Abstract
Metastasis continues to be the leading cause of mortality for patients with cancer. High expression of the chemokine receptor CXCR4 correlates with poor prognosis in many cancers, including osteosarcoma and melanoma. CXCL12, the ligand for CXCR4, is expressed at high levels in the lung and lymph node, which are the primary sites to which these tumors metastasize respectively. These findings suggest that therapy aimed at disruption of this specific receptor/ligand complex may lead to a decrease in metastases. CTCE-9908, a small peptide CXCR4 antagonist was utilized in two murine metastasis models to test this hypothesis. Treatment of osteosarcoma cells in vitro with CTCE-9908 led to the following changes: decreased adhesion, decreased migration, decreased invasion, and decreased growth rate. Following tail vein injection of osteosarcoma cells, mice that were treated with CTCE-9908 had a 50% reduction in the number of gross metastatic lung nodules and a marked decrease in micrometastatic disease. Similar findings were observed following injection of melanoma cells and treatment with CTCE-9908. However, these results could only be consistently reproduced when the cells were pre-treated with the inhibitor. A novel ex vivo luciferase assay showed decreased numbers of cells in the lung immediately after injection into mice, when treated with CTCE-9908, suggesting the importance of interactions between the receptor and the ligand. Our findings show that inhibition of the CXCR4/CXCL12 pathway decreases metastatic disease in two murine tumor models and expands on previous reports to describe potential mechanisms of action.
Keywords: Chemokines, CXCR4 inhibition, Melanoma, Osteosarcoma, Pulmonary metastasis
Introduction
Chemokines are chemotactic cytokines that play an important role in many normal physiological processes including fetal development and trafficking of naive lymphocytes [1]. Chemokines are secreted proteins that act in a coordinated fashion with specific G-protein coupled receptors [2]. To date, over 50 chemokines and 18 receptors have been identified [2]. One of these receptors, CXCR4, has been the target of diverse research interests due to its role in the recruitment of leukocytes to sites of inflammation and infection [3] and its function as a co-receptor for HIV infection [4]. Unlike many other chemokines, CXCR4 has only one recognized ligand, CXCL12 (formerly known as SDF-1, for stromal cell-derived factor-1) [2].
The importance of CXCR4 expression and metastatic cancer was first described by Muller et al. [5] In breast cancers, CXCR4 was expressed at high levels in primary tumors, but at low levels in normal breast tissue [5]. Its corresponding ligand, CXCL12, showed abundant expression in lung, lymph nodes, liver, and bone marrow, all of which are sites to which breast cancers metastasize. These findings have led to the development of a chemokine mediated metastasis model [6]. Cancerous cells express CXCR4 on the cell surface, detach from the primary tumor and enter the circulatory system. Organs that express high levels of the ligand CXCL12 have an increased chance of activating the receptor, triggering a series of events that eventually results in the formation of metastatic tumors.
CXCR4 is expressed in 67% of osteosarcomas, correlating inversely with survival [7]. In tumor samples that do not express CXCR4 mRNA, the survival rate is 90%, while in tumor samples that express CXCR4 mRNA, the survival rate is only 10%. Furthermore, the overwhelming majority of metastases from osteosarcoma are to the lung, a site that expresses high levels of the ligand CXCL12 [5]. Expression of CXCR4 also correlates with poor prognosis in patients with malignant melanoma [8]. Studies indicate that the percentage of melanoma cells that express CXCR4 correlates inversely with months of disease free survival. Animal models show that CXCR4 activation enhances the arrest of melanoma cells on endothelial cells in a β1 integrin dependent manner, suggesting that CXCR4 acts at an early step in metastasis [9].
The findings above led us to explore if the chemokine-mediated model of metastasis is relevant in either osteosarcoma or melanoma. At a certain point during tumorigenesis, cancer cells that are positive for the chemokine receptor CXCR4 enter the circulation. When they encounter a small capillary bed, there is a tendency for them to become trapped, at least transiently. If this event occurs within a vascular bed that has an abundant level of the ligand CXCL12, such as the lung, there is a higher probability that the receptor will bind the ligand. The coupling of CXCR4 to CXCL12 triggers a cascade of events that allows cancer cells to survive, invade, and finally proliferate, thereby leading to the formation of metastatic tumors. This model predicts that inhibition of the CXCR4/CXCL12 chemokine pathway would reduce lung metastases. To test this hypothesis, experimental metastasis models of murine osteosarcoma and melanoma were established. CTCE-9908, a 17 amino acid analog of CXCL12, was used to inhibit CXCR4. The sequence used to generate CTCE-9908 is identical in humans, rats and mice. Radioligand binding assays have shown that this peptide antagonizes both human and murine CXCR4 (D Wong personal communication). CTCE-9908 is currently being tested in clinical trials.
Materials and methods
Cell culture
The human osteosarcoma cell lines G292, HOS, MNNG/ HOS, MG63 and U2OS were obtained from American Type Culture Collection (Rockville, MD). Derivation of the K7M2 murine osteosarcoma cell line has been described previously [10]. The plasmid pMSCVpuro-Luciferase contains the Photinus pyralis luciferase gene under control of the constitutive murine stem cell virus promoter. pMSCVpuro-Luciferase was nucleofected into K7M2 cells using the Nucleofector II apparatus (Amaxa Biosystems, Rockville, MD). Nucleofection using the program A33 in solution V resulted in 30% transfection efficiency with 50% viability. Single cell clones were selected following the addition of 2.5 µg/ml of puromycin (Sigma-Aldrich, St. Louis, MO). These clones were then propagated into cell lines in the continued presence of puromycin at 2.5 µg/ml. Luminescence was tested by the addition of luciferin (Xenogen Biosciences, Cranbury, NJ) at a final concentration of 1 mg/ml. A highly luminescent clone K7M2-L10 was tested for its ability to metastasize. Although metastatic, this cell line had increased latency to metastases (66 days vs. 24 days for the parental K7M2 cell line). Therefore, a highly metastatic version of this clone was prepared as follows. A pulmonary nodule was harvested, minced into 1 mm fragments and added to a tissue culture plate in media containing puromycin at 2.5 µg/ml. Resulting single clones were expanded into cell lines and tested for both luminescence and metastatic potential. One of these K7M3-L10A was highly luminescent and resembled the parental K7M2 cell line in both the number of resulting metastatic nodules and the time to metastatic disease. This cell line was renamed K7M3-luciferase. Transduction of the B16 murine melanoma cell line with CXCR4 has been described previously [9]. All cell lines were cultured at 37°C in a 5% CO2 humidified tissue culture incubator in DMEM supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 units/ml penicillin and 100 µg/ml streptomycin (Invitrogen, Carlsbad, CA). CTCE-9908 powder was reconstituted with sterile water to a concentration of 40 mg/ml and then filtered through a 0.22 micron membrane vacuum filtration unit (Millipore, Billerica, MA). Appropriate amounts of CTCE-9908 solution were added to the media to obtain a final concentration of 100 µg/ml. Control scramble peptide was prepared similarly.
CXCL12 amino acid #22–29 sequence: KPVSLSYR
CTCE-9908 sequence: KGVSLSYR-K-RYSLSVGK
Scramble peptide sequence: LSYVKGRS-K-SRGKVYSL
Western blot analysis
Cells growing in 100cc tissue culture plates were washed twice with cold PBS. About 500 µl of 2X Laemmli lysis buffer (125 mM Tris, 20% glycerol, 4% SDS, pH 6.8) was added. Cells were collected with a cell scraper, boiled for 10 min and stored at −70°C. 40 µl of the lysate was added to 19.4 µl of 3X SDS Sample Buffer (Cell Signaling, Danvers, MA) and 0.6 µl of DTT (10 mM final concentration). The lysate was then loaded on a 4–12% NuPAGE Bis–Tris Gel (Invitrogen) and electrophoresed for 40 min at 200 V using MES Running Buffer (Invitrogen). The gel was transferred to a nitrocellulose membrane using an iBlot transfer apparatus (Invitrogen). After blocking, rabbit polyclonal CXCR4 antibody, ab2074 (Abcam, Cambridge, MA) at 1:500 dilution was added overnight followed by anti-rabbit IgG HRP secondary antibody (Cell Signaling). Signal was detected by chemiluminescence using the West Femto peroxidase system (Pierce, Rockford, IL). Images were taken on a Kodak 440 workstation using Kodak 1D software (Kodak Molecular Imaging, New Haven CT). The membrane was stripped using Stripping Buffer (Pierce), reblocked and then probed using β-actin HRP antibody (Abcam).
Q-RT-PCR analysis
Cells growing in 75cc tissue culture flasks at approximately 80% confluence were washed with PBS and lysed directly using the Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA). RNA was eluted in a total volume of 20 µl. First strand cDNA synthesis was performed using the SuperScript First-Strand Synthesis System for RT-PCR kit (Invitrogen). Quantitative RT-PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) with approximately 1 µg of template and 1.5 uM of primers (Integrated DNA Technologies, Coralville, IA). PCR was performed using a PTC-200 Peltier Thermal Cycler with a Chromo4 Continuous Fluorescence Detector and Opticon Monitor Analysis Software (MJ Research, Ramsey, MN). Primer sequences are as follows.
humanCXCR4.qpcr.f904: 5′-tat gct ttc ctt gga gcc aaa-3′
humanCXCR4.qpcr.r975: 5′-gct gga ccc tct gct cac a-3′
humanGAPDH.qpcr.f868: 5′-acc cac tcc tcc acc ttt ga-3′
humanGAPDH.qpcr.r943: 5′-cat acc agg aaa tga gct tga caa-3′
murineCXCR4.qpcr.f568: 5′-agg tac atc tgt gac cgc ctt t-3′
murineCXCR4.qpcr.r644: 5′-aga ccc acc att ata tgc tgg aa-3′
murineGAPDH.qpcr.f694: 5′-cgt gtt cct acc ccc aat gt-3′
murineGAPDH.qpcr.r766: 5′-tgt cat cat act tgg cag gtt tct-3′
FACS analysis
Cells growing in 100cc tissue culture dishes at approximately 80% confluence were washed twice with cold PBS. Cells were collected using a cell scraper and divided into two aliquots of 100 µl each. About 5 µl of one of the following antibodies was added. Mouse anti-human PE labeled CXCR4 antibody (R&D Systems, Minneapolis, MN). Goat anti-mouse PE labeled IgG antibody (R&D Systems). Rat anti-mouse PE labeled CXCR4 antibody (BD Biosciences, Franklin Lakes, NJ). Anti-rat PE labeled IgG2c antibody (BD Biosciences). After 10 min incubation, 5 µl of 7-AAD (BD Biosciences) was added and incubated for 5 min. Subsequently, 400 µl of PBS containing 1% formalin was added. Cells were analyzed on a FACSCalibur cell sorter (BD Biosciences) and mean log intensity was quantitated.
MTT growth assay
K7M2 cells at various densities were added to 96-well tissue culture plates and incubated at 37°C in a tissue culture incubator overnight. The cells were then left untreated or treated with media containing CTCE-9908 (100 µg/ml). At various time intervals 100 µl of MTT (Sigma-Aldrich, St. Louis, MO) at 0.5 mg/ml in RPMI 1640 without phenol red was added to the cells and incubated for 4 h. The mixture was solubilized with 100 µl of isopropanol and mixed thoroughly. The absorbance was measured at 570–690 nm using a VERSAmax tunable microplate reader (Molecular Devices, Sunnyvale CA). All results are the average of four replicates.
Adhesion assays
K7M2 cells growing in 100cc tissue culture plates were treated with CTCE-9908 (100 µg/ml) or left untreated overnight. 8-strip wells coated with fibronectin, vitronectin, laminin, collagen type I or collagen type IV (Chemicon, Temecula, CA) were hydrated with 200 µl of PBS for 15 min. Cells were harvested by the addition of Cellstripper (Mediatech, Herndon, VA) solution for 10 min. CTCE-9908 treated and untreated cells were centrifuged and then resuspended in media to a final concentration of 500,000 cells/ml. After removal of the PBS from the coated 8-well strips, 50,000 cells in a volume of 100 µl were added to each well, incubated for 1 h at 37°C in a tissue culture incubator and the media gently removed. The wells were washed twice gently with PBS containing calcium and magnesium. 100 µl of crystal violet solution (0.2% dissolved in 10% ethanol) was added and incubated for 5 min at room temperature. The wells were washed three times gently with PBS containing calcium and magnesium and 100 µl of solubilization buffer was added (50% NaH2PO4 0.1 M pH 4.5 and 50% ethanol). The absorbance was measured at 560 nm using a VERSAmax tunable microplate reader (Molecular Devices). All results are the average of seven replicates.
Ex vivo luminescent lung quantitation
K7M3-luciferase cells were harvested and washed twice with HBSS and then resuspended in HBSS to a final concentration of 10 million cells/ml. Half of the cells were treated with CTCE-9908 (100 µg/ml) for 30 min. About 100 µl (106 cells) was injected into the lateral tail vein of 6-week old female Balb/C mice (Taconic, Germantown, NY). Either five or six mice were used in each cohort. CTCE-9908 treated and untreated cohorts were euthanized 30 min and 6 h after injection of the cells. Twelve minutes prior to euthanization, mice were injected intraperitoneally with 100 µl (2.5 mg) of luciferin. The lungs were harvested and inflated with 1.5 ml of PBS containing 2.5 mg/ml luciferin and then added to wells of a 6-well plate containing 3 ml of PBS with 2.5 mg/ml luciferin. Samples were imaged for 1 min using the IVIS 100 (Xenogen) and quantitated as counts per sample, ×106.
Cytoskeletal examination
K7M2 cells were grown in 4-well glass slide chambers (Nunc, Rochester, NY) containing 0.5 ml of media. Cells were either left untreated or treated with CTCE-9908 (100 µg/ml) for 28 h. Cells were fixed with 3.7% formaldehyde for 10 min, made permeable with 0.2% Triton X-100 for 2 min and blocked with 0.2% BSA for 10 min. Texas Red-phalloidin (Invitrogen) was added at a dilution of 1:200 in PBS and incubated for 30 min at room temperature in the dark. DAPI nuclear stain (Invitrogen) was then added to a final concentration of 0.4 µg/ml and incubated for 10 min at room temperature in the dark. Cells were washed with PBS and then distilled water and VectaShield mounting media (Invitrogen) was applied prior to addition of a coverslip. The cells were observed using a fluorescent microscope at 40× and 100× magnifications.
Migration and invasion assays
The BD Biosciences HTS FluoroBlok System (BD Biosciences) employs a light-opaque polyethylene terephthalate microporous membrane that blocks wavelengths between 490 and 700 nm. The membrane has a pore size of 8 microns with a pore density of 105 pores/cm2 in 24-well tissue culture plates. Cells are added to the upper chamber and a chemo-attractant is added to the lower chamber. Cells are then labeled and when a bottom reading fluorescent plate reader is utilized, only the cells that have migrated through the membrane to the lower chamber are quantitated. The BioCoat™ Tumor Invasion System (BD Biosciences) was utilized to assay invasiveness in vitro. These plates consist of an 8-micron pore size membrane coated with a matrigel matrix, which mimics a basement membrane. Since the matrigel matrix occludes the pores, non-invasive cells are not able to invade through the coated membrane. In contrast, invasive cells are able to penetrate through the coated membrane.
K7M2 cells were treated with CTCE-9908 (100 µg/ml) or left untreated overnight. 105 cells, resuspended in media containing 1% serum, were added to the upper chamber of the system (either migration or invasion plates). Media containing 10% serum and CXCL12 (100 ng/ml) (R&D Systems) was added to the lower chamber. For CTCE-9908 treated cells, all of the media contained CTCE-9908 (100 µg/ml) throughout the course of the experiment. Cells were incubated overnight at 37°C in a tissue culture incubator. Cells were labeled with the fluorescent dye Calcein AM (4 µg/ml) (Invitrogen). Fluorescent intensity was then measured using a Victor3 bottom reading fluorescent plate reader (Perkin Elmer, Wellesley, MA) at excitation/emission wavelengths of 485/530 nm. All results are the average of six replicates. To guard against cytotoxic or growth inhibitory effects of CTCE-9908, all experiments included non-migratory/non-invasive controls. At the time of loading of treated or untreated cells in the upper chamber, an equal number of cells were added to regular 24-well tissue culture plates. These were subsequently labeled with Calcein AM and quantitated fluorescently. The intensity of both treated and untreated samples was analyzed to verify equal intensity in both samples.
Animal studies
Four-to-six week-old female Balb/C mice weighing approximately 20 g were obtained from Taconic (Germantown, NY). Protocols for animal care were reviewed and approved by the National Cancer Institute Animal Care and Use Committee. K7M2 murine osteosarcoma cells were resuspended in HBSS without phenol red and treated with CTCE-9908 (100 µg/ml) or an equal volume of PBS for 60 min. Following the pre-treatment period, 106 cells in a volume of 100 µl were injected into the lateral tail vein. The following day, mice began treatment with CTCE-9908 (67 mg/kg) or normal saline subcutaneously, once daily, 5 days on and two days off, for a period of 4 weeks. Stock CTCE-9908 solution was diluted in normal saline to obtain a final injection volume of 100 µl. At the first sign of morbidity, mice were euthanized. The remaining animals were sacrificed on day 25 and all organs were examined for the presence of metastatic disease. The lungs were inflated with a 2% solution of India ink diluted in PBS and stained overnight. The number of surface lung nodules was counted by two observers and in cases where the results were divergent, both observers counted the sample together. The samples were fixed in 10% formalin, sectioned and the slides were stained with H + E at American HistoLabs (Gaithersburg, MD). For experiments involving the B16 murine melanoma cell line, 8–12-week-old female B57BL/6 mice were obtained from the NCI-Frederick Cancer Research and Development Center (Frederick, MD). All parameters were similar to that described above with the following exceptions: 400,000 CXCR4-B16 cells were injected via tail vein, animals were sacrificed on day 14, and the lungs were not stained with India ink. For the second set of experiments, the mice began receiving CTCE-9908 or PBS on the day prior to injection of cells. After a control scramble peptide became available, mice were treated with PBS or the control scramble peptide.
Results
CXCR4 is expressed in murine and human osteosarcoma cell lines
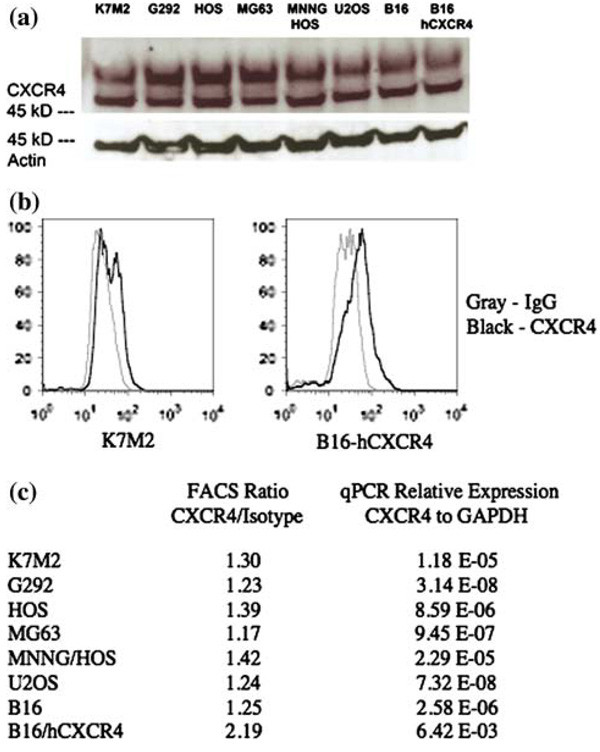
It has previously been reported that human osteosarcoma cell lines express CXCR4 at low levels as determined by western blot and FACS analysis [11]. To determine the CXCR4 expression status of our human and murine cell lines, we analyzed total cell lysates by western blot analysis. The results suggested that all osteosarcoma cell lines that were tested expressed CXCR4, but at very low levels (Fig. 1a). However, it was difficult to obtain consistent results in western blot studies, possibly due to the very low level of expression. Cells were then analyzed for cell surface CXCR4 expression by cell sorting. Again, all cells expressed CXCR4, however, at levels that were just slightly increased compared to IgG isotype control sorted cells (Fig. 1b). RT-PCR analysis confirmed low level expression of CXCR4 as compared to GAPDH housekeeping controls (Ct > 32.7 vs. Ct values between 15.1 and 18.1 for GAPDH). Quantitation of FACS and RT-PCR results are shown in Fig. 1c. These results confirmed published reports that CXCR4 expression is low in human osteosarcoma cell lines. Overexpression of CXCR4 in B16 melanoma cells has been reported previously [12].
Fig. 1. Osteosarcoma cell lines express low levels of CXCR4.
(a) Western blot analysis shows that all osteosarcoma cell lines express a similar level of CXCR4, which is known to be low from the literature. Actin is shown as a loading control. (b) FACS analysis demonstrates cell surface expression of CXCR4 in K7M2 osteosarcoma cells and B 16 melanoma cells, the latter was transfected with a human CXCR4 plasmid. (c) Quantitation of CXCR4 cell surface expression over that of IgG isotype control after FACS analysis and quantitation of CXCR4 mRNA levels relative to that of GAPDH control after quantitative RT-PCR analysis
CTCE-9908 decreases growth rate in vitro
K7M2 cells untreated and treated with CTCE-9908 were assessed for their ability to proliferate by MTT assay. Beginning at 25 h, cells treated with CTCE-9908 started to show a decrease in growth rate (Fig. 2a). The time to doubling of optical density for K7M2 cells was 19.5 ± 2.5 h. Cells treated with CTCE-9908 had an increase in doubling time to 26.5 ± 3.1 h (p = 0.016). Morphological assessment of cells treated with CTCE-9908 for 72 h revealed no difference in phenotype (Fig. 2b). The combination of the lack of a growth inhibitory effect prior to 25 h, and the lack of phenotypic changes even as late as 72 h, suggested that the growth inhibition induced by CTCE-9908 was not due to a cytotoxic effect. All subsequent experiments were performed at appropriate time points to insure that proliferation changes were not affecting parameters in other experiments.
Fig. 2. CTCE-9908 decreases the growth rate of K7M2 cells in vitro.
(a) K7M2 cells treated with CTCE-9908 have a decrease in growth rate as determined by optical density, shown on the Y-axis, following treatment with MTT. Control cells have a doubling time of 20 h whereas CTCE-9908 treated cells have a doubling time of 27 h. (b) Microscopic visualization of treated and untreated cells at 72 h show no phenotypic differences. This finding, and the fact that cells began their growth divergence after 25 h, suggests that the decrease in proliferative capability is not due to cytotoxicity of the compound
CTCE-9908 decreases adhesion to extracellular matrix proteins
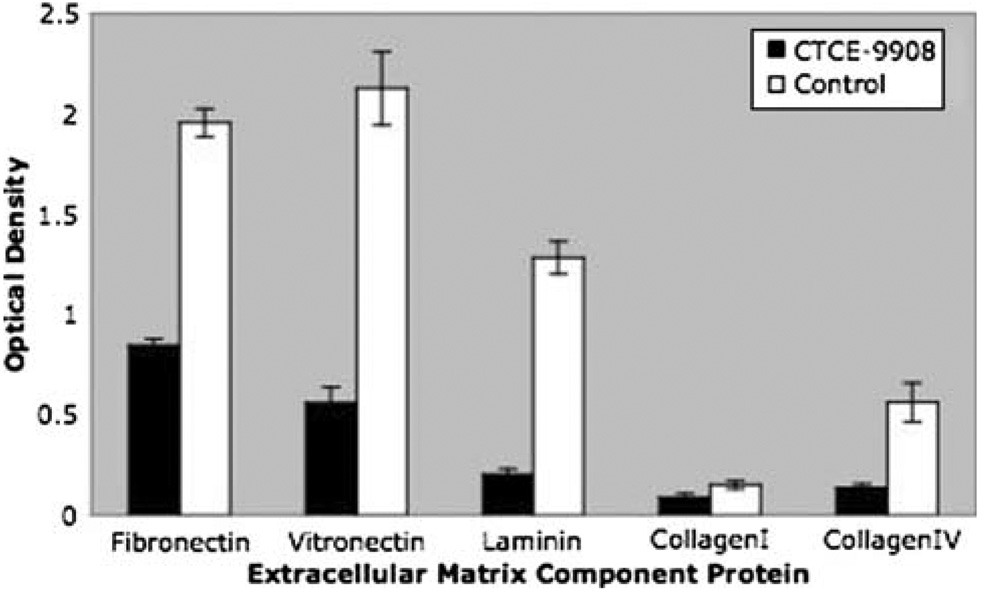
In an effort to understand the mechanism by which CXCR4 inhibition could decrease lung metastases, several of the steps that are believed to be necessary for successful metastasis, including adhesion, migration, and invasion were examined in vitro. The ability to adhere was examined by the addition of cells to plates coated with various extracellular matrix proteins. K7M2 cells treated with CTCE-9908 or left untreated overnight were added to plates coated with the following: fibronectin, vitronectin, laminin, collagen type I and collagen type IV. Unattached cells were removed by aspiration and adherent cells were stained with crystal violet, allowing for optical quantification. Cells that were treated with CTCE-9908 showed decreased binding to all of the extracellular matrix proteins that were tested (Fig. 3). All of these results were statistically significant with p-values <0.01. The effects on adhesion to fibronectin and laminin were studied further to determine the time course of CTCE-9908 mediated inhibition. After 1 h treatment with CTCE-9908, there was no difference in adhesion in either fibronectin or laminin coated plates. However, by 4 h, there was a two-fold decrease in adhesion in CTCE-9908 treated cells, an effect which continued to 25 h (data not shown).
Fig. 3. CTCE-9908 decreases adhesion to extracellular matrix proteins.
K7M2 cells treated with CTCE-9908 overnight have decreased adhesion to microtiters plates coated with various extracellular matrix component proteins compared to untreated control cells. The Y axis shows optical density following crystal violet staining of cells that had remained adherent to the plate. The percentage decreases were as follows: fibronectin (56%), vitronectin (73%), laminin (84%), collagen type I (41%) and collagen type IV (76%)
Ex vivo luminescent lung imaging
We utilized ex vivo luminescent imaging of lung samples to determine if the decreased adhesion seen in vitro with CTCE-9908 treatment could also be seen in vivo after the arrival of cells in the lung. K7M3-luciferase cells were divided into untreated and CTCE-9908 treated aliquots. Mice received cells by tail vein injection and the lungs were harvested 30 min or 6 h after injection of cells. Luminescence of the lungs was quantitated using the Xenogen luminescent detection system (Fig. 4a). At 30 min, the lungs of CTCE-9908 treated cells had a 2.5-fold decrease in luminescent intensity (CTCE-9908 average 22.7 ± 12.7 vs. control average 58.1 ± 25.6, p = 0.03, with values reported as counts × 105) (Fig. 4b). There was a slightly more pronounced 3-fold decrease at 6 h (CTCE-9908 average 2.6 ± 0.3 vs. control average 8.0 ± 1.6, p < 0.01, with values reported as counts × 105).
Fig. 4. CTCE-9908 treatment suggests decreased adhesion to the lung surface ex vivo.
K7M3-luciferase cells were injected into the tail vein of Balb/C mice. Following the intraperitoneal injection of luciferin, the lungs were harvested, inflated and soaked in PBS containing luciferin. Luminescent intensity was quantiated using the Xenogen IVIS system. (a) Bright-field and overlapping luminescent intensity of lungs, 6 h after injection of CTCE-9908 treated K7M3-luciferase cells, or PBS treated K7M3-luciferase cells. (b) Quantitation of luminescence at either 30 min or 6 h after injection of CTCE-9908 treated or untreated cells. Cells that were treated with CTCE-9908 had a 2.5-fold decrease in luminescent intensity at 30 min (p = 0.03) and a 3-fold decrease in luminescent intensity at 6 h (p < 0.01)
CTCE-9908 decreases migration of osteosarcoma cells
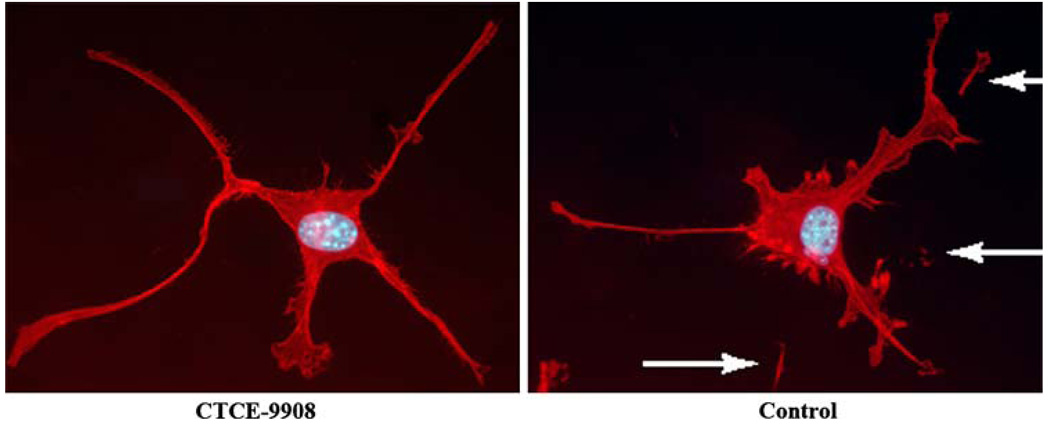
Cytoskeletal F-actin was examined by labeling with the F-actin specific stain, Texas Red-phalloidin, and visualized using fluorescent microscopy. There were no changes between CTCE-9908 treated and untreated cells in terms of the number and distribution of F-actin fibers. However, there was a marked difference in other aspects of the morphology. Untreated cells had many exosomes, which are thought to be remnants of the cell that remain attached to the glass slide as the cell moves (Fig. 5). This finding is consistent with the highly migratory nature of K7M2 cells. However, cells treated with CTCE-9908 did not show these exosomes, suggesting a much less aggressive migratory phenotype.
Fig. 5. CTCE-9908 decreases the migratory phenotype.
The photographs show CTCE-9908 treated and untreated K7M2 cells following labeling with the F-actin specific stain Texas Red-phalloidin and the nuclear stain DAPI. Untreated cells (right panel) have numerous pseudomembranous detachments (shown with arrows). These are thought to be remnants of the cell that remain attached to the glass slide as the cell moves, consistent with the highly migratory nature of K7M2 cells. CTCE-9908 treated cells (left panel) do not show this phenotype, suggesting decreased motility. There were no morphologically detectable differences in the F-actin cytoskeleton between treated and untreated cells
A BD Biosciences HTS FluoroBlok porous membrane system was utilized to quantitate the possible difference in migratory behavior observed above. Cells that were treated with CTCE-9908 had a decrease in their ability to migrate through the membrane towards CXCL12 (Fig. 6a). When 105 cells were plated, there was a 23% decrease in migration (CTCE-9908 average 30,244 ± 1,251 vs. control average 39,418 ± 2,619, p < 0.01). The above result is the average of six replicates and these results were verified in subsequent experiments. As an additional control, an equal amount of treated and untreated cells were added to a separate 24-well tissue culture plate. The fluorescent intensity of all of these samples were similar, indicating that the differences detected were due to a decrease in migration and not a function of cytotoxicity or decreased proliferation in the presence of CTCE-9908.
Fig. 6. CTCE-9908 decreases migration and invasion in the presence of CXCL12.
(a) K7M2 cells treated with CTCE-9908 have a decrease in their ability to migrate through an 8-micron membrane. The Y axis represents the aggregate fluorescent intensity of calcein stained cells that were able to penetrate the membrane to the lower chamber, which contained CXCL12 as the chemo-attractant. Cells that were treated with CTCE-9908 had a 23% decrease (p < 0.01) in their ability to migrate through the membrane. (b) An even greater decrease was detected when cells were added to a matrigel coated membrane. Cells treated with CTCE-9908 had a 45% decrease (p < 0.01) in their ability to invade through the matrigel layer
CTCE-9908 decreases invasion of osteosarcoma cells
The BD BioCoat™ system was used to assess the invasiveness of K7M2 cells treated with CTCE-9908. This system uses the same porous membrane plates described above, however, the membrane is coated with matrigel to mimic the basement membrane. K7M2 cells that were treated with CTCE-9908 or left untreated were added to the upper chamber and allowed to penetrate the matrigel coating and migrate through the porous membrane overnight. Cells that were treated with CTCE-9908 had a two-fold decrease in their ability to invade, as assessed by their presence on the other side of the membrane (Fig. 6b). When 105 cells were plated, there was a 45% decrease in invasion (CTCE-9908 average 1,983 ± 164 vs. control average 3,624 ± 303, p < 0.01). As in the migration assays, treated and untreated cells grown in regular tissue culture plates had similar fluorescent intensities.
CTCE-9908 decreases osteosarcoma lung metastases in mice
To determine if CTCE-9908 decreased lung metastases in vivo, K7M2 cells that were untreated or treated with CTCE-9908 were injected into the tail vein of Balb/C mice. The following day, the mice began treatment with CTCE-9908 (67 mg/kg) or normal saline by subcutaneous injection, 5 days on and 2 days off. By day 25, all eight of the mice in the control group had become dyspneic and lethargic, requiring euthanization. In contrast only one of eight mice in the CTCE-9908 treated group showed signs of morbidity by day 25.
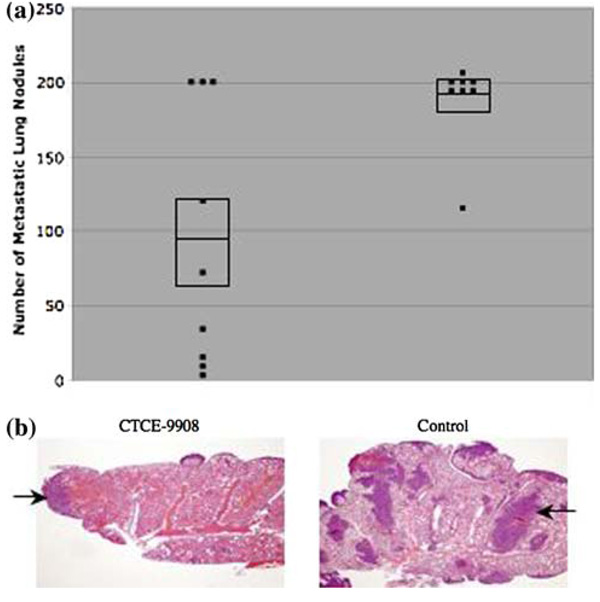
Lung samples were harvested from all of the mice, stained with India ink and the number of visible gross lung nodules counted. Mice that were treated with CTCE-9908 had a 50% decrease in the number of metastatic nodules. Mice treated with CTCE-9908 had an average number of 94.8 ± 86.7 lung nodules. In comparison, control mice had an average number of 189.4 ± 30.1 nodules (p = 0.01) (Fig. 7a). Histologic examination showed that all lung samples had evidence of micro-metastases, but the disease burden was much less in mice that were treated with CTCE-9908, supporting the data obtained by visual inspection (Fig. 7b). When viewed under high power, there was no difference in the histological features of successful osteosarcoma micrometastases in CTCE-9908 treated and untreated lung samples. Utilization of a control scramble peptide showed no difference between mice treated with PBS and those treated with control scramble peptide in the number of metastatic nodules.
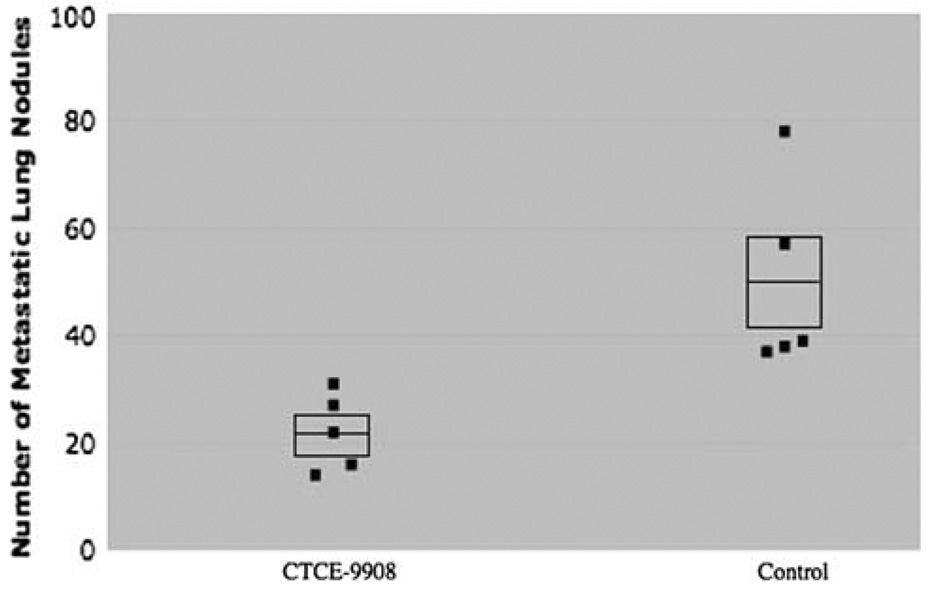
Fig. 7. CTCE-9908 decreases the number of lung nodules and micrometastatic disease in osteosarcoma.
(a) Mice that were treated with CTCE-9908 had a 50% decrease in the number of metastatic lung nodules, shown on the Y-axis, compared to control mice (CTCE-9908 average 94.8 ± 86.7 vs. control average 189.4 ± 30.1, p = 0.01). Each dot represents the number of nodules for one mouse. The middle line in the box represents the average number of nodules while the top and bottom lines of the box represent the standard error of the mean. (b) CTCE-9908 treated mice had a marked decrease in micrometastatic disease (dark purple/blue areas with H + E staining, two of which are shown with arrows). The samples in panel B are from a repeat experiment and not from the mice depicted in panel A
This experiment was consistently reproducible. However, attempts to perform this experiment without pre-treatment of cells with CTCE-9908 showed inconsistent results. In some attempts, a reduction in metastatic burden without pretreatment of the cells was observed, but was not statistically significant. This suggested that rapid blocking of CXCR4 receptor sites on the osteosarcoma cells is required to decrease metastatic disease in this experimental model. This may not be achievable when the animals are dosed 24 h after the administration of tumor cells.
Validation of in vivo results using a melanoma cell line
To determine if the inhibitory effects of CTCE-9908 on metastases could be generalized to other cancers that commonly metastasize to the lung, we tested B16 murine melanoma cells that overexpressed human CXCR4 [12]. Mice treated with the inhibitor had a 56% decrease in the number of lung nodules: CTCE-9908 (average 22.0 ± 7.2) versus control (average 49.8 ± 17.8, p = 0.02) (Fig. 8). To expand on these results, a variation of the experiment was performed in which the mice received one dose of CTCE-9908 prior to injection of the cells. In this setting, there was an 81% reduction in the number of lung nodules: CTCE-9908 (average 30.0 ± 15.0) versus control (average 158.0 ± 92.2, p = 0.035). These results further suggested that rapid blocking of receptor sites is important to reduce metastases in experimental models.
Fig. 8. CTCE-9908 decreases the number of lung nodules in mice injected with B16 melanoma cells.
Mice were injected intravenously with B16 melanoma cells transduced to overexpress CXCR4. Mice treated with CTCE-9908 had a 56% reduction in the number of metastatic lung nodules (CTCE-9908 average 22.0 ± 7.2 vs. control average 49.8 ± 17.8, p = 0.02)
Discussion
This study tested the hypothesis that disruption of the CXCR4/CXCL12 chemokine pathway could lead to a decrease in pulmonary metastases. The experimental design involved the use of a small peptide CXCR4 antagonist, CTCE-9908. In addition, it incorporated two experimental murine models of metastasis, osteosarcoma and melanoma. Our results demonstrate that mice treated with CTCE-9908 have a 50% decrease in the number of lung nodules in both models. These results show that CXCR4 inhibition is effective in decreasing metastases utilizing an experimental model.
The steps that are needed for a cancer cell to spread are complex. At a minimum, the cell must have the genetic components to be able to enter the systemic circulation either by hematogenous or lymphatic routes. Once the cell gains access to this avenue of spread, it should have the potential to land in any vascular bed, allowing for metastatic disease. However, it is clear, both clinically and in experimental models, that this is not true. In osteosarcoma, 80% of metastases are to the lungs, 20% are to bone, and only rarely does osteosarcoma metastasize to any other organ [13]. This clinical finding is mimicked in murine models in which lung metastases are common, bone metastases are infrequent, and no other affected organs are found, despite aggressive attempts to generate these latter types of metastases [10]. The interaction of chemokines and their receptors provides one explanation for organ specific metastatic behavior.
The first requirement for a cell to exit the bloodstream is arrest in a capillary bed. This may occur due to the large size of the cancer cell and the small caliber of the vessel. Physical arrest is thought to be non-specific. However, if it happens to occur in a vascular bed that has an abundance of a chemokine, then cells that express the appropriate receptor will have a high likelihood of interacting with the chemokine. It is known that binding of CXCR4 receptor on the cancer cell by the CXCL12 ligand secreted by cells in the end organ triggers the many subsequent steps required for metastasis, although the exact mechanisms remain to be elucidated.
Using an ex vivo luciferase assay, we have shown that lungs harvested after injection of cells that were pre-treated with CTCE-9908 had a 2–3 fold decrease in luminescent intensity, both immediately and 6 h after injection. These results suggested that direct interaction between the receptor and the ligand during the earliest steps of metastatic spread was crucial for subsequent formation of lung nodules. Although physical arrest would be equal in these two cases, the paucity of receptors that are available for interaction in the pre-treated cells most likely accounts for the difference in binding, potentially by decreasing the ability of the cells to remain adherent to the endothelial surface. This is supported by in vitro findings that show that adhesion to extracellular matrix proteins is markedly reduced in the presence of the inhibitor. This hypothesis would also explain the reduced ability to decrease metastases when the cells are not pre-treated with the inhibitor. By 24 h, when treatment with drug was started, the critical signals induced by CXCR4 and CXCL12 interaction may have already occurred. If so, treatment with the inhibitor at this point would not interfere with the early steps in the metastatic cascade.
Further support of this theory comes from our in vivo findings using the CXCR4 over-expressing B16 murine melanoma cell line. Mice that were injected following pretreatment of the cells with CTCE-9908 had a 50% decrease in lung nodules. A similar experiment was performed in mice that had received a systemic dose of CTCE-9908 one day prior to the injection of pre-treated cells. In this model, there was an 80% decrease in the number of lung nodules. It is possible that CTCE-9908 mediated inhibition led to priming of the lungs by interfering with the ligand. These findings suggest that the ability to remain adherent to the lung surface may play a critical role in CXCR4 mediated metastasis.
Our in vivo results suggest that CXCR4 blockade is most effective prior to the arrival of the cells, thereby precluding the establishment of micrometastatic disease. It would be ideal if CXCR4 blockade could be given to patients who do not have micrometastatic disease. In some cancer types this may be possible. For example, colon cancer is known to progress through stages, starting from hyperplasia to adenomas to carcinomas and then to metastatic disease. If initiated early enough in the process, CXCR4 may preclude the progression to metastatic disease.
Clinical observations suggest that at the time of diagnosis, osteosarcoma cells are already present in the lung despite the lack of overt nodules [14]. The question then becomes, what advantage will blocking CXCR4 provide in this setting? Studies in colon cancer models suggest a possible answer. CT-26 colon cancer cells that had been rendered CXCR4-negative and GFP-positive can be detected by RT-PCR in lung samples following intravenous injection of cells. However, only half of these mice progress to develop lung nodules, and many of those that do, regain CXCR4 expression [15]. Injection with control wildtype CXCR4-positive cells results in a similar number of cells on the lung surface early on. However, all mice progress to form visible metastases, suggesting the importance of CXCR4 for the outgrowth of colon carcinoma metastases.
Our in vitro data shows that CXCR4 inhibition also has the potential to affect later stages of metastases, such as migration, invasion, and proliferation. All of these steps are required for micrometastatic disease to subsequently progress to macrometastatic disease. In our models it is possible that CXCR4 inhibition may affect both early and late stages of metastases. Our data suggests that CXCR4 inhibition with CTCE-9908 is sufficient to influence metastatic progression when given before metastases have developed. Based on previous data, we remain interested in the possibility that CXCR4 inhibition can be potentiated if given as part of a combination strategy to control later steps in metastatic progression. Our results show that CXCR4 inhibition alone is not able to affect the later steps in metastases. But this may be overcome by potentiating CXCR4 inhibition with other therapy. In the B16 melanoma model, a different CXCR4 antagonist markedly reduced metastatic implantation, without altering the outgrowth of established lung metastases [9]. Further studies have shown that CXCR4 blockade sensitizes tumor cells, thereby increasing the efficacy of immunotherapy to decrease established metastases [16]. Thus, even if cells are able to evade CXCR4 blockade early in the course of metastases, they may still be amenable to targeting using combination therapy to prevent their progression to frank metastases. Studies to define the utility of CXCR4 antagonists later in metastatic progression are needed. The current use of CTCE-9908 in clinical trials makes this an ideal compound to define the role of CXCR4 inhibition in the process of metastasis.
Contributor Information
Su Young Kim, Pediatric Oncology Branch, National Cancer Institute, National, Institutes of Health, 10 Center Drive, Building 31, Room 3A11, Bethesda, MD 20892, USA.
Chih Hung Lee, Department of Dermatology, Kaohsiung Medical University, Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
Brieanne V. Midura, Pediatric Oncology Branch, National Cancer Institute, National, Institutes of Health, 10 Center Drive, Building 31, Room 3A11, Bethesda, MD 20892, USA
Choh Yeung, Pediatric Oncology Branch, National Cancer Institute, National, Institutes of Health, 10 Center Drive, Building 31, Room 3A11, Bethesda, MD 20892, USA.
Arnulfo Mendoza, Pediatric Oncology Branch, National Cancer Institute, National, Institutes of Health, 10 Center Drive, Building 31, Room 3A11, Bethesda, MD 20892, USA.
Sung Hyeok Hong, Pediatric Oncology Branch, National Cancer Institute, National, Institutes of Health, 10 Center Drive, Building 31, Room 3A11, Bethesda, MD 20892, USA.
Ling Ren, Pediatric Oncology Branch, National Cancer Institute, National, Institutes of Health, 10 Center Drive, Building 31, Room 3A11, Bethesda, MD 20892, USA.
Donald Wong, Chemokine Therapeutics Corp., Vancouver, BC, Canada.
Walter Korz, Chemokine Therapeutics Corp., Vancouver, BC, Canada.
Ahmed Merzouk, Chemokine Therapeutics Corp., Vancouver, BC, Canada.
Hassan Salari, Chemokine Therapeutics Corp., Vancouver, BC, Canada.
Hong Zhang, Dermatology Branch, National Cancer Institute, National, Institutes of Health, Bethesda, MD, USA.
Sam T. Hwang, Dermatology Branch, National Cancer Institute, National, Institutes of Health, Bethesda, MD, USA
Chand Khanna, Pediatric Oncology Branch, National Cancer Institute, National, Institutes of Health, 10 Center Drive, Building 31, Room 3A11, Bethesda, MD 20892, USA.
Lee J. Helman, Pediatric Oncology Branch, National Cancer Institute, National, Institutes of Health, 10 Center Drive, Building 31, Room 3A11, Bethesda, MD 20892, USA
References
- 1.Murdoch C. CXCR4: chemokine receptor extraordinaire. Immunol Rev. 2000;177:175–184. doi: 10.1034/j.1600-065x.2000.17715.x. [DOI] [PubMed] [Google Scholar]
- 2.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 3.Luster AD. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 4.Feng Y, Broder CC, Kennedy PE, et al. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 5.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 6.Murphy PM. Chemokines and the molecular basis of cancer metastasis. N Engl J Med. 2001;345:833–835. doi: 10.1056/NEJM200109133451113. [DOI] [PubMed] [Google Scholar]
- 7.Laverdiere C, Hoang BH, Yang R, et al. Messenger RNA expression levels of CXCR4 correlate with metastatic behavior and outcome in patients with osteosarcoma. Clin Cancer Res. 2005;11:2561–2567. doi: 10.1158/1078-0432.CCR-04-1089. [DOI] [PubMed] [Google Scholar]
- 8.Scala S, Ottaiano A, Ascierto PA, et al. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 2005;11:1835–1841. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- 9.Cardones AR, Murakami T, Hwang ST. CXCR4 enhances adhesion of B16 tumor cells to endothelial cells in vitro and in vivo via β1 integrin. Cancer Res. 2003;63:6751–6757. [PubMed] [Google Scholar]
- 10.Khanna C, Prehn J, Yeung C, et al. An orthotopic model of murine osteosarcoma with clonally related variants differing in pulmonary metastatic potential. Clin Exp Metastasis. 2000;18:261–271. doi: 10.1023/a:1006767007547. [DOI] [PubMed] [Google Scholar]
- 11.Perissinotto E, Cavalloni G, Leone F, et al. Involvement of chemokine receptor 4 / stromal cell-derived factor 1 system during osteosarcoma tumor progression. Clin Cancer Res. 2005;11:490–497. [PubMed] [Google Scholar]
- 12.Murakami T, Maki W, Cardones AR, et al. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002;62:7328–7334. [PubMed] [Google Scholar]
- 13.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 14.Dahlin DC, Coventry MB. Osteogenic sarcoma: a study of six hundred cases. J Bone Joint Surg Am. 1967;49:101–110. [PubMed] [Google Scholar]
- 15.Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63:3833–3839. [PubMed] [Google Scholar]
- 16.Lee CH, Kakinuma T, Wang J, et al. Sensitization of B16 tumor cells with a CXCR4 antagonist increases the efficacy of immunotherapy for established lung metastases. Mol Cancer Ther. 2006;5:2592–2599. doi: 10.1158/1535-7163.MCT-06-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]