Educational Objectives.
After reviewing this article, readers should be able to:
▪ Define the basic hematological defect in sickle cell disease.
▪ Identify the mechanisms of action and adverse events associated with standard treatment options.
▪ Review the protocol for preventing stroke and infection in patients with sickle cell disease.
▪ Identify treatment options currently under investigation.
Introduction
Sickle cell disease (SCD) is the most common inherited blood disorder in the U.S., affecting about 72,000 Americans. It is also the most common inherited disease among African-Americans and affects approximately one out of every 500 newborns. People of other races are also affected by SCD, with a rate of one of every 1,000 to 1,400 Hispanic-American births.1 A significant prevalence of the mutation responsible for sickle cell has been reported among other ethnic groups such as those native to Italy, Greece, Turkey, Saudi Arabia, India, Pakistan, Bangladesh, China, and Cyprus.2
In 2004, 83,149 hospitalizations were attributable to SCD in the U.S., at a cost of almost $488 million.3 Episodes of pain, chronic hemolytic anemia, and severe infections are some of the common characteristics of this disease that begin in early childhood.4 Management of SCD is geared toward preventing complications and reducing the number of sickle cell crises.
Pathogenesis
Sickle cell disease is characterized by a structural abnormality in the beta-globin chain of the hemoglobin molecule within the red blood cells (RBCs). The sickle mutation is a single base change (GAT → GTT) in the sixth codon of exon-1 of the beta-globin gene on chromosome 11. This change leads to the synthesis of the beta-globin polypeptide of the hemoglobin molecule. This mutation causes the replacement of the normal glutamic acid with valine acid, thus resulting in the formation of the sickle cell hemoglobin (HbS). This hydrophobic aminoacid substitution causes the hemoglobin to take on a “sickle” shape when in a deoxygenated state.
The ability of these sickled cells to adapt to their surroundings is impaired, especially in the microvasculature. These cells hemolyze prematurely, accounting for the chronic anemia frequently encountered by patients with SCD.5 The paucity of sickled cells in newborns with SCD led to the discovery that fetal hemoglobin (HgF) reduces the severity of SCD by preventing the formation of the hemoglobin S polymer.6
Fever, dehydration, hypoxia, acidosis, stress, and a cold environment may precipitate sickling, although a precursor event is not always identified.7,8 The pathophysiology of SCD is considerably complex, involving abnormalities of hemoglobin, the RBC’s membrane, erythrocyte hydration, the endothelium, vascular tone, inflammatory responses, leukocytes, and coagulation. This forceful combination of factors results in cell interactions, generating hemolysis and micro-vascular obstruction, ultimately leading to damage of nearly all organ systems.9
Risk Factors
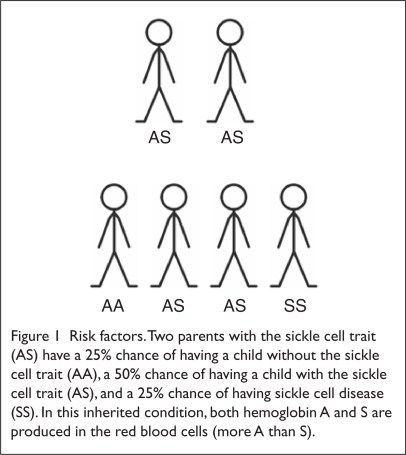
Two million people worldwide are carriers of or have the sickle cell trait. Carriers are usually asymptomatic and have a low percentage of sickle hemoglobin (HbS). Two parents who are carriers can both pass on the sickle cell trait to their offspring, resulting in SCD. There is a 50% chance with each pregnancy for the child of two sickle cell carriers to be born with the sickle cell trait, and there is a 25% chance for the child to be born with SCD (Figure 1).
Figure 1.
Risk factors. Two parents with the sickle cell trait (AS) have a 25% chance of having a child without the sickle cell trait (AA), a 50% chance of having a child with the sickle cell trait (AS), and a 25% chance of having sickle cell disease (SS). In this inherited condition, both hemoglobin A and S are produced in the red blood cells (more A than S).
Diagnosis
Screenings for SCD at birth are now performed in most states in the U.S. The presence of hemoglobin S (HbS) with elevated fetal hemoglobin (HbF) and the absence of hemoglobin A indicate either sickle cell anemia or beta thalassemia. It is imperative that sickle cell anemia be detected early, because preventive care must begin by the time a child is two months of age to improve survival.
The diagnosis of SCD is usually confirmed by electrophoresis. The sickle cell trait is also identified in screenings of newborns, who have a much lower percentage of hemoglobin S than other patients with SCD.
Treatment Options
The only cure for SCD is bone marrow transplantation, which usually necessitates a human lymphocyte antigen (HLA)-identical family member donor. There is an 85% disease-free survival rate, with a 7% transplant-related mortality rate and a 9% graft failure rate.10 Barriers to the widespread use of bone marrow transplantation in patients with SCD include a lack of suitable bone marrow donors and the need to identify patients with an adequate risk-to-benefit ratio. For these reasons, drug therapy for SCD continues to be the primary mode of disease management, focusing on decreasing the complications of this disease (Table 1).
Table 1.
Prophylaxis of Complications from Sickle Cell Disease
| Complication | ProphylacticTherapy |
|---|---|
| Streptococcus pneumoniae sepsis |
Newborns to 3 years:
|
| Bone marrow aplasia and megaloblastic erythropoiesis | 1 mg folic acid daily |
| Stroke | Exchange transfusions |
| Pain episodes | Hydroxyurea
Initiation of treatment:
|
Hydroxyurea
Hydroxyurea, the only agent that the Food and Drug Administration (FDA) has approved for the management of SCD, is indicated for sickle cell patients who have had at least three painful crises in the previous 12 months. Hydroxyurea prevents the complications of SCD by increasing HbF and total hemoglobin concentrations, by decreasing the adhesion of sickled cells to the endothelium in vitro, and by increasing polymerization time.11
The Multicenter Study of Hydroxyurea in Sickle Cell Anemia (MSH) was a randomized, double-blind, placebo-controlled clinical trial in which adult patients were assigned to receive hydroxyurea and placebo. Treatment with hydroxyurea resulted in a 44% difference in the median annual rate of painful crises: 2.5 crises per year in the hydroxyurea arm vs. 4.5 crises per year in the placebo arm. Because hydroxyurea reduced the frequency of episodes of pain, acute chest syndrome, and the need for blood transfusion, the study was stopped four months early.12
A nine-year follow-up of the participants in this trial showed a 40% reduction in mortality rates for patients taking hydroxyurea.13 In small clinical trials of hydroxyurea in children with SCD, the agent was found to be safe and efficacious, and these effects were sustained in long-term trials.14–16
Hydroxyurea’s potential benefits should be weighed against the risks of bone marrow suppression, which is reversible when the drug is discontinued. Complete blood counts are recommended every four to eight weeks after the hydroxyurea dose is stabilized.
Pain Medications
Pain, which is usually attributed to ischemia from the obstruction of blood vessels by sickled cells, is the most common symptom of SCD. It can be acute or chronic, and it varies among individuals in its frequency and intensity. Pain is the primary cause of hospitalization in patients with SCD, which is why proper management of pain in this population is essential.17
The general approach to the treatment of pain is to identify the causes, which include infection, extreme temperature, and emotional stress. Usually, however, there is not an identifiable cause, and the pain crisis occurs without warning. Milder pain is treated with general nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen and ketorolac tromethamine (Toradol, Roche) or analgesics like acetaminophen and tramadol (Ultram, PriCara).
Severe painful episodes should be treated with parenteral opiates at frequent intervals, not on an as-needed basis. In a study comparing intermittent intravenous (IV) injections and patient-controlled analgesia (PCA), PCA reduced the length of stay and was as efficacious as the injections.18
Acute episodes of pain may also be treated with IV hydration, and milder episodes may be treated with oral hydration—regardless of the patient’s state of hydration—to slow or stop the sickling process, which can be promoted by dehydration.19 After the pain has diminished and has tapered off, an oral analgesic can be given.
The opiate drugs that have been studied to treat SCD include morphine, hydromorphone, fentanyl (Duragesic, PriCara), and codeine-related agents.20 Morphine is considered the drug of choice for the treatment of acute sickle cell pain (Table 2), whereas meperidine (Demerol, Sanofi-Synthelabo) should be avoided because of the increased risk of seizures in patients with renal dysfunction, which can occur in patients with SCD.
Table 2.
Treatment Options for Complications from Acute Sickle Cell Disease
| Complication | Therapy | |
|---|---|---|
| Pain | Mild to moderate | Codeine with acetaminophen or aspirin
|
| Moderate to severe | Morphine sulfate immediate-release (MSIR)
|
|
| Infection | Fever without a source (rule out sepsis) |
Empirical therapy coverage for: Streptococcus pneumoniae, Salmonella, Haemophilus influenzae, gram-negative enterics |
| Meningitis | Streptococcus pneumoniae, Neisseria meningitides, H. influenzae | |
| Chest syndrome | S. pneumoniae, Legionella, Mycoplasma pneumoniae, respiratory syncytial virus, Chlamydia pneumoniae | |
| Osteomyelitis/septic arthritis | Salmonella, Staphylococcus aureus, S. pneumoniae | |
| Urinary tract infection | Escherichia coli, other gram-negative enterics |
IV = intravenously.
From National Institutes of Health. The Management of Sickle Cell Disease, 4th ed. revised, June 2002.22
Infection Prophylaxis
Patients with SCD are at an increased susceptibility to pneumococcal infection primarily because of the development of functional asplenia, which can occur at as early as six months of age.21 In the absence of a functional spleen, the organ can no longer serve its immunological functions of clearing bacteria from the blood and synthesizing antibodies, circumstances that can lead to an increased frequency of infection.22
In the PRophylaxis with Oral Penicillin in children with Sickle Cell Anemia (PROPS) study, when infants received prophylactic penicillin between three months and three years of age, pneumococcal infection rates decreased by 84%.23 PROPS II evaluated the consequences of discontinuing penicillin prophylaxis at five years of age, and there was no difference in the rates of infection in the penicillin arm, compared with the placebo arm (relative risk = 0.5).21
On the basis of the PROPS and PROPS II results, children younger than three years of age should receive 125 mg of penicillin orally twice daily, and children between three and five years of age should receive 250 mg of penicillin orally twice a day. For patients who are allergic to penicillin, erythromycin ethyl succinate (e.g., EryPed, Abbott) 20 mg/kg, divided into two daily doses, can provide adequate prophylaxis.24 Problems with penicillin prophylaxis include compliance, drug cost, patient tolerance, and resistant strains of micro-organisms.25
Immunizations in children with SCD should include all regular vaccines, with the addition of the flu vaccine yearly after six months of age; pneumococcal vaccine at two and five years of age; and, possibly, meningococcal vaccine.26
Stroke Prevention
The prevalence of stroke in patients with SCD was reported to be 11% before routine screening with transcranial Doppler ultrasonography (TCD) and monthly transfusions for primary stroke prevention in children at risk. Not all children with SCD are at equal risk for stroke; Doppler ultrasound should be performed to assess the patient’s blood flow velocity, because high blood flow velocity has been correlated with a subsequent stroke in children with SCD.27
In a randomized study of children with SCD, patients who had abnormal results (time-averaged mean blood flow exceeding 200 cm/second) received standard-of-care or transfusions with a target hemoglobin S concentration of less than 30%. The study was terminated early, because there was a 92% difference in cerebrovascular accidents (CVAs) between the standard-of-care group and the transfusion group.27
It is still not known when, if ever, it is optimal to discontinue prophylactic transfusions in patients with abnormal TCD results. The Optimal Primary Stroke Prevention in Sickle Cell Anemia (STOP 2) Trial Investigators randomly assigned children who were receiving prophylactic transfusions to either stop receiving them after 30 months or to continue with them. The endpoints evaluated were abnormal TCD findings or stroke; neither event occurred in the arm that continued transfusion therapy. The arm that discontinued therapy had 14 abnormal TCD results and two strokes within 4.5 ± 2.6 months of stopping transfusions.28 Children with abnormal TCD outcomes had a hydroxyurea-related decrease in TCD flow vel ocity from 216 ± 14 to 173 ± 31 cm/second, suggesting the possible benefit of switching to hydroxyurea for stroke prevention.29
With regular transfusions comes the burden of iron overload; until recently in the U.S., this condition was treated only with deferoxamine (Desferal, Novartis), which was administered by subcutaneous or IV infusion. A new once-daily oral chelator, deferasirox (Exjade, Novartis), when compared with deferoxamine in patients with SCD and iron overload, was found to be safe and effective in these patients.30
At the time of this writing, a study evaluating the role of aspirin in the stroke prophylaxis in children was recruiting participants.31
Folic Acid and Anemia
In patients with SCD, the RBC count is lower than normal because sickled cells usually die after 10 to 20 days, in contrast to 120 days for normal RBCs. Because of high cell turnover, folate stores are often depleted.
Folic acid replenishes the depleted folate stores necessary for erythropoiesis. Folic acid supplementation is well established in the treatment of chronic hemolytic anemia. Although it is proposed that folate in anemia raises hemoglobin levels and helps provide a healthy reticulocyte response,32 the use of folic acid in patients with SCD is not well supported by the primary literature. One prospective, randomized study, published in 1983, found no “striking effects” of folic acid supplementation in sickle cell anemia on the hematological profile or on growth in children with SCD who received this nutrient.33
In another study that measured folate stores in children with SCD who were not receiving folic acid, folate levels were found to be adequate.34 The National Heart, Lung, and Blood Institute guideline for SCD does recommend folic acid supplementation for patients with sickle cell anemia at the dose of 1 mg/day.21
Pulmonary Hypertension
Some studies have suggested that pulmonary hypertension (PHTN) is common in patients with sickle cell anemia. A prospective study of sickle cell anemia reported a 31% prevalence of PHTN in children 10 years of age and older.35 There is emerging evidence on the treatment of traditional PHTN with prostanoids such as epoprostenol (Flolan, GlaxoSmithKline) and phosphodiesterase-5 inhibitors such as sildenafil citrate (Revatio, Pfizer). The FDA approved sildenafil for the treatment of PHTN in 2005.
A study conducted at the Howard University Center for Sickle Cell Disease tested PHTN reversibility by giving prostacyclin infusions to eight patients with PHTN during cardiac catheterization.36 Pulmonary pressures were significantly reduced in six of the eight patients who received the infusions. An open-label, uncontrolled pilot trial of 12 patients with SCD and PHTN found that sildenafil improved exercise capacity and PHTN.37
Priapism
Priapism, possibly resulting from decreased blood flow to the corpus cavernosum, is a known problem in men with SCD.38 Usually precipitated by sexual activity, priapism has a prevalence of between 6% and 38% in SCD patients.39 There are two kinds of priapism: (1) “stuttering,” defined as repeated short episodes, with each episode lasting for between 30 minutes and three hours, and (2) “prolonged,” defined as episodes lasting for more than three hours.
Acute episodes of priapism lasting for more than two hours should be treated in the emergency department. Penile aspiration, followed by irrigation of the corpus cavernosum with a sympathomimetic agent, should be initiated if detumescence does not occur an hour after the patient’s arrival. Oral terbutaline (Brethine, aaiPharma) and pseudoephedrine (e.g., Sudafed, Pfizer) have demonstrated efficacy in the treatment of priapism.40
Investigational Treatment Options
Niprisan
As a phytopharmaceutical derived from a plant in its original state, Niprisan was developed by the Nigerian National Institute for Pharmaceutical Research and Development. This anti-sickling agent was granted orphan drug status by the FDA in 2003 under the name Hemoxin, made by Xechem International. Its anti-sickling properties are attributed to its ability to prolong or delay the time to polymerization of deoxy-Hb S.41
A phase 2, double-blind, placebo-controlled, six-month randomized crossover trial revealed that the frequency of sickle cell pain crisis in the Niprisan arm was significantly reduced.42 A phase 3 clinical trial is under way, and an investigational New Drug Application (NDA) is being prepared by Xechem for submission to the FDA.
Nitric Oxide
Nitric oxide (NO), a potent vasodilator thought to be deficient in patients with SCD, has been suggested as a therapeutic option.43 Its proposed mechanism of action is its ability to limit the sickling of RBCs by preventing them from sticking to vessel walls or by dilating peripheral blood vessels.44
Studies by Head et al. suggested that NO increases the hemoglobin oxygen affinity in homozygous HbS (SS) erythrocytes either when RBCs are exposed to NO in vitro or during NO inhalation in low concentrations in vivo.45 It is not clear how low concentrations of NO enhance oxygen affinity of erythrocytes in SCD, compared with normal erythrocytes, but research suggests that NO modifies HbS, thereby reducing polymerization and increasing oxygen affinity in sickled RBCs.
l-Arginine
The amino acid l-arginine is a required substrate for nitric acid synthesis by endothelial cells, platelets, and other cells. In addition to having a deficiency of NO, adults with SCD sometimes have significantly diminished arginine levels. This arginine deficiency may be the cause of PHTN in sickle cell patients; therefore, the infusion of l-arginine has been shown to reduce vascular resistance and improve blood oxygenation in infants with PHTN.46
In one study, l-arginine supplementation improved pulmonary artery pressures and hemodynamics in primary and secondary hypertension within one week of therapy. Overall, arginine was well tolerated with minimal adverse effects.47
l-Glutamine
l-Glutamine is a precursor for nicotinamide adenine di-nucleotide (NAD), which is deficient in patients with sickle cell anemia. A deficiency of glutamine may result in the possibility of skeletal muscle wasting, immunosuppression, and impaired wound healing.48 It is suggested that the deficiency in glutamine is a result of the rate of active transport of glutamate (a by-product of glutamine) in sickled RBCs.
Oral l-glutamine therapy can be beneficial in patients with sickle cell anemia by increasing the activity of NAD synthesis, thus countering the oxidant-dependent pathophysiology of sickled RBCs.49 In a four-week study of seven patients with sickle cell anemia who were 19 to 60 years of age, Niihara and colleagues found that given 30 g of oral l-glutamine for four weeks brought about a significant increase in NADH (the reduced form of NAD) and NAD redox potential (the ratio of NADH to NAD+ plus NADH).
These participants also experienced an overall improvement in energy, accompanied by an increased activity level and various degrees of decreases in chronic pain. Of the seven patients who participated in the study, six reported a decrease in daily narcotic usage. Overall, the oral administration of l-glutamine has shown a consistent and significant increase in red blood cell NADH and was well tolerated by the patients with no adverse effects.50
Magnesium
De Franceschi et al. suggested that a possible therapeutic strategy for SCD was based on reducing the cellular concentration of sickle cell hemoglobin (HbS) by preventing erythrocyte dehydration.51 The major determinant of sickle cell dehydration is the potassium chloride transporter, which is inhibited by increasing the erythrocyte magnesium content. Oral administration of magnesium showed considerable increases in sickle erythrocyte magnesium and potassium content and reductions in the number of dense sickle erythrocytes. In addition, the erythrocyte potassium chloride co-transport was reduced significantly, and the absolute reticulocyte count and the number of immature reticulocytes were greatly reduced.
The authors concluded that oral magnesium reduced the number of dense erythrocytes and improved the erythrocyte membrane transport abnormalities of patients with SCD, thus reducing erythrocyte dehydration. Transient diarrhea was the only significant side effect and was noted in one of 10 patients in the study.
Conclusion
Although bone marrow transplantation can cure SCD, it is an impractical solution for most Third World countries, which have a high disease burden. Even in the U.S., bone marrow transplantation is limited by the availability of donors. Pharmacological therapies are effective at reducing complications of SCD and are safe and easily administered, and they continue to prolong the life expectancy of patients.
Footnotes
Conflict of Interest (COI) Statement
The authors have no relationships to disclose. The article contains a discussion of off-label use. The content of this article has been reviewed under Jefferson’s COI policy.
References
- 1.American Stroke Association Sickle cell disease. Available at www.strokeassociation.org/presenter.jhtml?identifier=3034962. Accessed August 10, 2007.
- 2.Nietert PJ, Silverstein MD, Abboud MR. Sickle cell anemia: Epidemiology and cost of illness. Pharmacoeconomics. 2002;20:357–366. doi: 10.2165/00019053-200220060-00001. [DOI] [PubMed] [Google Scholar]
- 3.Steiner C, Miller J.Sickle cell disease patients in U.S. hospitals, 2004Healthcare Cost and Utilization Project (HCUP) Statistical Brief No. 21, December 2006.Rockville, Md: Agency for Health-care Research and Quality; Available at: www.hcup-us.ahrq.gov/reports/statbriefs/sb21.pdf. Accessed 2/24/2008. [PubMed] [Google Scholar]
- 4.Anemia, sickle cell. National Library of Medicine. Available at: www.ncbi.nlm.nih.gov/books/bv.fcgi?call=bv.View.ShowSection&rid=gnd.section.98 Accessed October 8, 2007.
- 5.Sickle cell anemiaACP PIER & AHFS DI Essentials,2007. American College of Physicians (Philadelphia, Pa.), Physician’s Information and Education Resource and American Hospital Formulary Service, Drug Information. Available at: http:/online.statref.com/titles/titleinfopage.aspx?titleid=92 Accessed December 14, 2007.
- 6.A brief history of sickle cell diseaseHarvard University Information Center for Sickle Cell and Thalassemic Disorders. Available at: http://sickle.bwh.harvard.edu/scd_history.html Accessed December 17, 2007.
- 7.Fosdal MB, Alexandrov AW. Events of hospitalization among children with sickle cell disease. J Pediatr Nurs. 2007;22:342–346. doi: 10.1016/j.pedn.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Driscoll CM. Sickle cell disease. Pediatr Rev. 2007;28:259–267. doi: 10.1542/pir.28-7-259. [DOI] [PubMed] [Google Scholar]
- 9.Raphael RI, Vichinsky EP. Pathophysiology and treatment of sickle cell disease. Clin Adv Hematol Oncol. 2005;3:492–505. [PubMed] [Google Scholar]
- 10.Iannone R, Ohene-Frempong K, Fuchs EJ, et al. Bone marrow transplantation for sickle cell anemia: Progress and prospects. Pediatr Blood Cancer. 2005;44:436–440. doi: 10.1002/pbc.20169. [DOI] [PubMed] [Google Scholar]
- 11.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337:762–769. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 12.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on morbidity and mortality in adult sickle cell anemia: Risks and benefits up to 9 years of treatment. JAMA. 2003;289:1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 14.Ferster A, Vermylen C, Cornu G, et al. Hydroxyurea for treatment of severe sickle cell anemia: A pediatric clinical trial. Blood. 1996;88(6):1960–1964. [PubMed] [Google Scholar]
- 15.Kinner TR, Helms RW, O’Branski EE, et al. Pediatric Hydroxyurea Group Safety of hydroxyurea in children with sickle cell anemia: Results of the HUG–KIDS study, a phase I/II trial. Blood. 1999;94(5):1550–1554. [PubMed] [Google Scholar]
- 16.Zimmerman SA, Schultz WH, Davis JS, et al. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood. 2004;103(6):2039–2045. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]
- 17.Marlowe KF, Chicella MF. Treatment of sickle cell pain. Pharmacotherapy. 2002;22(4):484–491. doi: 10.1592/phco.22.7.484.33675. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez ER, Bahal N, Hansen LA, et al. Intermittent injection vs. patient-controlled analgesia for sickle cell crisis pain: Comparison in patients in the emergency department. Arch Intern Med. 1991;151(7):1373–1378. [PubMed] [Google Scholar]
- 19.Okomo U, Meremikwu MM.Fluid replacement therapy for acute episodes of pain in people with sickle cell disease (Cochrane Review) The Cochrane LibraryIssue 2,2007. Article No. CD005406. [DOI] [PubMed]
- 20.Steinberg MH. Management of sickle cell disease. N Engl J Med. 1999;340:1021–1029. doi: 10.1056/NEJM199904013401307. [DOI] [PubMed] [Google Scholar]
- 21.Falletta JM, Woods GM, Verter JI, et al. Discontinuing penicillin prophylaxis in children with sickle cell anemia. J Pediatr. 1995;127:685–690. doi: 10.1016/s0022-3476(95)70154-0. [DOI] [PubMed] [Google Scholar]
- 22.The Management of Sickle Cell Disease 4th edrevised, June2002. National Institutes of Health/National Heart, Lung, and Blood Institute, Division of Blood Diseases and Resources, NIH Pub No. 02-2117.
- 23.Gatson MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia: A randomized trial. N Engl J Med. 1986;314:1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- 24.What is sickle cell anemia? National Heart, Lung, and Blood Institute (NHLBI) Available at: www.nhlbi.nih.gov/health/dci/Diseases/Sca/SCA_WhatIs.html. Accessed August 10, 2007.
- 25.Pai VB, Nahata MC. Duration of penicillin prophylaxis in sickle cell anemia: Issues and controversies. Pharmacotherapy. 2000;20(1):110–117. doi: 10.1592/phco.20.1.110.34660. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention (DCDC) Sickle cell disease: Five tips to help prevent infection. Available at: www.cdc.gov/ncbddd/sicklecell/tips_prevent.htm. Accessed October 16, 2007.
- 27.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 28.The Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP 2) Trial Investigators Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med. 2005;353:2769–2778. doi: 10.1056/NEJMoa050460. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman SA, Schultz WH, Burgett S, et al. Hydroxyurea therapy lowers transcranial Doppler flow velocities in children with sickle cell anemia. Blood. 2007;110:1043–1047. doi: 10.1182/blood-2006-11-057893. [DOI] [PubMed] [Google Scholar]
- 30.Vichinsky E, Onyekwere O, Porter J, et al. A randomised comparison of deferasirox versus deferoxamine for the treatment of transfusional iron overload in sickle cell disease. Br J Haematol. 2007;136(3):501–508. doi: 10.1111/j.1365-2141.2006.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lerner NB. Aspirin prophylaxis in sickle cell disease. Trial No. NCT00178464. Available at: http://clinicaltrials.gov/ct/show/NCT00178464?order=1. Accessed September 5, 2007.
- 32.Stuart MJ, Nagel RL. Sickle cell disease. Lancet. 2004;364(9442):1343–1360. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- 33.Rabb LM, Grandison Y, Mason K, et al. A trial of folate supplementation in children with homozygous sickle cell disease. Br J Haematol. 1983;54:589–594. doi: 10.1111/j.1365-2141.1983.tb02138.x. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Cortez HM, Griener JC, Hyland K, et al. Plasma homocysteine levels and folate status in children with sickle cell anemia. J Pediatr Hematol Oncol. 1999;21:219–223. doi: 10.1097/00043426-199905000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Nelson SC, Adade BB, McDonough EA, et al. High prevalence of pulmonary hypertension in children with sickle cell disease. J Pediatr Hematol Oncol. 2007;29:334–337. doi: 10.1097/MPH.0b013e31805d8f32. [DOI] [PubMed] [Google Scholar]
- 36.Castro O, Hoque M, Brown MD. Pulmonary hypertension in sickle cell disease: Cardiac catheterization results and survival. Blood. 2003;101:1257–1261. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- 37.Machado RF, Martyr S, Kato GJ, et al. Sildenafil therapy in patients with sickle-cell disease and pulmonary hypertension. Br J Haematol. 2005;130:445–453. doi: 10.1111/j.1365-2141.2005.05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adeyoju AB, Olujohunbge ABK, Morris J, et al. Priapism in sickle-cell disease: Incidence, risk factors and complications. An international multicentre study. BJU Int. 2002;90(9):898–902. doi: 10.1046/j.1464-410x.2002.03022.x. [DOI] [PubMed] [Google Scholar]
- 39.Fowler JE, Koshy M, Strub M, et al. Priapism associated with the sickle cell hemoglobinopathies: Prevalence, natural history and sequelae. J Urol. 1991;145(1):65–68. doi: 10.1016/s0022-5347(17)38248-4. [DOI] [PubMed] [Google Scholar]
- 40.Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1(1):116–120. doi: 10.1111/j.1743-6109.2004.10117.x. [DOI] [PubMed] [Google Scholar]
- 41.Iyamu EW, Turner EA, Asakura T. In vitro effects of niprisan (Nix-0699): A naturally occurring, potent antisickling agent. Br J Haematol. 2002;118(1):337–343. doi: 10.1046/j.1365-2141.2002.03593.x. [DOI] [PubMed] [Google Scholar]
- 42.Wambebe C, Khamofu H, Momoh JA, et al. Double-blind, placebo-controlled, randomized cross-over clinical trial of niprisan in patients with sickle cell disorder. Phytomedicine. 2001;8(4):252–261. doi: 10.1078/0944-7113-00040. [DOI] [PubMed] [Google Scholar]
- 43.Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. 2004;364:1343–1360. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- 44.Christensen D. Nitric oxide may help treat sickle cell anemia. Science News. 2000;157(5):78–81. [Google Scholar]
- 45.Head CA, Brugnara C, Martinez-Ruiz R, et al. Low concentrations of nitric oxide increase oxygen affinity of sickle erythrocytes in vitro and in vivo. J Clin Invest. 1997;100(5):1193–1198. doi: 10.1172/JCI119631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waugh WH, Daeschner CW, Files BA, et al. Evidence that l-arginine is a key amino acid in sickle cell anemia: A preliminary report. Nutr Res. 1999;19(4):501–518. [Google Scholar]
- 47.Morris CR, Morris SM, Hagar W, et al. Arginine therapy: A new treatment for pulmonary hypertension in sickle cell disease? Am J Respir Crit Care Med. 2003;168:63–69. doi: 10.1164/rccm.200208-967OC. [DOI] [PubMed] [Google Scholar]
- 48.Williams R, Olivi S, Li CS, et al. Oral glutamine supplementation decreases resting energy expenditure in children and adolescents with sickle cell anemia. J Pediatr Hematol Oncol. 2004;26(10):619–625. doi: 10.1097/01.mph.0000140651.65591.b8. [DOI] [PubMed] [Google Scholar]
- 49.Niihara Y, Zerez CR, Akiyama DS, et al. Increased red cell glutamine availability in sickle cell anemia: Demonstration of increased active transport, affinity, and increased glutamate level in intact red cells. J Lab Clin Med. 1997;130(1):83–90. doi: 10.1016/s0022-2143(97)90062-7. [DOI] [PubMed] [Google Scholar]
- 50.Niihara Y, Zerez CR, Akiyama DS, et al. Oral l-glutamine therapy for sickle cell anemia: I. Subjective clinical improvement and favorable change in red cell NAD redox potential. Am J Hematol. 1998;58:117–121. doi: 10.1002/(sici)1096-8652(199806)58:2<117::aid-ajh5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 51.De Franceschi L, Bachir D, Galacteros F, et al. Oral magnesium supplements reduce erythrocyte dehydration in patients with sickle cell disease. J Clin Invest. 1997;100(7):1847–1852. doi: 10.1172/JCI119713. [DOI] [PMC free article] [PubMed] [Google Scholar]