Abstract
This review provides an overview of the molecular mechanisms of K transport in the mammalian connecting tubule (CNT) and cortical collecting duct (CCD), both nephron segments responsible for the regulation of renal K secretion. Aldosterone and dietary K intake are two of the most important factors regulating K secretion in the CNT and CCD. Recently, angiotensin II (AngII) has also been shown to play a role in the regulation of K secretion. In addition, genetic and molecular biological approaches have further identified new mechanisms by which aldosterone and dietary K intake regulate K transport. Thus, the interaction between serum-glucocorticoid-induced kinase 1 (SGK1) and with-no-lysine kinase 4 (WNK4) plays a significant role in mediating the effect of aldosterone on ROMK (Kir1.1), an important apical K channel modulating K secretion. Recent evidence suggests that WNK1, mitogen-activated protein kinases such as P38, ERK, and Src family protein tyrosine kinase are involved in mediating the effect of low K intake on apical K secretory channels.
Keywords: Potassium transport, Potassium channel, Potassium secretion, Angiotensin, Aldosterone, K channel, Kidney
Overview of K transport mechanism in CNT and CCD
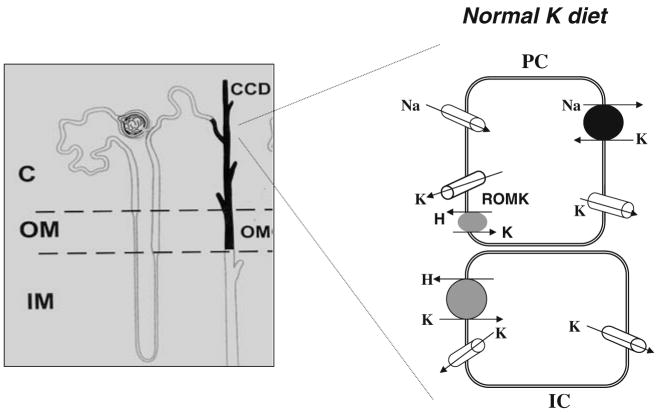
Maintaining plasma K within a narrow physiological range is essential for the function of neurons, cardiac myocytes, and skeletal muscles. The kidney plays a key role in regulating K excretion by completely filtering K in the glomerulus, reabsorbing K extensively along the proximal tubule and thick ascending limb, secreting K in the connecting tubule (CNT) and cortical collecting duct (CCD), and reabsorbing K in outer medullary collecting duct (OMCD). Two morphological distinct cells, principal cell (PC) and intercalated cell (IC), are present in the CNT and CCD [29, 75], and it is generally accepted that PC and IC are responsible for K secretion and for K absorption, respectively [27, 30]. Figure 1 is a cell model illustrating the K transport mechanism under control conditions (normal K intake) in both PC and IC in the CCD [29, 74, 75]. K secretion takes place by a two-step process: K enters the cell via the basolateral Na,K-ATPase and is secreted into the lumen through apical K channels along a favorable electrochemical gradient [76, 101]. K absorption is achieved by K entering the cell across the apical membrane through a luminal H,K-ATPase and leaving the cell across the basolateral membrane along a favorable K electrochemical gradient [19, 21, 33]. Although H,K-ATPase is mainly expressed in IC in collecting duct, colonic H,K-ATPase has been shown to be expressed in PC of connecting tubule and the CCD [115].
Fig. 1.
A model of principal cell (PC) and intercalated cell (IC) illustrates the K transport under control conditions (normal K intake)
Luminal Na transport provides normally an important driving force for K secretion [97], but recent evidence has shown that K secretion may continue when luminal Na transport is compromised. As demonstrated in microperfused rabbit CCD, K secretion may continue, albeit at a reduced rate, in the absence of luminal Na [69]. Since inhibition of basolateral Na/H exchanger significantly decreases such K secretion in the absence of luminal Na, it is most likely that Na recycling across the basolateral membrane through Na/H exchange supplies enough Na to sustain the activity of Na,K-ATPase which is essential for K secretion.
In addition to entering PC by Na,K-ATPase, K can also gain cell access across the basolateral membrane via K channels in the CCD provided the basolateral membrane hyperpolarizes to exceed the K equilibrium potential. This may occur as consequence of mineralocorticoid-induced stimulation of Na,K-ATPase [92]. Three types of K channels have been identified in the basolateral membrane of the CCD and shown to be activated by cGMP-dependent pathways [32, 37, 120, 123]. The regulation of Na,K-ATPase in the CCD has been covered in two review articles [24, 116] and will not be further discussed in the present review. Thus, this review paper is focused largely on the apical K transport mechanisms, especially apical K channels, in the CNT and CCD.
Apical K transport in the CNT and collecting duct
K channels
Several types of K channels including ROMK (Kir1.1), a Ca2+-activated big-conductance K channel (BK) and double-pore K channel, KCNK1, are expressed in the apical membrane of the CNT and CCD [25–27, 38, 78, 79, 95]. It is now well established that ROMK and BK channels are responsible for K secretion. In contrast, further experiments will be necessary to define the role of the two-pore K channels in K transport in the CNT or CCD. ROMK channels have similar biophysical properties and regulatory mechanism as that of the native small-conductance K (SK) channels identified in the mouse and rat CCD [35]. Thus, we terminate the SK as a ROMK-like SK channels in the present review. Since ROMK-like SK channels have a high open probability and are abundantly expressed in the apical membrane of the CNT and CCD under control conditions, ROMK channels are thought to play a major role for K secretion under normal dietary K intake [26, 31, 56, 93, 95, 100, 119]. However, when the tubule flow rate is high or dietary K intake increases [9, 94, 136], both BK channels and ROMK-like SK are involved in mediating K secretion.
ROMK
The ROMK channel [36] is a member of inwardly rectifying K (KIR) channels [72] that are functionally characterized by high K selectivity and either weak or strong inward rectification. The information about ROMK structure is largely obtained from the X-ray crystallographic structure of a K channel from Streptomyces lividans [20], demonstrating that each contains two membrane spanning segments and cytoplasmic N and C termini with high homology to the pore-forming H5 segment of voltage-gated K channels. Moreover, Minor et al. [66] employed a yeast genetic screening technique to analyze the packing structure of the M1 and M2 domains. They suggest that M2 segments line the pore and are surrounded by M1 segments which also participate in subunit–subunit interactions in the tetrameric channel complex.
ROMK channels are pH-sensitive, and a decrease in cell pH from 7.4 to 7.0 completely inhibits channel activity (which is defined by NPo, a product of channel number and open probability) [14, 85, 119]. Structure and function analysis have demonstrated that interaction between lysine residue 80 and alanine residue 177 on M2 domain is essential for the pH sensitivity of ROMK [85]. The native ROMK-like SK channel is sensitive to ATP [119], but ATP sensitivity is absent in ROMK channels expressed in oocytes. Several studies have demonstrated that ROMK channels may interact with CFTR or sulfonylurea receptor type 2B (SUR2B) and that such interaction is required for the ATP sensitivity [18, 59, 63]. ROMK channels have three putative PKA phosphorylation sites, and stimulation of PKA-induced phosphorylation increases ROMK channel activity [1, 64, 138]. This is achieved by either enhancing the insertion of ROMK channels into plasma membrane [73, 141] or by augmenting the effect of PIP2 [57] which has been shown to activate ROMK channels [40, 58]. Serum-glucocorticoid-induced kinase 1 (SGK1) also stimulates ROMK channel activity [143] by increasing the phosphorylation of a serine residue of the N terminus of ROMK (Ser 44 for ROMK1), a putative PKA phosphorylation site [140]. Thus, SGK1 stimulates the surface expression of ROMK1 channels through facilitating the export from endoplasmic reticulum. Recently, it has been suggested that SGK1 stimulates ROMK channels by phosphorylation of WNK4 [88]. The effect of PKC on ROMK channel is complex because PKC has both stimulatory and inhibitory effects [53, 144]. PKC-induced phosphorylation of ROMK channels is required for export of ROMK1 channels to the cell membrane [53]. However, stimulation of PKC inhibits ROMK channels by decreasing the sensitivity of ROMK channels to PIP2 [144]. ROMK1 channels are also a substrate for Src family protein tyrosine kinase (PTK) which increases the endocytosis of ROMK1 channels in the CCD by stimulation of tyrosine phosphorylation [54, 67].
Ca2+-activated BK
The BK channel is composed of a pore-forming α subunit (Slo 1) with six transmembrane segments and an accessory β subunit [60, 91]. BK channel activity has been detected at the apical membrane of both PC and IC of the CCD [25, 42]. Real-time polymerase chain reaction performed in the isolated single CCD has demonstrated that BK channel α, β2, and β4 subunits, but not β1, are expressed in rabbits fed a high K diet. Moreover, high K intake stimulates the transcription of BK channel β2 and 4 subunits [70]. In contrast, low Na intake, which increases aldosterone level, does not affect the messenger RNA level of BK α, β2, and β4 subunits in rabbit CCD, suggesting that aldosterone does not contribute to the regulation of BK channel expression [23]. BK channels are sensitive to cell pH and ATP at physiological Ca2+ levels [98, 99]. Their contribution to K secretion was initially deemed uncertain in light of their very low open probability in CCDs. Because such patch-clamp experiments were performed in split-open tubules with no fluid flow [25, 51], it is possible that in vivo BK channel activity may be higher. This possibility is suggested by two recent studies highlighting the role of BK channels in flow-stimulated K secretion in both the CNT [112] and the CCD [136]. The role of BK channel in flow-stimulated K secretion is also supported by the observation that an increase in flow failed to stimulate K secretion in the distal nephron in BK-α subunit knockout mice [87]. It is of interest that patch-clamp experiments have demonstrated high BK channel activity in IC [84]. Because IC has a low Na,K-ATPase activity, it is difficult to explain their possible contribution to significant rates of K secretion [10]. However, two lines of evidence suggest that BK channels in PC are involved in mediating renal K secretion: (1) BK channel activity in PC is increased in the CCD from rats on a high K diet and (2) BK channel activity is significantly augmented by inhibition of P38 and ERK, both of which are suppressed by HK intake [51]. Although BK channels play a role in flow-induced stimulation of K secretion, deletion of the BK channel α subunit does not affect the net K excretion in mice fed with high K. This suggests that HK-induced stimulation of ROMK channel expression and high plasma aldosterone level can compensate for deleting BK channels on K secretion [87].
KCl co-transport
The KCl co-transporter (KCC), most likely KCC1, has been shown to be expressed in the apical membrane of the distal nephron including CCD [52]. Several studies suggest a role for apical KCC in renal K secretion. A reduction in luminal Cl markedly increases K secretion in perfused rat distal tubules, a mixture of distal convoluted tubule, CNT, and initial CCD [22, 114]. This component of K secretion is not influenced by luminal Ba2+ or amiloride [22] and can be blocked by luminal inhibitors of KCl co-transporters [2]. These findings have been extended to the rabbit CCD where a decrease in luminal Cl from 112 to 5 mM increases K secretion by 48% [134]. A reduction in basolateral Cl also decreases K secretion without an effect on transepithelial voltage or Na transport. The direction of K flux can be reversed by a lumen-to-bath Cl gradient, resulting in K absorption. In perfused CCDs from rats treated with mineralocorticoid, vasopressin increases K secretion [96]. Since this increase in K secretion is resistant to luminal Ba2+, vasopressin may stimulate apical KCC in the distal tubule [2].
H,K-ATPase/K-ATPase
Two types of H,K-ATPase are expressed in the kidney: colonic H,K-ATPase, which is sensitive to both ouabain and Sch28080, and gastric H,K-ATPase, which is inhibited by Sch-28080 [6, 15, 21, 47, 103, 132]. Molecular cloning has revealed that colonic and gastric H,K-ATPase share 60–70% sequence homology and that gastric H,K-ATPase and colonic H,K-ATPase contain HKα1 (type I K-ATPase) and HKα2 (type III K-ATPase), respectively [39]. Several early studies have suggested that gastric H,K-ATPase was involved in renal K absorption from K-depleted animals [12, 28, 142]. It had been reported that Rb influx, an index of K transport, increased in the OMCD from rabbits on a K-deficient diet and that this effect was abolished by inhibition of gastric H,K-ATPase [147]. However, the late investigations have shown that colonic H,K-ATPase, rather than gastric H,K-ATPase, is mainly responsible for renal K reabsorption and that gastric H,K-ATPase is involved in mediating K-dependent proton secretion in collecting duct [39]. First, application of ouabain inhibited Sch-28080-sensitive Rb2+ absorption in OMCD [146]. Second, K restriction significantly increases the expression of type III K-ATPase [103]. Third, K-ATPase activity determined by ATP hydrolysis rate in the CCD and OMCD is not affected in HKα1 (−/−) mice on K-deficient diet, while no K-ATPase activity was detected in HKα2(−/−) mice on K-deficient diet [17]. However, deletion of colonic H,K-ATPase does not display the renal phenotype even in mice fed on K-deficient diet [65], suggesting kidney has an alternative mechanism to reabsorb K in HKα2(−/−) mice. Moreover, immunostaining has shown that colonic H,K-ATPase is also detected in the apical membrane of PC of the CCD during K restriction, suggesting that PC is also involved in K reabsorption [33].
Regulation of K excretion by K diet
K transport in the CNT and CCD is regulated by hormones such as aldosterone and dietary K intake. Dietary K intake plays a key role in the regulation of renal K secretion: High K intake stimulates, whereas low K intake decreases renal K secretion [79, 83, 121]. Recently, several new mechanisms by which K intake regulates apical secretory K channels in the CCD have been reported. They include WNK, SGK1, and CYP-epoxygenase-dependent metabolites of arachidonic acid.
Effect of high dietary K intake
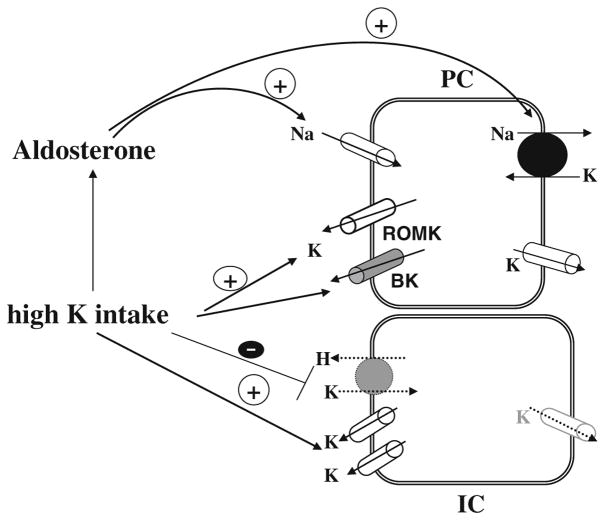
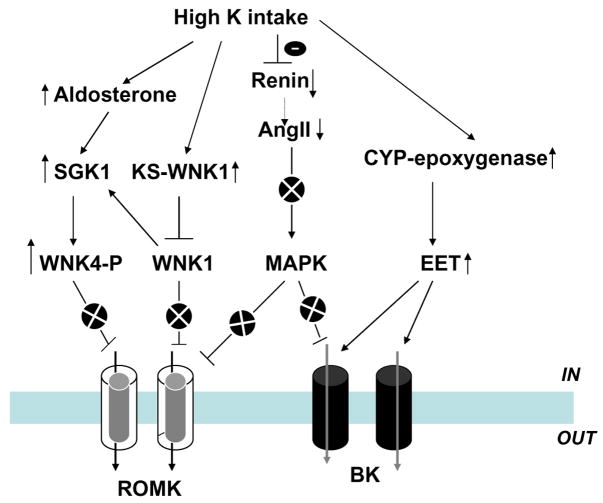
High K-intake-induced stimulation of renal K secretion is mediated by both aldosterone-dependent and an aldosterone-independent mechanism. Figure 2 is a cell model illustrating the current understanding of the effects of high K intake on K transport in PC and IC. HK intake stimulates aldosterone secretion which augments activity of both Na,K-ATPase and ENaC [24, 80, 89, 109, 116, 131]. Increased ENaC activity augments the driving force for K exit across the apical membrane of the CNT and CCD, whereas high Na,K-ATPase activity stimulates K secretion by increasing K uptake across the basolateral membrane. Mineralocorticoid receptor (MR) knockout mice have severe hyperkalemia and hyponatremia, underscoring the importance of aldosterone in the regulation of renal K secretion [11]. Moreover, high K intake significantly increases the activity of ROMK-like SK and BK channels, at least in part, by stimulating the expression of BK channel α subunit in the CCD [51, 70, 121]. However, the high K-intake-induced stimulation of ROMK-like SK channel activity may also require the involvement of factors other than aldosterone because infusion of aldosterone or application of low Na diet, a maneuver which increases circulated aldosterone level, fails to mimic the effect of high K intake [81, 82]. The notion that high K intake increases K secretion by an aldosterone-independent mechanism is also suggested by the observation that high K intake continues, albeit at a reduced rate, to stimulate K secretion in the isolated perfusion CCD from adrenalectomized rabbit [68]. HK intake inhibits K reabsorption not only by decreasing colonic H,K-ATPase expression [39] but also through enhancing apical K channel activity in IC [51]. This may indirectly affect the activity of H,K-ATPase by enhancing K recycling across the apical membrane, thereby diminishing net K reabsorption. This review is supported by the observation that inhibition of apical K channels in IC suppresses the H,K-ATPase activity (measured by proton extrusion) in K-repleted animals but has no effect in the K-restricted animals [145]. Although apical K channels in the IC play a role in the regulation of H, K-ATPase activity, their regulation mechanism has not yet been extensively explored. In contrast, advance in molecular biology has identified new signaling pathway regulating the apical K channels in PC. Figure 3 is a scheme illustrating the current understanding about mechanisms by which high K intake regulates apical K channels in the CCD through aldosterone-dependent and -independent signaling pathways. First, high K intake is expected to abolish the inhibitory effect of WNK4 on ROMK channels through aldosterone and SGK1 pathway. Second, high K intake suppresses renin-AngII signaling pathway which could decrease both ROMK and BK channel activity by a mitogen-activated protein kinases (MAPK)-dependent mechanism [7, 51]. Third, high K intake stimulates CYP epoxygenase activity and increases 11,12-EET production which stimulates BK channel activity [111]. However, the first possibility that aldosterone may stimulate ROMK channel by SGK1-dependent mechanism is only a speculation and needs to be examined in the future study.
Fig. 2.
A cell scheme illustrating the mechanism by which high K intake stimulates K secretion in the CCD by an aldosterone-dependent and -independent mechanisms. Solid arrow and dotted arrow indicate enhanced and diminished effect, respectively
Fig. 3.
A scheme showing the role of different signaling pathways in mediating the effect of high K intake on ROMK and BK channels in the CCD. The circle with x indicates the inhibition of a particular signaling pathway
WNKs and KS-WNK1
WNKs belong to a family of serine/threonine protein kinases. Four mammalian WNKs have been identified [46], of which WNK1, 3, and 4 are expressed in the CCD [45, 46, 50] and play an important role in the regulation of ROMK channels [16, 44, 48, 50, 88, 117]. Co-expression of WNK1, 3, and 4 inhibits the ROMK channel activity in Xenopus oocytes, and the effect of WNKs on ROMK is mediated by stimulation of clathrin-dependent endocytosis [44]. Recently, it has also been demonstrated that intersectin, a scaffold protein containing two Eps15 homology domains and four or five tandem SH3 domains, is required for the interaction between WNK4 and clathrin [34]. In addition, a kidney-specific splice form of WNK1 (KS-WNK1), in which an alternative 5′ exon replaces the first four exons of WNK1, is expressed in the CCD [77]. Unlike the long form of WNK1 which inhibits ROMK channels [16], KS-WNK1 lacks kinase activity and does not block ROMK channels. Moreover, KS-WNK1 can antagonize the inhibitory effect of WNK1 [48, 117]. It has been reported that high K intake increases the expression of KS-WNK1 and accordingly attenuates the inhibitory effect of WNK1 on ROMK channels [48, 117]. Thus, the alteration of the ratio between long and short form of WNK1 may be an important mechanism by which high K intake stimulates ROMK channel activity. Moreover, WNK1 has also been reported to stimulate SGK1 through PI3 kinase [137]. Because high K intake is expected to increase SGK1, which stimulates K secretion [113], WNK1-mediated activation of SGK1 could also play a role in mediating the effect of high K intake on K secretion.
Aldosterone and SGK
A large body of evidence suggests that SGK1 mediates, at least in part, the effect of aldosterone on renal K secretion [41, 88, 113, 116]. This notion is supported by studies performed in SGK1 knockout mice demonstrating that the phenotype of SGK1 deletion is similar to MR knockout mice and displays impaired renal K secretion in response to high dietary K intake [41]. The mechanism by which SGK1 stimulates renal K secretion includes enhancing the export of ROMK channels from the ER [140]. Recently, it has also been shown that WNK4 is the substrate of SGK1 which phosphorylates serine residue 1169 of its C terminus. Moreover, such SGK1-induced phosphorylation of WNK4 abolishes its inhibitory effect on ROMK channels in Xenopus oocytes [88]. However, the immunostaining study performed in SGK1 knockout mice shows that apical staining of ROMK channels in the CNT and CCD is normal or even intensified [41], suggesting that SGK1 is not essential for the export of ROMK channels. Thus, the stimulatory effect of aldosterone and SGK1 on K secretion may be mediated mainly by increasing Na transport. But it should be noted that in chronic experiments, aldosterone or SGK1 may play a permissive role in mediating the effect of high K intake on ROMK channels because high K intake failed to stimulate ROMK channels in adrenalectomized rats [81].
Role of AngII
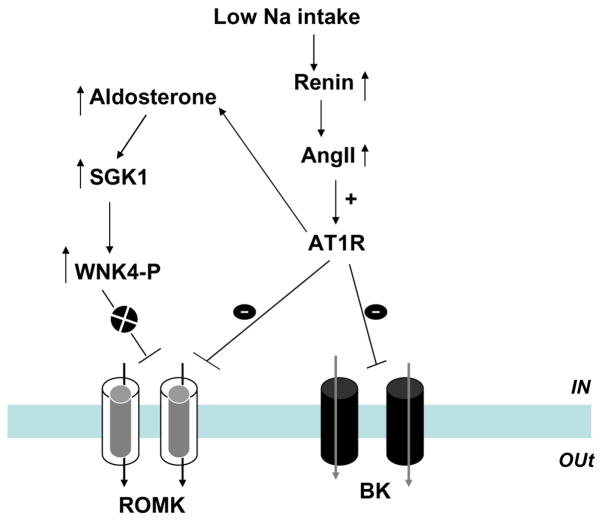
Because high K intake suppresses the renin and AngII system [90, 102], it may play a role in modulating the effect of aldosterone or SGK1 on ROMK channels [46]. Figure 3 is a cell model illustrating the possible mechanism by which aldosterone-induced stimulation of SGK1 activates ROMK channels when the AngII system is down-regulated by high K intake. Thus, when AngII is suppressed, SGK1 stimulates the phosphorylation of WNK4 and thereby abolishes the WNK4-mediated inhibition of ROMK channels. In contrast, when the AngII signaling pathway is active under conditions of low Na intake, the stimulatory effect of SGK1 on ROMK channel is compromised.
Figure 4 is a scheme illustrating the role of the interaction between AngII and aldosterone/SGK1 in the regulating ROMK channels and K secretion during low Na intake. Unlike the condition of high K intake which suppresses renin–AngII pathway, low Na intake stimulates renin and AngII system. Because AngII has been shown to inhibit ROMK channels [130], the stimulatory effect of SGK1 on ROMK channels may be suppressed. Alternatively, AngII signaling pathway could directly modulate SGK1-WNK4 interaction and hence abolish the stimulatory effect of SGK1 on ROMK channels. This model could explain that infusion of aldosterone or low Na intake fails to stimulate ROMK channels and also that high K intake alone is not able to stimulate ROMK channels in the absence of aldosterone [81]. But the role of AngII in interacting with SGK1-WNK4 pathway is not explored and needs future experiments to prove the hypothesis.
Fig. 4.
A scheme showing the mechanism by which aldosterone and AngII regulate ROMK and BK channels in the CCD during low Na intake. The circle with x indicates the inhibition of a particular signaling pathway
Cytochrome P450 (CYP) epoxygenase
CYP epoxygenases such as CYP2C23 or CYP2J are expressed in the CNT and CCD [61, 71, 110]. Two lines of evidence suggest that CYP epoxygenase plays an important role in mediating the effect of high K intake on K secretion in the CCD [111]: (1) high K intake increased epoxyeicosatrienoic acid (EET) levels in the CCD and (2) 11,12-EET stimulates BK channel activity in the CCD. The expression of CYP2C23 is also increased in response to a high K diet [111]. The effect of high K on CYP2C23 is specific because high K intake does not increase CYP2J expression. The effect of high K intake on CYP2C23 expression is not due to high aldosterone level because low Na intake has been shown to decrease the expression of CYP2C23 [110]. Thus, CYP epoxygenase-dependent metabolism of arachidonic acid stimulates BK channels in response to a high K diet by an aldosterone-independent mechanism. This pathway may play a role in BK-dependent K secretion in the CCD and possibly in the CNT (Fig. 3).
Effect of low dietary K intake
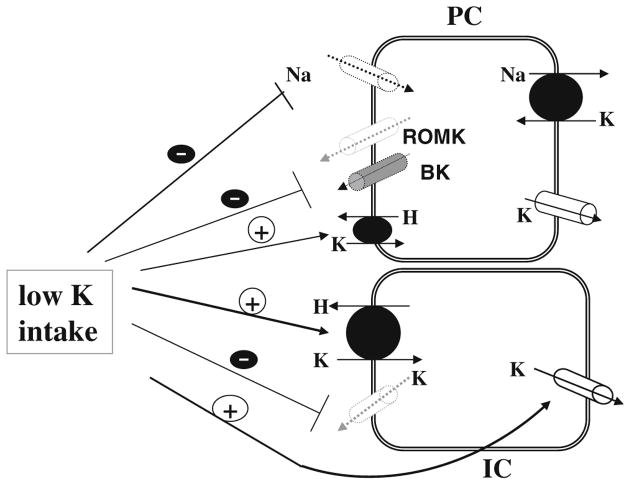
K restriction decreases renal K secretion by inhibiting both apical ROMK and BK channels in PC [51, 127] as well as by stimulating K absorption [133, 135]. Figure 5 is a scheme of a cell model illustrating the current understanding regarding the effect of low K intake on apical K channels, Na channels, H,K-ATPase, and basolateral K channels in the CCD. Low K intake increases superoxide anion production which stimulates the expression of Src family PTK and the phosphorylation of p38 and ERK MAPKs [8]. Single-channel analysis in the CCD of mice and rats has demonstrated that the activity of both BK and ROMK-like SK decreases following activation of PTK and MAPKs [124, 129]. In addition, stimulation of ERK has been shown to inhibit epithelial Na channels (ENaC) [106] and accordingly diminish the driving force for K secretion. Moreover, K restriction stimulates K absorption [12, 28, 142] through enhancing colonic H,K-ATPase transcription [62, 103], and it is possible that PC may also be involved in K reabsorption [32]. K-restriction-induced increase in K reabsorption may also be the result of inhibiting apical K channels in IC [145, 148] because blocking K channels in IC prevents K recycling into the lumen and favors K absorption [145]. The molecular mechanism by which low K intake inhibits apical K channels in PC has been extensively studied. Figure 6 is a scheme illustrating the signaling pathway by which low K intake inhibits apical K channels in the CCD.
Fig. 5.
A cell scheme illustrating the mechanism by which low K intake inhibits K secretion in PC and stimulates K absorption in IC of the CCD. Solid arrow and dotted arrow indicate the enhanced and attenuated effect, respectively
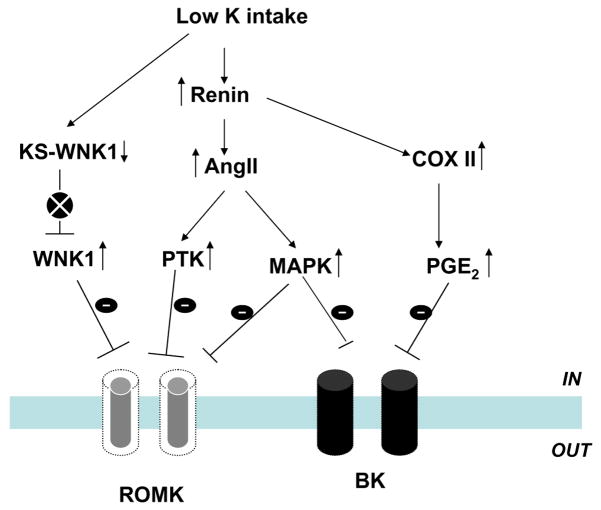
Fig. 6.
A scheme showing the role of different signaling pathways in mediating the effect of low K intake on ROMK and BK channels in the CCD. The circle with x indicates the inhibition of a particular signaling pathway
Role of AngII
K restriction has been shown to stimulate renin and the AngII system [86, 90, 102]. Moreover, micropuncture studies have revealed that luminal perfusion of AngII inhibited K secretion in the distal nephron [118]. Patch-clamp experiments have further demonstrated that AngII down-regulates ROMK channels in the CCD and that such inhibition could be demonstrated only in the CCD from K-restricted rats [130]. Furthermore, the suppression of ROMK channels by AngII was absent by blocking NADPH oxidase or attenuated by inhibiting Src family PTK. This suggests that superoxide anions and PTK are involved in mediating the effect of angiotensin II.
MAPK
Low K intake has also been shown to activate MAPK such as p38 and ERK [7]. This effect is possibly mediated by superoxide anions because suppression of superoxide anions production abolished the effect of low K intake on MAPK activity [7,8]. The inhibitory role of p38 and ERK in the regulation of K secretion has been suggested by the observations that blocking p38 and ERK increases the ROMK channel activity and BK channel activity in the CCD [7, 51]. Moreover, the effect of MAPK on ROMK channels is independent of Src family PTK.
Src family PTK
Both ROMK and Src family PTK, c-Src, are expressed in the CCD [55]. ROMK channels are the substrate of Src family PTK, and it has been shown that tyrosine residue 337 of ROMK1 is a phosphorylation site of PTK. The tyrosine phosphorylation of ROMK is regulated by dietary K intake: A low K intake increases, whereas a high K intake decreases the level of the tyrosine-phosphorylated ROMK. Moreover, the level of Src family PTK such as c-Src and c-Yes increased in the renal cortex and outer medulla obtained from rats on a K-deficient diet and significantly decreased by high K intake [126]. Thus, it is possible that PTK is involved in mediating the inhibitory effect of low dietary K intake on the ROMK-like SK channels.
The role of PTK in mediating the effect of low K intake on K secretion is further supported by the finding that blocking PTK with herbimycin A significantly increased the activity of the ROMK-like SK channels in CCDs from rats on a KD diet [126]. However, the inhibitory effect of PTK on ROMK-like SK channels is not a direct consequence of the tyrosine phosphorylation because addition of exogenous c-Src does not inhibit the channel activity in excised patches [122]. Several lines of evidence indicate that stimulation of tyrosine phosphorylation of ROMK1 facilitates internalization of ROMK-like SK channels. First, inhibition of protein tyrosine phosphatase (PTP) decreases ROMK-like SK channel activity. This effect is absent in the presence of sucrose-containing bath or in CCDs treated with concanavalin A, an agent which inhibits endocytosis, [125], suggesting that inhibition of PTP increases the internalization of ROMK-like SK channels. Second, inhibition of PTP significantly increases tyrosine phosphorylation of ROMK1 and reduces the number of ROMK1 detected by confocal microscopic image and surface biotin labeling in HEK293 cells transfected with ROMK1 and c-Src [107]. Third, inhibition of PTP has no effect on K channel activity in cells transfected with the ROMK1 mutant, R1Y337A, indicating that phosphorylation of tyrosine residue 337 is essential for initiating the internalization of ROMK1 [107].
While stimulation of tyrosine phosphorylation enhances the internalization of ROMK1 channels, facilitating dephosphorylation has an opposite effect on ROMK1 channels. It has been demonstrated that stimulating tyrosine dephosphorylation increases the surface density of ROMK1 channels [67]. Moreover, the observation that inhibition of microtubule formation or application of tetanus toxin abolished the effect of herbimycin A on the ROMK channels in CCDs indicates that the effect of inhibiting PTK results from the stimulation of exocytosis [108, 128].
KS-WNK1 and WNK1
As discussed above, KS-WNK1 antagonizes effect on WNK1 which inhibits ROMK channels. K restriction has been reported to decrease the expression of KS-WNK1 and increase long form WNK1. Consequently, the antagonizing effect of KS-WNK1 on WNK1 is diminished, and WNK1-mediated inhibition of ROMK channels is enhanced in the CCD from animals fed on a low K diet [48].
Regulation of K transport in the CCD by hormones other than aldosterone and AngII
Vasopressin
Vasopressin plays an important role in stimulating renal K excretion during dehydration. A decrease in extracellular volume is expected to increase the plasma level of vasopressin which stimulates renal K excretion. The stimulatory effect of vasopressin on renal K excretion is partially due to increasing apical ROMK channel activity in the CCD by activation of V2 receptor and cAMP-dependent pathway [13]. Moreover, it has been shown that vasopressin increases K secretion in the distal tubule, including CNT and initial CCD, by stimulation of V1 receptor [3]. It has been reported that luminal vasopressin stimulates K secretion in distal tubule, and the effect of luminal vasopressin is abolished in the presence BK channel blocker, iberiotoxin. Thus, it is possible that stimulation of luminal V1 receptor activates BK channels by a Ca2+- and PKC-dependent mechanism [4].
PGE2
Cyclooxygenase (COX) 1 and 2 are expressed in the CCD [139]. We have previously demonstrated that low K intake stimulates the COX2 expression and PGE2 production in the rat kidney and that PGE2 inhibits ROMK channels by a PKC-MAPK-dependent pathway [43], as shown in Fig. 6. Low K intake has been shown to stimulate renin production which increases PGE2 production [86, 90, 102]. This mechanism may play a role in suppressing apical K channels during K restriction.
Uroguanylin and guanylin
Guanylin and uroguanylin have been shown to cause both hyperpolarization and depolarization of cultured CCD principal cells. The peptide-induced hyperpolarization and depolarization are blocked by protein kinase G and phospholipase A2, respectively [104, 105]. Micropuncture study has demonstrated that urogunylin stimulates BK-channel-dependent K secretion in rat distal nephron [5].
Prospect
Although our understanding regarding K transport in the CNT and CCD has been significantly extended recently, an integrated mechanism by which hormone and dietary K intake regulates K transport is still not completely understood. In the future study, it would be important to understand the connection and interaction among different kinases and pathways which regulate K secretion in the CCD. In addition, another focus would be to identify new molecular mechanisms and paradigm of renal K handling in the distal nephron. Recently, a study which measure simultaneously changes in plasma K and urinary K excretion demonstrates that increasing K loading with food intake in stomach stimulates renal K secretion even if plasma K concentration remains unchanged by K loading [49]. However, if K loading is not companied by stomach food feeding, raising plasma K concentration is observed before renal K secretion increases. This suggests a possible K sensing mechanism or gut factor present in the gastric tissue. Thus, it would be interesting to determine the nature of such a gut factor and the signaling mechanism by the gut factor regulates K transport in the collecting duct.
Acknowledgments
We dedicate this manuscript to Dr. Steven H. Hebert, our friend and colleague who died quite unexpectedly on April 15th 2008. We lost with him a long-standing collaborator, friend, and an investigator who made major contributions in the field of potassium transport. The authors also thank Drs. D. Mount and R. B. Silver for their insightful comments. The work is supported by NIH grants DK 47402, DK54983 and HL34100.
Contributor Information
Wen-Hui Wang, Department of Pharmacology, BSB 537, New York Medical College, 15 Dana Road, Valhalla, NY 10595, USA, e-mail: wenhui_wang@nymc.edu.
Gerhard Giebisch, Department of Cellular & Molecular Physiology, Yale University School of Medicine, New Haven, CT 06511, USA.
References
- 1.Ali S, Chen X, Lu M, Xu J-C, Lerea KM, Hebert SC, Wang W. A kinase anchoring protein (AKAP) is required for mediating the effect of PKA on ROMK1. Proc Natl Acad Sci U S A. 1998;95:10274–10278. doi: 10.1073/pnas.95.17.10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amorim JB, Bailey MA, Musa-Aziz R, Giebisch G, Malnic G. Role of luminal anion and pH in distal tubule potassium secretion. Am J Physiol Renal Physiol. 2003;284:F381–F388. doi: 10.1152/ajprenal.00236.2002. [DOI] [PubMed] [Google Scholar]
- 3.Amorim JBO, Malnic G. V1 receptors in luminal action of vasopressin on distal K+ secretion. AJP–Renal Physiol. 2000;278:F809–F816. doi: 10.1152/ajprenal.2000.278.5.F809. [DOI] [PubMed] [Google Scholar]
- 4.Amorim JBO, Musa-Aziz R, Mello-Aires M, Malnic G. Signaling path of the action of AVP on distal K+ secretion. Kidney Int. 2004;66:696–704. doi: 10.1111/j.1523-1755.2004.00800.x. [DOI] [PubMed] [Google Scholar]
- 5.Amorim JBO, Musa-Aziz R, Lessa LMA, Malnic G, Fonteles MC. Effect of uroguanylin on potassium and bicarbonate transport in rat renal tubules. Can J Physiol Pharmacol. 2006;84:1003. doi: 10.1139/y06-044. [DOI] [PubMed] [Google Scholar]
- 6.Armitage FE, Wingo CS. Luminal acidification in K-replete OMCDi: contributions of H-K-ATPase and bafilomycin-A1-sensitive H-ATPase. Am J Physiol. 1994;267:F450–F458. doi: 10.1152/ajprenal.1994.267.3.F450. [DOI] [PubMed] [Google Scholar]
- 7.Babilonia E, Li D, Wang ZJ, Sun P, Lin DH, Wang WH. Mitogen-activated protein kinase (MAPK) inhibits ROMK-like small conductance K channels in the CCD of K-restricted rats. J Am Soc Nephrol. 2006;17:2687–2696. doi: 10.1681/ASN.2006050426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babilonia E, Lin D, Zhang Y, Wei Y, Yue P, Wang WH. Role of gp91phox-containing NADPH oxidase in mediating the effect of K restriction on ROMK channels and renal K excretion. J Am Soc Nephrol. 2007;18:2037–2045. doi: 10.1681/ASN.2006121333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey MA, Cantone A, Yan QS, MacGregor GG, Leng Q, Amorim JB, Wang T, Hebert SC, Giebisch G, Malnic G. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of type II Bartter’s syndrome and in adaptation to a high K diet. Kidney Int. 2006;70:51–59. doi: 10.1038/sj.ki.5000388. [DOI] [PubMed] [Google Scholar]
- 10.Beck F-X, Dorge A, Giebisch G, Thurau K. Effect of diuretics on cell potassium transport: an electron microprobe study. Kidney Int. 1990;37:1423–1428. doi: 10.1038/ki.1990.132. [DOI] [PubMed] [Google Scholar]
- 11.Berger S, Bleich M, Schmid W, Cole TJ, Peters J, Watanabe H, Kriz W, Warth R, Greger R, Schutz G. Mineralocorticoid receptor knockout mice: pathophysiology of Na+ metabolism. Proc Natl Acad Sci. 1998;95:9424–9429. doi: 10.1073/pnas.95.16.9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buffin-Meyer B, Younes-Ibrahim M, Barlet-Bas C, Cheval L, Marsy S, Doucet A. K depletion modifies the properties of Sch-28080-sensitive K-ATPase in rat collecting duct. Am J Physiol. 1997;272:F124–F131. doi: 10.1152/ajprenal.1997.272.1.F124. [DOI] [PubMed] [Google Scholar]
- 13.Cassola AC, Giebisch G, Wang W. Vasopressin increases density of apical low-conductance K+ channels in rat CCD. Am J Physiol. 1993;264:F502–F509. doi: 10.1152/ajprenal.1993.264.3.F502. [DOI] [PubMed] [Google Scholar]
- 14.Choe H, Zhou H, Palmer LG, Sackin H. A conserved cytoplasmic region of ROMK modulates pH sensitivity, conductance, and gating. Am J Physiol. 1997;273:F516–F529. doi: 10.1152/ajprenal.1997.273.4.F516. [DOI] [PubMed] [Google Scholar]
- 15.Codina J, Wall SM, DuBose TD., Jr Contrasting functional and regulatory profiles of the renal H+,K+-ATPases. Sems Nephrol. 1999;19:399–404. [PubMed] [Google Scholar]
- 16.Cope G, Murthy M, Golbang AP, Hamad A, Liu CH, Cuthbert AW, O’Shaughnessy KM. WNK1 affects surface expression of the ROMK potassium channel independent of WNK4. J Am Soc Nephrol. 2006;17:1867–1874. doi: 10.1681/ASN.2005111224. [DOI] [PubMed] [Google Scholar]
- 17.Dherbecourt O, Cheval L, Bloch-Faure M, Meneton P, Doucet A. Molecular identification of Sch28080-sensitive K-ATPase activities in the mouse kidney. Pflugers Arch. 2006;451:769–775. doi: 10.1007/s00424-005-1508-1. [DOI] [PubMed] [Google Scholar]
- 18.Dong K, Xu J, Vanoye CG, Welch R, MacGregor GG, Giebisch G, Hebert SC. An amino acid triplet in the NH2 terminus of rat ROMK1 determines interation with SUR2B. J Biol Chem. 2001;276:44347–44353. doi: 10.1074/jbc.M108072200. [DOI] [PubMed] [Google Scholar]
- 19.Doucet A, Marsy S. Characterization of K-ATPase activity in distal nephron: stimulation by potassium depletion. Am J Physiol. 1987;253:F418–F423. doi: 10.1152/ajprenal.1987.253.3.F418. [DOI] [PubMed] [Google Scholar]
- 20.Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 21.DuBose TD, Jr, Gitomer J, Codina J. H+,K+-ATPase. Curr Opin Nephrol Hypertens. 1999;8:597–602. doi: 10.1097/00041552-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Ellison DH, Velazquez H, Wright FS. Stimulation of distal potassium secretion by low lumen chloride in the presence of barium. Am J Physiol. 1985;248:638–649. doi: 10.1152/ajprenal.1985.248.5.F638. [DOI] [PubMed] [Google Scholar]
- 23.Estilo G, Liu W, Pastor-Soler N, Mitchell P, Carattino MD, Kleyman TR, Satlin LM. Effect of aldosterone on BK channel expression in mammalian cortical collecting duct. AJP–Renal Physiol. 2008;295:F780–F788. doi: 10.1152/ajprenal.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eric F, Davi M, Sand G, Geor D, Manl V, Alai D, Alai V, Vane S, Fran V, Martin PY. Mechanism of control of Na,K-ATPase in principal cells of the mammalian collecting duct. Ann NY Acad Sci. 2003;986:570–578. doi: 10.1111/j.1749-6632.2003.tb07255.x. [DOI] [PubMed] [Google Scholar]
- 25.Frindt G, Palmer LG. Ca-activated K channels in apical membrane of mammalian CCT, and their role in K secretion. Am J Physiol (Renal Fluid Electrolyte Physiol) 1987;252–221:F458–F467. doi: 10.1152/ajprenal.1987.252.3.F458. [DOI] [PubMed] [Google Scholar]
- 26.Frindt G, Palmer LG. Low-conductance K channels in apical membrane of rat cortical collecting tubule. Am J Physiol (Renal Fluid Electrolyte Physiol) 1989;256–225:F143–F151. doi: 10.1152/ajprenal.1989.256.1.F143. [DOI] [PubMed] [Google Scholar]
- 27.Frindt G, Palmer LG. Apical potassium channels in the rat connecting tubule. Am J Physiol Renal Physiol. 2004;287:F1030–F1037. doi: 10.1152/ajprenal.00169.2004. [DOI] [PubMed] [Google Scholar]
- 28.Garg LC. Respective role of H-ATPase and H–K-ATPase in ion transport in the kidney. JASN. 1991;2(5):949–960. doi: 10.1681/ASN.V25949. [DOI] [PubMed] [Google Scholar]
- 29.Giebisch G. Renal potassium transport: mechanisms and regulation. Am J Physiol. 1998;274:F817–F833. doi: 10.1152/ajprenal.1998.274.5.F817. [DOI] [PubMed] [Google Scholar]
- 30.Giebisch G, Hebert SC, Wang WH. New aspects of renal potassium transport. Pflugers Arch. 2003;446:289–297. doi: 10.1007/s00424-003-1029-8. [DOI] [PubMed] [Google Scholar]
- 31.Gray DA, Frindt G, Palmer LG. Quantification of K+ secretion through apical low-conductance K channels in the CCD. Am J Physiol Renal Physiol. 2005;289:F117–F126. doi: 10.1152/ajprenal.00471.2004. [DOI] [PubMed] [Google Scholar]
- 32.Gray DA, Frindt G, Zhang YY, Palmer LG. Basolateral K+ conductance in principal cells of rat CCD. Am J Physiol Renal Physiol. 2005;288:F493–F504. doi: 10.1152/ajprenal.00301.2004. [DOI] [PubMed] [Google Scholar]
- 33.Guntupalli J, Onuigbo M, Wall S, Alpern RJ, DuBose TD., Jr Adaptation to low-K+ media increases H(+)–K(+)-ATPase but not H(+)-ATPase-mediated pHi recovery in OMCD1 cells. Am J Physiol. 1997;273:C558–C571. doi: 10.1152/ajpcell.1997.273.2.C558. [DOI] [PubMed] [Google Scholar]
- 34.He G, Wang HR, Huang SK, Huang C-L. Intersectin links WNK kinase to endocytosis of ROMK1. J Clin Invest. 2007;117:1078–1087. doi: 10.1172/JCI30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev. 2005;85:319–371. doi: 10.1152/physrev.00051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hebert SC, Wang W-H. Structure and function of the low conductance KATP channel, ROMK. Wien Klin Wochenschr. 1997;109:471–476. [PubMed] [Google Scholar]
- 37.Hirsch J, Schlatter E. K+ channels in the basolateral membrane of rat cortical collecting duct. Kidney Int. 1995;48:1036–1046. doi: 10.1038/ki.1995.387. [DOI] [PubMed] [Google Scholar]
- 38.Ho K. The ROMK-cystic fibrosis transmembrane conductance regulator connection: new insights into the relationship between ROMK and cystic fibrosis transmembrane conductance regulator channels. Curr Opin Nephrol Hypertens. 1998;7:49–58. doi: 10.1097/00041552-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Horisberger J-D, Doucet A. Renal ion-translocating ATPase: the P-type family. In: Alpern RJ, Hebert SC, editors. The kidney: physiology and pathophysiology. Elsevier; Amsterdam: 2008. pp. 57–90. [Google Scholar]
- 40.Huang C-L, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbg. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 41.Huang DY, Wulff P, Volkl H, Loffing J, Richter K, Kuhl D, Lang F, Vallon V. Impaired regulation of renal K+ elimination in the sgk1-knockout mouse. J Am Soc Nephrol. 2004;15:885–891. doi: 10.1097/01.asn.0000120368.59693.a8. [DOI] [PubMed] [Google Scholar]
- 42.Hunter M, Lopes AG, Boulpaep EL, Giebisch G. Single channel recordings of calcium-activated potassium channels in the apical membrane of rabbit cortical collecting tubules. Proc Natl Acad Sci U S A. 1984;81:4237–4239. doi: 10.1073/pnas.81.13.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin Y, Wang Z, Zhang Y, Yang B, Wang WH. PGE2 inhibits apical K channels in the CCD through activation of the MAPK pathway. AJP–Renal Physiol. 2007;293:F1299–F1307. doi: 10.1152/ajprenal.00293.2007. [DOI] [PubMed] [Google Scholar]
- 44.Kahle KT, Wilson FH, Leng Q, Lalioti MD, O’Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet. 2003;35:372–376. doi: 10.1038/ng1271. [DOI] [PubMed] [Google Scholar]
- 45.Kahle KT, Gimenez I, Hassan H, Wilson FH, Wong RD, Forbush B, Aronson PS, Lifton RP. WNK4 regulates apical and basolateral Cl-flux in extrarenal epithelia. Proc Natl Acad Sci. 2004;101:2064–2069. doi: 10.1073/pnas.0308434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kahle KT, Ring AM, Lifton RP. Molecular physiology of the WNK kinases. Annu Rev Physiol. 2008;70:329–355. doi: 10.1146/annurev.physiol.70.113006.100651. [DOI] [PubMed] [Google Scholar]
- 47.Kone BC. Renal H,K-ATPase: structure, function and regulation. Miner Electrolyte Metab. 1996;22:349–365. [PubMed] [Google Scholar]
- 48.Lazrak A, Liu Z, Huang CL. Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc Natl Acad Sci. 2006;103:1615–1620. doi: 10.1073/pnas.0510609103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee FN, Oh G, McDonough AA, Youn JH. Evidence for gut factor in K+ homeostasis. AJP–Renal Physiol. 2007;293:F541–F547. doi: 10.1152/ajprenal.00427.2006. [DOI] [PubMed] [Google Scholar]
- 50.Leng Q, Kahle KT, Rinehart J, MacGregor GG, Wilson FH, Canessa CM, Lifton RP, Hebert SC. WNK3, a kinase related to genes mutated in hereditary hypertension with hyperkaelemia, regulates the K+ channel ROMK1 (Kir1.1) J Physiol. 2006;571:275–286. doi: 10.1113/jphysiol.2005.102202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li DM, Wang ZJ, Sun P, Jin Y, Lin DH, Hebert SC, Giebisch G, Wang WH. Inhibition of mitogen-activated protein kinase stimulates the Ca2+-dependent big conductance K channels (BK) in cortical collecting duct. Proc Natl Acad Sci U S A. 2006;103:19569–19574. doi: 10.1073/pnas.0609555104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liapis H, Nag M, Kaji DM. K–Cl cotransporter expression in the human kidney. AJP–Cell Physiol. 1998;275:C1432–C1437. doi: 10.1152/ajpcell.1998.275.6.C1432. [DOI] [PubMed] [Google Scholar]
- 53.Lin DH, Sterling H, Lerea KM, Giebisch G, Wang WH. Protein kinase C (PKC)-induced phosphorylation of ROMK1 is essential for the surface expression of ROMK1 channels. J Biol Chem. 2002;277:44332–44338. doi: 10.1074/jbc.M203702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin DH, Sterling H, Lerea KM, Welling P, Jin L, Giebisch G, Wang WH. K depletion increases the protein tyrosine-mediated phosphorylation of ROMK. Am J Physiol Renal Physiol. 2002;283:F671–F677. doi: 10.1152/ajprenal.00160.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin DH, Sterling H, Yang B, Hebert SC, Giebisch G, Wang WH. Protein tyrosine kinase is expressed and regulates ROMK1 location in the cortical collecting duct. Am J Physiol Renal Physiol. 2004;286:F881–F892. doi: 10.1152/ajprenal.00301.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ling BN, Hinton CF, Eaton DC. Potassium permeable channels in primary cultures of rabbit cortical collecting tubule. Kidney Int. 1991;40:441–452. doi: 10.1038/ki.1991.231. [DOI] [PubMed] [Google Scholar]
- 57.Liou HH, Zhou SS, Huang CL. Regulation of ROMK1 channel by protein kinase A via a phosphatidylinositol 4,5-bisphosphate-dependent mechanism. Proc Natl Acad Sci U S A. 1999;96:5820–5825. doi: 10.1073/pnas.96.10.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu M, Hebert SC, Giebisch G. Hydrolyzable ATP and PIP2 modulate the small-conductance K channel in apical membrane of rat cortical collecting duct. J Gen Physiol. 2002;120:603–615. doi: 10.1085/jgp.20028677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu M, Leng Q, Egan ME, Caplan MJ, Boulpaep E, Giebisch G, Hebert SC. CFTR is required for PKA-regulated ATP sensitivity of Kir1.1 potassium channels in mouse kidney. J Clin Invest. 2006;116:797–806. doi: 10.1172/JCI26961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu R, Alioua A, Kumar Y, Eghbali M, Stefani E, Toro L. MaxiK channel partners: physiological impact. J Physiol. 2006;570:65–72. doi: 10.1113/jphysiol.2005.098913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma J, Qu W, Scarborough PE, Tomer KB, Moomaw CR, Maronpot R, Davis LS, Breyer MD, Zeldin DC. Molecular cloning, enzymatic characterization, developmental expression, and cellular localization of a mouse cytochrome P450 highly expressed in kidney. J Biol Chem. 1999;274:17777–17788. doi: 10.1074/jbc.274.25.17777. [DOI] [PubMed] [Google Scholar]
- 62.Marsy S, Elalouf J-M, Doucet A. Quantitative RT-PCR analysis of mRNA encoding a colonic H,K-ATPase alpha subunit along the rat nephron: effect of K+ depletion. Pflugers Arch. 1996;432:494–500. doi: 10.1007/s004240050161. [DOI] [PubMed] [Google Scholar]
- 63.McNicholas CM, Nason MW, Guggino WB, Schwiebert EM, Hebert S, Giebisch G, Egan ME. The functional CFTR-NBF1 is required for ROMK2-CFTR interaction. Am J Physiol. 1997;273:F843–F848. doi: 10.1152/ajprenal.1997.273.5.F843. [DOI] [PubMed] [Google Scholar]
- 64.McNicholas CM, Wang W, Ho K, Hebert SC, Giebisch G. Regulation of ROMK1 K+ channel activity involves phosphorylation processes. Proc Natl Acad Sci U S A. 1994;91:8077–8081. doi: 10.1073/pnas.91.17.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meneton P, Schultheis PJ, Greeb J, Nieman ML, Liu LH, Clarke LL, Duffy JJ, Doetschman T, Lorenz JN, Shull GE. Increased sensitivity to K+ deprivation in colonic H,K-ATPase-deficient mice. J Clin Invest. 1998;101:536–542. doi: 10.1172/JCI1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Minor DL, Masseling SJ, Jan YN, Jan LY. Transmembrane structure of an inwardly rectifying potassium channel. Cell. 1999;97:879–891. doi: 10.1016/s0092-8674(00)80597-8. [DOI] [PubMed] [Google Scholar]
- 67.Moral Z, Deng K, Wei Y, Sterling H, Deng H, Ali S, Gu RM, Huang XY, Hebert SC, Giebisch G, Wang WH. Regulation of ROMK1 channels by protein tyrosine kinase and tyrosine phosphatase. J Biol Chem. 2001;276:7156–7163. doi: 10.1074/jbc.M008671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muto S, Sansom S, Giebisch G. Effects of a high potassium diet on electrical properties of cortical collecting duct from adrenalectomized rabbits. J Clin Invest. 1988;81:376–380. doi: 10.1172/JCI113329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muto S, Tsuruoka S, Miyata Y, Fujimura A, Kusano E, Wang WH, Seldin D, Giebing G. Basolateral Na+/H+ exchange is involved in maintaining K+ secretion during diminished Na+ transport in rabbit CCD. Kid Int. 2008 doi: 10.1038/ki.2008.447. [DOI] [PubMed] [Google Scholar]
- 70.Najjar F, Zhou H, Morimoto T, Bruns JB, Li HS, Liu W, Kleyman TR, Satlin LM. Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. Am J Physiol Renal Physiol. 2005;289:F922–F932. doi: 10.1152/ajprenal.00057.2005. [DOI] [PubMed] [Google Scholar]
- 71.Nakagawa K, Holla VR, Wei Y, Wang WH, Gatica A, Wei S, Mei S, Miller CM, Cha DR, Price EJ, Zent R, Pozzi A, Breyer MD, Guan Y, Falck JR, Waterman MR, Capdevila JH. Salt sensitive hypertension is associated with a dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J Clin Invest. 2006;116:1696–1702. doi: 10.1172/JCI27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nichols CG, Lopatin AN. Inward rectifier potassium channels. Ann Rev Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- 73.O’Connell AD, Leng Q, Dong K, MacGregor GG, Giebisch G, Hebert SC. Phosphorylation-regulated endoplasmic reticulum retention signal in the renal outer-medullary K+ channel (ROMK) Proc Natl Acad Sci. 2005;102:9954–9959. doi: 10.1073/pnas.0504332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Neil RG. Potassium secretion by the cortical collecting tubule. Federation Proc. 1981;40:2403–2407. [PubMed] [Google Scholar]
- 75.O’Neil RG, Hayhurst AR. Functional differentiation of cell types of cortical collecting duct. Am J Physiol. 1985;248:449–453. doi: 10.1152/ajprenal.1985.248.3.F449. [DOI] [PubMed] [Google Scholar]
- 76.O’Neil RG, Sansom SC. Characterization of apical cell membrane Na+ and K+ conductances of cortical collecting duct using microelectrode techniques. Am J Physiol. 1984;247(Renal 16):F14–F24. doi: 10.1152/ajprenal.1984.247.1.F14. [DOI] [PubMed] [Google Scholar]
- 77.O’Reilly M, Marshall E, Speirs HJL, Brown RW. WNK1, a gene within a novel blood pressure control pathway, tissue-specifically generates radically different isoforms with and without a kinase domain. J Am Soc Nephrol. 2003;14:2447–2456. doi: 10.1097/01.asn.0000089830.97681.3b. [DOI] [PubMed] [Google Scholar]
- 78.Orias M, Velazquez H, Tung F, Lee G, Desir GV. Cloning and localization of a double-pore K channel, KCNK1: exclusive expression in distal nephron segments. AJP–Renal Physiol. 1997;273:F663–F666. doi: 10.1152/ajprenal.1997.273.4.F663. [DOI] [PubMed] [Google Scholar]
- 79.Palmer LG. Potassium secretion and the regulation of distal nephron K channels. Am J Physiol. 1999;277:F821–F825. doi: 10.1152/ajprenal.1999.277.6.F821. [DOI] [PubMed] [Google Scholar]
- 80.Palmer LG, Antonian L, Frindt G. Regulation of the Na–K pump of the rat cortical collecting tubule by aldosterone. J Gen Physiol. 1993;102:43–57. doi: 10.1085/jgp.102.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol. 1994;104:693–710. doi: 10.1085/jgp.104.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palmer LG, Frindt G. Regulation of apical membrane Na and K channels in rat renal collecting tubules by aldosterone. Sems Nephrol. 1992;12:37–43. [PubMed] [Google Scholar]
- 83.Palmer LG, Frindt G. Regulation of apical K channels in rat cortical collecting tubule during changes in dietary K intake. Am J Physiol. 1999;277:F805–F812. doi: 10.1152/ajprenal.1999.277.5.F805. [DOI] [PubMed] [Google Scholar]
- 84.Palmer LG, Frindt G. High-conductance K channels in intercalated cells of the rat distal nephron. AJP–Renal Physiol. 2007;292:F966–F973. doi: 10.1152/ajprenal.00191.2006. [DOI] [PubMed] [Google Scholar]
- 85.Rapedius M, Haider S, Browner KF, Shang L, Sanson MSP, Baukroitz T, Tucker SJ. Structural and functional analysis of the putative pH sensor in the Kir1.1 (ROMK) potassium channel. EMBO Rep. 2006;7:611–616. doi: 10.1038/sj.embor.7400678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ray PE, Suga SI, Liu XH, Huang X, Johnson RJ. Chronic potassium depletion induces renal injury, salt sensitivity, and hypertension in young rats. Kidney Int. 2001;59:1850–1858. doi: 10.1046/j.1523-1755.2001.0590051850.x. [DOI] [PubMed] [Google Scholar]
- 87.Rieg T, Vallon V, Sausbier M, Sausbier U, Kaissling B, Ruth P, Osswald H. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int. 2007;72:566–573. doi: 10.1038/sj.ki.5002369. [DOI] [PubMed] [Google Scholar]
- 88.Ring AM, Leng Q, Rinehart J, Wilson FH, Kahle KT, Hebert SC, Lifton RP. An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proc Natl Acad Sci. 2007;104:4025–4029. doi: 10.1073/pnas.0611728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rossier BC, Canessa CM, Schild L, Horisberger J-D. Epithelial sodium channels. Curr Opin Nephrol Hyperten. 1994;3:487–496. doi: 10.1097/00041552-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 90.Saikaley A, Bichet D, Kucharczyk J, Peterson LN. Neuroendocrine factors mediating polydipsia induced by dietary Na, Cl, and K depletion. Am J Physiol Regul Integr Comp Physiol. 1986;251:R1071–R1077. doi: 10.1152/ajpregu.1986.251.6.R1071. [DOI] [PubMed] [Google Scholar]
- 91.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 92.Sansom SC, O’Neil RG. Effects of mineralocorticoids on transport properties of the cortical collecting duct basolateral membrane. Am J Physiol. 1986;251:743–757. doi: 10.1152/ajprenal.1986.251.4.F743. [DOI] [PubMed] [Google Scholar]
- 93.Satlin LM. Postnatal maturation of potassium transport in rabbit cortical collecting duct. Am J Physiol. 1994;266:F57–F65. doi: 10.1152/ajprenal.1994.266.1.F57. [DOI] [PubMed] [Google Scholar]
- 94.Satlin LM. Developmental regulation of expression of renal potassium secretory channels. Curr Opin Nephrol Hypertens. 2004;13:445–450. doi: 10.1097/01.mnh.0000133979.17311.21. [DOI] [PubMed] [Google Scholar]
- 95.Satlin LM, Palmer LG. Apical K+ conductance in maturing rabbit principal cell. Am J Physiol. 1997;272:F397–F404. doi: 10.1152/ajprenal.1997.272.3.F397. [DOI] [PubMed] [Google Scholar]
- 96.Schafer JA, Troutman SL. Potassium transport in cortical collecting tubules from mineralocorticoid-treated rat. Am J Physiol (Renal Fluid Electrolyte Physiol) 1987;253:F76–F88. doi: 10.1152/ajprenal.1987.253.1.F76. [DOI] [PubMed] [Google Scholar]
- 97.Schafer JA, Troutman SL, Schlatter E. Vasopressin and mineralocorticoid increase apical membrane driving force for K+ secretion in rat CCD. Am J Physiol. 1990;258:F199–F210. doi: 10.1152/ajprenal.1990.258.1.F199. [DOI] [PubMed] [Google Scholar]
- 98.Schlatter E. Regulation of ion channels in the cortical collecting duct. Renal Physiol Biochem. 1993;16:21–36. doi: 10.1159/000173749. [DOI] [PubMed] [Google Scholar]
- 99.Schlatter E, Haxelmans S, Hirsch J, Leipziger J. pH dependence of K+ conductances of rat cortical collecting duct principal cells. Pflugers Archiv. 1994;428:631–640. doi: 10.1007/BF00374587. [DOI] [PubMed] [Google Scholar]
- 100.Schlatter E, Lohrmann E, Greger R. Properties of the potassium conductances of principal cells of rat cortical collecting ducts. Pflugers Arch. 1992;420:39–45. doi: 10.1007/BF00378639. [DOI] [PubMed] [Google Scholar]
- 101.Schlatter E, Schafer JA. Electrophysiological studies in principal cells of rat cortical collecting tubules. Pflugers Arch. 1987;409:81–92. doi: 10.1007/BF00584753. [DOI] [PubMed] [Google Scholar]
- 102.Sealey JE, Clark I, Bull MB, Laragh JH. Potassium balance and the control of renin secretion. J Clin Invest. 1970;49:2119–2127. doi: 10.1172/JCI106429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Silver RB, Soleimani M. H–K-ATPase regulation and role in pathophysiological states. Am J Physiol Renal Physiol. 1999;276:F799–F811. doi: 10.1152/ajprenal.1999.276.6.F799. [DOI] [PubMed] [Google Scholar]
- 104.Sindic A, Hirsch JR, Velic A, Piechota H, Schlatter E. Guanylin and uroguanylin regulate electrolyte transport in isolated human cortical collecting ducts. Kidney Int. 2005;67:1420–1427. doi: 10.1111/j.1523-1755.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 105.Sindic A, Velic A, Basoglu C, Hirsch JR, Edemir B, Kuhn M, Schlatter E. Uroguanylin and guanylin regulate transport of mouse cortical collecting duct independent of guanylate cyclase C. Kidney Int. 2005;68:1008–1017. doi: 10.1111/j.1523-1755.2005.00518.x. [DOI] [PubMed] [Google Scholar]
- 106.Soundararajan R, Zhang TT, Wang J, Vandewalle A, Pearce D. A novel role for glucocorticoid-induced leucine zipper protein in epithelial sodium channel-mediated sodium transport. J Biol Chem. 2005;280:39970–39981. doi: 10.1074/jbc.M508658200. [DOI] [PubMed] [Google Scholar]
- 107.Sterling H, Lin DH, Gu RM, Dong K, Hebert SC, Wang WH. Inhibition of protein-tyrosine phosphatase stimulates the dynamin-dependent endocytosis of ROMK1. J Biol Chem. 2002;277:4317–4323. doi: 10.1074/jbc.M109739200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sterling H, Lin DH, Wei Y, Wang WH. Tetanus toxin abolishes exocytosis of ROMK1 induced by inhibition of protein tyrosine kinase. Am J Physiol Renal Physiol. 2003;284:F510–F517. doi: 10.1152/ajprenal.00309.2002. [DOI] [PubMed] [Google Scholar]
- 109.Summa V, Camargo SMR, Bauch C, Zecevic M, Verrey F. Isoform specificity of human Na+,K+-ATPase localization and aldosterone regulation in mouse kidney cells. J Physiol. 2004;555:355–364. doi: 10.1113/jphysiol.2003.054270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun P, Lin DH, Wang T, Babilonia E, Wang ZJ, Jin Y, Kemp R, Nasjletti A, Wang WH. Low Na intake suppresses the expression of CYP2C23 and the arachidonic acid-induced inhibition of ENaC. Am J Physiol Renal Physiol. 2006;291:1192–1200. doi: 10.1152/ajprenal.00112.2006. [DOI] [PubMed] [Google Scholar]
- 111.Sun P, Liu W, Lin DH, Yue P, Kemp R, Satlin LM, Wang WH. Epoxyeicosatrienoic acid (EET) activates the Ca2+-dependent bid conductance K channel in the cortical collecting duct. J Am Soc Nephrol. 2008 doi: 10.1681/ASN.2008040427. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taniguchi J, Imai M. Flow-dependent activation of maxi K+ channels in apical membrane of rabbit connecting tubule. J Membr Biol. 1998;164:35–45. doi: 10.1007/s002329900391. [DOI] [PubMed] [Google Scholar]
- 113.Vallon V, Wulff P, Huang DY, Loffing J, Volkl H, Kuhl D, Lang F. Role of Sgk1 in salt and potassium homeostasis. Am J Physiol Regul Integr Comp Physiol. 2005;288:R4–R10. doi: 10.1152/ajpregu.00369.2004. [DOI] [PubMed] [Google Scholar]
- 114.Velazquez H, Ellison DH, Wright FS. Luminal influences on potassium secretion: chloride, sodium, and thiazide diuretics. Am J Physiol (Renal Fluid Electrolyte Physiol) 1992;262–231:F1076–F1082. doi: 10.1152/ajprenal.1992.262.6.F1076. [DOI] [PubMed] [Google Scholar]
- 115.Verlander JW, Moudy RM, Campbell WG, Cain BD, Wingo CS. Immunohistochemical localization of H–K-ATPase alpha 2c-subunit in rabbit kidney. AJP–Renal Physiology. 2001;281:F357–F365. doi: 10.1152/ajprenal.2001.281.2.F357. [DOI] [PubMed] [Google Scholar]
- 116.Fran V, Vane S, Dirk H, Davi M, Alai V, Eric F, Mari Z. Short-term aldosterone action on Na,K-ATPase surface expression: role of aldosterone-induced SGK1? Ann NY Acad Sci. 2003;986:554–561. doi: 10.1111/j.1749-6632.2003.tb07253.x. [DOI] [PubMed] [Google Scholar]
- 117.Wade JB, Fang L, Liu J, Li D, Yang CL, Subramanya AR, Maouyo D, Mason A, Ellison DH, Welling PA. WNK1 kinase isoform switch regulates renal potassium excretion. Proc Natl Acad Sci. 2006;103:8558–8563. doi: 10.1073/pnas.0603109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang T, Giebisch G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol. 1996;271:F143–F149. doi: 10.1152/ajprenal.1996.271.1.F143. [DOI] [PubMed] [Google Scholar]
- 119.Wang W, Schwab A, Giebisch G. Regulation of small-conductance K channel in apical membrane of rat cortical collecting tubule. Am J Physiol. 1990;259:F494–F502. doi: 10.1152/ajprenal.1990.259.3.F494. [DOI] [PubMed] [Google Scholar]
- 120.Wang WH. The cGMP-dependent protein kinase stimulates the basolateral 18 pS K channel of the rat CCD. Am J Physiol Renal Physiol. 2000;278:C1212–C1217. doi: 10.1152/ajpcell.2000.278.6.C1212. [DOI] [PubMed] [Google Scholar]
- 121.Wang WH. Regulation of Renal K transport by dietary K intake. Annu Rev Physiol. 2004;66:547–569. doi: 10.1146/annurev.physiol.66.032102.112025. [DOI] [PubMed] [Google Scholar]
- 122.Wang WH, Lerea KM, Chan M, Giebisch G. Protein tyrosine kinase regulates the number of renal secretory K channel. Am J Physiol Renal Physiol. 2000;278:F165–F171. doi: 10.1152/ajprenal.2000.278.1.F165. [DOI] [PubMed] [Google Scholar]
- 123.Wang WH, McNicholas CM, Segal AS, Giebisch G. A novel approach allows identification of K channels in the lateral membrane of rat CCD. Am J Physiol. 1994;266:F813–F822. doi: 10.1152/ajprenal.1994.266.5.F813. [DOI] [PubMed] [Google Scholar]
- 124.Wei Y, Bloom P, Gu R, Wang W. Protein-tyrosine phosphatase reduces the number of apical small conductance K+ channels in the rat cortical collecting duct. J Biol Chem. 2000;275:20502–20507. doi: 10.1074/jbc.M000783200. [DOI] [PubMed] [Google Scholar]
- 125.Wei Y, Bloom P, Gu RM, Wang WH. Protein-tyrosine phosphatase reduces the number of apical small conductance K channels in the rat cortical collecting duct. J Biol Chem. 2000;275:20502–20507. doi: 10.1074/jbc.M000783200. [DOI] [PubMed] [Google Scholar]
- 126.Wei Y, Bloom P, Lin DH, Gu RM, Wang WH. Effect of dietary K intake on the apical small-conductance K channel in the CCD: Role of protein tyrosine kinase. Am J Physiol Renal Physiol. 2001;281:F206–F212. doi: 10.1152/ajprenal.2001.281.2.F206. [DOI] [PubMed] [Google Scholar]
- 127.Wei Y, Bloom P, Lin DH, Gu RM, Wang WH. Effect of dietary K intake on the apical small-conductance K channel in the CCD: Role of protein tyrosine kinase. Am J Physiol Renal Physiol. 2001;281:F206–F212. doi: 10.1152/ajprenal.2001.281.2.F206. [DOI] [PubMed] [Google Scholar]
- 128.Wei Y, Wang WH. The role of cytoskeleton in mediating the effect of vasopressin and herbimycin A on the secretory K channels in the CCD. Am J Physiol Renal Physiol. 2001;282:F680–F686. doi: 10.1152/ajprenal.00229.2001. [DOI] [PubMed] [Google Scholar]
- 129.Wei Y, Wang ZJ, Babilonia E, Sterling H, Sun P, Wang WH. Effect of hydrogen peroxide on ROMK channels in the cortical collecting duct. Am J Physiol Renal Physiol. 2006;0:0. doi: 10.1152/ajprenal.00389.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wei Y, Zavilowitz B, Satlin LM, Wang WH. Angiotensin II inhibits the ROMK-like small-conductance K channel in renal cortical collecting duct during dietary K restriction. J Biol Chem. 2007;0:0. doi: 10.1074/jbc.M607477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Welling PA, Caplan M, Sutters M, Giebisch G. Aldosterone-mediated Na/K-ATPase expression is alpha 1 isoform specific in the renal cortical collecting duct. J Biol Chem. 1993;268:23469–23476. [PubMed] [Google Scholar]
- 132.Wingo CS, Cain BD. The renal H–K-ATPase: physiological significance and role in potassium homeostasis. Annu Rev Physiol. 1993;55:323–347. doi: 10.1146/annurev.ph.55.030193.001543. [DOI] [PubMed] [Google Scholar]
- 133.Wingo CS. Potassium transport by medullary collecting tubule of rabbit: effects of variation in K intake. Am J Physiol (Renal Fluid Electrolyte Physiol) 1987;253–22:F1136–F1141. doi: 10.1152/ajprenal.1987.253.6.F1136. [DOI] [PubMed] [Google Scholar]
- 134.Wingo CS. Reversible chloride-dependent potassium transport in cortical collecting tubule. Am J Physiol. 1989;256:F697–F704. doi: 10.1152/ajprenal.1989.256.4.F697. [DOI] [PubMed] [Google Scholar]
- 135.Wingo CS, Armitage FE. Rubidium absorption and proton secretion by rabbit outer medullary collecting duct via H–K-ATPase. Am J Physiol (Renal Fluid Electrolyte Physiol) 1992;263:F849–F857. doi: 10.1152/ajprenal.1992.263.5.F849. [DOI] [PubMed] [Google Scholar]
- 136.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol. 2001;280:F786–F793. doi: 10.1152/ajprenal.2001.280.5.F786. [DOI] [PubMed] [Google Scholar]
- 137.Xu B, Stippec S, Lazrak A, Huang CL, Cobb MH. WNK1 Activates SGK1 by a phosphatidylinositol 3-kinase-dependent and non-catalytic mechanism. J Biol Chem. 2005;280:34218–34223. doi: 10.1074/jbc.M505735200. [DOI] [PubMed] [Google Scholar]
- 138.Xu ZC, Yang Y, Hebert SC. Phosphorylation of the ATP-sensitive, inwardly rectifying K+ channel, ROMK, by cyclic AMP-dependent protein kinase. J Biol Chem. 1996;271:9313–9319. doi: 10.1074/jbc.271.16.9313. [DOI] [PubMed] [Google Scholar]
- 139.Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann JB, Briggs JP. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. AJP–Renal Physiol. 1998;274:F481–F489. doi: 10.1152/ajprenal.1998.274.3.F481. [DOI] [PubMed] [Google Scholar]
- 140.Yoo D, Kim BY, Campo C, Nance L, King A, Maouyo D, Welling PA. Cell surface expression of the ROMK (Kir 1.1) channel is regulated by the aldosterone-induced kinase, SGK-1, and protein kinase A. J Biol Chem. 2003;278:23066–23075. doi: 10.1074/jbc.M212301200. [DOI] [PubMed] [Google Scholar]
- 141.Yoo D, Fang L, Mason A, Kim BY, Welling PA. A phosphorylation-dependent export structure in ROMK (Kir 1.1) channel overrides an endoplasmic reticulum localization signal. J Biol Chem. 2005;280:35281–35289. doi: 10.1074/jbc.M504836200. [DOI] [PubMed] [Google Scholar]
- 142.Younes-Ibrahim M, Barlet-Bas C, Buffin-Meyer B, Cheval L, Rajerison R, Doucet A. Ouabain-sensitive and -insensitive K-ATPases in rat nephron: effect of K depletion. Am J Physiol. 1995;268:F1141–F1147. doi: 10.1152/ajprenal.1995.268.6.F1141. [DOI] [PubMed] [Google Scholar]
- 143.Yun CC, Palmada M, Embark HM, Fedorenko O, feng Y, Henke G, Setiawan I, Boehmer C, Weinman EJ, Sandrasagra S, Korbmacher C, Cohen P, Pearce D, Lang F. The serum and glucocorticoid-inducible kinase SGK1 and Na/H exchange regulating factor NHERF2 synergize to stimulate the renal outer medullary K channel ROMK1. JASN. 2002;13:2823–2830. doi: 10.1097/01.asn.0000035085.54451.81. [DOI] [PubMed] [Google Scholar]
- 144.Zhen WZ, Li XJ, Hilgemann DW, Huang CL. Protein kinase C inhibits ROMK1 channel activity via phosphotidylinositol-4,5-bisphosphosphate-dependent mechanism. J Biol Chem. 2003;276:16852–16856. doi: 10.1074/jbc.M300619200. [DOI] [PubMed] [Google Scholar]
- 145.Zhou X, Lynch IJ, Xia SL, Wingo CS. Activation of H-K-ATPase by CO2 requires a basolateral Ba2+-sensitive pathway during K restriction. Am J Physiol Renal Physiol. 2000;279:F153–F160. doi: 10.1152/ajprenal.2000.279.1.F153. [DOI] [PubMed] [Google Scholar]
- 146.Zhou X, Wingo CS. H-K-ATPase enhancement of Rb efflux by cortical collecting duct. Am J Physiol (Renal Fluid Electrolyte Physiol) 1992;263:F43–F48. doi: 10.1152/ajprenal.1992.263.1.F43. [DOI] [PubMed] [Google Scholar]
- 147.Zhou X, Wingo CS. Mechanisms of rubidium permeability by rabbit cortical collecting duct during potassium restriction. Am J Physiol. 1992;263:F1134–F1141. doi: 10.1152/ajprenal.1992.263.6.F1134. [DOI] [PubMed] [Google Scholar]
- 148.Zhou X, Wingo CS. Stimulation of total CO2 flux by 10% CO2 in rabbit CCD: role of an apical Sch-28080- and Ba-sensitive mechanism. Am J Physiol. 1994;267(Renal 36):F114–F120. doi: 10.1152/ajprenal.1994.267.1.F114. [DOI] [PubMed] [Google Scholar]