Abstract
Three major flavonoid chamomile components (quercetin, apigenin-7-O-glucoside and rutin) were subjected to oxidative metabolism by cytochrome P-450 of rat liver microsomal preparations. Changes over time in their respective concentrations were followed using reversed-phase HPLC with UV detection. No clean-up had to be applied as only the specific flavonoid had to be separated from the background components originating from the rat liver microsome.
Neither the concentration of apigenin-7-O-glucoside nor that of the diglycoside rutin decreased during one hour of exposure to rat microsomal treatment. In contrast, the concentration of quercetin, a lipophilic aglycon, decreased.
Our analytical HPLC results complement the in silico calculated lipophilicity (logP) of these compounds; the relatively high lipophilicity of quercetin appears to predispose it to oxidative metabolism in order to decrease its fat solubility. In contrast the much less lipophilic compounds apigenin-7-O-glucoside and rutin were resistant in vitro to microsomal treatment.
Keywords: Microsomal treatment, cytochrome P-450 dependent oxidation, HPLC, quercetin, apigenin-7-O-glucoside, rutin.
INTRODUCTION
Chamomile is one of the oldest and best known favourites amongst medicinal herbs and its position as a therapeutical plant with multiple uses is well established. The positive effects derived from chamomile use are related to the presence of its numerous flavonoid constituents. Regarding their core structure many of these constituents are either flavone- (apigenin, luteolin) or flavonol- derivatives (quercetin, patuletin, isorhamnetin) [1]. They appear in various forms such as aglyca, mono- and diglycosides, and/or acyl-derivatives [2].
Some of these constituents have been directly associated with specific therapeutic effects; luteolin and the quercetin aglyca for instance have demonstrated protective and delaying effects on the development of diabetic complications [3].
Furthermore apigenin and its -7-O-glucoside derivative are among the most important therapeutic flavonoids; their spasmolytic and antiphlogistic effects are especially prominent [4], in addition to anti proliferative and apoptotic effects in various human cancer cell lines [5].
The presence of apigenin in chamomile is however a matter of some debate as its presence has not been proved unequivocally. Carle et al. [6] and Schreiber et al. [7] compared extracts derived from freshly collected wild plants with those obtained from greenhouse plants. They concluded that apigenin is not originally present in fresh chamomile, but the result of secondary enzymatic processes. This would explain the great differences in the reported ratios of apigenin and apigenin-7-O-glycoside in the literature, differences which might also be due to different harvesting methods and storage conditions used. Repčak and Martonfi [8] observed that the content of apigenin aglyca in various parts of the flower increases by polyploidization. In di- and tetraploid samples the amount of apigenin aglyca increased by about 15 %. According to both the European and Hungarian Pharmacopoeias [9] the content of apigenin-7-O-glucoside in the Matricaria flower should be at least 0,25 %. This content is measured by HPLC. The HPLC method is suitable for the determination of other flavones too, irrespective of their state of glycosylation and/or acylation [9-15].
Our aim was to establish whether any of the major matricaria components [quercetin, apigenin-7-O-glucoside and rutin] is subjected to hepatic oxidative microsomal metabolism.
EXPERIMENTAL
Materials:
HPLC grade acetonitrile, methanol and trifluoroacetic acid were purchased from Merck (Darmstadt, Germany). Water was double-distilled and deionized. Rutin, apigenin-7-O-glucoside and quercetin were purchased from Sigma-Aldrich (St. Louis, MO, USA). The chemical structures of rutin, apigenin-7-O-glucoside and quercetin are shown in Table 1. The Zorbax RX-C-18 column and precolumn were purchased from Kromat Kft. (Budapest, Hungary).
Table 1.
Name, Chemical Structure, Molecular Size and Composition Calculated Value of Lipophilicity (logP) and Total Polar Surface Area (TPSA, in Angström) of Certain Flavonoids Derived from Chamomile
| # | Name | Structure | MW | logP | TPSA |
|---|---|---|---|---|---|
| 01 | Rutin | 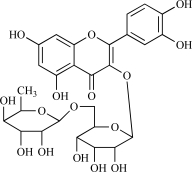 |
C28H10O16 610.57 | 0.68 | 265.52 |
| 02 | Apigenin-7-O-glucoside | 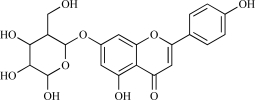 |
C21H20O10 432.41 | -0.01 | 166.14 |
| 03 | Quercetin | 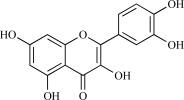 |
C15H10O7 302.25 | 1.75 | 127.45 |
| 04 | Apigenin | 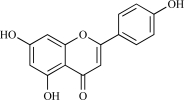 |
C15H10O5 270.25 | 2.79 | 86.99 |
| 05 | Apigenin-7-O-neohesperidosid | 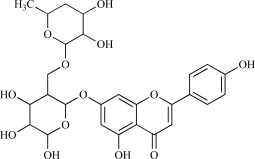 |
C27H30O13 562.57 | 0.68 | 204.83 |
| 06 | Axillarin | 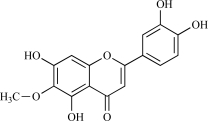 |
C16H12O7 316.28 | 2.49 | 116.45 |
| 07 | Chrysoeriol | 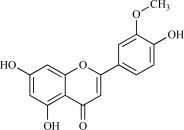 |
C16H12O6 300.28 | 2.71 | 96.22 |
| 08 | Chrysoplenol | 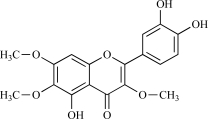 |
C18H16O8 360.34 | 2.53 | 114.68 |
| 09 | Chrysoplenetin | 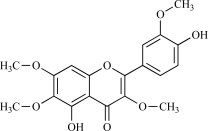 |
C18H19O8 374.34 | 2.80 | 103.68 |
| 10 | Eupatoletin | 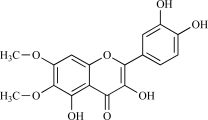 |
C17H14O8 346.31 | 1.87 | 125.68 |
| 11 | Eupaletin | 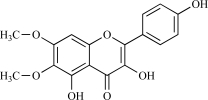 |
C17H14O7 330.31 | 2.42 | 105.45 |
| 12 | Isorhamnetin | 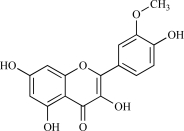 |
C16H12O7 316.28 | 2.05 | 116.45 |
| 13 | Jaceidin | 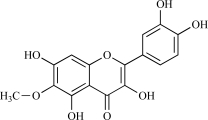 |
C18H16O8 360.34 | 2.53 | 114.68 |
| 14 | Luteolin | 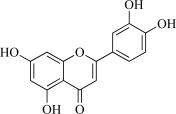 |
C15H10O8 286.25 | 2.39 | 107.22 |
| 15 | Patuletin | 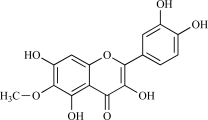 |
C16H12O8 332.28 | 1.64 | 136.68 |
| 16 | Spinacetin | 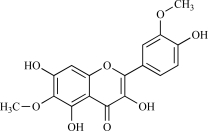 |
C17H14O8 346.31 | 1.87 | 125.68 |
Microsomal Preparation:
Quercetin, apigenin-7-O-glucoside and rutin (25 µM, each) were incubated separately for 0, 15, 30, 45 and 60 minutes with rat liver microsome (Gedeon Richter Plc., Budapest, Hungary, Lot no. 081022, 0.5 mg mL-1) in 4 mL incubation medium also consisting of Tris-HCl buffer (0.12 mM, pH 7.4 at 37°C), MgCl2 (5 mM), sodium phosphate (6.25 mM), D-glucose-6-phosphate (5 mM), D-glucose 6-phosphate dehydrogenase (1 UmL-1), and the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH, 0.5 mM). Parallel experiments were carried out using each substance (quercetin, apigenin-7-O-glucoside, rutin) in the buffered medium but without any D-glucose-6-phosphate, D-glucose 6-phosphate dehydrogenase and the reduced form of nicotinamide adenine dinucleotide phosphate. A control experiment that subjected the entire mixture to microsomal treatment (rat liver microsome, in the solution of Tris-HCl buffer at pH 7.4, MgCl2, sodium phosphate, D-glucose-6-phosphate, D-glucose 6-phosphate dehydrogenase, and the reduced form of nicotinamide adenine dinucleotide phosphate) but without any substrate to be metabolized was also performed. Microsomal treatment was done using a rotating, thermostat-regulated water bath at 37°C. Incubation with microsomal preparation was terminated after 0, 15, 30 and 60 min respectively, by adding a 4-fold excess of methanol. Each experiment was performed in duplicate. Incubated samples were placed at -20 oC for 10 minutes, and centrifuged using 2500 x g at 4 oC (Sigma Laborzentrifugen, Osterode am Harz, Germany) and finally stored at -80 oC before their reversed-phase HPLC measurement.
Chromatography:
A JASCO made HPLC system was used that contained a solvent delivery system (PU 1580 Pump), degasser (DG-2080-54), an autoinjector (AS-2057 Plus), and a UV detector (UV-1575). Elution of rutin, apigenin-7-O-glucoside and quercetin was monitored at 257 nm.
The samples were not subjected to any clean-up. Fifty µL of the samples were injected. The separation was done on a 250 mm x 4.6 mm I.D., 5-µm particle size Zorbax RX-C-18 column protected by a 12.5 mm x 4.6 mm, I.D. precolumn containing the same packing material. The mobile phases were acetonitrile—water 40:60, 35:65, 30:70, 25:75, 20:80, 15:85 and 10:90, and each mobile phase contained 0.1% of trifluoroacetic acid (TFA). The mobile phase flow rate was 1 mL min-1, the separations were done at ambient temperature (24°C ± 1°C). Chromatograms were electronically stored and evaluated by the use of Borwin 1.50 chromatography software (JMBS, Le Fontanil, France).
Calibration:
Calibrations of the substances were done covering 0.5 µg mL-1 through 20 µg mL-1 range for quercetin, apigenin-7-O-glucoside and rutin.
Lipophilicity Calculations:
The logP value, and the total polar surface area (TPSA) were calculated using the Pallas program of CompuDrug [16,17].
RESULTS
The physico-chemical parameters of several chamomile flavonoids belonging to three major groups [aglycons (flavonoid compounds without any substituting sugar), monoglycosylated and diglycosylated] were calculated using the Pallas program. Chemical structures, molecular composition, molecular size, logP and total polar surface area (TPSA in Angström) are given in Table 1. Flavonoid aglycons were rather lipophilic with a logP value higher than 1.5.
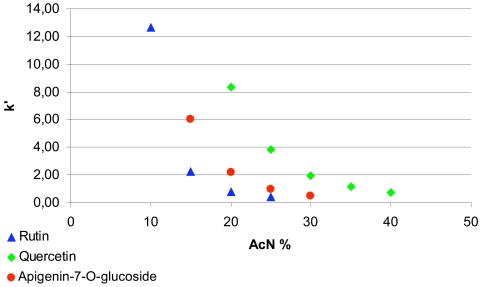
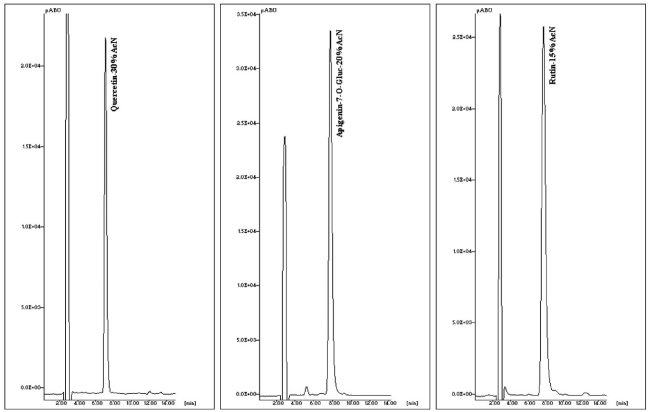
The mobile phase composition for HPLC of quercetin, apigenin-7-O-glucoside and rutin was optimized to avoid any interference with the background signal of the microsomal incubation medium. Different ratios of the organic modifier and water in the mobile phases were used, such as acetonitrile—water 50:50, 45:55, 40:60, 35:65, 30:70, 25:75, 20:80, 15:85, 10:90 (Fig. 1). Using the optimum mobile phase compositions, the peaks of quercetin, apigenin-7-O-glucoside and rutin eluted at 7.10, 7.72 and 7.73 minutes with the ratio of acetonitrile and water (30:70) for separation of qurcetin, apigenin-7-O-glucoside (20:80) and rutin (15:85), respectively. (Fig 2, from left to right).
Fig. (1).
The elution of quercetin, apigenin-7-O-glucoside and rutin are characterized by their k’ values when using various compositions of the mobile phases.
Fig. (2).
Typical chromatograms of the microsomal medium spiked with the appropriate amount of quercetin, apigenin-7-O-glucoside and rutin in panels a, b and c respectively.
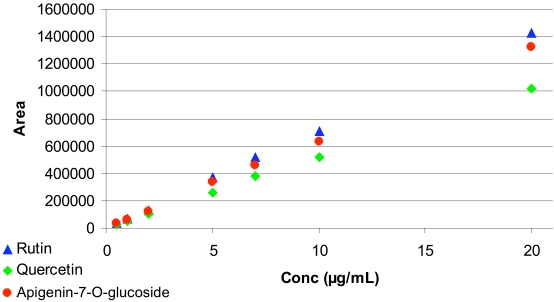
No interference of any one of the flavonoid compound peaks with those from the microsomal medium was found. Fig. (3) shows that calibration for all substances produced a straight line characterized by their respective parameters, a, y0 and R2 (from the equation of the straight line: y = ax + y0), as shown in Table 2.
Fig. (3).
Calibration curve for quercetin, apigenin-7-O-glucoside and rutin using the adequate mobile phase.
Table 2.
Characteristics of the Calibration Lines (y = ax + y0) of RP-HPLC Quantitative Determination of Quercetin, Apigenin-7-O-Glucoside and Rutin
| Compound | a (Slope) | y0 | R2 |
|---|---|---|---|
| Quercetin (03) | 51077 | 3701 | 0.9993 |
| Apigenin-7-O-glucoside (02) | 66236 | 8101 | 0.9991 |
| Rutin (01) | 71362 | 2246 | 0.9995 |
Molecular size (MW), lipophilicity (logP) and total polar surface area (TPSA) of several flavonoids were calculated using the Pallas Program of CompuDrug Inc. These values are given in Table 1.
CONCLUSIONS
Chamomile [the dried flower heads of Matricaria recutita L. (Chamomilla recutita [L.] Reuschert] has been used for centuries as a home remedy; its therapeutic value is well established as documented by the inclusion in numerous Pharmacopoeias.
In traditional medicine the plant is mostly used as chamomile tea, which is administered p.o. for alleviation of gastro-intestinal complaints [18]. Externally chamomile is applied on skin and/or mucous membranes at sites of minor inflammation, including the oral cavity and the gums (mouthwashes), the respiratory tract (inhalations), and the anal and genital areas (baths, ointments) [19,20].
The main constituents of chamomile are the essential oil and the flavone derivatives [21-23].
In vitro liver microsomal treatment of organic compounds serves as a model for the first pass effect of the liver in a living animal. Information may be obtained both on the putative fate of the compound in the body, and also the preferred way of administration. Liver microsomes generally metabolize xenobiotics (foreign compounds ingested) using oxidative metabolism via the cytochrome P-450 system. The lipophilicity of xenobiotics decreased via these reactions.
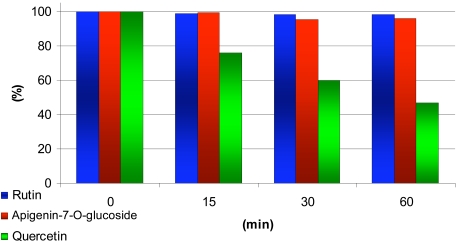
Fig. (4) compares the relative changes of the concentrations of quercetin, apigenin-7-O-glucoside and rutin during microsomal treatment. While the concentration of rutin and that of apigenin-7-O-glucoside remain unchanged, that of quercetin decreases.
Fig. (4).
Concentration of quercetin, apigenin-7-glucoside and rutin through the microsomal treatment at 0, 15, 30 and 60 min of the treatment.
The most lipophilic flavonoid, the aglycon quercetin undergoes cytochrome P-450-dependent microsomal metabolism, while the two less lipophilic compounds examined (apigenin-7-O-glucoside and rutin) were resistant to the effect of rat liver microsome (Fig. 4).
REFERENCES
- 1.Carle R, Isaac Q. Fortschritte in der Kamillenforschung in den Jahren 1974 bis 1984. Dtsch. Apoth. Ztg. 1985;125(Suppl 1):2–8. [Google Scholar]
- 2.Švehliková V, Bennett RN, Mellon FA, Needs PW, Piacente S, Kroon PA, Bao Z. Isolation, identification and stability of acylated derivatives of apigenin 7-O-glucoside from chamomile (Chamomilla recutita [L.] Rauschert) Phytochemistry. 2004;65:2323–2332. doi: 10.1016/j.phytochem.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Kato A, Minoshima Y, Yamamoto J, Adachi I, Watson AA, Nash RJ. Protective effects of dietary chamomile tea on diabetic complications. J. Agricult. Food Chem. 2008;56:8206–8211. doi: 10.1021/jf8014365. [DOI] [PubMed] [Google Scholar]
- 4.Petri G. Gyógynövények és készítményeik a terápiában (Medicinal Plants and their Products in Therapy, in Hungarian) Budapest: Galenus Publisher; 2006. [Google Scholar]
- 5.Srivastava JK, Gupta S. Anti proliferative and apoptotic effects of chamomile extract in various human cancer cells. J. Agricult Food Chem. 2007;55:9470–9478. doi: 10.1021/jf071953k. [DOI] [PubMed] [Google Scholar]
- 6.Carle R, Dölle B, Müller W, Baummeister U. Thermospray liquid-chromatography mass-spectrometry (tsp lc/ms) - analysis of acetylated apigenin-7-glucosides from chamomilla-recutita. Pharmazie. 1993;48:304–306. [Google Scholar]
- 7.Schreiber A, Carle R, Reinhard E. On the accumulation of apigenin in chamomile flowers. Planta Med. 1991;56:179–181. doi: 10.1055/s-2006-960920. [DOI] [PubMed] [Google Scholar]
- 8.Repčak M, Martonfi P. The variability pattern of apigenin glucosides in chamomilla-recutita diploid and tetraploid cultivars. Pharmazie. 1995;50:696–699. [Google Scholar]
- 9.VIII Tomus II., editor. Pharmacopoea Hungarica. Medicina, Budapest: 2004. pp. 1944–1946.pp. 2203–5. [Google Scholar]
- 10.Carle R. In: Hagers Handbuch der Pharmazeutischen Praxis. 5th. Hänsel R, Keller K, Rimpler H, Schneider G, editors. Vol. 4. Berlin: Springer; 1992. pp. 817–831. [Google Scholar]
- 11.Kakasy A, Marczal G, Héthelyi É, Lemberkovics É. Dracocephalum fajok mikromorfológiai és fitokémiai jellemzése (Micromorphology and phytochemistry of certain Dracocephalum species, in Hungarian) Botanikai Közlemények. 2004;91:140. [Google Scholar]
- 12.Papp I, Apáti P, Andrasek V, Blázovics A, Balázs A, Kursinszki L, Kite GC, Houghton PJ, Kéry Á. LC-MS analysis of antioxidant plant phenoloids. Chromatographia. 2004;60:S93–S100. [Google Scholar]
- 13.Kakasy A, Fűzfai Z, Kursinszki L, Molnár-Perl I, Lemberkovics É. Analysis of non-volatile constituents in Dracocephalum species by HPLC and GC-MS. Chromatographia. 2006;63:S17–S22. [Google Scholar]
- 14.Weber B, Herrmann M, Hartmann B, Joppe H, Schmidt CO, Bertram HJ. HPLC/MS and HPLC/NMR as hyphenated techniques for accelerated characterization of the main constituents in Chamomile (Chamomilla recutita [L.] Rauschert) Eur. Food Res. Technol. 2008;226:755–760. [Google Scholar]
- 15.Szőke É, Kéry Á, Lemberkovics É. Schweiz Pharmacognosy. Pharmacobotanical and Phytochemical Investigations of Plant Drugs. Budapest: Semmelweis Kiadó; 2009. (in press) [Google Scholar]
- 16.Pallas Program: www.compudrug.com
- 17.Molnár L, Keserű GM, Papp Á, Gulyás Z, Darvas F. A neural network based classification scheme for cytotoxicity predictions: validation on 30,000 compounds. Bioorg. Med. Chem. Lett. 2006;16:81037–81039. doi: 10.1016/j.bmcl.2005.10.079. [DOI] [PubMed] [Google Scholar]
- 18.Franke R, Schilcher H. Chamomile Industrial Profiles. Boca Raton: CRC Press; 2005. [Google Scholar]
- 19.Carle R, Isaac O. Die Kamille – Wirkung und Wirksamkeit. Z. Phytotherapie. 1987;8:67. [Google Scholar]
- 20.Förster CF, Süssmann HE, Patzelt-Wenczler R. Schweiz. Rundschau Med. 1996;85:1476. [PubMed] [Google Scholar]
- 21.Schilcher H. Die Kamilla – Handbuch für Ärzte, Apotheker und anderen Naturwissenschaftler. Stuttgart, Germany: Wiss. Verlagsges; 1987. p. 152. [Google Scholar]
- 22.Szőke É, Máday E, Tyihák E, Kuzovkina IN, Lemberkovics É. New terpenoids in cultivated and wild chamomile (in vivo and in vitro) J. Chromatogr. B. 2004;800:231–238. doi: 10.1016/j.jchromb.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 23.Shikov AN, Laakso I, Pozharitskaya ON, Darman HJD, Makarov VG, Tikhonov VP, Hiltunen R. Identification of spiroketal polyacetylenes as the main components of an oil extract of chamomile (Chamomilla recutita L. Rausch.) flowers. Planta Med. 2006;72:1026. [Google Scholar]