Abstract
Astrocytes comprise a large proportion of the CNS neural support cells and play a critical role in neural injury and repair. The present study examined the impact of ovarian aging using an ex vivo model system, where astrocytes were derived from the olfactory bulb of young, reproductively competent females and reproductive senescent females. Cellular morphology and the spatial pattern of laminin deposition was altered in astrocyte cultures derived from reproductive senescent females. Young adult astrocytes had a flattened polygonal shape with actin bundles at the cell edges, while reproductive senescent astrocytes had a contractile appearance with thick stress fibers visible throughout the cell. Moreover, in reproductive senescent astrocytes, BDNF was elevated with a concomitant reduction in expression of the BDNF receptor, TrkB. To examine the ability of astrocytes derived from young adult and reproductive senescent females to promote neuronal differentiation, neural progenitor cells (NPCs) were co-cultured with astrocytes derived from these groups. At 4 days in vitro, MAP-2+ NPCs were located in smaller clusters when co-cultured with young adult astrocytes and in large clusters when co-cultured with older astrocytes. At day-6 and -10, neuronal differentiation was significantly reduced in reproductive senescent astrocyte:NPC co-cultures, as determined by NeuN+ cell numbers and MAP-2+ process lengths. Further, estrogen only enhanced neuronal differentiation in young adult:NPC co-cultures. The ovarian age-related astrocyte phenotype thus limits the ability of this cell to promote neuronal differentiation in NPC populations and suggest that the astrocyte-mediated microenvironment in older acyclic females is less conducive to repair following neurovascular injury.
Keywords: laminin, stress fibers, neurospheres, neural progenitor cells, estrogen, PC12 cells
INTRODUCTION
Young adult and reproductive senescent female rats differ in their constitutive expression of neurotrophins (Jezierski & Sohrabji, 2001), their neuroinflammatory response to brain injury (Nordell et al., 2003) and blood-brain barrier permeability (Bake & Sohrabji, 2004). Further, estrogen replacement to reproductive senescent females is associated with a poor outcome in the presence or absence of injury (Sohrabji, 2005). To determine the cellular target of these ovarian aging effects, astrocytes were examined as they are involved in neuronal support (Wujek & Akeson, 1987; Liesi & Silver, 1988; Ullian et al., 2001; Parish et al., 2002), blood-brain barrier integrity (Janzer & Raff, 1987; Kim et al., 2006 recent review) and regulation of the inflammatory response (Fontana et al., 1982; Robbins et al., 1987). Thus, several astrocyte functions overlap with cellular activities where our laboratory has observed ovarian age-related differences.
Astrocytes may participate in the inflammatory response and neuronal repair through growth promoting factors. In astrocyte:adult retinal ganglion cell co-cultures, astrocytes reduced neuronal oxidative stress and increased axonal regeneration through release of astrocyte-specific soluble factors (Lucius & Sievers, 1996). Astrocytes secrete and/or express several soluble factors that might contribute to neuronal health, such as nerve growth factor (Yamakuni et al., 1987; Schwartz & Mishler, 1990; Lu et al., 1991), brain-derived neurotrophic factor (Riley et al., 2004), leukemia inhibiting factor (Banner et al., 1997) and bone morphogenic factor (Zhang et al., 2006). Moreover, astrocytes also produce and secrete extracellular matrix molecules such as laminin (Powell et al., 1997). Laminin, in particular is crucial for neuronal migration (Dodd & Jessell, 1988), survival and sprouting following injury (Liesi et al., 1984).
Estrogen may play a key role in regulating astrocyte activity. Astrocyte morphology varies with estrogen levels (Parducz et al., 1993; Amateau & McCarthy, 2002) and estrogen increases glial-derived neurotrophic factor (Platania et al., 2005) and transforming growth factor beta-1 and beta-2 (Platania et al., 2005) following injury in astrocytes. Moreover, in cultured astrocytes estrogen increased expression of the glial glutamate transporters, resulting in enhanced glutamate uptake (Pawlak et al., 2005).
In view of the evidence that astrocytes are responsive to estrogen, ovarian decline resulting in the menopause or reproductive senescence may affect these cells and their ability to promote neuronal differentiation. To test this hypothesis, astrocytes from young adult (= young adult astrocytes) and reproductive senescent (= reproductive senescent astrocytes) female rats were studied in an ex vivo culture. Our results indicate that young adult astrocytes differ from reproductive senescent astrocytes in their geometry, laminin deposition and growth factor systems. Since these factors are known to affect neuronal differentiation, astrocyte:neural progenitor cell (NPC) co-cultures were studied.
Neural progenitor cells were chosen as a model co-culturing system due to their importance in the adult brain following injury (Gould & Tanapat, 1997; Liu et al., 1998; Magavi et al., 2000; Jin et al., 2001; Zhang et al., 2001; Ohab et al., 2006). Our studies show that differentiation of fetal NPCs was impaired in co-cultures with reproductive senescent astrocytes and facilitated in young adult astrocytes.
RESULTS
Characteristics of Young Adult and Ovarian-Aged Astrocytes
Laminin Expression in Astrocytes
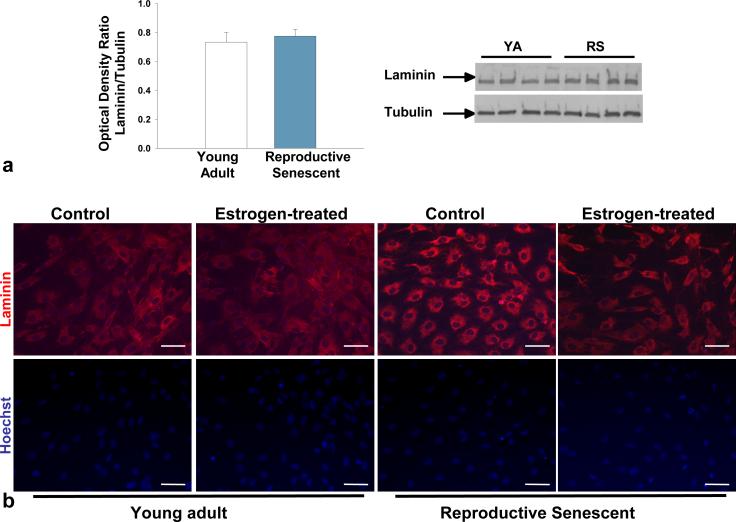
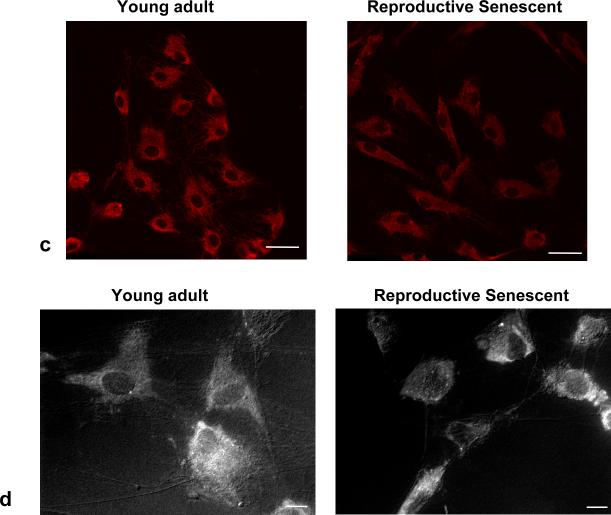
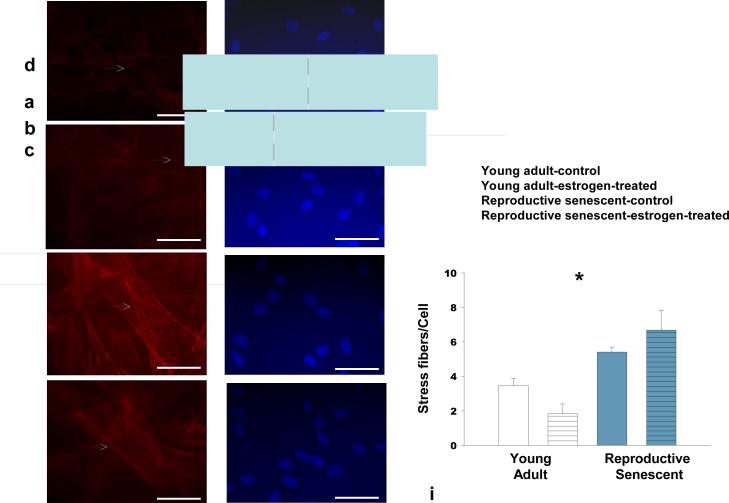
Laminin, a matrix molecule crucial for neuronal outgrowth of NPCs (Camarillo et al., 2007) is reduced in astrocytes derived from chronologically aged (24 mos.) male rats (Rozovsky et al., 2005). In this study, no significant differences in laminin expression were observed in young adult or reproductive senescent astrocytes as determined by immunoblotting (Fig. 1a). However, the pattern of laminin deposition, as visualized by light (Fig. 1b) and confocal (Fig. 1c) microscopy, differed markedly with ovarian age. In reproductive senescent astrocytes, laminin was concentrated in the perinuclear regions, while in young adult astrocytes, laminin was widely dispersed. Additionally in the latter group, laminin immunoreactivity was observed in astrocyte processes, spanning the spaces between neighboring cells (Fig. 1b, 1c). In reproductive senescent astrocytes, laminin deposition was tightly concentrated around the nucleus, with minimal immunopositive labeling between adjacent astrocytes (Fig. 1b, 1c). To determine if the pattern of laminin deposition was a reflection of age-related differences in cell geometry, young adult and reproductive senescent astrocyte cultures were examined under combined epifluoresence:phase-contrast illumination. This approach revealed that laminin expression closely overlapped with the astrocyte morphology and indicated obvious age-related differences in the cell geometry (Fig. 1d). Young adult astrocytes were more polygonal in shape and flattened, with actin bundles mainly seen at the cell edges. Reproductive senescent astrocytes were more contractile, with strongly stained, thickly-bundled F-actin-positive stress fibers visible throughout the cell body. As shown in Fig 2 (a-d), the number of stress fibers increased by 1.6-fold in the absence of estrogen and 3.7-fold in the presence of estrogen in reproductive senescent astrocytes as compared to young adult astrocytes (Fig. 2i; F(1,14): 5.249, p≤ 3.80×10-2, interaction effect: age x hormone).
FIG 1.
Protein expression of laminin from young adult and reproductive senescent astrocytes. No differences in laminin protein expression were observed in young adult or reproductive senescent astrocytes by Western blot analysis (a). Laminin expression as examined by immunohistochemistry in young adult and reproductive senescent astrocytes displayed a different localization pattern (b,c). Astrocyte laminin expression (b, top panel) was analyzed in the presence or absence of estrogen (40 nM dose) and counter-stained with the nuclear dye Hoechst 33258 (b, bottom panel). A highly dispersed pattern of laminin deposition was seen in the young adult astrocyte cultures in contrast to the largely perinuclear concentration of laminin in reproductive senescent astrocyte cultures, both here and in 1c by confocal microscopy. Combined epifluorescence-phase contrast light microscopy (1d) indicates that laminin organization closely approximates the morphology of the cell. Bars represent mean ± SEM of a single representative experiment with n=4 for each treatment group. Bar: 50 μm.
FIG 2.
F-actin stress fibers are significantly increased in reproductive senescent astrocytes as compared to young adult astrocytes. Stress fiber formation was analyzed in astrocytes cultured in the presence and absence of 40 nM estrogen by Alexa Fluor 594® phalloidin staining in young (a,b,i) and reproductive senescent astrocytes (c,d,i) and counter-stained with the nuclear dye Hoechst 33258 (e-h). Estrogen treatment attenuated stress fiber formation in young adults but not reproductive senescent astrocyte cultures. White arrow indicates presence of stress fibers. Bars represent mean ± SEM of a single representative experiment with n=4-5 for each treatment group. Interaction of age and hormone is statistically significant at p<0.05 and indicated by (*). Bar: 50 μm.
BDNF Expression in Astrocytes
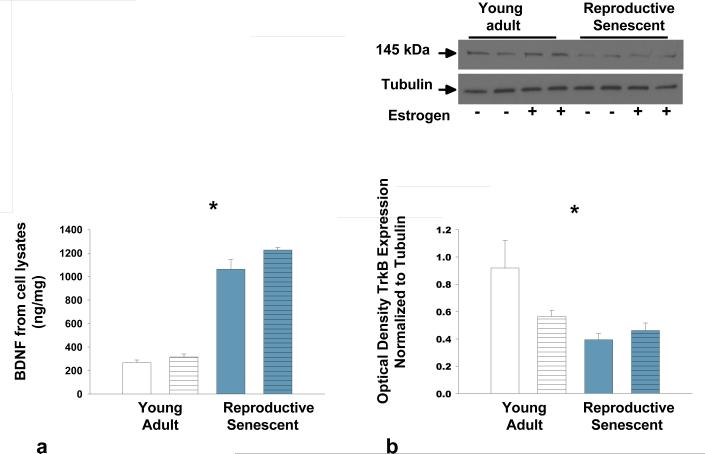
BDNF is important for neuronal growth and our laboratory has shown that in olfactory bulb homogenates, the BDNF receptor TrkB, decreases with ovarian age (Jezierski & Sohrabji, 2001). To determine the expression of BDNF and TrkB, young adult and reproductive senescent astrocyte cell lysates were subjected to ELISA and immunoblotting, respectively. BDNF expression in astrocyte cell lysates was inversely correlated with expression of TrkB such that BDNF was significantly increased (by 3-fold) with age (Fig. 3a; F(1,22): 323.62, p< 1.20×10-14, main effect age) while TrkB decreased by approximately 1.7-fold with age (Fig. 3b; F(1,12): 8.3021, p< 2.29×10-2, main effect age). No differences in the truncated form of the TrkB receptor were observed (data not shown).
FIG 3.
Protein expression of BDNF and TrkB in young adult and reproductive senescent astrocyte cell lysates. BDNF protein expression was determined by ELISA and TrkB expression was determined by immunoblotting. TrkB expression was normalized to α-tubulin. BDNF expression (a) increased with age while expression of TrkB (b) decreased with age in astrocyte cell lysates. Bars represent mean ± SEM of a single representative experiment with n=4-8 for each treatment group. Main effect of age was statistically significant at p<0.05 and is indicated by (*).
Astrocytes and Neuronal Differentiation
Astrocyte:neural progenitor cell co-cultures
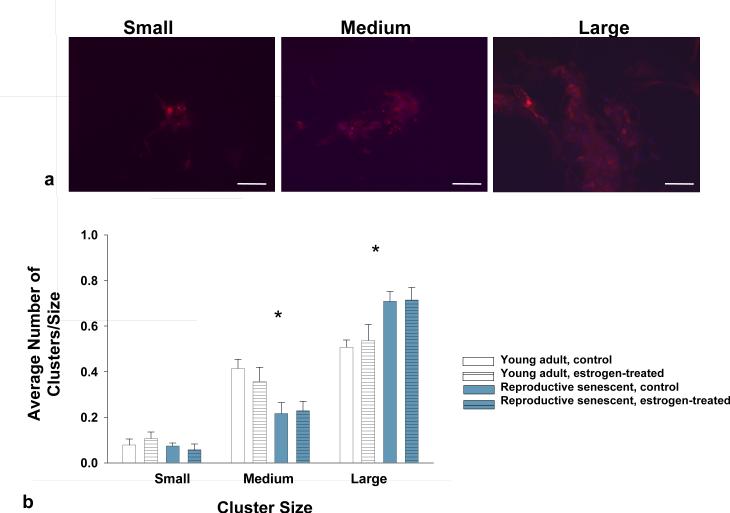
Since migration and neuronal differentiation of progenitor cells is enhanced with laminin (Camarillo et al., 2007) and BDNF (Shetty & Turner, 1998), we next examined the ability of young adult and reproductive senescent astrocytes to promote neuronal differentiation of fetalderived cortical NPCs. The NPCs used in this study (Camarillo et al., 2007) and those used in other studies (Reynolds & Weiss, 1992; Vescovi et al., 1993; Gritti et al., 1999) proliferate in serum-free mitogenic media and differentiate rapidly into neurons following mitogenic factor withdrawal and laminin (Camarillo et al., 2007). In vitro, NPCs form neurospheres when grown in differentiating media and over time, differentiated neurons migrate away from these clusters (Reynolds et al., 1992; Camarillo et al., 2007). In this study, astrocyte:NPC co-cultures were tracked over a 4-10 day period. At day 4, most of the MAP-2+ cells were localized to clusters, however, the cluster size was affected by the astrocyte monolayer (F(2,63): 17.68, p<0.0001, interaction effect). When MAP-2+ NPCs were compared in young adult and reproductive senescent astrocyte:NPC co-cultures, large clusters significantly increased by 30% in reproductive senescent astrocyte co-cultures (Fig. 4b) whereas, in young adult astrocyte:NPC co-cultures a 60% increase in the frequency of medium-sized clusters was observed (Fig. 4b).
FIG 4.
Neurosphere cluster size was used as a measure of putative neuronal maturity in NPCs co-cultured with young adult or reproductive senescent astrocytes (a). Small neurosphere clusters were defined as (a, far left) aggregates of NPCs consisting of 5-10 visible grouped nuclei, medium clusters (a, middle panel) as consisting of 11-20 visible grouped nuclei and large clusters (a, far right) as containing greater than 20 visible grouped nuclei. Cells were immunoreactive for MAP-2 (red, a) and counter-stained for the nuclear dye Hoechst 33258 (blue, a). The frequency of small cluster size did not differ between those cultured with young adult or reproductive senescent astrocytes (b). However, neurosphere aggregates co-cultured with young adult astrocytes had significantly more medium size clusters while reproductive senescent astrocyte co-cultures had predominantly large clusters (b). Bars represent mean ± SEM of a single representative experiment with n=5-6 for each treatment group. Statistical significance at p< 0.05 is indicated by (*). Bar: 50 μm.
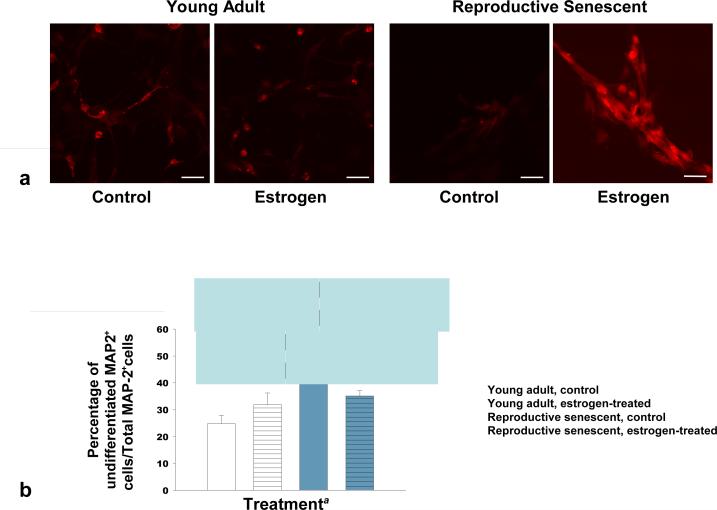
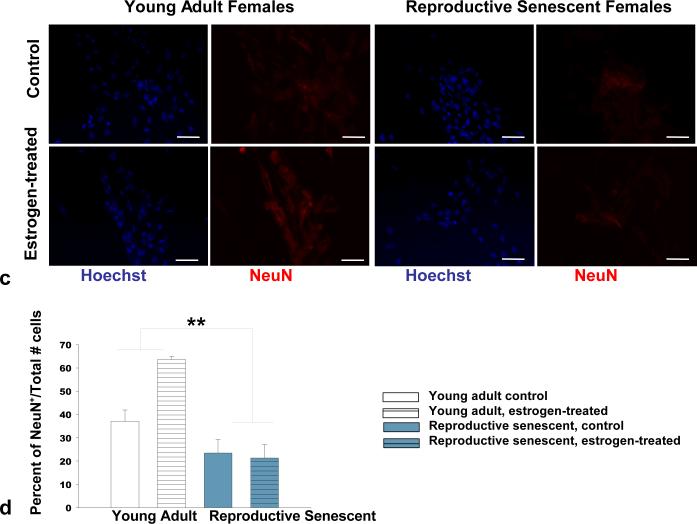
At 6 days, quantitative analysis of immunolabeled MAP-2, an early neuronal marker, and NeuN, a later neuronal marker, confirmed that the marked difference in neuronal differentiation was associated with the reproductive age of the astrocyte donors (Figures 5a, 5b). Both MAP-2 (Fig. 5a, 5b) and NeuN (Fig. 5c, 5d) immunolabeling indicated a significant increase in neuronal maturity of the differentiated NPCs when co-cultured with young adult astrocytes. A significantly higher percentage of MAP-2+ cells lacking processes was observed in reproductive senescent astrocyte:NPC co-cultures (Fig. 5b; F(1,14): 7.05, p≤ 1.89×10-2, main effect age). Further, estrogen caused a 3-fold increase in NeuN+ cells in young adult astrocyte:NPC co-cultures as compared to estrogen-treated reproductive senescent astrocyte:NPC co-cultures (Fig. 5d; F(1,16): 6.08, p≤ 2.53×10-2, interaction effect: hormone x age).
FIG 5.
Neuronal differentiation was significantly increased in co-cultures of NPCs with young adult astrocytes at day-6 as compared to co-cultures with reproductive senescent astrocytes (a-d). The percentage of non-process bearing MAP-2+ progenitor cells was increased in co-cultures with reproductive senescent astrocytes (a,b). Immunolabeling for the late stage neuronal marker, NeuN, was enhanced with estrogen only in young adult astrocyte:NPC co-cultures (c,d). Bars represent mean ± SEM of a single representative experiment with n=3-6 for each treatment group. Arrows indicate individual cell bodies. The main effect of age was statistically significant at p<0.05 and is indicated by (*). The interaction effect of age with estrogen exposure was statistically significant at p<0.05 and is indicated by (**). Bar: 50 μm.
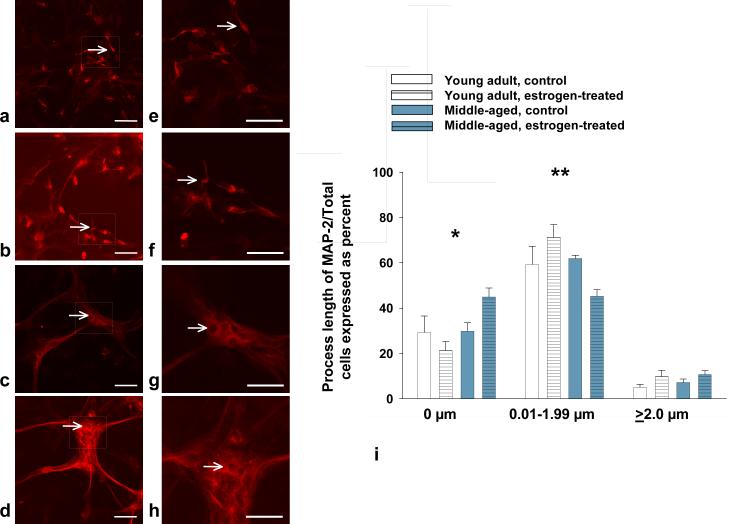
In astrocyte:NPC co-cultures grown for 10 days, the percentage of MAP-2+ cells with no visible processes was greater in reproductive senescent astrocyte:NPC co-cultures indicating delayed neuronal outgrowth. This effect was exacerbated 2.1-fold with estrogen when reproductive senescent astrocyte:NPC co-cultures were compared to NPCs co-cultured with young adult astrocytes (Fig. 6a-d,6i; F(1,19): 6.03, p≤ 2.39×10-2, interaction effect: hormone x age). Moreover, estrogen enhanced the percentage of MAP-2+ cells with process lengths of 0.01-1.99 mM by 1.2-fold in young adult astrocytes but not reproductive senescent astrocyte co-cultures (Fig. 6i; F(1,20): 5.32, p≤ 3.18×10-2, interaction: hormone x age).
FIG 6.
Estrogen enhanced the length of MAP-2+ processes in NPCs co-cultured with young adult astrocytes (a,b,i) as compared to co-cultures with reproductive senescent astrocytes (c,d,i). Arrows indicate individual cell bodies. In the reproductive senescent astrocyte:NPC co-cultures, significantly more of non-process bearing cells were observed. Thus, the percentage of MAP-2+ cells with no visible processes was significantly increased in reproductive senescent astrocyte:NPC co-cultures and was exacerbated with estrogen treatment (i,*). Likewise, estrogen enhanced the percentage of MAP-2+ cells with processes between 0.01 and 1.99 μm in length in young adult astrocyte:NPC co-cultures as compared to co-cultures with reproductive senescent astrocytes (i, **). Boxed regions in image (a-d) are enlarged to show process detail for young adult (e,f) and reproductive senescent astrocytes (g,h). Bars represent mean ± SEM of a single representative experiment with n=4-6 for each treatment group. Interaction of age and hormone treatment was statistically significant at p<0.05 and is indicated by (*,**). Bar: 50 μm (a-d) or 20 μm (e-h).
To determine if decreased neuronal differentiation was due to ovarian aging or simply chronological age of the donor, NPCs were co-cultured with astrocytes derived from age-matched males. In age-matched male astrocyte:NPC co-cultures, no significant differences were observed between young and middle-aged male astrocytes in the number of NeuN+ cells at Day 6 (Supplemental Fig. 1) or in the process lengths at Day 10 (Supplemental Fig. 2).
Astrocyte:PC12 Co-Cultures
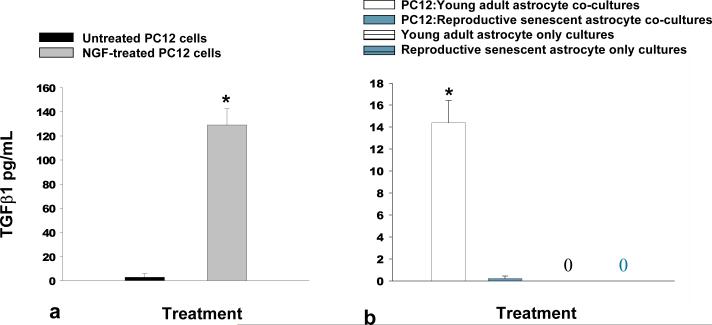
Neuroepithelial progenitors are derived from the neural tube (Rao, 2004; Mokry & Karbanova, 2006) and have the capability of transitioning into a glial or neuronal fate. To determine if the differences in the ability of astrocytes to promote neuronal differentiation affected other cell types, young adult and reproductive senescent astrocytes were co-cultured with pheochromocytoma (PC12) cells, a neural crest-derived cell (Fujita et al., 1989) for 6 days. PC12 cell differentiation was determined indirectly through TGFβ1expression, a growth factor known to increase when PC12 cells differentiate into neurons (Kim et al., 1994). TGFβ1 expression was significantly increased by 43-fold when PC12 cells were cultured in the presence of NGF (Fig. 7a; F(1,4): 85.42, p≤ 7.62×10-4). TGFβ1 expression was also enhanced by 14-fold when co-cultured in the presence of young adult but not reproductive senescent astrocytes or in astrocyte only cultures (Fig. 7b; F(1,14): 20.376, p≤ 3.0×10-3, main effect of age). Estrogen did not enhance TGFβ1 expression in PC12 co-cultures with either young adult or reproductive senescent astrocytes (data not shown).
FIG 7.
DiI-Labeled PC12 cells co-cultured with young adult astrocytes for 6 days exhibited increased levels of TGFβ1 as compared to co-cultures with reproductive senescent astrocytes. NGF-induced neuronal differentiation of PC12 cells significantly increased the protein expression of TGFβ1 (a). An increase in TGFβ1 protein expression was also observed when PC12 cells were co-cultured with young adult astrocytes but not when PC12 co-cultured with reproductive senescent astrocytes or in astrocyte only cultures (b). 0 indicates that no detectable level of TGFβ1 was observed. Bars represent mean ± SEM of a single representative experiment with n=3-5 for each treatment group. Statistical significance at p< 0.05 is indicated by (*, **). Bar: 50 μm.
One possible explanation for the impairment in neuronal differentiation of NPCs in reproductive senescent astrocytes could be that these “aged” astrocytes are promoting proliferation over differentiation. However, no significant differences were observed in the percentage of NPCs that were immunopositive for Ki67, a marker of proliferation (Supplemental Fig. 3) when co-cultured with either young adult or reproductive senescent astrocytes.
DISCUSSION
Our results show that the phenotype of reproductive senescent astrocytes was associated with an impaired ability to promote neurite outgrowth and differentiation of embryonic NPCs as compared to young adult astrocytes. Laminin deposition and cell morphology were distinctly different in young adult and reproductive senescent astrocytes such that laminin was widely dispersed in young adult astrocytes but more focally distributed in reproductive senescent astrocytes and in each case, the laminin distribution correlated with the cell's geometry. Young adult astrocytes were flattened out while reproductive senescent astrocytes were contractile, thus it is possible that less surface laminin is available for neuronal outgrowth in reproductive senescent astrocytes. Further, stress fiber formation was increased in reproductive senescent astrocytes as compared to young adult astrocytes indicating impairments in cell spreading and adherence with ovarian age (Hedin et al., 1989). Increases in actin stress fibers are also indicative of increased inflammation (Singh et al., 1999; Bailly et al., 2004). Thus, laminin deposition and the cytoskeletal architecture, which is likely influenced by actin stress fiber formation, may underlie the astrocyte age-associated variations in neuronal differentiation.
Astrocytes synthesize and secrete many factors that influence neuronal health and enhance repair following injury. In the CNS, inflammatory cytokines like interleukin-1beta and interleukin-6 modulate the astrocyte response and play an active role in sprouting of dopaminergic neurons in the substantia nigra following injury (Parish et al., 2002). In retinal ganglion cells, astrocytes enhanced the frequency and amplitude of postsynaptic currents (Pfrieger & Barres, 1997) and increased synapse number on neurons (Ullian et al., 2001). Astrocytes express other growth factors such as nerve growth factor, fibroblast growth factor, ciliary neurotrophic factor, insulin-like growth factors I & II (Muller et al., 1995), brain-derived neurotrophic factor, glial-derived neurotrophic factor and leukemia inhibitory factor (Yoshida & Toya, 1997). Several of these factors are regulated by estrogen, and in many cases, the expression of these growth factors change with age.
This study also showed that estrogen increased the process length and neuronal maturity of NPCs when co-cultured with young adult but not reproductive senescent astrocytes. Estrogen-mediated actions are not unexpected in astrocyte cultures as estrogen receptors (ER) are present on astrocytes in several areas of the brain. In explant and primary cultures of rat hippocampus, neocortex, preoptic area, and spinal cord both ERα and ERβ co-localize along with cholinergic and muscarinic receptors on astrocytes (Hösli & Hösli, 2000). Further, in the developing rat prepoptic area (Amateau & McCarthy, 2002) and the adult rat arcuate nucleus (Parducz et al., 1993) astrocyte morphology varies with estrogen levels. Estrogen may also regulate neurite outgrowth in concert with the growth factor insulin-like growth factor 1 (Topalli & Etgen, 2004). The present study showed that estrogen affected specific aspects of astrocyte:NPC co-cultures and that these effects were restricted to young adults. Specifically, estrogen increased the proportion of NeuN expressing cells and the percentage of short, process-bearing MAP-2+ neurons in these cultures.
The present study also suggests that BDNF signaling may be impaired in reproductive senescent astrocytes. Although both young and reproductive senescent astrocytes expressed BDNF in cell lysates, there was an inverse relationship between BDNF and its receptor, TrkB, in reproductive senescent astrocytes. BDNF was highly expressed while TrkB was significantly decreased as compared to young adult astrocytes. This is consistent with a previous observation from this laboratory showing that expression of the full length forms of TrkB were virtually undetectable in olfactory bulb homogenates (Jezierski & Sohrabji, 2001). BDNF/TrkB signaling plays a key role in both astrocytes and neurons. BDNF and TrkB expression was detected along the rostral migratory pathway in post-natal day 4 mice and inhibition of BDNF/TrkB signaling resulted in impaired migration of neuroblasts from the subventricular zone in vitro (Chiaramello et al., 2007). In retinal progenitor cells, inhibiting TrkB signaling resulted in increased differentiation of cells in the inner nuclear layer and decreased differentiation of photoreceptor cells (Turner et al., 2006). Astrocyte-conditioned media stimulated TrkB signaling in primary hippocampal neurons leading to increased post-synaptic clustering of GABAA receptors, thus influencing inhibitory synapse formation (Elmariah et al., 2005), and TrkB activation of brain-derived endothelial cells resulted in increased production of angiogenic factors (Li et al., 2006). Further, astrocyte-derived glutamate release, which would be important for modulation of synaptic transmission and plasticity, has been shown to be mediated through TrkB (Pascual et al., 2001). In this study, we propose that the increased BDNF expression observed in reproductive senescent astrocytes may well be a compensatory mechanism to the reduced expression of the TrkB receptor in these cells and may suggest impaired growth factor signaling.
Ovarian-age related differences affecting neuronal differentiation were examined in astrocytes using two different models, neuroepithelial progenitor cell:astrocyte co-cultures and pheochromocytoma (PC12) cell:astrocyte co-cultures. Both of these in vitro models have characteristics of progenitor cells that are present during development. Embryonic neuroepithelial progenitor cells are derived from the neural tube (Rao, 2004; Mokry & Karbanova, 2006) and the cells used in this study have been previously shown to contain a mixed population of cells expressing several stem and progenitor cell markers such as CD133/prominin-1, Sca-1 and CD117/c-kit (Santillano et al., 2005). In this study, young adult astrocytes enhanced neuronal differentiation of both PC12 cells and NPCs.
An important feature of this work is that the donor astrocytes were derived from female rats that were not “chronologically-aged”, but “reproductively-aged” as they were only 9-11 mos. old but were acyclic and in continuous diestrus. Interestingly, when 9-11 mos. old male astrocytes were compared to 3-4 mos. old young adult males neuronal differentiation was not impaired in astrocyte:neurosphere co-cultures indicating that middle age per se, was not detrimental to the ability of adult astrocytes to promote differentiation. This comparison further supports the hypothesis that ovarian aging impairs the ability of astrocytes to promote neuronal differentiation in much the same way as chronological aging. In males, Rozovsky et al. (2005) showed that neurite outgrowth was reduced in co-cultures of embryonic day 18 neurons with astrocytes derived from chronologically-aged (24 mos.) Fischer rats. Collectively, these studies suggest that the capacity of astrocytes to support neuronal differentiation may be lost at an earlier age in females, possibly due to the loss of cyclic estrogen.
The present studies have important implications for neuronal repair and differentiation in the aging brain especially following neurovascular injury. Recent papers have shown that following stroke or ischemia, neurogenesis and migration of neural progenitor cells occurs in the adult brain (Liu et al., 1998; Jin et al., 2001; Zhang et al., 2001; Ohab et al., 2006) and that estrogen increases neurogenesis in the dorsal subventricular zone of ovariectomized adult female mice (Suzuki et al., 2007). Our data would suggest that the molecular environment, created in part by astrocytes, significantly impacts neuronal differentiation of embryonic neural progenitor cells and that estrogen was not beneficial in promoting this process in reproductive senescent astrocytes. Thus hormone-replacement therapy should be carefully considered in reproductive senescent females so as to not undermine potential endogenous repair mechanisms or the success of transplantation therapies targeted at ameliorating neurodegeneration resulting from diseases such as Parkinson's and Alzheimer's disease or neurovascular injury.
EXPERIMENTAL PROCEDURES
Animals
Adult Sprague Dawley rats were purchased from Harlan Laboratories (IN) as young adults (3-4 mos.; females - 250g, males - 375g) or middle-aged animals (9-11 mos.; females - 300g; males - 475g). Middle-aged females (= reproductive senescent) were purchased as retired breeders. The estrus cycle was determined by vaginal smears taken each morning for a period of 14-20 days. Vaginal smears were obtained with a cotton swab, placed on a slide and examined by microscopy (Olympus, BX2) using a 20X objective. Rats that persisted in one stage for 7 days were considered acyclic and for these studies astrocytes were collected only from retired breeders in constant diestrus. The animals used for fetal, murine, cortical neuroepithelial progenitor cell collection were derived from timed-pregnant mice (Jackson Laboratories, ME) generated from timed-matings of either wild type C57BL/6 mice or a transgenic mouse line with an enhanced green fluorescent protein [GFP; C57BL/6-Tg(ACTB-EGFP)1Osb/J] under the control of a chicken beta-actin promoter and cytomegalovirus enhancer. All animals were maintained in an AALAC-approved facility on a 12h light:dark cycle with lights on at 06:00h and with food and water available ad libitum. All procedures were in accordance with NIH and institutional guidelines governing animal welfare. For collection of the olfactory bulb, the rats were deeply anesthetized (ketamine: 87 mg/kg; xylazine: 13 mg/kg) and decapitated. For collection of the GFP+ neural progenitor cells, timed-pregnant mice were deeply anesthetized (ketamine: 100 mg/kg; xylazine: 5 mg/kg) and fetuses were isolated from a gravid uterus at gestational day 12.5 as previously described (Camarillo et al., 2007; Prock & Miranda, 2007).
Tissue Culture
Astrocyte cultures were established using our previously published laboratory protocol (Lewis et al., 2008) and is similar to those methods in other published studies using astrocyte cultures (Wu et al., 2004). Briefly, astrocytes were collected from the olfactory bulbs of young adult, reproductive senescent and age-matched male rats in a semi-sterile hood and rinsed in Optimem:N2 supplement (99:1, Invitrogen, CA) in a sterile hood. To make direct comparisons between astrocyte cultures derived from young adult females and reproductive senescent females, astrocytes collected from these two distinct groups were always cultured simultaneously. The olfactory bulb tissue was mechanically dissociated with a surgical blade and chemically dissociated with 1X trypsin/EDTA (Invitrogen). The cell suspension was pelleted by centrifuging at 700 rpm for 5 minutes. The supernatant was removed from the cell pellet and the cells were re-suspended in an astrocyte-specific growth media (DMEM/F12 [1:1], 10% FBS, 4.6g/L glucose) and plated onto poly-d-lysine hydrobromide (0.05 mg/ml. Sigma, MO) coated dishes. The cells were culture for 10 days at 37° C with 5% CO2. At day 10, cells were shaken at 400 rpm on a plate shaker at room temperature for 10 minutes and washed 3 times with DMEM to remove microglia and oligodendrocytes. The astrocytes were trypsinized for 2-3 minutes and re-plated at a density of 150,000 cells for 35 mm culture dishes. Astrocytes used for immunohistochemistry were plated onto poly-d-lysine coated glass coverslips. The purity of the astrocyte population was determined in a previous study by examining the ratio of the astrocyte specific marker, glial fibrillary acidic protein (GFAP), the neuronal marker, microtubule-associated protein (MAP-2, Chemicon, CA) and two microglial markers, ionized-calcium binding adapter molecule 1 (Iba1, Santa Cruz, CA) and the cell surface glycoprotein Mac-1 (CD11b, Serotec, NC) relative to the total number of stained nuclei. The primary astrocyte cultures are mainly GFAP-positive cells with approximately 5-8% microglial contamination and no significant differences have been observed in the level of microglial contamination in young adult or reproductive senescent astrocyte cultures (Lewis et al., 2008). Further, no significant differences have been observed in the growth rate of young adult or reproductive senescent astrocyte cultures (Lewis et al., 2008). In some experiments, 40nM of 17b-estradiol was added to cultures concurrent with the switch from serum-containing medium (DMEM/F12 [1:1], 10% FBS, 4.6g/L glucose) to the treatment medium that was serum free (Neurobasal Medium, 1X N2 supplement, 0.2M L-glutamine).
Protein Collection
Proteins were isolated by harvesting the cells from 6 well plates containing 100 μL of lysis buffer as previously reported (Sohrabji et al., 2000). Protein concentrations were determined using the bicinchoninic acid method (Pierce, IL) with BSA as a standard. Total protein content was determined as a product of protein concentration (μg/μL) x total fluid content of sample.
Immunoblotting
Laminin and TrkB expression were analyzed by immunoblotting. Total protein from astrocyte cell lysates were separated by SDS-PAGE and transferred to a Hybond-C membrane (Amersham Pharmacia Biotech, NJ) for Western blot analysis using standard laboratory techniques (Jezierski & Sohrabji, 2000; Jezierski & Sohrabji, 2001). Protein expression of laminin (1:150, Sigma, MO) and TrkB (1:1000, BD Biosciences, CA) were normalized to α-tubulin (1:2000, Sigma). An HRP-conjugated secondary (laminin: anti-rabbit, 1:2000, Santa Cruz; α-tubulin, TrkB: anti-mouse, 1:2500, Transduction Laboratories) was used to capture the primary signal. Western Lightening® Chemiluminescence Reagent (Perkin Elmer, MA) was utilized for immunodetection and X-ray film was used to capture the intensity of the signal and the densitometry program Quantity One (BioRad, CA) for quantifying the signal.
Enzyme-linked Immunosorbent Assay (ELISA) For Trophic Factors
BDNF protein expression was determined in astrocyte cell lysates using a commercial kit, BNDF Emax® ImmunoAssay System (Promega, WI). To determine the level of differentiation in PC12:astrocyte co-cultures, a commercial kit was used to quantify the protein expression of TGFβ1 using the Emax® ImmunoAssay System (Promega) in media from PC12:astrocyte co-cultures. Manufacturer's instructions were followed for both kits. The plates were read at 450 nm in a microplate reader (Bio-Tek, VT) and standard curves were established from optical densities of wells containing known dilutions of standard, using KC3 software (Bio-Tek, VT). Sample measurements were interpolated from standard curves.
Immunohistochemistry
In astrocytes or astrocyte co-culture experiments, the presence of laminin (Sigma), microtubule associated protein-2 (MAP-2, Chemicon, MA) and neuronal nuclei (NeuN, Chemicon) were determined. Cultures were washed briefly in 1X phosphate-buffered saline (dPBS, Invitrogen, CA) and fixed for one hour in 2% paraformaldehyde. Astrocytes or astrocyte co-cultures were washed 3 × 10 minutes and incubated in block solution for 1 hour followed by incubation with the primary overnight at 4° C (Laminin, 1:75; MAP-2, 1:500; NeuN, 1:100). The primary was diluted in dPBS, 1% normal goat serum and 0.3% Triton. Controls were incubated in diluent only. Following washes, astrocytes or astrocyte co-cultures were labeled with a fluorescent secondary Alexa Fluor 594 (Laminin and MAP-2 1:2000; NeuN, 1:5000, Molecular probes, CA) to visualize the labeling for 1 hour at room temperature. Astrocytes were counterstained with the nuclear stain, Hoechst 33258 (Polysciences Inc., PA) for 1 hour at room temperature and cover-slipped with ProLong® Antifade kit (Molecular Probes). Slides were visualized by epifluorescence or light microscopy and photographed using an Olympus BX2 microscope and QCapture imaging software (v2.7.3, QImaging Corporation, Canada). Additionally, confocal microscopy was also used to visualize and photograph the images using a Biorad Radiance 2000MP with LaserSharp 2000 Software and combined phase-contrast:epifluorescent imaging was performed using a Zeiss Axiovert 200M microscope and Stallion digital imaging software (Image Analysis Laboratory, Texas A & M University, Dept. of Veterinary Integrative Biosciences).
F-Actin Staining
Actin fibers were visualized using a stain for phalloidin (Verderame et al., 1980). Briefly cells were fixed as described above. Alexa Fluor® 594 phalloidin (1:40, Molecular probes) was incubated with the cells for 20 minutes to visualize the F-actin fiber bundles. Thick, F-actin stained cells were counted using a 40X objective lens and camera settings were gated to capture only the intensely stained fibers. At least 100+ cells were counted per condition. Hoechst 33258 nuclear dye was used as a marker to identify individual cells. The total number of phalloidin-stained stress fibers was normalized to the total number of nuclei for each condition to calculate the number of stress fibers per cell.
Astrocyte-Neural Progenitor Cell Co-Cultures
Astrocytes were grown to confluence on poly-d-lysine hydrobromide-coated glass coverslips and switched to the non-serum containing treatment medium in the presence and absence of 40 nM 17β-estradiol for 24 hours. On the following day, gestational day 12.5 (E12.5)-derived fetal murine cortical neuroepithelial cells, maintained as neurosphere cultures (kind gift from Dr. R.C. Miranda, see (Santillano et al., 2005; Camarillo et al., 2007; Prock & Miranda, 2007) were plated onto an astrocyte monolayer derived from young adult females, reproductive senescent females or age-matched males at a density of 50,000 cells per 35 mm dish. These neurosphere aggregates have been previously shown to contain a mixed population of cells expressing several stem and progenitor cell markers (Santillano et al., 2005), but have the capacity to rapidly differentiate into neurons (Camarillo et al., 2007). Neural progenitor cells (NPCs) were co-cultured with astrocytes in the presence or absence of estrogen for either 4, 6 or 10 days. Cells were labeled for the early-stage neuronal marker, MAP-2 or the late-stage neuronal marker, NeuN by immunohistochemistry as described above. Immunohistochemical analysis of young adult and senescent astrocyte:NPC co-cultures were performed simultaneously. Experiments using age-matched male astrocyte co-cultures were performed separate from the female astrocyte co-cultures, thus these two experiments cannot be compared directly.
Quantitation of Neuronal Maturity in Astrocyte:Neural Progenitor Cell Co-Cultures
As neuronal differentiation was limited on day 4, the measure used for this time point was the neurosphere aggregate or cluster size. Thus, the number of clusters and the size of the neurosphere were counted in 5 fields of view using a 20X objective lens by two individuals blind to the treatments. Cluster sizes were defined as follows: small: 0-10 visible grouped nuclei, medium: 11-20 visible grouped nuclei, large: 21+ visible grouped nuclei (Fig. 5a).
At day 6, neuronal differentiation was determined by counting the total number of NeuN positive cells in 5 fields of view using a 20X objective lens and normalizing to the total number of Hoechst-labeled NPCs. A second measure employed for the day-6 analysis was to count the total number of MAP-2+ cells that did not contain any visible processes and normalize this value to the total number of MAP-2+ cells in 5 fields of view using a 40X objective lens. For both measures, at least 100 cells were counted per culture and values were expressed as a percentage.
Given the obvious differences in neuronal differentiation at day 6, two measures were employed for the day 10 analyses. First, the number of MAP-2+ cells that did not contain any visible processes were counted and secondly, the process length was measured on each MAP-2+ cell containing visible processes. Process lengths were determined by measuring from the cell body of the MAP-2+ cell to the end of the process (white arrow, Supplemental Fig. 4) using a 40X objective lens and an Olympus BX2 microscope equipped with a 10 micron reticle. Hoescht labeling was used to define each individual cell. One hundred cells from 5 different fields were analyzed from each culture and all MAP-2+ processes on a cell were measured.
Astrocyte-PC12 Cell Co-Cultures
Astrocytes were grown to confluence on poly-d-lysine hydrobromide-coated glass coverslips and switched to the non-serum containing treatment medium in the presence and absence of 40 nM 17β-estradiol for 24 hours. On the following day, pheochromocytoma (PC12) cells were labeled with DiI (2.5 mg/mL, Molecular Probes) and plated onto an astrocytic monolayer derived from either young adult or reproductive senescent females at a density of 270,000 cells per 35 mm dish. Control PC12 cells were grown in the absence of astrocytes on poly-d-lysine-coated coverslips or on laminin-coated coverslips in the presence of nerve growth factor (NGF, 25ng/mL). PC12 cells are known to differentiate into a neuronal phenotype in the presence of exogenous nerve growth factor (Greene et al., 1983). Six days following the addition of PC12 cells, astrocyte:PC12 cells were rinsed briefly and fixed with 2% paraformaldehyde for 1 hour at room temperature. Laminin-coated controls were fixed in 4% paraformaldehyde. Following washing, the cells were mounted onto microscope slides as described above.
Astrocyte:GFP+ Neural Progenitor Cell Co-Cultures
To quantitate the proliferation rate of the NPCs when co-cultured with young or reproductive senescent astrocytes GFP+ cortical NPCs were immunolabeled for Ki67 following co-culturing with young adults or reproductive senescent astrocytes in the presence and absence of estrogen for 6- or 10-days. Cells were rinsed and briefly fixed with 2% paraformaldehyde for 1 hour at room temperature and immunolabeled for Ki67 (1:250, BD Pharmigen) using Alexa Fluor 594 as the secondary (1:2000, Molecular Probes). The percentage of Ki67+:GFP+ cells was determined. At least 100 cells were counted per slide.
Statistical Analysis
Statistical analysis was performed using a statistical software package (SPSS Inc., IL), and group differences were considered significant at p≤0.05. The day-10 MAP-2 process length data was analyzed using a two-way ANOVA [independent variables: hormone (estrogen vs vehicle control) and age (young vs reproductive senescence)], with post-hoc analyses for each process length category (0, 0.01-1.99, 2+). The cluster size for the day-4 astrocyte:neurosphere experiment was first analyzed using a two-way ANOVA for hormone treatment and cluster size separately for young adult- and reproductive senescent astrocyte co-cultures. Since no estrogen effects were detected, the data was grouped and a combined two-way ANOVA was performed, with post-hoc analyses for each cluster size. The BDNF/TrkB experiment, the day-6 astrocyte:NPC co-culture experiments, the astrocyte:PC12 co-culture experiments and the astrocyte:GFP+ NPC co-cultures experiments were analyzed using a two-way ANOVA with hormone and age as independent variables. All experiments were performed at least twice and a total of 3-6 replicates were used for each iteration.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Dr. R.C. Miranda for his generous gift of neural progenitor cells and Anita Mantri for her contributions to the project. This work was supported by NIH AG19515 and AG028303 to FS, the John Warren Brain Tumor foundation grant and P30-ES09106 from the National Institute of Environmental Health Sciences for support of the Confocal Imaging Lab.
REFERENCES
- Amateau SK, McCarthy MM. Sexual differentiation of astrocyte morphology in the developing rat preoptic area. J. Neuroendocrinol. 2002;14:904–910. doi: 10.1046/j.1365-2826.2002.00858.x. [DOI] [PubMed] [Google Scholar]
- Bailly K, Ridley AJ, Hall SM, Haworth SG. RhoA activation by hypoxia in pulmonary arterial smooth muscle cells is age and site specific. Circ. Res. 2004;94:1383–1391. doi: 10.1161/01.RES.0000128405.83582.2e. [DOI] [PubMed] [Google Scholar]
- Bake S, Sohrabji F. 17β-Estradiol differentially regulate blood-brain barrier permeability in young and aging female rats. Endocrinol. 2004;145:5471–5475. doi: 10.1210/en.2004-0984. [DOI] [PubMed] [Google Scholar]
- Banner LR, Moayeri NN, Patterson PH. Leukemia inhibitory factor is expressed in astrocytes following cortical brain injury. Exp. Neurol. 1997;147:1–9. doi: 10.1006/exnr.1997.6536. [DOI] [PubMed] [Google Scholar]
- Camarillo C, Kumar LS, Bake S, Sohrabji F, Miranda RC. Ethanol regulates angiogenic cytokines during neural development: evidence from an in vitro model of mitogenwithdrawal-induced cerebral cortical neuroepithelial differentiation. Alcohol Clin. Exp. Res. 2007;31:324–335. doi: 10.1111/j.1530-0277.2006.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaramello S, Dalmasso G, Bezin L, Marcel D, Jourdan F, Peretto P, Fasolo A, Marchis S. BDNF/TrkB interaction regulates migration of SVZ precursor cells via PI3-K and MAP-K signalling pathways. Eur. J. Neurosci. 2007;26:1780–1790. doi: 10.1111/j.1460-9568.2007.05818.x. [DOI] [PubMed] [Google Scholar]
- Dodd J, Jessell TM. Axon guidance and the patterning of neuronal projections in vertebrates. Science. 1988;242:692–699. doi: 10.1126/science.3055291. [DOI] [PubMed] [Google Scholar]
- Elmariah S, Oh E, Hughes E, Balice-Gordon R. Astrocytes Regulate Inhibitory Synapse Formation via Trk-Mediated Modulation of Postsynaptic GABAA Receptors. J Neurosci. 2005;25:3638–3650. doi: 10.1523/JNEUROSCI.3980-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A, Kristensen F, Dubs R, Gemsa D, Weber E. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J. Immunol. 1982;129:2413–2419. [PubMed] [Google Scholar]
- Fujita K, Lazarovici P, Guroff G. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ. Health Perspect. 1989;80:127–142. doi: 10.1289/ehp.8980127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Lesion-induced proliferation of neuronal progenitors in the dentate gyrus of the adult rat. Neurosci. 1997;80:427–436. doi: 10.1016/s0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- Greene L, Liem R, Shelanski M. Regulation of a high molecular weight microtubule-associated protein in PC12 cells by nerve growth factor. J. Cell Biol. 1983;96:76–83. doi: 10.1083/jcb.96.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti A, Frolichsthal-Schoeller P, Galli R, Parati EA, Cova L, Pagano SF, Bjornson CR, Vescovi AL. Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. J. Neurosci. 1999;19:3287–3297. doi: 10.1523/JNEUROSCI.19-09-03287.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedin U, Bottger BA, Luthman J, Johansson S, Thyberg J. A substrate of the cellattachment sequence of fibronectin (Arg-Gly-Asp-Ser) is sufficient to promote transition of arterial smooth muscle cells from a contractile to a synthetic phenotype. Dev. Biol. 1989;133:489–501. doi: 10.1016/0012-1606(89)90052-3. [DOI] [PubMed] [Google Scholar]
- Hösli E, Hösli W. Histochemical and electrophysiological evidence for estrogen receptors on cultured astrocytes: colocalization with cholinergic receptors. Int. J. Devl. Neurosci. 2000;18:101–111. doi: 10.1016/s0736-5748(99)00074-x. [DOI] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Jezierski M, Sohrabji F. Region- and peptide-specific regulation of the neurotrophins by estrogen. Mol. Brain Res. 2000;85:77–84. doi: 10.1016/s0169-328x(00)00244-8. [DOI] [PubMed] [Google Scholar]
- Jezierski M, Sohrabji F. Neurotrophin expression in the reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiol. Aging. 2001;22:309–319. doi: 10.1016/s0197-4580(00)00230-x. [DOI] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc. Natl. Acad. Sci. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim J, Park J, Lee S, Kim W, Yu Y, Kim K. Blood-neural barrier: intercellular communication at glio-vascular interface. J Biochem Mol Biol. 2006;39:339–345. doi: 10.5483/bmbrep.2006.39.4.339. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Park K, Rudkin BB, Dey BR, Sporn MB, Roberts AB. Nerve growth factor induces transcription of transforming growth factor- beta 1 through a specific promoter element in PC12 cells. J. Biol. Chem. 1994;269:3739–3744. [PubMed] [Google Scholar]
- Lewis DK, Johnson AB, Stoehlgren S, Harms A, Sohrabji F. Effects of estrogen receptor agonists on regulation of the inflammatory response in astrocytes from young adult and middle-aged female rats. J. Neuroimmunol. 2008;195:47–59. doi: 10.1016/j.jneuroim.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Ford MC, Lavik EB, Madri JA. Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: an in vitro study. J. Neurosci. Res. 2006;84:1656–1668. doi: 10.1002/jnr.21087. [DOI] [PubMed] [Google Scholar]
- Liesi P, Kaakkola S, Dahl D, Vaheri A. Laminin is induced in astrocytes of adult brain by injury. EMBO J. 1984;3:683–686. doi: 10.1002/j.1460-2075.1984.tb01867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P, Silver J. Is astrocyte laminin involved in axon guidance in the mammalian CNS? Dev. Biol. 1988;130:774–785. doi: 10.1016/0012-1606(88)90366-1. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing R, Sharp F. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J. Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Yokoyama M, Dreyfus CF, Black IB. NGF gene expression in actively growing brain glia. J. Neurosci. 1991;11:318–326. doi: 10.1523/JNEUROSCI.11-02-00318.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucius R, Sievers J. Postnatal retinal ganglion cells in vitro: protection against reactive oxygen species (ROS)-induced axonal degeneration by cocultured astrocytes. Brain Res. 1996;743:56–62. doi: 10.1016/s0006-8993(96)01029-3. [DOI] [PubMed] [Google Scholar]
- Magavi S, Leavitt B, Macklis J. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- Mokry J, Karbanova J. Foetal mouse neural stem cells give rise to ependymal cells in vitro. Folia Biol. (Praha) 2006;52:149–155. [PubMed] [Google Scholar]
- Muller HW, Junghans U, Kappler J. Astroglial neurotrophic and neurite-promoting factors. Pharm. Ther. 1995;65:1–18. doi: 10.1016/0163-7258(94)00047-7. [DOI] [PubMed] [Google Scholar]
- Nordell V, Scarborough M, Buchanan A, Sohrabji F. Differential effects of estrogen in the injured forebrain of young adult and reproductive senescent animals. Neurobiol. Aging. 2003;5798:1–11. doi: 10.1016/s0197-4580(02)00193-8. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J. Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parducz A, Perez J, Garcia-Segura L. Estradiol induces plasticity of GABAergic synapses in the hypothalamus. Neurosci. 1993;53:395–401. doi: 10.1016/0306-4522(93)90203-r. [DOI] [PubMed] [Google Scholar]
- Parish C, Finkelstein D, Tripanichkul W, Satoskar A, Drago J, Horne M. The Role of Interleukin-1, Interleukin-6, and Glia in Inducing Growth of Neuronal Terminal Arbors in Mice. J. Neurosci. 2002;22:8034–8041. doi: 10.1523/JNEUROSCI.22-18-08034.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Climent E, Guerri C. BDNF induces glutamate release in cerebrocortical nerve terminals and in cortical astrocytes. Neuroreport. 2001;12:2673–2677. doi: 10.1097/00001756-200108280-00017. [DOI] [PubMed] [Google Scholar]
- Pawlak J, Brito V, Kuppers E, Beyer C. Regulation of glutamate transporter GLAST and GLT-1 expression in astrocytes by estrogen. Mol. Brain Res. 2005;138:1–7. doi: 10.1016/j.molbrainres.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Pfrieger F, Barres B. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- Platania P, Seminara G, Aronica E, Troost D, Vincenza C, Angela S. 17beta-estradiol rescues spinal motoneurons from AMPA-induced toxicity: a role for glial cells. Neurobiol. Dis. 2005;20:461–470. doi: 10.1016/j.nbd.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Powell E, Meiners S, DiProspero N, Geller H. Mechanisms of astrocyte-directed neurite guidance. Cell Tissue Res. 1997;290:385–393. doi: 10.1007/s004410050945. [DOI] [PubMed] [Google Scholar]
- Prock TL, Miranda RC. Embryonic cerebral cortical progenitors are resistant to apoptosis, but increase expression of suicide receptor DISC-complex genes and suppress autophagy following ethanol exposure. Alcohol Clin. Exp. Res. 2007;31:694–703. doi: 10.1111/j.1530-0277.2007.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M. Stem and precursor cells in the nervous system. J Neurotrauma. 2004;21:415–427. doi: 10.1089/089771504323004566. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J. Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Riley C, Cope T, Buck C. CNS neurotrophins are biologically active and expressed by multiple cell types. J. Mol. Histol. 2004;35:771–783. doi: 10.1007/s10735-004-0778-9. [DOI] [PubMed] [Google Scholar]
- Robbins DS, Shirazi Y, Drysdale BE, Lieberman A, Shin HS, Shin ML. Production of cytotoxic factor for oligodendrocytes by stimulated astrocytes. J Immunol. 1987;139:2593–2597. [PubMed] [Google Scholar]
- Rozovsky I, Wei M, Morgan T, Finch C. Reversible age impairments in neurite outgrowth by manipulations of astrocytic GFAP. Neurobiol. Aging. 2005;26:705–715. doi: 10.1016/j.neurobiolaging.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Santillano DR, Kumar LS, Prock TL, Camarillo C, Tingling JD, Miranda RC. Ethanol induces cell-cycle activity and reduces stem cell diversity to alter both regenerative capacity and differentiation potential of cerebral cortical neuroepithelial precursors. BMC Neurosci. 2005;6:59. doi: 10.1186/1471-2202-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JP, Mishler K. Beta-adrenergic receptor regulation, through cyclic AMP, of nerve growth factor expression in rat cortical and cerebellar astrocytes. Cell Mol Neurobiol. 1990;10:447–457. doi: 10.1007/BF00711186. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. In vitro survival and differentiation of neurons derived from epidermal growth factor-responsive postnatal hippocampal stem cells: inducing effects of brain-derived neurotrophic factor. J. Neurobiol. 1998;35:395–425. doi: 10.1002/(sici)1097-4695(19980615)35:4<395::aid-neu7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Singh R, Wang B, Shirvaikar A, Khan S, Kamat S, Schelling JR, Konieczkowski M, Sedor JR. The IL-1 receptor and Rho directly associate to drive cell activation in inflammation. J. Clin. Invest. 1999;103:1561–1570. doi: 10.1172/JCI5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F. Estrogen: a neuroprotective or proinflammatory hormone? Emerging evidence from reproductive aging models. Ann. N. Y. Acad. Sci. 2005;1052:75–90. doi: 10.1196/annals.1347.006. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Peeples K, Marroquin O. Local and cortical effects of olfactory bulb lesions on trophic support and cholinergic function and their modulation by estrogen. J. Neurobiol. 2000;45:61–74. doi: 10.1002/1097-4695(20001105)45:2<61::aid-neu1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Gerhold LM, Bottner M, Rau SW, Dela Cruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS, Merchenthaler I, Gage FH, Wise PM. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J Comp. Neurol. 2007;500:1064–1075. doi: 10.1002/cne.21240. [DOI] [PubMed] [Google Scholar]
- Topalli I, Etgen AM. Insulin-like growth factor-I receptor and estrogen receptor crosstalk mediates hormone-induced neurite outgrowth in PC12 cells. Brain Res. 2004;1030:116–124. doi: 10.1016/j.brainres.2004.09.057. [DOI] [PubMed] [Google Scholar]
- Turner BA, Sparrow J, Cai B, Monroe J, Mikawa T, Hempstead BL. TrkB/BDNF signaling regulates photoreceptor progenitor cell fate decisions. Dev. Biol. 2006;299:455–465. doi: 10.1016/j.ydbio.2006.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian E, Sapperstein S, Christopherson K, Barres B. Control of synapse number by glia. Science. 2001;291:657–660. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Verderame M, Alcorta D, Egnor M, Smith K, Pollack R. Cytoskeletal F-actin patterns quantitated with fluorescein isothiocyanate-phalloidin in normal and transformed cells. PNAS. 1980;77:6624–6628. doi: 10.1073/pnas.77.11.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11:951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- Wu H, Friedman W, Dreyfus C. Differential regulation of neurotrophin expression in basal forebrain astroctyes by neuronal signals. J. Neurosci. 2004;76:76–85. doi: 10.1002/jnr.20060. [DOI] [PubMed] [Google Scholar]
- Wujek J, Akeson R. Extracellular matrix derived from astrocytes stimulates neuritic outgrowth from PC12 cells in vitro. Dev Brain Res. 1987;34:87–97. doi: 10.1016/0165-3806(87)90198-2. [DOI] [PubMed] [Google Scholar]
- Yamakuni T, Ozawa F, Hishinuma F, Kuwano R, Takahashi Y, Amano T. Expression of beta-nerve growth factor mRNA in rat glioma cells and astrocytes from rat brain. FEBS Lett. 1987;223:117–121. doi: 10.1016/0014-5793(87)80520-3. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Toya S. Neurotrophic activity in cytokine-activated astrocytes. Keio J Med. 1997;46:55–60. doi: 10.2302/kjm.46.55. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neurosci. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Trautmann K, Artelt M, Burnet M, Schluesener HJ. Bone morphogenetic protein-6 is expressed early by activated astrocytes in lesions of rat traumatic brain injury. Neurosci. 2006;138:47–53. doi: 10.1016/j.neuroscience.2005.11.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.