of their initial treatment (Table 1). There were 27 male (77.1%) and 8 female patients (22.9%), with a mean age of 55.3 yr (range 33-81). We included patients whose brain lesions were diagnosed before or within 2 months from the diagnosis of the primary tumors. The cytological or histological diagnosis of the primary site tumor was carried out by means of open biopsy and/or resection, fine needle aspiration biopsy, or bronchial lavage during bronchoscopy. The histologic subtypes were adenocarcinoma in 29 patients, large cell carcinoma in 2, and unclassified in 4. In terms of the extent of the disease excluding the brain lesions, as measured by the international staging system (12), there were 3 patients with stage I, 11 with stage II, 14 with stage III, and 7 with stage IV. The patients' general condition was assessed using the Karnofsky performance status (KPS) score. Thirty patients had a KPS score of 70 or more and five patients had a KPS score less than 70. All of the patients underwent GKS as a part of their initial treatment using Leksell model B and C gamma knife. A total of 166 brain lesions were treated initially and the numbers of brain lesions were one in 9 patients, 2 in 7 patients, 3 in 7 patients and more than 3 in 12 patients. The mean number of lesions per patient was 4.7 (range 1-27). The mean intracranial total tumor volume was 10.6 cm3 (0.2-50.9 cm3). The mean marginal dose was 18.8 Gy (11-25 Gy) and the median marginal isodose was 50% (36-75%) of the maximal dose. GKS was performed in the several cases with extraordinary large volume or number of lesions if they would have no available treatment options in case of progression after WBRT only and their performance status was favorable. Although dose planning was compromised to acquire acceptable level of predictable complication, it was assumed that even low dose of boost would provide marginal benefit with acceptable risk and cost. GKS was performed more than once in 11 patients with local recurrence or the occurrence of new lesions during the follow up period.
Table 1.
Characteristics of 35 patients with synchronous brain metastases from non-small cell lung cancer treated with gamma knife radiosurgery
Twenty seven patients received whole-brain radiotherapy WBRT) (a total dose of 3,000 cGy in 10 fractions) with interval not longer than two weeks before or after GKS. Five patients underwent surgical excision of the primary site tumor. Twenty patients were given systemic chemotherapy after GKS. The mean 5 cycles of chemotherapy, consisting of taxotere/cisplatinum, were given and second-line chemotherapy protocol consisting of gemcitabine or gefitinib (Iressa) was administered additionally in 5 patients among them.
Survival curves were obtained by the Kaplan-Meier method using a statistical software program (SPSS, Inc., Chicago, IL, U.S.A.). Comparisons of survival from the univariate analysis were conducted using the log-rank test, while the multivariate analysis was conducted using the Cox proportional hazards model. The results were considered significant when the p value was less than 0.05.
RESULTS
Survival time and causes of death
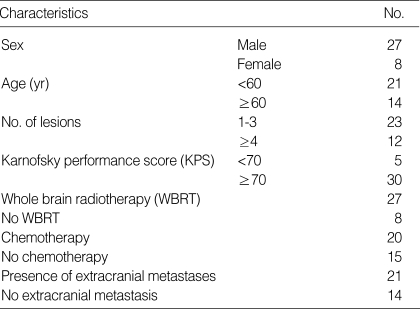
The mean follow up period was 12.5 months (0.75-43 months) and, at the conclusion of the study, 21 patients had died and 14 were still alive. The overall median survival time was 12 months (0.75-43 months) from the diagnosis of brain metastasis. The survival rates were 47.7% at 1 yr and 32.5% at 2 yr, as shown in Fig. 1. The causes of death were progression of the brain lesions in 7 patients (33.3%), progression of extracranial disease in 12 patients (57.2%), and unknown origin in 2 patients (9.5%).
Fig. 1.
Overall survival time from the diagnosis of the brain metastases in the 35 patients who underwent gamma knife surgery.
Local control, recurrence, development of new lesions and complications after GKS
Local recurrence or development of new lesions in brain occurred in 6 (17.1%) and 11 (31.4%) patients, respectively. The actuarial rates without local recurrence were 93.1% at 6 months and 68.6% at 1 yr after the first GKS. Mean marginal dose and tumor volume in the patient with local recurrence were 18.8 Gy and 7.5 cm3each respectively. The actuarial rates without development of new lesions in the brain were 86.9% at 6 months and 59.7% at 1 yr. Therefore, the survival rates without any progression of brain lesions were 80.3% at 6 months and 36.9% at 1 yr from the diagnosis. Eleven patients (31.4%) needed second or more GKS up to five times and the mean interval between the first and second GKS was 8.3 months. Adverse effects of radiosurgery, mostly radiation necrosis, developed in 5 patients (14.3%) who were treated with mean marginal doses of 18.8 Gy for mean tumor volume of 6.0 cm3. Major permanent disability occurred in 1 patient (2.9%) who was treated with GKS twice for the same lesion. Statistically significant relationship between dose/volume and local control/toxicity could not be identified.
Prognostic factors for surviva
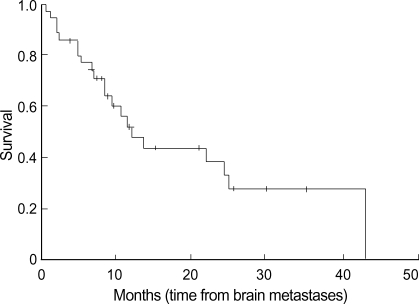
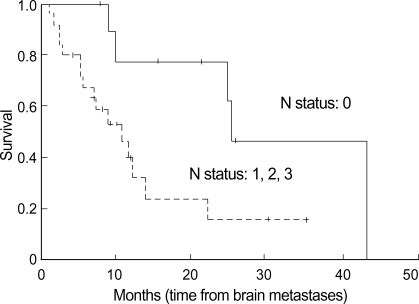
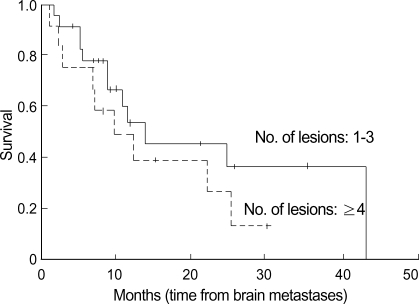
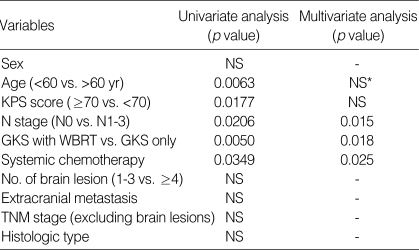
The results of the univariate analysis for those factors that may influence survival are summarized in Table 2. Age, N stage, KPS score, WBRT, and systemic chemotherapy were found to affect survival. Age younger than 60 yr old, the absence of regional lymph node involvement (N0) (Fig. 2), WBRT (+) (Fig. 4), chemotherapy (+) (Fig. 3), and KPS score 70 or more were favorable factors for longer survival. Meanwhile, survival was not significantly influenced by sex, extracranial metastasis, number of brain lesions (Fig. 5), intracranial tumor volume, TNM stage and the histologic type. The median survival time for patients with one to three lesions was 12.2 months from the diagnosis of brain metastasis, while that for patients with four or more lesions was 9.8 months. However, the difference did not reach statistical significance (p>0.05).
Fig. 2.
Survival time according to the N status of the primary lung cancer. The survival time is significantly shorter in those patients with a later N status.
Fig. 4.
Survival time according to the whole brain radiotherapy. Longer survival is shown in patients who underwent WBRT+GKS than those who did GKS only.
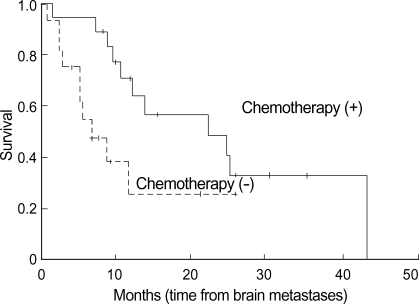
Fig. 3.
Survival time according to the systemic chemotherapy. Patients who received chemotherapy show longer survival time significantly than those who did not.
Fig. 5.
Survival time according to the number of brain lesions. Survival tended to be shorter in patients with four or more lesions, but the difference did not achieve statistical significance.
Multivariate analysis, designed to clarify the independent role of those prognostic factors which were identified as having a significant influence in the univariate analysis, revealed that N stage, WBRT, and chemotherapy were significant predictors for survival (p<0.05) (Table 2).
Table 2.
Uni- and multi-variate analysis of 35 patients with nonsmall cell lung cancer with synchronous brain metastasis treated by gamma knife surgery
*NS, not significant.
DISCUSSION
Recently, stereotactic radiosurgery has become a widely used treatment modality for brain metastases. However, there is still some controversy surrounding the proper indications for radiosurgery, because improved local control of brain lesions does not guarantee the improved survival of patients with a short life expectancy, due to the progression of extracranial disease. The recently reported results of an randomized trial of WBRT vs. WBRT and radiosurgery revealed that radiosurgery provided a survival gain in recursive partitioning analysis (RPA) (14)class 1 patients of 25 months vs. 5 months (8,15). This study was limited to patients with a small number (1 to 3) of brain lesions, however, there have been several reports which insisted that radiosurgery in patients with larger numbers of metastases is beneficial (16-19), although they were not randomized trials. In this series, we did not limit the number of brain lesions in the radiosurgical treatment. Predictably, those patients with one to three lesions had a longer survival time than those with four or more lesions (12.2 vs. 9.8 months), even if this difference was not statistically significant probably due to the small number of patients in this study. However, the survival of both groups in this series is still better than the comparable data in the literature in which median survival was 3.6 months with WBRT only (3,5). At the same time, our results show that the prognosis of patients with synchronous brain metastases is not worse than those with metachronous lesions. A similar result was reported by Flannery et al. (20). The major cause of death in this series was the progression of systemic disease (57.2%), regardless of the initial status of the brain lesions (e.g. multiplicity or volume), and it is suggested that effective local treatment modality for brain lesions could prevent death from neurologic causes resulting in survival gain.
In this series, the prognostic factors identified by the multivariate analysis were N stage, WBRT, and chemotherapy. N stage is a well known prognostic factor of NSCLC and our results show that it is still significant in the patients with metastatic bran lesions. Most of the patients in this study underwent WBRT and survival was longer with GKS and WBRT than GKS only. Recently there are several reports suggesting that radiosurgery only may produce survival outcome comparable to radiosurgery plus WBRT in selected patients particularly with small number of lesions with controlled primary tumor (15,19). Somewhat contradictory result of our study may be caused by the different characteristics of the patients. One third of the cases included in this study had more than 3 brain lesions and all had the primary tumor that was not controlled at the time of GKS. It is thought that WBRT is still an effective treatment option in the situation of synchronous brain metastases. The better survival with systemic chemotherapy can be interpreted in two ways. One may be that it is a reflection of selection bias. Those patients with a better general condition and a strong motivation to recover might be preferentially selected for chemotherapy, thereby resulting in their having a longer survival. The other, more optimistic interpretation is that the delayed progression of the disease resulted from the systemic chemotherapy. Systemic chemotherapy might influence not only the extracranial disease, but also the brain lesions. However, it is not possible to draw conclusions, because this study is not a randomized trial and the number of patients included is too small. Whatever the right interpretation is, it is clear that the adequate control of brain lesions can prolong the patient's survival, until death occurs as a result of systemic disease. In other words, control of the primary tumor is the most important for the further prolongation of survival, if adequate local treatment (e.g. radiosurgery with WBRT) has already been administered for the brain lesions.
Quality of life is an issue as important as survival and only one randomized trial reported KPS outcomes for WBRT alone vs. WBRT with radiosurgery boost (21). Concerning the issue, our study has limitation as a retrospective study mainly focused on survival outcome. However, most of the patients died due to systemic causes rather than neurological ones and it seems that major disabling symptoms in these patients were not neurological problems.
Our results have some practical implications in making decision of treatment strategy when the patient was diagnosed as having lung cancer with simultaneous brain lesions. If the poor prognosis is expected due to the presence of brain lesions, it is reasonable to withhold aggressive local or systemic treatment to the primary site tumor or extracranial disease. Several previous studies reported improved survival with surgical excision of both the primary tumor and intracranial lesions (survival rate 56-66.4% at 1 yr), mostly in case of a few metastases in brain and resectable lung lesion (10,22,23). It is thought that a similar principle may be applied when the use of radiosurgery and chemotherapy is considered for patients with multiple brain lesions and advanced extracranial disease. Because the treatment of brain lesions with radiosurgery can afford a substantial period of freedom from disease progression in the brain, trial of aggressive treatment to extracranial disease may improve survival. Currently, the response rate of advanced stage NSCLC to chemotherapy is known to be in the range of 42-60%, depending on the regimen (24,25). It means that at least a subgroup of patients may benefit from the aggressive treatment of their brain lesions followed by systemic chemotherapy. Recently it was reported that median survival was longer with WBRT and chemotherapy for NSCLC with synchronous brain metastases (58.1 weeks) than WBRT and best supportive care only (19.0 weeks) (26). This data support benefit of chemotherapy. In this study, the main cause of chemotherapeutic failure was extracranial progression, however, still there was aggravation of neurologic status in 19.6%. It suggests that effectively combined local and systemic modality may further improve the outcome. This issue needs to be further investigated through randomized trial and quality of life as well as survival would be another important point of consideration.
In conclusion, radiosurgery should be considered as a part of initial treatment in NSCLC patients with metastatic brain lesions of synchronous onset. Systemic treatment following the aggressive local treatment of brain lesions may provide further survival gain.
References
- 1.Peto R, Chen ZM, Boreham J. Tobacco--the growing epidemic. Nat Med. 1999;5:15–17. doi: 10.1038/4691. [DOI] [PubMed] [Google Scholar]
- 2.Delattre JY, Krol G, Thaler HT, Posner JB. Distribution of brain metastases. Arch Neurol. 1988;45:741–744. doi: 10.1001/archneur.1988.00520310047016. [DOI] [PubMed] [Google Scholar]
- 3.Coia LR. The role of radiation therapy in the treatment of brain metastases. Int J Radiat Oncol Biol Phys. 1992;23:229–238. doi: 10.1016/0360-3016(92)90567-2. [DOI] [PubMed] [Google Scholar]
- 4.Posner JB. Diagnosis and treatment of metastases to the brain. Clin Bull. 1974;4:47–57. [PubMed] [Google Scholar]
- 5.Lagerwaard FJ, Levendag PC, Nowak PJ, Eijkenboom WM, Hanssens PE, Schmitz PI. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999;43:795–803. doi: 10.1016/s0360-3016(98)00442-8. [DOI] [PubMed] [Google Scholar]
- 6.Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, Tans JT, Lambooij N, Metsaars JA, Wattendorff AR, Brand R, Hermans J. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583–590. doi: 10.1002/ana.410330605. [DOI] [PubMed] [Google Scholar]
- 7.Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 8.Kaal EC, Niel CG, Vecht CJ. Therapeutic management of brain metastasis. Lancet Neurol. 2005;4:289–298. doi: 10.1016/S1474-4422(05)70072-7. [DOI] [PubMed] [Google Scholar]
- 9.Cho KT, Kim DG. Stereotactic radiosurgery fro brain metastases: Prognostic factors for survival and local control. J Korean Neurosurg Soc. 2003;33:333–338. [Google Scholar]
- 10.Iwasaki A, Shirakusa T, Yoshinaga Y, Enatsu S, Yamamoto M. Evaluation of the treatment of non-small cell lung cancer with brain metastasis and the role of risk score as a survival predictor. Eur J Cardiothorac Surg. 2004;26:488–493. doi: 10.1016/j.ejcts.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 11.Nugent JL, Bunn PA, Jr, Matthews MJ, Ihde DC, Cohen MH, Gazdar A, Minna JD. CNS metastases in small cell bronchogenic carcinoma: increasing frequency and changing pattern with lengthening survival. Cancer. 1979;44:1885–1893. doi: 10.1002/1097-0142(197911)44:5<1885::aid-cncr2820440550>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Schuette W. Treatment of brain metastases from lung cancer: chemotherapy. Lung Cancer. 2004;45(Suppl 2):S253–S257. doi: 10.1016/j.lungcan.2004.07.967. [DOI] [PubMed] [Google Scholar]
- 13.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 14.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 15.Kocher M, Maarouf M, Bendel M, Voges J, Muller RP, Sturm V. Linac radiosurgery versus whole brain radiotherapy for brain metastases. A survival comparison based on the RTOG recursive partitioning analysis. Strahlenther Onkol. 2004;180:263–267. doi: 10.1007/s00066-004-1180-y. [DOI] [PubMed] [Google Scholar]
- 16.Amendola BE, Wolf A, Coy S, Amendola MA. Radiosurgery as palliation for brain metastases: a retrospective review of 72 patients harboring multiple lesions at presentation. J Neurosurg. 2002;97:511–514. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 17.Nam TK, Lee JI, Jung YJ, Im YS, An HY, Nam DH, Park K, Kim JH. Gamma knife surgery for brain metastases in patients harboring four or more lesions: survival and prognostic factors. J Neurosurg. 2005;102(Suppl):147–150. doi: 10.3171/jns.2005.102.s_supplement.0147. [DOI] [PubMed] [Google Scholar]
- 18.Yang CC, Ting J, Wu X, Markoe A. Dose volume histogram analysis of the gamma knife radiosurgery treating twenty-five metastatic intracranial tumors. Stereotact Funct Neurosurg. 1998;70(Suppl 1):41–49. doi: 10.1159/000056405. [DOI] [PubMed] [Google Scholar]
- 19.Serizawa T, Iuchi T, Ono J, Saeki N, Osato K, Odaki M, Ushikubo O, Hirai S, Sato M, Matsuda S. Gamma knife treatment for multiple metastatic brain tumors compared with whole-brain radiation therapy. J Neurosurg. 2000;93(Suppl 3):32–36. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 20.Flannery TW, Suntharalingam M, Kwok Y, Koffman BH, Amin PP, Chin LS, Nicol B, Fowler Z, Young AB, Regine WF. Gamma knife stereotactic radiosurgery for synchronous versus metachronous solitary brain metastases from non-small cell lung cancer. Lung Cancer. 2003;42:327–333. doi: 10.1016/s0169-5002(03)00357-x. [DOI] [PubMed] [Google Scholar]
- 21.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ., Jr Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 22.Ambrogi V, Tonini G, Mineo TC. Prolonged survival after extracranial metastasectomy from synchronous resectable lung cancer. Ann Surg Oncol. 2001;8:663–666. doi: 10.1007/s10434-001-0663-7. [DOI] [PubMed] [Google Scholar]
- 23.Bonnette P, Puyo P, Gabriel C, Giudicelli R, Regnard JF, Riquet M, Brichon PY. Surgical management of non-small cell lung cancer with synchronous brain metastases. Chest. 2001;119:1469–1475. doi: 10.1378/chest.119.5.1469. [DOI] [PubMed] [Google Scholar]
- 24.Hotta K, Kiura K, Ueoka H, Tabata M, Fujiwara K, Kozuki T, Okada T, Hisamoto A, Tanimoto M. Effect of gefitinib ('Iressa', ZD1839) on brain metastases in patients with advanced non-small-cell lung cancer. Lung Cancer. 2004;46:255–261. doi: 10.1016/j.lungcan.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 25.Ishida A, Kanoh K, Nishisaka T, Miyazu Y, Iwamoto Y, Kohno N, Miyazawa T. Gefitinib as a first line of therapy in non-small cell lung cancer with brain metastases. Intern Med. 2004;43:718–720. doi: 10.2169/internalmedicine.43.718. [DOI] [PubMed] [Google Scholar]
- 26.Kim DY, Lee KW, Yun T, Kim DW, Kim TY, Heo DS, Bang YJ, Kim NK. Efficacy of platinum-based chemotherapy after cranial radiation in patients with brain metastasis from non-small cell lung cancer. Oncol Rep. 2005;14:207–211. [PubMed] [Google Scholar]