Abstract
Methamphetamine, a commonly seen substance of abuse, has been reported to exert detrimental effect on bodily function including the cardiovascular system although its mechanism of action is poorly understood. This study was designed to examine the direct impact of methamphetamine on isolated whole heart and single cardiomyocyte contractile function. Murine hearts and isolated cardiomyocytes from adult FVB mice were exposed to various concentrations of methamphetamine for 30 min prior to the assessment of mechanical function using a Langendroff apparatus and an IonOptix Myocam® system, respectively. Cardiac contractile properties analyzed included maximal velocity of left ventricular pressure development and decline (± dP/dt), peak shortening amplitude (PS), maximal velocity of shortening/relengthening (± dLdt), time-to-PS (TPS), time-to-90% relengthening (TR90), resting and electrically-stimulated increase of intracellular Ca2+ as well as intracellular Ca2+ decay. Our results revealed that acute methamphetamine exposure depressed ± dP/dt, PS and rise of intracellular Ca2+ without affecting ± dLdt, TPS, TR90, resting intracellular Ca2+ and intracellular Ca2+ decay. Furthermore, methamphetamine nullified the adrenergic agonist norepinephrine-elicited positive cardiomyocyte contractile response, including elevated PS, ± dLdt and shortened TR90 without affecting TPS. Western blot analysis showed unchanged expression of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a) and phospholamban, associated with upregulated Na+-Ca2+ exchanger levels following acute methamphetamine exposure. In addition, methamphetamine promoted overt cardiomyocyte protein damage evaluated by carbonyl formation. Taken together, these results demonstrate direct cardiac depressant effect of methamphetamine in myocardium and isolated cardiomyocytes, possibly associated with protein damage and dampened adrenergic response.
Keywords: methamphetamine, myocardium, cardiomyocyte, protein damage, adrenergic response
INTRODUCTION
Methamphetamine, a synthetic amine, is derived from amphetamine with profound effect on the central nervous system. It is a potent additive stimulant illegally manufactured, distributed and abused in the United States (NIDA, 1998). It has been usually referred to as “speed”, “crystal”, “crank”, “go”, and “ice”. An estimated US population of 12.3 million are believed to consume the illegal drug somewhere during their life time, representing 5.2% of the American population of age 12 years and older (Office of Applied Studies, 2003). An alarming 54% increase of the emergency department visit was documented between 1995 to 2002 involving use of amphetamines or methamphetamines, with more than half the visits involving young adults aged 18 to 34 (Office of Applied Studies, 2003). The main cardiovascular manifestations of methamphetamine abuse encompass tachycardia, atrioventricular arrhythmias, myocardial ischemia and hypertension (Derlet and Horowitz, 1995; Anglin et al., 2000). Both experimental and clinical examinations especially those from autopsy and individual case reports suggest that methamphetamine exposure is associated with structural and functional changes of hearts, leading to cardiac hypertrophy, disarrangement of myofibers, fibrosis, dilated cardiomyopathy, congestive heart failure and sudden death (Jacobs, 1989; Islam et al., 1995; He et al., 1996; Maruta et al., 1997; Karch et al., 1999; Maeno et al., 2000; Wijetunga et al., 2003). More recently, a case-control study revealed that young methamphetamine users had an alarming 3.7-fold increase in the odds ratio for cardiomyopathy, after adjusting for age, body mass index, and renal failure (Yeo et al., 2007). Nonetheless, the precise mechanism behind methamphetamine exposure-elicited change in cardiac function remains elusive. The aim of the present study was to examine the effect of methamphetamine on cardiac contractile function at the levels of both whole heart and isolated cardiomyocytes. Multicellular cardiac contractile function measured as pre-load recruitable stroke work and dP/dt exhibits significantly decreased myocardial contractile function following methamphetamine treatment (Yu et al., 2002). However, assessment of methamphetamine exposure-induced cardiac electromechanical response in multicellular preparations suffers from the limitation that the heart encompasses a heterogeneous population of cells and may not accurately represent functional changes of the working cardiomyocytes. Thus, the action of methamphetamine on the hearts may be influenced by non-myocyte factors such as interstitial connective tissue or fibrous growth. For example, increased ventricular stiffness may reflect a greater amount of interstitial fibrosis and a shift in collagen content rather than a direct reflection on the mechanical properties of myocytes themselves. To the best of our knowledge, no study has utilized isolated cardiomyocytes to examine the impact of methamphetamine exposure in cardiac contractile function.
MATERIALS AND METHODS
Isolated heart Langendorff study
The experimental procedures described in this study were approved by the institutional animal care and use committee of University of Wyoming (Laramie, WY). In brief, adult male FVB mice (22 – 25 g) were anesthetized with ketamine/xylazine (3:5, 1.32 mg/kg, i.p.). Hearts were removed, the aorta was cannulated, placed on a Langendorff Apparatus (AD Instruments Model ML870B2), and perfused with KHB (95% O2, 5% CO2 bubbled; 5 ml/min at 37°C). A micro-balloon tipped catheter was inserted for left ventricular pressure measurements (Verma et al., 2003).
Isolation of murine cardiomyocytes
Single cardiomyocytes were isolated from adult male FVB mice (22 – 25 g) as described (Ren et al., 2008). In brief, hearts were removed and perfused with Krebs-Henseleit bicarbonate (KHB) buffer containing (in mM): 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 10 HEPES, and 11.1 glucose. Hearts were digested with collagenase D for 20 min. Left ventricles were removed and minced before being filtered. The percentage of the rod-shaped viable cardiomyocytes was ~ 70%.
Myocyte shortening and relengthening
Mechanical properties of cardiomyocytes were assessed by an IonOptix Myocam system (IonOptix Inc., Milton, MA, USA). Cells were placed in a chamber mounted on the stage of an inverted microscope and superfused (at 25oC) with a buffer containing (in mM): 131 NaCl, 4 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, 10 HEPES, at pH 7.4. The cells were field stimulated at a frequency of 0.5 Hz. Cell shortening and relengthening were assessed using the following indices: peak shortening (PS), time-to-PS (TPS) and time-to-90% relengthening (TR90), maximal velocities of shortening (+ dLdt) and relengthening (− dLdt) (Ren et al., 2008).
Intracellular Ca2+ fluorescence measurement
A cohort of cardiomyocytes was loaded with fura-2/AM (0.5 μM) for 10 min at 25oC, and fluorescence measurements were recorded with a dual-excitation fluorescence photomultiplier system (Ionoptix) as previously described (Ren et al., 2008). Myocytes were imaged through an Olympus IX-70 Fluor oil objective. Cells were exposed to light emitted by a 75W lamp and passed through either a 360 or a 380 nm filter (bandwidths were ± 15 nm), while being stimulated to contract at 0.5 Hz. Fluorescence emissions were detected between 480–520 nm by a photomultiplier tube after first illuminating the cells at 360 nm for 0.5 sec then at 380 nm for the duration of the recording protocol (333 Hz sampling rate). The 360 excitation scan was repeated at the end of the protocol and qualitative changes in intracellular Ca2+ concentration was inferred from the ratio of the fura-2 fluorescence intensity (FFI) at the two wavelengths. Fluorescence decay time (single and bi-exponential decay rates) was also measured as an indication of intracellular Ca2+ clearing rate.
Western blot analysis
Expression of SERCA2a, Na+-Ca2+ exchanger (NCX) and phospholamban (PLB) were examined by western blot. Following a 30-min exposure with methamphetamine (1.0 mM), cardiomyocytes were collected and sonicated in a lysis buffer containing 20 mM tris (ph 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% triton, 0.1% SDS and protease inhibitor cocktail. The protein concentration of the supernatant was assessed using the protein assay reagent (Bio-Rad Laboratories, Inc, Hercules, CA, USA). The extracted proteins were separated on 10 – 15% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes. After blocking the membrane was incubated with rabbit anti-SERCA2a (1:1000, Affinity Bioreagents Inc., Golden, CO, USA), rabbit anti-NCX polyclonal (1:1000, Swant, Bellinzona, Switzerland) and mouse anti-PLB monoclonal antibody (1:2000, Abcam inc, Cambridge, MA, USA) antibodies overnight at 4°c followed by incubation with second antibodies. The antigens were detected by the luminescence method. Quantification of band density was determined with the Quantity One software (Bio-Rad, version 4.4.0, build 36) and reported in optical density per square millimeter (Ren et al., 2008).
Protein carbonyl assay
Following methamphetamine treatment (1.0 mM, 30 min), proteins were extracted from cardiomyocytes. Nucleic acids were eliminated by treating the samples with 1% streptomycin sulphate for 15 min, followed by a 10 min centrifugation (11,000×g). Protein was precipitated by adding an equal volume of 20% trichloroacetic acid (TCA) to protein (0.5 mg) and centrifuged for 1 min. The TCA solution was removed and the sample resuspended in 10 mM 2, 4-dinitrophenylhydrazine (2,4-DNPH) solution. Samples were incubated at room temperature for 15–30 min. After adding 500 μl of 20% TCA, samples were centrifuged for 3 min. The supernatant was discarded, the pellet washed in ethanol: ethyl acetate and allowed to incubate at room temperature for 10 min. The samples were centrifuged again for 3 min and the ethanol: ethyl acetate steps repeated twice or more times. The precipitate was resuspended in 6 M guanidine solution, centrifuged for 3 min and any insoluble debris removed. The maximum absorbance (360–390 nm) of the supernatant was read against appropriate blanks (water, 2 M HCl) and the carbonyl content was calculated using the molar absorption coefficient of 22,000 M−1 cm−1(Ren et al., 2008).
Data analysis
Data were expressed as Mean ± SEM. Statistical significance (p < 0.05) for each variable was estimated by analysis of variance (ANOVA) followed by Tukey’s test for post hoc analysis.
RESULTS
Effect of methamphetamine on isolated heart function and cardiomyocyte contractile function
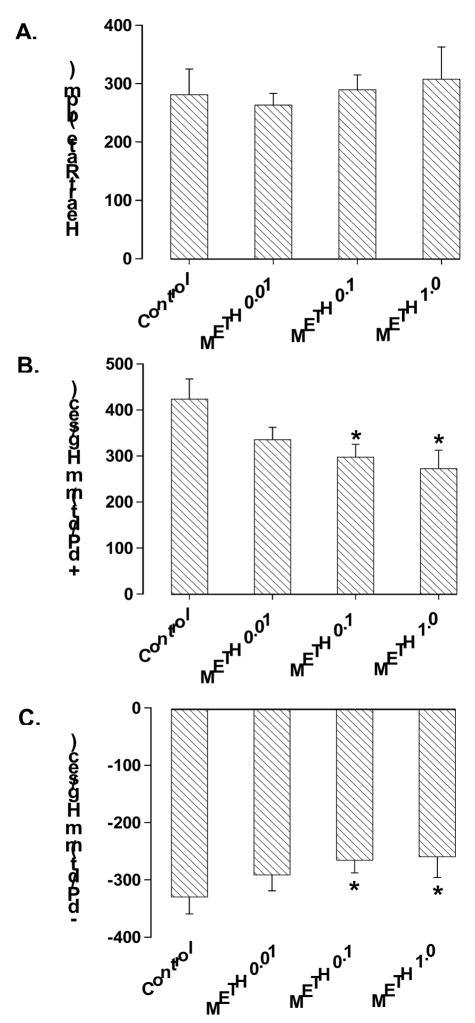
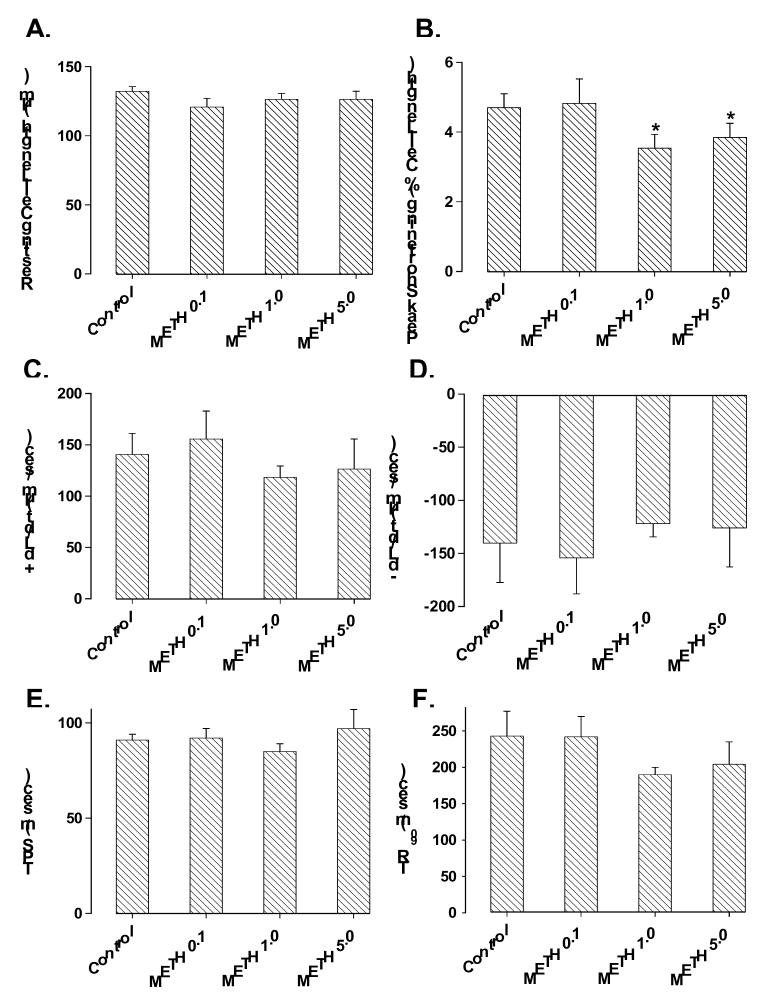
Acute exposure of methamphetamine for 30 min did not affect the heart rate although it significantly inhibited the maximal velocity of left ventricular pressure development (+ dP/dt) and decline (− dP/dt). At the end of a 30-min exposure, methamphetamine inhibited + dP/dt by ~30% and 37% at 0.1 mM and 1.0 mM, respectively. Similarly, methamphetamine inhibited − dP/dt by ~20% and 21% at 0.1 mM and 1.0 mM, respectively. The threshold of methamphetamine-induced suppression on heart function was between 0.01 mM and 0.1 mM (Fig. 1). The methamphetamine-induced inhibition in ± dP/dt was maximal within 15 min of exposure, maintainable up to 60 min and was reversible upon min washout (data not shown). The inhibitory effect of methamphetamine on isolated heart contractility was associated with reduced peak shortening in freshly isolated cardiomyocytes. However, the threshold required for the methamphetamine-elicited inhibition on cardiomyocyte contractile capacity was shifted to a higher concentration range (between 0.1 mM and 1.0 mM). The resting cell length, maximal velocity of shortening and relengthening (± dLdt), duration of shortening (TPS) and relengthening (TR90) were unaffected in cardiomyocytes by methamphetamine at the concentration range tested (Fig. 2).
Fig. 1.
Influence of acute methamphetamine (METH, 0.01 – 1.0 mM for 30 min) exposure on heart rate (panel A), maximal velocity of left ventricular pressure development (+ dP/dt, panel B) and decline (− dP/dt, panel C) in isolated mouse hearts. Mean ± SEM, n = 6, * p < 0.05 vs. control value.
Fig. 2.
Murine cardiomyocyte contractile function in response to acute methamphetamine (METH, 0.1 – 5.0 mM for 30 min) exposure. Panel A: Resting cell length; Panel B: peak shortening (% of cell length); Panel C: Maximal velocity of shortening (+ dL/dt); Panel D: Maximal velocity of relengthening (− dL/dt); Panel E: Time-to-PS (TPS); and Panel F: Time-to-90% relengthening (TR90). Mean ± SEM, n = 28 –35 cells per group, * p < 0.05 vs. control value.
Effect of methamphetamine on norepinephrine-elicited cardiomyocyte contractile response
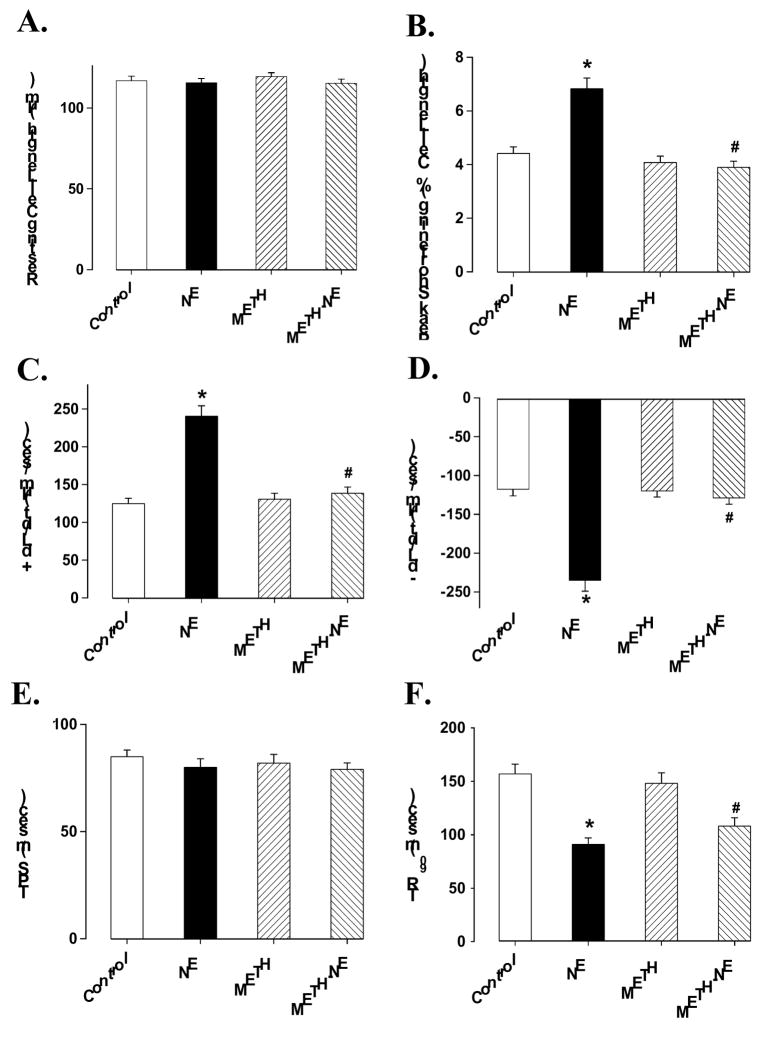
Methamphetamine is known to interfere with cardiac adrenergic responsiveness (Urabe, 1982), although cardiac norepinephrine content may not be depleted following chronic methamphetamine administration (Ruffoli et al., 2008). To examine the impact of acute methamphetamine exposure on cardiomyocyte adrenergic response, isolated cardiomyocytes were incubated with the adrenergic agonist norepinephrine (1 μM) in the absence or presence of methamphetamine (0.1 mM) for 30 min before mechanical function was assessed. Although this concentration of methamphetamine did not elicit any overt effect on cardiomyocyte contractile function, it effectively blunted norepinephrine-elicited increase in peak shortening (PS) as well as maximal velocities of shortening and relengthening (± dL/dt). Furthermore, norepinephrine elicited shortened cardiomyocyte relengthening duration (TR90), which was also abrogated by methamphetamine. The duration of shortening (TPS) was not affected by either norepinephrine or methamphetamine (Fig. 3).
Fig. 3.
Influence of methamphetamine (METH) on norepinephrine (NE)-induced cardiomyocyte contractile response. Isolated murine cardiomyocytes were incubated with NE (1 μM), METH (0.1 mM) or both for 30 min prior to assessment of mechanical function. Panel A: Resting cell length; Panel B: peak shortening (% of cell length); Panel C: Maximal velocity of shortening (+ dL/dt); Panel D: Maximal velocity of relengthening (− dL/dt); Panel E: Time-to-PS (TPS); and Panel F: Time-to-90% relengthening (TR90). Mean ± SEM, n = 107 cells per group, * p < 0.05 vs. control group, * p < 0.05 vs. NE group.
Effect of methamphetamine on intracellular Ca2+ transients
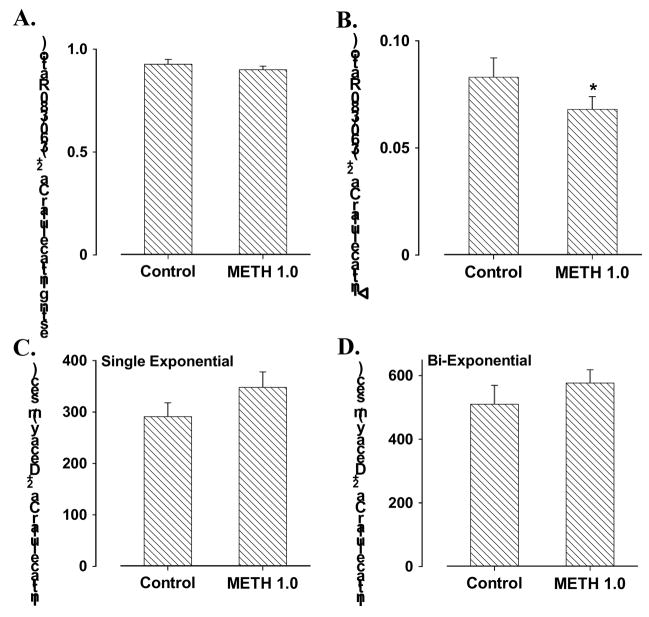
To explore the possible mechanism underlying methamphetamine-induced cardiomyocyte mechanical dysfunction, we used the membrane permeable intracellular Ca2+ fluorescent dye fura-2 to evaluate intracellular Ca2+ homeostasis in cardiomyocytes. Our results shown in Fig. 4 depicted comparable resting intracellular Ca2+ levels and intracellular Ca2+ transient decay rates (either single or bi-exponential) associated with a decrease in electrically-stimulated rise in intracellular Ca2+ in cardiomyocytes following methamphetamine (1.0 mM) exposure. These data indicated existence of intracellular Ca2+ handling defect in cardiomyocytes following methamphetamine exposure.
Fig. 4.
Murine cardiomyocyte intracellular Ca2+ property in response to acute methamphetamine (METH, 1.0 mM for 30 min) exposure. Panel A: Resting intracellular Ca2+ levels; Panel B: electrically-stimulated increase in intracellular Ca2+ levels; Panel C: Intracellular Ca2+ transient decay rate (single exponential); and Panel D: Intracellular Ca2+ transient decay rate (Bi-exponential). Mean ± SEM, n = 47 –50 cells per group, * p < 0.05 vs. control value.
Protein expression of intracellular Ca2+ regulatory proteins and carbonyl formation
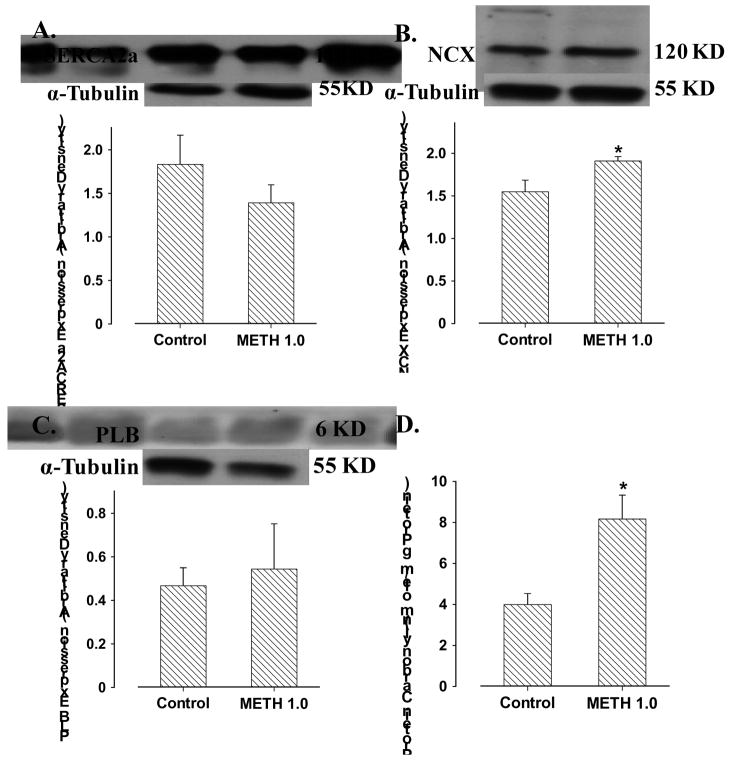
To better understand the mechanism of action responsible for methamphetamine-induced intracellular Ca2+ dysregulation, Western blot analysis was performed to assess the expression of the key Ca2+ regulatory proteins. Our data revealed that acute methamphetamine (1.0 mM) exposure unregulated the expression of NCX without affecting that of SERCA2a and phospholamban. Our data further revealed that significantly elevated formation of protein carbonyl in myocytes following methamphetamine exposure (Fig. 5).
Fig. 5.
Levels of cardiomyocyte Ca2+ regulatory proteins and myocardial protein carbonyl formation in response to acute methamphetamine (METH, 1.0 mM for 30 min) exposure. Panel A: SERCA2a; Panel B: Na+-Ca2+ exchanger (NCX); Panel C: Phospholamban (PLB); and Panel D: Protein carbonyl formation. Inset: Representative gel blots depicting the expression of SERCA2a, NCX and PLB using specific antibodies. Mean ± SEM, n = 6 – 7 cell isolation or myocardium per group, * p < 0.05 vs. control value.
DISCUSSION
Our study provided evidence for the first time that the physically and psychologically additive substance methamphetamine directly depresses cardiac contractile function, intracellular Ca2+ handling and adrenergic response in isolated murine cardiomyocytes, in parallel to its cardiac depressant action on the whole heart Langendorff apparatus setting. The methamphetamine-induced cardiac depression may involve enhanced protein damage evidenced by enhanced carbonyl formation. Intracellular Ca2+ homeostasis was disturbed by acute methamphetamine exposure supported by dampened intracellular Ca2+ release in response to electrical stimuli and upregulated NCX but not SERCA2a and phospholamban expression. These results favor a direct detrimental effect of methamphetamine on cardiomyocyte mechanical function, which may contribute to the methamphetamine-induced impairment of myocardial contractile function following exposure of the abused substance.
Although the mechanism(s) of action underscoring reduced cardiomyocyte contraction in response to acute methamphetamine exposure is not fully clear at this time, several speculations may be considered. First, the observation that methamphetamine abolished norepinephrine-induced positive cardiac contractile response suggests potential involvement of adrenergic response in methamphetamine-elicited inhibitory effect in the heart. This is consistent with the previous finding that methamphetamine directly interferes with cardiac adrenergic responsiveness (Urabe, 1982). Methamphetamine exposure was reported to promote catecholamine including norepinephrine release (Yu et al., 2002), the later may directly stimulate cardiac adrenergic receptor, improved cardiac contractility (albeit transiently), cardiac remodeling leading to cardiac hypertrophy and fibrosis. Excessive stimulation of catecholamine release in response to methamphetamine exposure may quickly exhaust the catecholamine storage (Ruffoli et al., 2008), leading to a reduced adrenergic response. Catecholamine desensitization is a hallmark of heart failure (Brodde et al., 2006). It has been shown that heart failure may trigger classical catecholamine desensitization to both isoproterenol and norepinephrine in the presence and absence of ganglionic blockade (Brodde et al., 2006). Data from our current study is in line with the desensitized adrenergic response and cardiac contractile function, supporting a role of adrenergic desensitization in methamphetamine-elicited heart anomalies and ultimately heart failure. Nonetheless, it should be mentioned that direct action of methamphetamine on the cardiac adrenergic signaling cascade (such as at the levels of membrane receptor, G protein and G protein coupled effectors) is essentially unknown. Secondly, the observation that methamphetamine inhibits the electrically-stimulated intracellular Ca2+ release and upregulates Na+-Ca2+ exchanger depicts that methamphetamine may interferes with intracellular Ca2+ homeostasis. The reduced intracellular Ca2+ release in response to electrical stimuli is consistent with the compromised myocardial contractility (± dP/dt and PS) following acute exposure of methamphetamine. Expression of SERCA2a and phospholamban was unchanged following methamphetamine exposure, not favoring a role of the sarco(endo)plasmic Ca2+-ATPase (Ca2+ pump) in methamphetamine-induced cardiomyocyte mechanical response. This observation of unchanged SERCA2a/phospholamban expression is in line with the unaffected duration of shortening and relengthening (TPS and TR90) following acute methamphetamine exposure. Upregulated NCX expression/activity has been shown to be associated with compromised myocardial contractile function due to its possible role in reduced cytosolic Ca2+ ion (Goldhaber et al., 2005). Homozygous overexpression of NCX has been shown to lead to modest hypertrophy at baseline and are more susceptible to cardiac failure during the stress of pregnancy or aortic banding (Goldhaber et al., 2005).
Data from our current study revealed enhanced protein carbonyl formation, indicative of protein damage, in cardiomyocytes following methamphetamine exposure. Although exactly how methamphetamine induces protein damage warrants further study, methamphetamine has been shown to promote apoptosis and accumulation of reactive oxygen species. Methamphetamine is known to trigger neuronal, splenic and myocardial damage via apoptosis (Iwasa et al., 1996; Davidson et al., 2001). In addition, methamphetamine may facilitate the formation of reactive oxygen species including ONOO−, which in turn can cause tissue and cell damage (Wang et al., 2001). Although it is beyond the scope of the current study, methamphetamine has been demonstrated to promote p53 expression and necrosis (Imam et al., 2001). These apoptotic and necrotic as well as pro-oxidation properties of methamphetamine are consistent with the detrimental effect of methamphetamine on protein integrity.
Measurement of contractile performance in isolated cardiomyocytes has been established to provide a fundamental assessment of cardiac contractile function in pathological states, in this case acute methamphetamine exposure. However, as in any study of this nature, caution needs to be taken when correlating the cellular findings to whole heart function, as the latter is composed of heterogeneous cell types, including nerve terminals and fibroblasts. These heterogeneous cell types may have contributed to the subtle discrepancies between our cardiomyocyte and Langendorff heart studies (e.g., in the threshold of methamphetamine).
In conclusion, our study provides the first laboratory evidence at the level of cardiomyocytes to confirm the clinical findings that methamphetamine use is linked to a distinctive form of dilated cardiomyopathy (Jacobs, 1989; Wijetunga et al., 2003; Yeo et al., 2007). Our results demonstrated that direct cardiomyocyte contractile depression by methamphetamine, possibly through protein damage and intracellular Ca2+ dysregulation. These findings not only reveal an essential role of the basic myocardial working element, cardiomyocytes, in methamphetamine-induced cardiac anomalies but also provide new insights for better therapeutic remedy against methamphetamine-induced global cardiac pathology. Nonetheless, the precise nature behind methamphetamine-induced depression on cardiomyocyte contractile function is still unclear. Awaiting future studies include cardiac excitation-contraction coupling and membrane ion channels in response to methamphetamine exposure. These approaches will be crucial to further our understanding of the pathological profiles of methamphetamine.
Acknowledgments
This work was supported in part by the University of Wyoming North Rockies Regional INBRE 5P20RR016474.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anglin MD, Burke C, Perrochet B, Stamper E, Dawud-Noursi S. History of the methamphetamine problem. Journal of psychoactive drugs. 2000;32:137–141. doi: 10.1080/02791072.2000.10400221. [DOI] [PubMed] [Google Scholar]
- Brodde OE, Bruck H, Leineweber K. Cardiac adrenoceptors: physiological and pathophysiological relevance. Journal of pharmacological sciences. 2006;100:323–337. doi: 10.1254/jphs.crj06001x. [DOI] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain research. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Derlet RW, Horowitz BZ. Cardiotoxic drugs. Emergency medicine clinics of North America. 1995;13:771–791. [PubMed] [Google Scholar]
- Goldhaber JI, Henderson SA, Reuter H, Pott C, Philipson KD. Effects of Na+-Ca2+ exchange expression on excitation-contraction coupling in genetically modified mice. Annals of the New York Academy of Sciences. 2005;1047:122–126. doi: 10.1196/annals.1341.011. [DOI] [PubMed] [Google Scholar]
- He SY, Matoba R, Fujitani N, Sodesaki K, Onishi S. Cardiac muscle lesions associated with chronic administration of methamphetamine in rats. Am J Forensic Med Pathol. 1996;17:155–162. doi: 10.1097/00000433-199606000-00014. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Itzhak Y, Cadet JL, Islam F, Slikker W, Jr, Ali SF. Methamphetamine-induced alteration in striatal p53 and bcl-2 expressions in mice. Brain Res Mol Brain Res. 2001;91:174–178. doi: 10.1016/s0169-328x(01)00139-5. [DOI] [PubMed] [Google Scholar]
- Islam MN, Kuroki H, Hongcheng B, Ogura Y, Kawaguchi N, Onishi S, Wakasugi C. Cardiac lesions and their reversibility after long term administration of methamphetamine. Forensic Sci Int. 1995;75:29–43. doi: 10.1016/0379-0738(95)01765-b. [DOI] [PubMed] [Google Scholar]
- Iwasa M, Maeno Y, Inoue H, Koyama H, Matoba R. Induction of apoptotic cell death in rat thymus and spleen after a bolus injection of methamphetamine. International journal of legal medicine. 1996;109:23–28. doi: 10.1007/BF01369597. [DOI] [PubMed] [Google Scholar]
- Jacobs LJ. Reversible dilated cardiomyopathy induced by methamphetamine. Clin Cardiol. 1989;12:725–727. doi: 10.1002/clc.4960121211. [DOI] [PubMed] [Google Scholar]
- Karch SB, Stephens BG, Ho CH. Methamphetamine-related deaths in San Francisco: demographic, pathologic, and toxicologic profiles. J Forensic Sci. 1999;44:359–368. [PubMed] [Google Scholar]
- Maeno Y, Iwasa M, Inoue H, Koyama H, Matoba R, Nagao M. Direct effects of methamphetamine on hypertrophy and microtubules in cultured adult rat ventricular myocytes. Forensic Sci Int. 2000;113:239–243. doi: 10.1016/s0379-0738(00)00216-4. [DOI] [PubMed] [Google Scholar]
- Maruta T, Nihira M, Tomita Y. Histopathological study on acute poisoning of methamphetamine, morphine or cocaine. Nihon Arukoru Yakubutsu Igakkai Zasshi. 1997;32:122–138. [PubMed] [Google Scholar]
- NIDA. Methamphetamine abuse and addiction. NIDA Research Report 1998 [Google Scholar]
- Office of Applied Studies. NSDUH Series H-25. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Office of Applied Studies; Rockville, MD: 2003. Results from the 2002 National Survey on Drug Use and Health:National Findings. [Google Scholar]
- Ren J, Privratsky JR, Yang X, Dong F, Carlson EC. Metallothionein alleviates glutathione depletion-induced oxidative cardiomyopathy in murine hearts. Critical care medicine. 2008;36:2106–2116. doi: 10.1097/CCM.0b013e31817bf925. [DOI] [PubMed] [Google Scholar]
- Ruffoli R, Soldani P, Pasquali L, Ruggieri S, Paparelli A, Fornai F. Methamphetamine fails to alter the noradrenergic integrity of the heart. Annals of the New York Academy of Sciences. 2008;1139:337–344. doi: 10.1196/annals.1432.017. [DOI] [PubMed] [Google Scholar]
- Urabe M. Inhibitory mechanism of methamphetamine in the isolated myocardium of bullfrog. Arch Int Pharmacodyn Ther. 1982;257:239–254. [PubMed] [Google Scholar]
- Verma S, Yuen VG, Badiwala M, Anderson TJ, McNeill JH. Working heart function in diabetes is not improved by spironolactone treatment. Canadian journal of physiology and pharmacology. 2003;81:493–496. doi: 10.1139/y03-041. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hayashi T, Chang CF, Chiang YH, Tsao LI, Su TP, Borlongan C, Lin SZ. Methamphetamine potentiates ischemia/reperfusion insults after transient middle cerebral artery ligation. Stroke; a journal of cerebral circulation. 2001;32:775–782. doi: 10.1161/01.str.32.3.775. [DOI] [PubMed] [Google Scholar]
- Wijetunga M, Seto T, Lindsay J, Schatz I. Crystal methamphetamine-associated cardiomyopathy: tip of the iceberg? J Toxicol Clin Toxicol. 2003;41:981–986. doi: 10.1081/clt-120026521. [DOI] [PubMed] [Google Scholar]
- Yeo KK, Wijetunga M, Ito H, Efird JT, Tay K, Seto TB, Alimineti K, Kimata C, Schatz IJ. The association of methamphetamine use and cardiomyopathy in young patients. Am J Med. 2007;120:165–171. doi: 10.1016/j.amjmed.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Yu Q, Montes S, Larson DF, Watson RR. Effects of chronic methamphetamine exposure on heart function in uninfected and retrovirus-infected mice. Life sciences. 2002;71:953–965. doi: 10.1016/s0024-3205(02)01769-1. [DOI] [PubMed] [Google Scholar]