Abstract
Family, twin, and adoption studies have demonstrated that genes play an important role in the development of alcoholism. We investigated the association between alcoholism and the genetic polymorphisms of the GABAA receptor genes on chromosome 5q33-34 in Korean population. The genotype of the GABAA receptor gene polymorphisms were determined by performing polymerase chain reaction genotyping for 172 normal controls and 162 male alcoholics who are hospitalized in alcoholism treatment institute. We found a significant association between the genetic polymorphisms of the GABAA α1 and GABAA α6 receptor gene and alcoholism. The GG genotype of the GABAA α1 receptor gene was associated with the onset age of alcoholism and alcohol withdrawal symptoms, and a high score on the Korean version of the ADS. However, there was no association between the genetic polymorphisms of the GABAA β2 and γ2 receptor gene and alcoholisms. Our finding suggest that genetic polymorphisms of the GABAA α1 and GABAA α6 receptor gene may be associated with the development of alcoholism and that the GG genotype of the GABAA α1 receptor gene play an important role in the development of the early onset and the severe type of alcoholism.
Keywords: Alcoholism; Polymorphism, Genetic; GABA-A Receptor Gene
INTRODUCTION
Alcoholism is one of the most common psychiatric diseases that have been well defined clinically (1), and has a strong genetic influence. Family, twin and adoption studies have convincingly demonstrated that genes play an important role in the development of alcoholism, accounting for approximately 40-60% of the population variance (2,3). The alleged candidate genes of alcoholism are the γ-aminobutyric acid (GABA) gene, the serotonin receptor gene, the monoamine oxidase A promoter, the serotonin transporter regulatory gene, the dopamine type 2 receptor gene, the catechol-O-methyltransferase gene, the cannabinoid receptor gene, and the µ-opioid receptor gene (4). Despite the strong evidence that genetic effects contribute to the susceptibility of alcoholism, detecting the specific genes that increase or decrease the risk for alcoholism has proven difficult.
GABA is the major inhibitory neurotransmitter in the human central nervous system and is involved in many of the behavioral effects of alcohol, including motor incoordination, anxiolysis, sedation, withdrawal signs and ethanol preference (5,6). There are two primary GABA receptor subtypes: the GABAA and GABAB receptors. The GABAA receptors act through intrinsic ion channels and are composed of multiple subunits, designated α, β, γ, δ, and p, with several identified genes coding for these subunits (5). The GABAA receptor agonists tend to potentiate the behavioral effects of alcohol, whereas the GABAA receptor antagonists attenuate these effects. The GABAA receptors have also been implicated in ethanol tolerance and dependence (6). However, the precise mechanisms by which the GABAA receptors are involved in these actions of ethanol remain unknown. A role for the GABAB receptors in the actions of ethanol has not been studied nearly as extensively as that of the GABAA receptors. Most of the GABAA receptor genes are organized into clusters; the GABAA α2, GABAA α4, GABAA β1 and GABAA γ1 gene cluster on chromosome 4p13-p12 (7); the GABAA α5, GABAA β3 and GABAA γ3 gene cluster on chromosome 15q11-q13 (8); and the GABAA α1, GABAA α6, GABAA β2 and GABAA γ2 gene cluster on chromosome 5q33-34 (9).
Several recent genetic studies have addressed the importance of the GABAA receptor genes in the development of alcoholism. Animal studies have demonstrated that alterations in the response to drugs and alcohol may be caused by amino acid differences at the GABAA α6 and GABAA γ2 subunits (9). Several loci related to alcohol withdrawal were also identified on the mouse chromosome 11, which corresponds to the region containing four GABAA receptor genes on human chromosome 5q33-34 (10). However, human genetic studies have reported conflicting results about the role of the GABAA receptor genes on chromosome 5q33-34 in the development of alcoholism (11-14). Therefore, we explored the association between the genetic polymorphisms of the GABAA receptor genes on chromosome 5q33-34 and alcoholism in Korean population.
MATERIALS AND METHODS
Subjects and clinical assessments
The study subjects consisted of 162 male alcoholics who were hospitalized in alcoholism treatment institutes (mean age=45.9 yr, SD=7.0 yr) and 172 control subjects (mean age=43.1 yr, SD=11.4 yr). The study was approved by the Institutional Review Board of Gyeongsang National University.
The 334 participants were interviewed by two psychiatrists. Individuals were diagnosed with alcohol dependence using DSM-IV criteria (15), and alcoholic subjects who had major psychiatric histories, including schizophrenia, major depressive disorders, bipolar disorders, anxiety disorder and organic mental disorder, or who had difficulties participating in the objective interview procedures due to cognitive and communication impairments, were excluded from the study.
The control subjects were recruited from the staff and the men who visited the Health Examination Center at Gyeongsang National University Hospital in Jinju City. All the subjects were asked by a physician to participate in the study, and a written informed consent was obtained from every subject. None of control subjects had a family history of alcoholism and a lifetime history of any other psychiatric, neurological or medical disorders. The control and alcoholic subjects were age-matched.
Patients were interviewed to collect demographic data, including age, gender, occupation, educational level, economic status, marital status and alcohol related questions that inquired about the subjects' alcohol drinking history and the alcohol-associated problematic history. Several standardized research scales were used for the clinical assessment of alcoholism, including the Alcohol Dependence Scale (ADS) (16), the Beck Depression Inventory (BDI) (17), and the Obsessive-Compulsive Drinking Scale (OCDS) (18). All of the above scales had been already standardized in Korean language.
Genomic DNA extraction
We collected a 3-4 mL venous blood sample, which were poured into 7.5% potassium-EDTA containing tubes, were extracted immediately or stored at -20℃ for later use. Genomic DNA was extracted by using QIAmp DNA blood midi kit according to the manufacturer's recommendations (QIAmp DNA blood midi kit, QIAGEN Inc., CA, U.S.A.). Briefly, 200 µL of Qiagen protease and 2.4 mL of buffer AL were added to 2 mL of specimen. Samples were incubated at 70℃ for 10 min after which time 2 mL of 100% ethanol was added to each. Each mixture was vortexed and pipetted into a QIAamp Spin column, which was then centrifuged at 1,850×g for 3 min at room temperature. Samples were washed by the adding 2 mL of AW1 to each spin column, followed by centrifugation at 4,500×g for 5 min. Washing was repeated with 2 mL of AW2, followed by centrifugation at 4,500×g for 5 min. Spin columns were then placed in clean centrifuge tubes, and a 300 µL volume of AE was added to elute the DNA. Elution was accomplished with a final centrifugation step at 4,500×g for 5 min. DNA yield is determined by measuring the concentration of DNA in the eluate by absorbance at 260 nm and 280 nm, an eluate containing 50-100 ng DNA/µL. Pure DNA has an A260/A280 ratio of 1.6-1.9.
Determination of GABAA α1 receptor gene polymorphism
PCR was carried out with the primers, forward (5'-GCT ATG GAT TGG TTT ATT GCC GTG TG-3') and reverse (5'-ATA ATA TTG ATG TAC TAC AGG GAC-3'). A 50 µL total volume of the reaction mixture contained the following; 100 ng genomic DNA, 30 pmole of each primer, 1.25 mM dNTP, 2.5 mM MgCl2, and 1 unit of DNA Taq polymerase. After an initial 5 min denaturation at 95℃, the reaction mix was processed by use of a Perkin Elmer thermocycler according to the following program; 40 cycles of 20 sec at 95℃, 30 sec at 55℃, 40 sec at 72℃, with final extension for 10 min at 72℃. The PCR product (15 µL) was then electrophoresed in 2% agarose gel and visualized with ethidium bromide and ultraviolet light. If the nucleotide 15 of the last intron of the GABAA α1 is a G, the restriction enzyme cuts the 165 bp fragments into two fragments of 141 and 24 bp. If the nucleotide 15 is an A, the PCR fragment remains uncut.
Determination of the GABAA α6 receptor gene polymorphism
Forward exon primer, 5'-GGA GGC ACC AGT AAA ATA GAC CAG-3'and reverse intronic primer, 5'-AAT ACT GAA CAA TGG AAG ACA AAA G-3', were used to amplify a specific region of 423 bp fragment. The reaction mixture contained PCR buffer, 2.5mM MgCl2, 1 unit of Taq DNA polymerase, 1.25 mM dNTP, 30 pmole of each primer and 100 ng of genomic DNA. After an initial 5 min denaturation at 95℃, there followed 40 cycles of PCR (denaturation, 1 min 30 sec at 95℃; annealing, 30 sec at 56℃; polymerization, 40 sec at 72℃) with final extension for 10 min at 72℃ on a Perkin Elmer GeneAmp 9600 thermocycler. The PCR product was subjected to 2% agarose gel electrophoresis and stained with ethidium bromide for viewing. Two units of Nhe I (Roche, Mannhein, Germany) were digested with 22.3 µL of the PCR products at 37℃ for 4 hr. Electrophoresis of the digest was carried out with 3% agarose gel, and the bands were visualized by ethidium bromide staining. If the nucleotide 1,519 in the non-coding region of the the GABAA α6 receptor gene is a T, the restriction enzyme cuts the 423 bp fragments into two fragments of 257 and 166 bp. If the nucleotide 1,519 is a C, the PCR fragment remains uncut.
Determination of the GABAA β2 receptor gene polymorphism
Forward exon primer, 5'-AAG CAC AAT GCT AGC CTA TGG TGC-3'and reverse intronic primer, 5'-GTT CAC ATA ATA AAG CCA ATA GAC GAT-3', were used to amplify a specific region of 253 bp fragment. The reaction mixture contained PCR buffer, 1.5 mM MgCl2, 1 unit of Taq DNA polymerase, 1.25 mM dNTP, 30 pmole of each primer and 100 ng of genomic DNA. After an initial 5 min denaturation at 95℃, the reaction mix was processed by use of a Perkin Elmer thermocycler according to the following program; 40 cycles of 30 sec at 95℃, 30 sec at 56℃, 40 sec at 72℃, with final extension for 10 min at 72℃. The PCR product was subjected to % agarose gel electrophoresis and stained with ethidium bromide for viewing. Two units of Nhe I (Roche, Mannhein, Germany) were digested with 22.3 µL of the PCR products at 37℃ for 4 hr. Electrophoresis of the digest was carried out with 3% agarose gel, and the bands were visualized by ethidium bromide staining. If the nucleotide 1,412 in the last exon before the non-coding region of the GABAA β2 receptor gene is a C, Ban I digests the 253 bp fragments into two fragments of 229 and 24 bp. If the nucleotide 1,412 is a T, the PCR fragment remains uncut.
Determination of the GABAA γ2 receptor gene polymorphism
Forward exon primer, 5'-AAA GAT AAA AAG AAG AAA AAC CCT-3'and reverse intronic primer, 5'-CAC AGA AAA TAG AAA CAG ACT TGA-3', were used to amplify a specific region of 224 bp fragment. The reaction mixture contained PCR buffer, 1.5 mM MgCl2, 1 unit of Taq DNA polymerase, 1.25 mM dNTP, 30 pmole of each primer and 100 ng of genomic DNA. After an initial 5 min denaturation at 95℃, the reaction mix was processed by use of a Perkin Elmer thermocycler according to the following program; 40 cycles of 30 sec at 95℃, 30 sec at 50℃, 40 sec at 72℃, with final extension for 10 min at 72℃. The PCR product was subjected to 2% agarose gel electrophoresis and stained with ethidium bromide for viewing. Two units of Nhe I (Roche, Mannhein, Germany) were digested with 22.3 µL of the PCR products at 37℃ for 4 hr. Electrophoresis of the digest was carried out with 3% agarose gel, and the bands were visualized by ethidium bromide staining. If the nucleotide 123 in the upstream intron of 24 bp exon of the GABAA γ2 is an A, the restriction enzyme cuts the 224 bp fragments into two fragments of 103 and 99 bp. If C is present at this position, the PCR fragment does not digest.
Statistical analysis
For the case-control genetic comparisons, differences in genotype and allele frequencies between groups were evaluated by using the chi-square test or Fisher's exact test, where appropriate. Univariate comparisons of clinical data between groups were conducted with independent t-tests or ANOVA were used. The Duncan test was used for the post-hoc analysis. Since we compare genotypic and allelic distributions of four independent polymorphic markers between healthy comparison subjects and alcoholism, a Bonferroni correction was applied to correct for multiple testing level of significant was set to alpha<0.05/4=0.0125). The Hardy-Weinberg equilibrium was tested for by the chi-square test. All significance levels were set at p<0.05 and the statistical analyses were run on SPSS software, version 11.0 (SPSS, Chicago, IL, U.S.A.).
RESULTS
Genotype and allele frequencies of the GABAA receptor gene polymorphisms
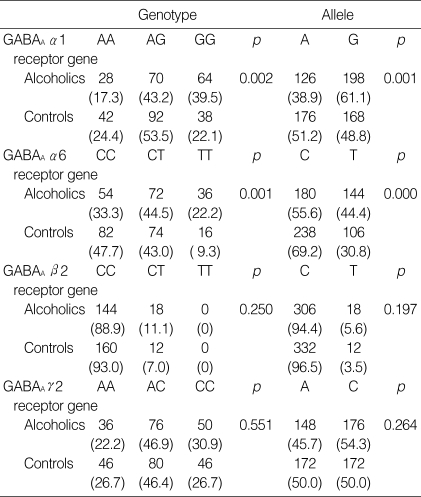
The genotype frequency of the polymorphisms did not deviate from that expected according to Hardy-Weinberg equilibrium (GABAA α1, p=0.893; GABAA α6, p=0.892; GABAA β2, p=0.650; GABAA γ2, p=0.918). When compared with the controls, an association between polymorphisms of the GABAA α1 and α6 receptor gene was with alcohol dependence. Allelic distribution of polymorphisms of the GABAA α1 and α6 receptor gene was associated with alcohol dependence. These associations remained statistically significant even after Bonferroni correction. However, there were no significant associations between alcohol dependence and the genetic polymorphisms of the GABAA β2 and GABAA γ2 receptor genes (Table 1).
Table 1.
Genotype and allele frequencies of the GABAA receptor gene polymorphisms on chromosome 5q33-34 in alcoholic and control subjects
*Values are presented as number (proportion in the sample in %).
Association of the clinical characteristics with genetic polymorphisms of the GABAA receptor genes
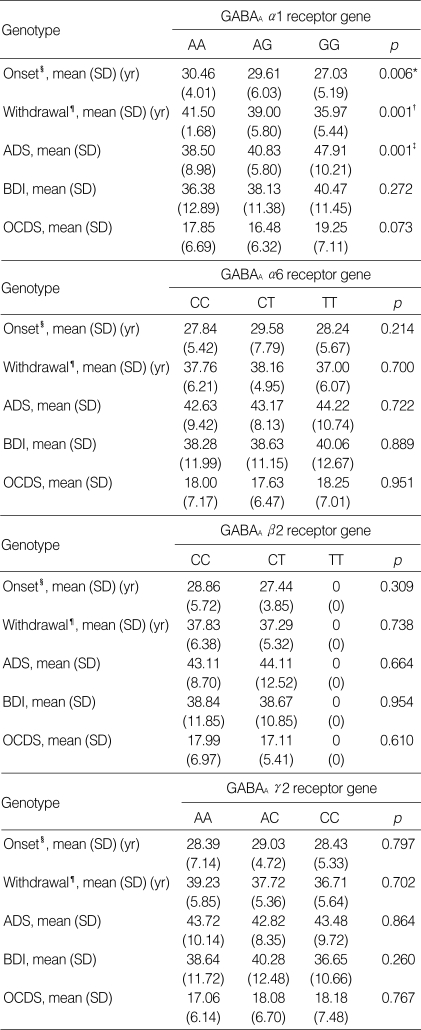
Genetic polymorphisms of the GABAA α1 receptor gene were associated with the mean onset age of alcoholism and the alcohol withdrawal symptoms and mean score of the ADS. The GG genotype of the GABAA α1 receptor gene was associated with the early onset of alcoholism and the alcohol withdrawal symptoms. The ADS mean score was significantly higher for alcoholics with the GG genotype than for the alcoholics with the AA and AG genotypes of the GABAA α1 receptor gene. However, the GABAA α1 receptor gene polymorphisms were not associated with the scores of the BDI and OCDS. There were no associations found between the other GABAA receptor gene polymorphisms and the clinical characteristics of the alcoholics (Table 2).
Table 2.
Clinical characteristics and genetic polymorphisms of the GABAA receptor genes on chromosome 5q33-34
*, group difference, AA-AG (p=0.641), AA-GG (p=0.000), AG-GG (p=0.000); †, group difference, AA-AG (p=1.000), AA-GG (p=0.002), AG-GG (p=0.026); ‡, group difference, AA-AG (p=0.452), AA-GG (p=0.004), AG-GG (p=0.013); §, Onset age of alcoholism; ¶, Onset age of the alcohol withdrawal symptoms; ADS, alcohol dependence scale; BDI, Beck depression inventory; OCDS, Obsessive-Compulsive Drinking Scale.
DISCUSSION
Alcoholism is a complex, multifaceted disorder that has long been recognized to run in families. There is substantial evidence from twin research and adoption studies that a major genetic component operates in the development of alcoholism. In this study, we investigated the association between alcoholism and the genetic polymorphisms of the GABAA receptor genes, and the association between the genetic polymorphisms and the clinical characteristics of alcoholics. We found that genetic polymorphisms of the GABAA α1 and α6 receptor genes were associated with alcoholism, but the GABAA β2 and γ2 receptor genes were not. A number of groups have investigated the role of the GABAA receptor genes polymorphisms that are located on chromosome 5, and these studies have reported mixed results. In research on several populations, revealed a possible association between alcoholism and the genetic polymorphisms of the GABAA α1 or α6 receptor genes (11,12). Although there was some evidence for association, previous studies also showed no association of the GABAA α6, β2 and γ2 receptor genes polymorphisms with alcoholism or familial alcoholism (13,14). These controversial results from other studies might be due to either ethnic differences in the allele frequencies or the difference of the phenotype definition. Because Koreans are a unitary race that is genetically homogeneous (19), the subjects in this study have the merit of minimizing the stratification bias that can originate from association studies when the subjects of these studies consist of diverse races. Therefore, our results support the evidence that genetic polymorphisms of the GABAA α1 and α6 receptor genes may have crucial roles for the development of alcoholism in Koreans.
The GG genotype of the GABAA α1 receptor gene was significantly associated with the early onset of alcoholism and the alcohol withdrawal symptoms. Furthermore, the GG genotype was associated with a high mean ADS score, which suggests a tendency toward severe alcoholism. Typology studies have suggested a way of reducing the etiological heterogeneity. The best known of these typologies is the Type 1/Type 2 distinction developed by Cloninger from the studies that were done on the adopted sons of Swedish alcoholics (2). Type 1 alcoholics are characterized by the late onset of problem drinking, few childhood risk factors, a prominence of guilt and anxiety related to drinking, relatively mild dependence, few alcohol related problems and little psychopathology. In contrast, type 2 alcoholics are characterized by the early onset of alcoholism, many childhood risk factors, significant psychopathology, a strong family history of alcohol abuse and the absence of guilt and fear concerning drinking (20). An adoption study based on Cloninger's typology reported that the lifetime risk of severe alcoholism was increased sixfold for the adopted sons having the type 2 genetic background compared with type 1 subjects, regardless of their postnatal environment (21). In contrast, neither the genetic nor environmental risk factors for type 1 alcoholism were sufficient enough to cause alcoholism. These finding indicate that the age of onset and the antisocial behavior represent two characteristics that are susceptible to the genetic components of alcoholism. Our results support possibility that the GG genotype of the GABAA α1 receptor gene may be associated with Cloninger's type 2 alcoholics. However, further evaluations for the antisocial behavior and the family history of alcoholics will be required to elucidate a positive association between Cloninger's type 2 alcoholics and the genetic polymorphisms of the GABAA α1 receptor gene.
A possible limitation in case-control association studies is the risk of spurious associations as a result of population admixture. However, this objection is less likely in the present study, as our study group was composed of subjects of Korean descent for generations and because the Italian population is relatively homogeneous. In addition, the size of our sample is relatively small and replication of our results on a larger and independent sample is required. Moreover, another limitation was that this study lacked a structured diagnostic interview. Finally, only male patients were genotyped. Therefore, the generalization of the results to all alcoholics is limited.
In conclusion, there was a significant association between the genetic polymorphisms of the GABAA α1 and α6 receptor genes and alcoholism. The GG genotype of the GABAA α1 receptor gene was associated with not only the onset age of alcoholism and the alcohol withdrawal symptom, but also the ADS score in Korean population. These results indicate that genetic polymorphisms of the GABAA α1 and α6 receptor genes may have a crucial role in the development of alcoholism. In particular, the GG genotype of the GABAA α1 receptor gene may play an important role in the development of early onset drinking and the more severe alcoholism in Korean population.
References
- 1.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 2.Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse: cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- 3.Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. A population-based twin study of alcoholism in women. JAMA. 1992;268:1877–1882. [PubMed] [Google Scholar]
- 4.Dick DM, Foroud T. Candidate genes for alcohol dependence: a review of genetic evidence from human studies. Alcohol Clin Exp Res. 2003;27:868–879. doi: 10.1097/01.ALC.0000065436.24221.63. [DOI] [PubMed] [Google Scholar]
- 5.Buck KJ. Molecular genetic analysis of the role of GABAergic systems in the behavioral and cellular actions of alcohol. Behav Genet. 1996;26:313–323. doi: 10.1007/BF02359387. [DOI] [PubMed] [Google Scholar]
- 6.Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA-A receptors in the acute and chronic effects of ethanol. Psychophamacology. 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- 7.McLean PJ, Farb DH, Russek SJ. Mapping of the alpha 4 subunit gene (GABRA4) to human chromosome 4 defines an alpha 2-alpha 4-beta 1-gamma 1 gene cluster: further evidence that modern GABAA receptor gene clusters are derived from an ancestral cluster. Genomics. 1995;26:580–586. doi: 10.1016/0888-7543(95)80178-o. [DOI] [PubMed] [Google Scholar]
- 8.Greger V, Knoll JH, Woolf E, Glatt K, Tyndale RF, DeLorey TM, Olsen RW, Tobin AJ, Sikela JM, Nakatsu Y, et al. The gamma-aminobutyric acid receptor gamma 3 subunit gene (GABRG3) is tightly linked to the alpha 5 subunit gene (GABRA5) on human chromosome 15q11-q13 and is transcribed in the same orientation. Genomics. 1995;26:258–264. doi: 10.1016/0888-7543(95)80209-5. [DOI] [PubMed] [Google Scholar]
- 9.Loh EW, Ball D. Role of the GABA(A)beta2, GABA(A)alpha6, GABA (A)alpha1 and GABA(A)gamma2 receptor subunit genes cluster in drug responses and the development of alcohol dependence. Neurochem Int. 2000;37:413–423. doi: 10.1016/s0197-0186(00)00054-1. [DOI] [PubMed] [Google Scholar]
- 10.Buck KJ, Hood HM. Genetic association of a GABA(A) receptor gamma2 subunit variant with severity of acute physiological dependence on alcohol. Mammalian Genome. 1998;9:975–978. doi: 10.1007/s003359900909. [DOI] [PubMed] [Google Scholar]
- 11.Song J, Koller DL, Foroud T, Carr K, Zhao J, Rice J, Nurnberger JI, Jr, Begleiter H, Porjesz B, Smith TL, Schuckit MA, Edenberg HJ. Association of GABA-A receptors and alcohol dependence and the effects of genetic imprinting. Am J Med Genet. 2003;117:39–45. doi: 10.1002/ajmg.b.10022. [DOI] [PubMed] [Google Scholar]
- 12.Dick DM, Edenberg HJ, Xuei X, Goate A, Hesselbrock V, Schuckit M, Crowe R, Foroud T. No association of the GABAA receptor genes on chromosome 5 with alcoholism in the collaborative study on the genetics of alcoholism sample. Am J Med Genet B Neuropsychiatr Genet. 2005;132:24–28. doi: 10.1002/ajmg.b.30058. [DOI] [PubMed] [Google Scholar]
- 13.Loh EW, Smith I, Murray R, McLaughlin M, McNulty S, Ball D. Association between variants at the GABAAbeta2, GABAAalpha6 and GABAAgamma2 gene cluster and alcohol dependence in a Scottish population. Mol Psychiatry. 1999;4:539–544. doi: 10.1038/sj.mp.4000554. [DOI] [PubMed] [Google Scholar]
- 14.Radel M, Vallejo RL, Iwata N, Aragon R, Long JC, Virkkunen M, Goldman D. Haplotype-based localization of an alcohol dependence gene to the 5q34 gamma-aminobutyric acid type A gene cluster. Arch Gen Psychiatry. 2005;62:47–55. doi: 10.1001/archpsyc.62.1.47. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edition (DSM-IV) Washington, DC: American Psychiatric Association; 1994. pp. 195–196. [Google Scholar]
- 16.Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- 17.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An Inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 18.Anton RF. Obsessive-compulsive aspects of craving: development of the Obsessive Compulsive Drinking Scale. Addiction. 2000;95:211–217. doi: 10.1080/09652140050111771. [DOI] [PubMed] [Google Scholar]
- 19.Han GR, Lee YW, Lee HL, Kim SM, Ku TW, Kang IH, Lee HS, Hwang JJ. A Korean population study of the nine STR loci FGA, VWA, D3S1358, D18S51, D21S11, D8S1179, D7S820, D13S317 and D5S818. Int J Legal Med. 2000;114:41–44. doi: 10.1007/s004140000137. [DOI] [PubMed] [Google Scholar]
- 20.Schuckit MA. Alcohol-related disorders. In: Sadock BJ, Sadock VA, editors. Kaplan and Sadock's comprehensive textbook of psychiatry. 8th edition. Philadelphia: Lippincott Willains and Wilkins; 2004. pp. 1168–1188. [Google Scholar]
- 21.Sigvardsson S, Bohman M, Cloninger CR. Replication of the Stockholm Adoption Study of alcoholism: Confirmatory cross-fostering analysis. Arch Gen Psychiatry. 1996;53:681–687. doi: 10.1001/archpsyc.1996.01830080033007. [DOI] [PubMed] [Google Scholar]