INTRODUCTION
Hypertension is defined as a systolic blood pressure (BP) of 140 mm Hg or higher, a diastolic BP of 90 mm Hg or higher, or the need to take an antihypertensive drug.1 The prevalence of hypertension in America has reached epidemic proportions; almost 72 million adults (nearly one in three) have hypertension.2
The implications of this prevalence are devastating. Hypertension is strongly associated with ischemic heart disease and stroke, and the risk of mortality from these conditions is doubled with every increase of 20 mm Hg in systolic BP or of 10 mm Hg in diastolic BP. Even small increases in BP (values up to 130–139/85–89 mm Hg), when compared to normotensive levels (120/80 mm Hg or below), result in a doubling of the relative risk of cardiovascular disease.1
CLASSIFICATION OF HYPERTENSION
As a result of the Seventh Report of the Joint National Committee in 2004, hypertension is now stratified into four classes: normal, pre-hypertension, stage 1, and stage 2 (Table 1). The addition of a pre-hypertension class resulted from evidence that patients with BP in the range of 130–139/80–89 mm Hg had twice the likelihood of later developing hypertension than patients with lower BP values.3
Table 1.
Classification of Hypertension
|
Systolic Blood Pressure (mm Hg) |
Diastolic Blood Pressure (mm Hg) |
|
|---|---|---|
| Normal | Below 120 | and below 80 |
| Pre-hypertension | 120–139 | or 80–89 |
| Stage 1 hypertension | 140–159 | or 90–99 |
| Stage 2 hypertension | 160 or higher | 100 or higher |
Data from Chobanian AV, Bakris GL, Black HR, et al. JAMA 2003;289(19):2560–2572.1
PATHOPHYSIOLOGY OF HYPERTENSION
The key components contributing to BP are (1) cardiac output, as determined by heart rate and the volume contained in the intravascular space, and (2) the degree of constriction, or resistance in the vascular walls. Most patients with elevated BP are considered to have “essential” hypertension, whereas a small percentage have secondary hypertension resulting from underlying renal or adrenal insufficiencies.
For patients with essential hypertension, increased BP typically results from increased peripheral resistance, whereas cardiac output remains normal. Increased peripheral resistance is a result of contraction of the smooth muscle in the small arterioles; prolonged contraction, in turn, results in a thickening of the vascular walls, yielding an irreversibly increased resistance.
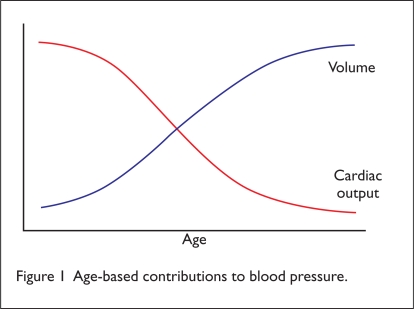
The pathophysiology of hypertension varies among age groups (Figure 1). Younger, healthier patients with elevated BP are more likely to have a normal-volume, high-cardiac-output form of hypertension. These patients usually benefit from medications that decrease cardiac output by decreasing heart rate and decrease peripheral resistance by causing vasodilation. As people age, cardiac output naturally declines, as does kidney function, leading to low-cardiac-output, high-volume hypertension. These patients obtain little benefit from drugs that lower heart rate, because they already have a reduced cardiac output. Medications that reduce volume are usually more effective.
Figure 1.
Age-based contributions to blood pressure.
All current antihypertensive therapies decrease BP by modifying one or more of the aforementioned components. Older treatments, such as beta blockers, alpha blockers and agonists, direct vasodilators, and calcium-channel blockers, were aimed primarily at reducing the heart rate and peripheral vascular resistance. Diuretics are used to reduce volume directly. Unfortunately, monotherapy with any of these agents is seldom sufficient, and patients typically require two or more antihypertensive medications from different classes before their BP can be considered controlled.
Most recent on the antihypertensive forefront are drugs targeted at the renin-angiotensin-aldosterone system (RAAS). These drugs are classified as angiotensin-converting enzyme (ACE)–inhibitors and angiotensin-receptor blockers (ARBs).
The Renin–Angiotensin–Aldosterone System
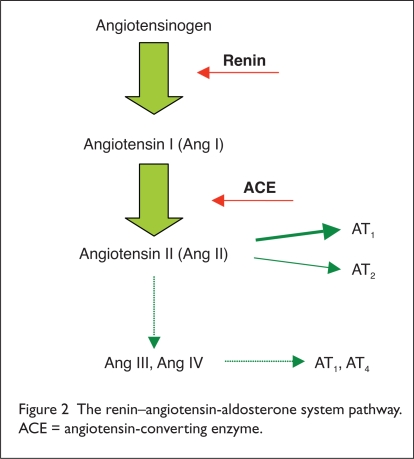
The importance of the RAAS is well established; the RAAS affects cardiovascular, cerebrovascular, and renal function. Drugs that inhibit the RAAS have proven ability to reduce BP, to prevent cardiovascular and cerebrovascular events, and to protect the kidneys.4–7. This pathway begins with angiotensinogen, which renin acts upon to form inactive angiotensin I (Ang I). Ang I is converted by ACE to form angiotensin II (Ang II) (Figure 2).
Figure 2.
The renin–angiotensin-aldosterone system pathway. ACE = angiotensin-converting enzyme.
Ang II then acts at its own receptors to constrict blood vessels, to increase production of aldosterone and antidiuretic hormone (ADH), and to increase the thirst reflex of the hypothalamus. Ang II also inhibits renin, providing negative feedback and completing the cycle.
We have gained more in-depth knowledge of this pathway in the last 10 years. For instance, we now know of at least three angiotensin receptors:8
The AT1 receptor is responsible for many of the effects associated with Ang II.
The AT2 receptor has an organ-protective role (i.e., it protects the brain from ischemia) and antagonizes the AT1 receptor.
The AT4 receptor affects renal tubular function and may have a role in memory.
In a phenomenon referred to as “ACE-escape,” the body is capable of forming Ang II even when ACE is inhibited. This occurs via non-ACE enzymes in the kidney, heart, and vascular tissues. This is where the primary benefit of directly blocking renin comes in; it appears to inhibit the entire pathway thereafter.
Unlike an ACE-inhibitor, a product called aliskiren (Tekturna, Rasilez, Novartis) does not prevent the breakdown of bradykinin.9,10 Increased levels of bradykinin cause the release of local vasodilators and may be part of the additional protective benefit of ACE-inhibitors. In addition, ARBs block only the AT1 receptor, leaving AT2 open to cause vasodilation when stimulated by circulating Ang II.11 The full effect of preventing the stimulation of all angiotensin receptors remains to be seen.
PHARMACOLOGY
Aliskiren is a highly selective competitive inhibitor of renin (Figure 3). As a result, the conversion of angiotensinogen to angiotensin I, as well as the later production of angiotensin II, is blocked. When Ang II levels are inhibited, the feedback loop is suppressed, resulting in further reductions in plasma renin concentrations.
Figure 3.
Chemical structure of aliskiren (Tekturna).
PHARMACOKINETICS
Aliskiren has low oral bioavailability (about 3%), and absorption is further decreased when it is taken with a high-fat meal; that is, the area-under-the-curve (AUC) concentration is decreased by 71% and the peak concentration (Cmax) is decreased by 85%. The extent of metabolism is unknown. Aliskiren is eliminated unchanged in the urine (25%) and in the feces via biliary excretion. The time to sustained antihypertensive effect is approximately two weeks (Table 2).
Table 2.
Pharmacokinetics of Aliskiren
| Parameter | Measure |
|---|---|
| Absorption | Poor (3%) |
| Distribution | 49.5% protein binding |
| Metabolism | Cytochrome P450 (CYP 3A4) |
| Elimination | Urine, feces |
| Time to peak concentration | 1–3 hours |
| Elimination half-life | 24 hours |
CLINICAL STUDIES
The approval of aliskiren by the Food and Drug Administration (FDA) was based on the efficacy shown in six placebo-controlled, eight-week clinical trials that included more than 2,000 patients with mild-to-moderate hypertension. Doses of aliskiren 150 mg and 300 mg were more effective in reducing BP than placebo, and the effect was maintained for up to one year. Safety was evaluated in almost 6,500 patients; 1,740 of these patients took aliskiren for longer than six months, and 1,250 were treated for longer than one year.10
Comparative Studies
As of this writing, few comparative studies of efficacy have been completed. One study showed that aliskiren doses of 75 mg, 150 mg, and 300 mg do not differ significantly from once-daily losartan potassium 100 mg (Cozaar, Merck). Aliskiren 150 mg is also as effective as irbesartan 150 mg (Avapro, Bristol-Myers Squibb/Sanofi). Aliskiren has been compared to lisinopril for tolerability, and when used alone or in combination with hydrochlorothiazide (HCTZ), it was determined to have similar antihypertensive effects and tolerability as lisinopril treatment (Zestril, AstraZeneca; Prinvil, Merck).12
Stanton et al.11
Losartan was studied in a four-week, multicenter, double-blind, randomized, active-comparator, parallel-group trial in 226 patients with mild-to-moderate hypertension. After a washout period of one to three weeks, patients receiving aliskiren experienced reductions in systolic BP of −0.4 (±11.7) mm Hg with 37.5 mg, −5.3 (±11.3) mm Hg with 75 mg, −8 (±11) mm Hg with 150 mg, and −11 (±11) with 300 mg. Statistical analysis using pairwise group comparison with analysis of covariance (ANCOVA) revealed no significant difference between aliskiren 75, 150, or 300 mg and losartan 100 mg (P > 0.05).
Gradman et al.13
The irbesartan study was an eight-week randomized, multicenter, double-blind, placebo-controlled, active-comparator trial of 652 patients. Patients with severe hypertension (BP ≥ 180/110 mm Hg), type-1 diabetes, poor glucose control, or a history of cardiovascular disease were excluded. After a two-week washout period, patients were randomly assigned to receive aliskiren 150, 300, or 600 mg; irbesartan 150 mg; or placebo. Once-daily aliskiren was as effective as irbesartan at 150 mg.
At doses of 300 mg and 600 mg, aliskiren lowered diastolic BP but not systolic BP more than irbesartan 150 mg (12.5/8.9 mm Hg ± 1.2 ± 0.7 mm Hg) (P = 0.05). Aliskiren 600 mg was no more effective in further reducing BP than the 300-mg dose.
Combination Therapies
Villamil et al.14
Aliskiren in combination with hydrochlorothiazide (HCTZ) produced greater BP reductions than aliskiren alone in an eight-week, international, double-blind, randomized, placebo-controlled, dose-ranging multifactorial trial of 2,752 patients. The patients were randomly assigned to receive aliskiren 75, 150, or 300 mg; HCTZ monotherapy 6.25, 12.5, or 25 mg; or aliskiren/HCTZ combinations (except 300/6.25 mg) in a factorial manner.
Aliskiren/HCTZ was more effective than placebo (P ≤ 0.0001) or any monotherapy regimen (P < 0.05) in reducing BP, excluding the combinations of aliskiren/ HCTZ 150/6.25 mg and 75/12.5 mg. Treatment was well tolerated.
The most frequently reported side effects were head ache (7.2%) and nasopharyngitis (3.8%).
O’Brien et al.15
Three individual open-label, nonrandomized studies examined aliskiren in combination with HCTZ, ramipril (Altace, King), and irbesartan.
In the aliskiren/diuretic study, 23 patients received once-daily oral aliskiren 150 mg for three weeks. HCTZ 25 mg once daily was added to the aliskiren in patients (17 out of 23) whose daytime ambulatory BP remained greater than or equal to 130/85 mm Hg. This addition resulted in an overall reduction in systolic BP from a baseline value of 150.6 ± 8.9 mm Hg to 133.8 ± 7 mm Hg (P = 0.0007).
In the ACE inhibitor/aliskiren (n = 21) and ARB/aliskiren (n = 23) combination studies, patients received a three-week treatment of oral ramipril 5 mg once daily or irbesartan 150 mg once daily. Aliskiren 75 mg was then given for an additional three-week period. Finally, the aliskiren dose was increased to 150 mg daily for three weeks.
The addition of aliskiren 75 mg to ramipril 5 mg yielded a decrease in systolic BP from 143.4 ± 11.1 mm Hg to 139.4 ± 12.7 mm Hg (P = 0.03). The dose increase of aliskiren to 150 mg resulted in a further reduction to 136.4 ± 10.9 mm Hg (P = 0.0006). In the irbesartan trial, the addition of aliskiren 75 mg did not yield a significant decrease in BP.
Pool et al.16
Another eight-week multicenter, randomized, double-blinded, placebo-controlled trial investigated aliskiren monotherapy as well as combination therapy with valsartan (Diovan, Novartis) in 1,123 patients with mild-to-moderate hypertension. After a tapering off of current antihypertensive treatment and a three- to four-week placebo run-in period, patients received either once-daily placebo, aliskiren monotherapy 75, 150, or 300 mg; valsartan monotherapy 80, 160, or 320 mg; a combination of aliskiren/valsartan 75/80 mg; 50/160 mg, or 300/320 mg; or a combination of valsartan/HCTZ 160/12.5 mg.
Compared with placebo, aliskiren 300 mg monotherapy significantly reduced mean systolic BP (P < 0.0001) and diastolic BP (P = 0.0001).
The aliskiren/valsartan combination significantly decreased mean diastolic BP and systolic BP relative to placebo at all dose combinations: aliskiren 75 mg/valsartan 80 mg lowered diastolic BP by 3.25 ± 1.21 mm Hg (placebo-corrected mean, P < 0.01) and systolic BP by 4.49 ± 1.87 mm Hg (placebo-corrected mean, P < 0.05), compared with placebo.
Aliskiren 300 mg/valsartan 320 mg resulted in a placebo-corrected mean lowering in both diastolic BP and systolic BP of 4.34 ± 1.23 mm Hg and 8.07 ± 1.90 mm Hg (P < 0.001 for both measures versus placebo), respectively. There was no significant reduction in either diastolic or systolic BP with valsartan 160 mg/HCTZ 12.5 mg, compared with aliskiren 150 mg/valsartan 160 mg or aliskiren 300 mg/valsartan 320-mg combinations.
ADVERSE DRUG EVENTS
In clinical trials, 2.2% of patients discontinued therapy because of adverse drug events; this figure was less than the 3.5% of those receiving placebo. Angioedema of the face, extremities, lips, tongue, glottis or larynx has been reported and may occur at any time during treatment. Hypotension was rarely experienced with aliskiren alone (0.1% of patients) or with aliskiren combination therapy (in fewer than 1% of patients). Hyperkalemia, defined by a potassium level above 5.5 mEq/L, was rare with aliskiren monotherapy (0.9% with aliskiren vs. 0.9% with placebo); however, hyperkalemia occurred more frequently when aliskiren was combined with an ACE-inhibitor (5.5%).
Dose-related gastrointestinal (GI) side effects were reported during clinical trials. Most of these events were mild and did not result in discontinuation of therapy. The most common GI side effect was diarrhea, in 2.3% of patients. Cough occurred slightly more often in patients receiving aliskiren (1.1%) than placebo (0.6%). In trials comparing aliskiren with an ACE-inhibitor, however, cough occurred one-third to one-half as often in the aliskiren arm. Two patients experienced a single episode of tonic–clonic seizure with a loss of consciousness, although one patient had had a history of seizures. Aliskiren was discontinued and was not re-introduced.
Other adverse events occurring at rates higher than 1% included headache, nasopharyngitis, dizziness, fatigue, upper respiratory tract infection, and back pain. These events were experienced at the same rate or at greater rates in the placebo arms.
DRUG INTERACTIONS
Aliskiren is metabolized by cytochrome P450 (CYP 3A4). Concurrent administration with agents that are CYP 3A4 substrates, such as atorvastatin calcium (Lipitor, Pfizer) and CYP 3A4 inhibitors such as ketoconazole (Nizoral, PriCara), may increase aliskiren plasma concentrations. Patients should be monitored for an increased incidence of adverse events such as diarrhea, dyspepsia, and hypotension.
The concurrent administration of aliskiren and furosemide (Lasix, Sanofi-Aventis) resulted in a substantial decrease in furosemide’s AUC and peak concentrations; therefore, patients taking both agents should be monitored for a decreased effect of furosemide.10
SPECIAL POPULATIONS
Aliskiren has not been studied in patients younger than 18 years of age. In patients older than 65 years of age, the AUC concentration is increased, but dose adjustments are not necessary. No dose adjustment is required in patients with mild-to-moderate renal or hepatic impairment. Aliskiren has not been evaluated in patients with more severe renal impairment (a creatinine clearance below 30 mL/minute).
PRECAUTIONS AND WARNINGS
Aliskiren is classified as a Pregnancy Category C agent for the first trimester and as a Category D drug for the second and third trimesters. It has not been evaluated in pregnant women, but based on evidence from other drugs that influence the RAAS, aliskiren should be discontinued as soon as pregnancy is detected.
Drugs that act on the RAAS have caused fetal and neonatal mortality and morbidity. Reports of fetal and neonatal morbidity following the use of RAAS agents in the second and third semesters include hypotension, neonatal skull hypoplasia, anuria, reversible or irreversible renal failure, oligohydramnios, and death.
The use of ACE-inhibitors in the first trimester has also been linked to birth defects. Risks and benefits should be carefully weighed for pregnant women; if no alternative treatment is available, the fetus should be closely monitored.
Aliskiren is excreted in the breast milk of rats, but it has not been evaluated in humans. Women who plan to nurse should discontinue their use of the drug.10
CONTRAINDICATIONS
Specific contraindications have not been determined.
DOSING AND ADMINISTRATION
The starting dose of aliskiren is 150 mg by mouth once daily. If desired BP reduction is not attained, the dose may be increased to 300 mg once daily. No additional BP-lowering effects are observed at doses higher than 300 mg daily. The antihypertensive effect of aliskiren is usually evident in two weeks.
Aliskiren may be taken without regard to meals, but it is advisable to take it at the same time each day with the same meal. It is best for patients to avoid high-fat meals while they are taking aliskiren.
In January 2008, the FDA approved aliskeren with HCTZ (Tekturna HCT); it is available in dosage strengths of 150 mg/12.5 mg, 150 mg/25 mg, 300 mg/12.5 mg, and 300 mg/25 mg.
COST
As of January 2008, the average wholesale price (AWP) for a one-month supply of aliskiren 150 mg was $69.99. The AWP for 300 mg was $87.99.17
CONCLUSION
Aliskiren is suggested as a new approach to reducing BP via mediation of the RAAS pathway. The direct inhibition of renin eliminates the ACE escape phenomenon, because it inhibits angiotensin I, thereby preventing the need for ACE.
Aliskiren offers another option for antihypertensive treatment, and it may be useful in patients who are unable to tolerate ACE-inhibitors. Although no data are available in terms of the drug’s potential cardiac and renal protective effects as a renin inhibitor, on the basis of evidence from other drugs affecting the RAAS, aliskiren offers promise.
Footnotes
Disclosure: The authors have no commercial or industrial relationships to disclose in regard to this article.
REFERENCES
- 1.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics–2007 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 3.Vasan RS, Larson MG, Leip EP, et al. Assessment of frequency of progression to hypertension in nonhypertensive participants in the Framingham Heart Study: A cohort study. Lancet. 2001;358:1682–1686. doi: 10.1016/S0140-6736(01)06710-1. [DOI] [PubMed] [Google Scholar]
- 4.The European Trial on Reduction of Cardiac Events with Perindopril in Stable Coronary Artery Disease Investigators Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: Randomised, double-blind, placebo-controlled, multicentre trial (EUROPA study) Lancet. 2003;362:782–788. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 5.Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint Reduction in Hypertension Sudy (LIFE): A randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 7.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 8.Schmieder RE, Hilgers KF, Schlaich MP, et al. Renin–angiotensin system and cardiovascular risk. Lancet. 2007;369:1208–1219. doi: 10.1016/S0140-6736(07)60242-6. [DOI] [PubMed] [Google Scholar]
- 9.Azizi M, Webb R, Nussberger J, et al. Renin inhibition with aliskiren: Where are we now, and where are we going? J Hypertens. 2006;24(2):243–256. doi: 10.1097/01.hjh.0000202812.72341.99. [DOI] [PubMed] [Google Scholar]
- 10.East Hanover, NJ: Novartis; 2007. Product information: Tekturna tablets (aliskiren) [Google Scholar]
- 11.Stanton A, Jensen C, Nussberger J, et al. Blood pressure lowering in essential hypertension with an oral renin inhibitor, aliskiren. Hypertension. 2003;42(6):1137–1143. doi: 10.1161/01.HYP.0000101688.17370.87. [DOI] [PubMed] [Google Scholar]
- 12.Strasser RH, Puig JG, Farsang C, et al. A comparison of the tolerability of the direct renin inhibitor aliskiren and lisinopril in patients with severe hypertension. J Hum Hypertens. 2007;21(10):780–787. doi: 10.1038/sj.jhh.1002220. [DOI] [PubMed] [Google Scholar]
- 13.Gradman AH, Schmieder RE, Lins RL, et al. Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation. 2005;111:1012–1018. doi: 10.1161/01.CIR.0000156466.02908.ED. [DOI] [PubMed] [Google Scholar]
- 14.Villamil A, Chrysant SG, Calhoun D, et al. Renin inhibition with aliskiren provides additive antihypertensive efficacy when used in combination with hydrochlorothiazide. J Hypertens. 2007;25(1):217–226. doi: 10.1097/HJH.0b013e3280103a6b. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien E, Barton J, Nussberger J, et al. Aliskiren reduces blood pressure and suppresses plasma renin activity in combination with a thiazide diuretic, an angiotensin-converting enzyme inhibitor, or an angiotensin receptor blocker. Hypertension. 2007;49(2):276–284. doi: 10.1161/01.HYP.0000253780.36691.4f. [DOI] [PubMed] [Google Scholar]
- 16.Pool JL, Schmieder RE, Azizi M, et al. Aliskiren, an orally effective renin inhibitor, provides antihypertensive efficacy alone and in combination with valsartan. Am J Hypertens. 2007;20(1):11–20. doi: 10.1016/j.amjhyper.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Pharmacy Society of Wisconsin Available at: www.pswi.org/irx/Tekturna.pdf. Accessed December 21, 2007.