Abstract
Ixabepilone (Ixempra) is a member of a new class of cytotoxic agents, the epothilones. Epothilones promote tubulin polymerization in vitro and demonstrate antitumor activity. This article reviews the preclinical and clinical data that have led to the approval of ixabepilone for patients with locally advanced or metastatic breast cancer for whom anthracycline and taxane treatments have failed.
Keywords: ixabepilone, breast cancer, metastatic, locally advanced, epothilones, taxanes, anthracyclines, capecitabine
INTRODUCTION
In 2007, it was estimated that breast cancer would account for more than 40,000 deaths in the U.S.1 The outcome for patients with advanced breast cancer has improved significantly in recent years. Mortality rates have declined as a result of better mammography screening and improved therapies with the introduction of new medications.2–5 Of these agents, the taxanes and anthracyclines have emerged as the cornerstones of therapy for advanced disease as well as for early-stage breast cancer. Unfortunately, although taxanes and anthracyclines are highly active initially, treatment failure occurs in a substantial number of patients, and median survival for metastatic breast cancer is two to three years.3,6–8
In more than 90% of patients with metastatic cancer, treatment failure occurs as a result of the development of cross-resistance to antineoplastic agents.9 This multidrug resistance phenotype is thought to be conferred via the overexpression of efflux transporters, such as P-glycoprotein (P-gp), and other multidrug-resistant proteins that serve as efflux pumps, effectively removing anticancer agents from targeted tumor cells. Other mechanisms of anticancer drug resistance include alterations in target proteins, such as beta-tubulin, as in the case of taxanes.9–12 The fact that both anthracyclines and taxanes are susceptible to a range of multidrug resistance mechanisms represents a considerable limiting factor in breast cancer therapy.13–15 The increased use of these agents in the adjuvant setting for earlier-stage breast cancer means that fewer effective options are available for patients with advanced disease.2,4
For patients who no longer respond to anthracyclines and taxanes, capecitabine (Xeloda, Roche) is commonly used, but objective response rates (ORRs) are reported to be low (9%–14%).16,17 Until recently, capecitabine was the only approved agent for patients with metastatic breast cancer that was resistant to paclitaxel (Taxol, Bristol-Myers Squibb) and to anthracyclines. Therefore, there is a significant unmet need for better therapeutic agents for late-stage breast cancer.
Ixabepilone (Ixempra, Bristol-Myers Squibb), a member of the epothilone class, was approved by the U.S. Food and Drug Administration (FDA) on October 16, 2007, as monotherapy for patients with locally advanced or metastatic breast cancer in whom anthracyclines, taxanes, and capecitabine have failed and in combination with capecitabine for patients in whom an anthracycline and a taxane have failed.18 Ixabepilone was specifically developed for patients with disease that is resistant to other chemotherapies, because it has a low susceptibility to multiple mechanisms of drug resistance.10,19 This review summarizes results from the large clinical program for this promising agent.
DATA SOURCES
PubMed and the Proceedings of the American Society of Clinical Oncology were searched for any relevant material published between 2001 and September 2007. Ixabepilone and BMS-247550 were used as search terms.
PHARMACOLOGY AND PRECLINICAL ACTIVITY
The antineoplastic properties of taxanes are mediated through their ability to bind to and stabilize the tubulin subunits of cellular microtubules, resulting in mitotic arrest in the G2/M phase and apoptosis. The efficacy of taxanes in breast cancer has suggested that targeting of cell microtubules plays a critical role in treating this malignancy. This knowledge has led to a new generation of microtubule-targeted agents, of which epothilones represent a promising class.20
Natural epothilones are isolated from the soil-dwelling myxobacterium Sorangium cellulosum. It was believed that the natural epothilones occupied a common or overlapping binding site with the taxanes on beta-tubulin. However, electron crystallography data show that epothilones bind to a common tubulin-binding site in a manner qualitatively different from that used by the taxanes.21,22 Because of this altered binding, epothilones are less susceptible to drug-resistance mechanisms that limit the efficacy of taxanes.22–25 Various point mutations in beta-III tubulin may also confer resistance to taxanes but not to epothilones.23 In addition, P-gp over-expression does not affect the cytotoxicity of epothilones to the same degree as it affects other chemotherapeutic agents.26
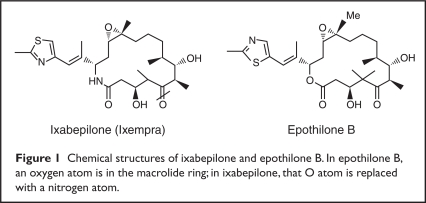
A novel microtubule-stabilizing agent, ixabepilone, was developed to optimize the properties of naturally occurring epothilone B (Figure 1).19,21,23,27 Of the epothilones currently in development, ixabepilone is the most clinically advanced.21
Figure 1.
Chemical structures of ixabepilone and epothilone B. In epothilone B, an oxygen atom is in the macrolide ring; in ixabepilone, that O atom is replaced with a nitrogen atom.
PHARMACOKINETICS
In patients with cancer, the pharmacokinetic properties of ixabepilone are linear at doses of 15 to 57 mg/m2.18 The drug disposition of ixabepilone is characterized by a rapid distributive phase, followed by a more prolonged terminal elimination phase.28,29 Ixabepilone has a terminal elimination half-life of approximately 52 hours.18 At the recommended dose and schedule (40 mg/m2 administered intravenously over three hours every three weeks), no accumulation of ixabepilone within the plasma is expected, because the cycle length is approximately 10 times the terminal elimination half-life.18
Ixabepilone is typically distributed across a large volume at steady state (in excess of 1,000 L). This is consistent with extensive tissue uptake and high binding (range, 67%–77%) to serum proteins.18
Ixabepilone is extensively metabolized in the liver by oxidative metabolism via cytochrome P450 (CYP 3A4) to more than 30 metabolites, none of which has been shown to have clinically relevant cytotoxic activity.18 Elimination occurs primarily via the liver. After an intravenous (IV) dose of 14[C]-ixabepilone was administered to patients, 65% of the dose was eliminated in the feces and 21% of the dose was excreted in the urine.18
CLINICAL TRIALS
Phase 1 Clinical Studies
In the phase 1 setting, a number of IV infusion schedules of ixabepilone have been evaluated. These include a single dose every three weeks, a daily dose for three or five days every three weeks, and a weekly schedule.
Several authors have investigated the once-every-three-week regimen.29–31 In total, 63 patients with a range of solid tumors were treated according to this schedule at dose levels ranging from 7.4 to 65 mg/m2. Responses to therapy were observed in patients with melanoma, non–small-cell lung cancer, ovarian cancer, and breast cancers (taxanenaive and taxane-refractory). Dose-limiting toxicities included grade 4 neutropenia, peripheral neuropathy, gastrointestinal (GI) discomfort, fatigue, and emesis. Of note, the incidence of neuropathy in these trials was theorized to be related to peak concentrations; therefore, a three-hour instead of a one-hour infusion schedule was recommended for phase 2 studies.31 For phase 2 development of this schedule, 40 mg/m2 was established as the recommended dose of ixabepilone.
Phase 1 studies have also established the maximum tolerated dose for ixabepilone when administered as a one-hour daily infusion on three or five consecutive days every three weeks.28,32 On these schedules, the maximum tolerated dose was 6 or 8 mg/m2 per day.28,32 Weekly schedules of ixabepilone have also been investigated.33–35
Phase 2 Clinical Studies
The clinical efficacy of ixabepilone as a single agent has been studied for a variety of tumor types and has been extensively evaluated in phase 2 trials of both advanced and metastatic breast cancer (Table 1).36–41 In these studies, ixabepilone has demonstrated promising clinical activity and good tolerability across a broad spectrum of patients. In addition, given the significant negative impact that drug resistance may have on outcome, it is particularly encouraging to note that in these trials, ixabepilone activity was observed against anthracycline-resistant, taxane-resistant, and capecitabine-resistant tumors. The primary outcome measure for these studies was the ORR. ORRs ranged from 11.5% to 57% and were dependent on previous therapy and the line of therapy.36–41
Table 1.
Response Rates in Clinical Trials of Ixabepilone Monotherapy in Metastatic Breast Cancer: Key Phase 2 Trials
| Trial | Eligibility Criteria | No. | Dose and Schedule | Response Rate |
|---|---|---|---|---|
| Baselga et al.41 | Neoadjuvant therapy in patients with locally advanced breast cancer | 164 | 40 mg/m2 q3w | pCR rate, 19% |
| Denduluri et al.40 | 1st- or >2nd-line therapy in patients with taxanenaive MBC | 23 | 6 mg/m2 days 1–5 q3w | ORR, 57%; SD rate, 26% |
| Roché et al.39 | 1st-line therapy in patients with MBC previously treated with an adjuvant anthracycline | 65 | 40 mg/m2 q3w | ORR, 41.5%; SD rate, 35% |
| Low et al.38 | 1st- 2nd-, or >3rd-line therapy in patients previously treated with a taxane as prior neoadjuvant, adjuvant, or metastatic therapy | 37 | 6 mg/m2 days 1–5 q3w | ORR, 22%; SD rate, 35% |
| Thomas et al.37 | 1st-, 2nd-, 3rd-, or 4th-line therapy in patients with taxane-resistant MBC | 49 | 40 mg/m2 q3w | ORR, 12%; SD rate, 41% |
| Perez et al.36 | 3rd- or 4th-line therapy in patients with MBC resistant to anthracyclines, taxanes, and capecitabine | 126 | 40 mg/m2 q3w | ORR, 11.5%; SD rate, 50% |
| Denduluri et al.42 | Patients with MBC who have received prior taxane therapy | 12 | 8–10 mg/m2 q3w | ORR, 0%; SD rate for ≥6 weeks, 83% |
MBC = metastatic breast cancer; ORR = objective response rate; q3w = every 3 weeks; pCR = pathological complete response; SD = stable disease.
As neoadjuvant therapy, ixabepilone appears to compare favorably in activity with that of other cytotoxic monotherapies.41 Data have been reported from a phase 2 trial in 164 patients with locally advanced breast cancer. Up to four cycles of ixabepilone (40 mg/m2 infused over three hours every three weeks) were administered as primary systemic (neoadjuvant) therapy to patients with invasive breast cancer (stages IIA–IIIB) before surgery. A pathological complete response (pCR) rate of 18% (29/161) was observed, and 17 of these patients (11%) also achieved pCRs in the axillary lymph nodes. The pCR rate in patients with triple-negative (estrogen receptor/progesterone receptor/human epidermal growth factor receptor-2 [ER/PR/HER-2–negative]) tumors was 26% (11/42).41
As a first-line therapy, ixabepilone was evaluated in 65 patients with metastatic breast cancer who had received one (92%) or two (8%) prior anthracycline-based adjuvant regimens.39 In this single-arm, phase 2 study, ixabepilone monotherapy was given as a 40- or 50-mg/m2 infusion over one or three hours every three weeks. Patients who had received a taxane as part of their anthracycline-based adjuvant regimen were not excluded from the study, provided that more than one year had elapsed since the completion of their treatment. However, most patients (83%) had not received a taxane previously. The ORR was 41.5% (95% confidence interval [CI], 29.4%–54.4%) with a median duration of 8.2 months (95% CI, 5.7–10.2 months). In addition, 35% of patients achieved stable disease. Median overall survival was 22 months (95% CI, 15.6–27 months).39
The highest response rate was noted in a single-arm, phase 2 study in which ixabepilone (6 mg/m2 infused over one hour on five consecutive days every three weeks) was given as a first-line or a subsequent therapy to 23 patients with metastatic breast cancer.40 These patients were taxane-naive, but 70% had received anthracycline and/or capecitabine earlier.40 In total, 13 patients (57%) had partial responses and six patients (26%) had stable disease, resulting in an ORR of 57% (95% CI, 34.5%– 76.8%) with a median duration of 5.6 months. Median time to disease progression was 5.5 months.40
In these phase 2 studies, lower response rates were observed with tumors that were refractory to taxane; however, given the highly unresponsive nature of this type of disease, the response to ixabepilone remains clinically relevant.
An additional phase 2 trial was conducted in patients with metastatic breast cancer who had experienced disease progression while receiving, or within four months of receiving, a taxane (or within six months if they had received adjuvant taxane therapy) and who had been given a taxane as their most recent regimen.37 In total, 49 patients received ixabepilone as a three-hour infusion of 40 mg/m2 every three weeks, resulting in a response rate of 12% (95% CI, 4.7%–26.5%). All six responses were partial, and five of these occurred in patients who had not responded to prior taxane therapy. The median response duration was 10.4 months. In addition, 20 patients achieved stable disease. The median time to progression was 2.2 months (95% CI, 1.4–3.2 months), and median survival was 7.9 months.37
In another phase 2 study, 37 patients with breast cancer with measurable disease who had received paclitaxel, docetaxel (Taxotere, Sanofi-Aventis), or both, as prior neoadjuvant, adjuvant, or metastatic therapy received IV ixabepilone at 6 mg/m2 per day on days one through five every three weeks.38 The best responses included a complete response in one patient (3%), partial responses in seven patients (19%), and stable disease in 13 patients (35%).
A small phase 2 study evaluated ixabepilone (8–10 mg/m2 per day for three days every three weeks) in patients with metastatic breast cancer who had previously received taxanes.42 In this trial, at least one response among the first 12 patients was required for accrual to continue to a total of 37 patients. No complete or partial responses were observed (N = 12). The authors concluded that these dosages of ixabepilone were not effective in this heavily pretreated population. This outcome might have resulted from the fact that the patient population had not achieved adequate dose density.
In the phase 2 registration trial,36 ixabepilone was administered as a 40-mg/m2 IV infusion given over three hours every three weeks in a multicenter, single-arm study of patients with metastatic breast cancer that was resistant to earlier therapy with anthracyclines, taxanes, and capecitabine. The primary endpoint was the ORR. Secondary endpoints included time to response, response duration, progression-free survival, and tolerability.
In this study, patients were heavily pre-treated and had disease resistance to multiple earlier therapies. Of 126 enrolled patients, 88% had received two or more previous antineoplastic regimens in the metastatic setting, 64% had three or more metastatic sites, 77% had visceral disease, and 33% had triple-negative tumors. Triple-negative tumors are common in premenopausal women, and they are aggressive and resistant to standard antineoplastic agents used to treat metastatic breast cancer. An ORR was achieved in 11.5% (95% CI, 6.3%–18.9%) of patients, as assessed by an independent radiology facility, as well as in 18% (95% CI, 11.9%–27.0%), as assessed by the investigator. An ORR was achieved in 12% (5/42) of patients with triple-negative tumors whose responses were assessable by the radiology facility.36 The response rate in patients with progressive disease as a best response to prior taxanes was 11%, according to the manufacturer.
The median time to response was 6.1 weeks (range, 5–19 weeks). The median progression-free survival was 3.1 months (95% CI, 2.7–4.2 months); the median response duration was 5.7 months (95% CI, 4.4–7.3 months).
The potential for combined therapy with ixabepilone and capecitabine was initially highlighted through preclinical studies, which demonstrated synergy between these compounds.43 Subsequently, a phase 1/2 study confirmed the activity and feasibility of this regimen and identified appropriate doses for a phase 3 study.44–46 It is encouraging that in this phase 1/2 trial, ixabepilone and capecitabine had non-overlapping toxicity profiles.45
In phase 2 studies, ixabepilone monotherapy demonstrated activity and good tolerability with a range of tumor types, including non–small-cell lung cancer, squamous cell cancer of the head and neck, and prostate cancer.47–59 Many of the patients in these studies had advanced pretreated tumors that were resistant to other antineoplastic agents.
Phase 3 Clinical Trials
The value of ixabepilone combined with capecitabine was confirmed in a randomized phase 3 controlled trial, conducted in patients with anthracyclinepretreated/resistant and taxane-resistant metastatic breast cancer.44 In total, 752 patients were randomly assigned: 375 received ixabepilone (40 mg/m2 IV over three hours, every three weeks) plus capecitabine (1,000 mg/m2 orally twice daily every 14 days), and 377 received capecitabine (1,250 mg/m2 twice daily orally every 14 days). The primary endpoint was progression-free survival. Secondary endpoints included ORR, overall survival, duration of response, and time to response.
Progression-free survival was found to be significantly longer for the experimental arm (5.8 months) than for the control arm (4.2 months) (hazard ratio = 0.75; 95% CI, 0.64–0.88 months; P = 0.00003), with a 25% reduction in estimated risk of disease progression. A subset analysis also revealed that this advantage was maintained across a range of predefined patient types, including patients whose tumors were triple-negative (ER–, PR–, and HER-2–) and those whose tumors were HER-2 positive (HER-2+). The ORR was 35% in the ixabepilone/capecitabine arm and 14% in the capecitabine arm (P < 0.0001).44
In patients who exhibited progression of disease as a best response to prior taxane therapy, the ORR in the experimental arm was 33% (95% CI, 26%–42%); in the control arm, the ORR was 14% (95% CI, 8%–20%). The median duration of response was 6.4 months for ixabepilone/capecitabine (95% CI, 5.6–7.1 months) and 5.6 months for capecitabine (95% CI, 4.2–7.5 months). The time to response was similar for the two treatment arms: 11.7 and 12 weeks, respectively. In the ixabepilone/capecitabine arm, 41% of patients achieved stable disease; in the capecitabine monotherapy arm, 46% of patients achieved stable disease.44
Table 2 lists grade 3/4 adverse events for this phase 3 study. Hematological toxicity was common and consisted primarily of leukopenia and neutropenia, with a 4% incidence of febrile neutropenia. Growth factor support was not required but was administered to 20% of patients who received ixabepilone/capecitabine and to 3% of the capecitabine patients. Anemia and thrombocytopenia were most often grade 1/2 in both treatment groups.44 Peripheral neuropathy was common, as it is with any of the tubulinactive drugs. The peripheral neuropathy associated with ixabepilone in this study occurred in 65% of patients in the combination arm and was primarily sensory and cumulative but generally reversible. Peripheral neuropathy was related mainly to the maximum plasma concentration (Cmax) and, to a lesser extent, to the area-under-the-curve (AUC) concentration. Patients received a median of four cycles before the onset of grade 3/4 neuropathy. After dose reductions, the patients were able to receive a median of three additional cycles of therapy. The median time to resolution (a return to baseline or to grade 1) of grade 3/4 neuropathy was six weeks.44
Table 2.
Incidence of Grade 3 and 4 Adverse Events in the Pivotal Phase 3 Trial of Ixabepilone/Capecitabine or Capecitabine Alone In Patients with Metastatic Breast Cancer Previously Treated with Or Resistant to Anthracycline and Resistant to Taxanes
| Ixabepilone plus Capecitabine (n = 369) | Capecitabine (n = 368) | |
|---|---|---|
| Hematological toxicities | % | % |
| Leukopenia | 57 | 6 |
| Anemia | 10 | 4 |
| Neutropenia | 68 | 11 |
| Thrombocytopenia | 8 | 4 |
| Febrile neutropenia | 4 | <1 |
| Nonhematological toxicities | ||
| Peripheral neuropathy | 23 | 0 |
| Hand–foot syndrome | 18 | 17 |
| Fatigue | 9 | 3 |
| Myalgia | 8 | .3 |
| Diarrhea | 6 | 9 |
| Vomiting | 4 | 2 |
| Nausea | 3 | 2 |
| Mucositis | 3 | 2 |
| Arthralgia | 3 | 0 |
Data derived from Thomas ES, Gomez HL, Li RK, et al. J Clin Oncol 2007;25:5210–5217.44
A second phase 3 trial of ixabepilone plus capecitabine in taxane-pretreated patients (trial NCT00082433) has completed enrollment, and data are now being analyzed. Patients received a maximum of two prior antineoplastic regimens, or if they had been treated for metastatic disease, they relapsed within one year of treatment. The primary outcome measure is overall survival; secondary outcome measures include time to progression, ORR, duration of response in patients with measurable disease, and quality of life.
DOSAGE, ADMINISTRATION, AND DOSE MODIFICATIONS
The FDA-approved dose of ixabepilone is 40 mg/m2 given intravenously over three hours every three weeks. In clinical studies of ixabepilone, doses for patients with a body surface area (BSA) greater than 2.2 m2 were to have been based on a BSA of 2.2 m2. Because few patients in the clinical studies of ixabepilone had a BSA greater than 2.2 m2, data on these patients are limited. Ixabepilone is commercially available as 15-mg and 45-mg kits; each kit contains two vials consisting of lyophilized drug and diluent for constitution. Ixabepilone kits must be stored in a refrigerator at 2°C to 8°C (36°F–46°F). The diluent used with ixabepilone contains Cremophor EL (BASF Aktiengesellschaft) and dehydrated alcohol. After constitution with the diluent, the concentration of ixabepilone is 2 mg/mL. The constituted solution must be further diluted with lactated Ringer’s solution USP in non-di(2-ethylhexyl)-phthalate (DEHP) IV bags to a final concentration of between 0.2 and 0.6 mg/mL. The infusion solution must be administered with a non-DEHP infusion set via an in-line filter with a microporous membrane of 0.2 to 1.2 microns. Diluted solutions are stable at room temperature and remain stable in light for up to six hours.18 Because of the potential for neurotoxicity, ixabepilone should be infused over three or more hours.60
Dose modifications are required for patients with liver impairment (Table 3). Ixabepilone was evaluated in 56 patients with mild-to-severe hepatic impairment, as defined by bilirubin and aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels. Compared with patients with normal hepatic function (n = 17), the AUC0–∞of ixabepilone increased by 22% in patients with either bilirubin 1 to 1.5 times above the upper limit of normal (ULN) or an AST level above the ULN but with bilirubin below 1.5 times the ULN. The AUC concentration increased by 30% in patients with bilirubin above 1.5 to 3 times the ULN and any AST level and by 81% in patients with bilirubin greater than 3 times the ULN and any AST level.61
Table 3.
Dose Adjustments for Ixabepilone
| Adjustment | Suggested Dose or Dose Modification |
|---|---|
| Nonhematological toxicity* | |
| Grade 2 neuropathy (moderate) lasting ≥7 days | Decrease dose by 20% |
| Grade 3 neuropathy (severe) lasting <7 days | Decrease dose by 20% |
| Grade 3 neuropathy (severe) lasting ≥7 days or disabling neuropathy | Discontinue treatment |
| Any grade 3 toxicity (severe) | Decrease dose by 20% |
| Transient grade 3 arthralgia/myalgia or fatigue | No change in ixabepilone dose |
| Grade 3 hand–foot syndrome | No change in ixabepilone dose |
| Any grade 4 toxicity (disabling) | Discontinue treatment |
| Hematological toxicity* | |
| Neutrophils <500 cells/mm3 for ≥7 days | Decrease dose by 20% |
| Febrile neutropenia | Decrease dose by 20% |
| Platelets <25,000/mm3or platelets <50,000/mm3 with bleeding | Decrease dose by 20% |
| Hepatic Impairment | |
| Ixabepilone monotherapy | |
| AST and ALT ≤2.5 × ULN and bilirubin ≤1 × ULN | Recommended dose: 40 mg/m2 |
| AST and ALT ≤10 × ULN and bilirubin ≤1.5 × ULN | Recommended dose: 32 mg/m2 |
| AST and ALT ≤10 × ULN and bilirubin >1.5 × ULN ≤3 × ULN | Recommended dose: 20–30 mg/m2 |
| Ixabepilone + capecitabine combination therapy | |
| AST or ALT >2.5 × ULN or bilirubin >1 × ULN | Contraindicated |
| Coadministration with other drugs | |
| Strong inhibitors of CYP 3A4†‡ | Starting dose: 20 mg/m2 |
ALT = alanine aminotransferase;AST = aspartate aminotransferase; CYP 3A4 = cytochrome P450 isoenzyme 3A4; ULN = upper limit of normal.
Toxicities graded in accordance with the National Cancer Institute Common Toxicity Criteria (CTC) for adverse events.
Inhibiting oxidative metabolism of ixabepilone may significantly increase its plasma concentrations.
Examples of strong cytochrome CYP 3A4 inhibitors: ketoconazole, itraconazole, clarithromycin, atazanavir, nefazodone, saquinavir, telithromycin, ritonavir, amprenavir, indinavir, nelfinavir, delavirdine, and voriconazole.
Hepatic impairment results in increased exposure to ixabepilone and greater toxicity when ixabepilone is given with capecitabine or as monotherapy. Dose modification of ixabepilone used as monotherapy is based on the degree of hepatic impairment (see Table 1). Patients should not receive ixabepilone monotherapy if AST or ALT levels are more than 10 times the ULN or if bilirubin is 3 times the ULN.18
CONTRAINDICATIONS
The most commonly described adverse effect of drugs formulated with Cremophor EL is hypersensitivity. Ixabepilone diluent contains a highly purified form of Cremophor EL. It is believed that this purity might be one reason that hypersensitivity reactions are less pronounced than with paclitaxel (Taxol).18 Of 1,323 patients mentioned in the prescribing information (PI) for ixabepilone, 1% (n = 9) experienced severe hypersensitivity reactions.18 Three of these nine patients were able to receive re-treatment.
Ixabepilone should not be given to patients who have a history of severe hypersensitivity (grade 3/4) to Cremophor EL or its derivatives. Premedication with H1 and H2 antihistamines is advocated for all patients to reduce the risk of their experiencing any such reactions.18,39 In contrast to clinical practice with the taxanes,62,63 corticosteroid pre-medication is not necessary unless the patient has experienced a hypersensitivity reaction to ixabepilone. Because patients are likely to have been heavily pretreated, antiemetic therapy should be used as part of the premedication protocol before treatment with ixabepilone.
The use of ixabepilone is contraindicated in patients with neutropenia or thrombocytopenia (neutrophil count below 1,500/mm3 or platelet counts of less than 100,000/mm3). Grade 4 neutropenia (below 500 cells/mm3) occurred in 36% of patients treated with ixabepilone plus capecitabine and in 23% of those receiving ixabepilone alone.18,44
The neutropenia-related death rate was higher in patients with significant liver impairment. Because of this adverse event, ixabepilone/capecitabine is contraindicated in patients with AST or ALT levels above 2.5 times the ULN or with bilirubin above 1 times the ULN, as stated in the boxed warning.18
ADVERSE EFFECTS
In phase 2 monotherapy trials, ixabepilone demonstrated a manageable safety profile when given at the recommended schedule of a 40-mg/m2 IV infusion over three hours every three weeks to appropriately selected patients.36,37,39,60 Adverse events commonly reported with ixabepilone monotherapy are summarized in Table 4. Grade 3 and 4 treatment-related adverse events included peripheral sensory neuropathy, fatigue and asthenia, myalgia, stomatitis, mucositis, and neutropenia, although febrile neutropenia was uncommon. Among lower-grade events, alopecia occurred at a notably high rate.36,37,39
Table 4.
Treatment-Related Adverse Events (%) Occurring in 10% of More of Patients in Clinical Trials Of Ixabepilone Monotherapy in Metastatic Breast Cancer: Key Phase 2 Trials
| Adverse Event | Denduluri et al.40 (n = 23) | Roché et al.39 (n = 65) | Low et al.38 (n = 37) | Thomas et al.37 (n = 49) | Perez et al.36 (n = 126) |
|---|---|---|---|---|---|
| Nonhematological toxicity | |||||
| Peripheral sensory neuropathy | 52 (Grade 3/4: 0) | 71 (Grade 3/4: 20) | 54 (Grade 3/4: 3) | 63 (Grade 3/4: 12) | 60 (Grade 3/4: 14) |
| Fatigue/asthenia | 78 (Grade 3/4: 13) | 68 (Grade 3/4: 6) | 64 (Grade 3/4: 13) | 76 (Grade 3/4: 27) | 50 (Grade 3/4: 14) |
| Myalgia, arthralgia | 30 | 97 | 52 | 84 | 49 |
| Alopecia | 87 | 92 | 54 | 43 | 48 |
| Nausea | 61 | 54 | 54 | 57 | 42 |
| Stomatitis, mucositis, pharyngitis | – | 32 | – | 28 | 29 |
| Vomiting | 39 | 26 | 21 | 41 | 29 |
| Diarrhea | 48 | 29 | 35 | 31 | 22 |
| Rash | – | 22 | – | 12 | – |
| Musculoskeletal pain | – | – | – | – | 20 |
| Anorexia | – | 18 | – | 18 | 19 |
| Constipation | 56 | 20 | 27 | 20 | 16 |
| Nail changes | 56 | 17 | 30 | 8 | 9 |
| Fever | – | 14 | – | 16 | – |
| Abdominal pain, cramping | – | 8 | – | 10 | 13 |
| Headache | – | 14 | – | – | 11 |
| Neuropathic pain | – | 12 | – | 8 | – |
| Pain, other | – | 14 | – | 65 | 8 |
| Infection without neutropenia | – | 14 | – | 12 | – |
| Infection, febrile neutropenia | 0 | 6 | 14 | 6 | – |
| Motor neuropathy | 9 | 6 | – | – | 10 |
| Taste disturbance, dysgeusia | 65 | 11 | 33 | – | 6 |
| Hematological toxicity | |||||
| Neutropenia | 87 (Grade 3/4: 22) | 89 (Grade 3/4: 58) | 67 (Grade 3/4: 35) | – (Grade 3/4: 53) | 79 (Grade 3/4: 54) |
| Leukopenia | – | 92 (Grade 3/4: 50) | – | 6 (Grade 3/4: 2) | 90 (Grade 3/4: 49) |
| Anemia | 83 (Grade 3/4: 0) | 92 (Grade 3/4: 3) | 73 (Grade 3/4:) | 6 (Grade 3/4: 4) | 84 (Grade 3/4: 8) |
| Thrombocytopenia | 52 (Grade 3/4: 4) | 40 (Grade 3/4: 0) | 40 (Grade 3/4: 8) | – | 44 (Grade 3/4: 8) |
In the pivotal phase 3 study, adverse events related to ixabepilone plus capecitabine were usually mild to moderate and generally manageable (see Table 2), although a higher rate of treatment-related mortality was reported in patients with liver dysfunction.44 The addition of ixabepilone to capecitabine significantly increased grade 3/4 hematological toxicity, predominantly neutropenia, even though the rates of febrile neutropenia and infection were low. Grade 3/4 non-hematological events also occurred more frequently with the combination (including neuropathy, fatigue, and myalgia), but the addition of ixabepilone to capecitabine did not increase the incidence of grade 3/4 hand–foot syndrome or diarrhea, the predominant toxicities associated with capecitabine.
Caution is advised when ixabepilone is administered to patients with pre-existing neuropathy. The presence of grade 1 neuropathy and prior treatment with neurotoxic chemotherapy do not predict the development or worsening of neuropathy.18 Patients with grade 2 neuropathy were excluded from clinical trials. Neuropathy is perhaps the most clinically relevant adverse event associated with ixabepilone; on average, 23% of patients experience grade 3/4 neuropathy. Ixabepilone-associated neuropathy is cumulative and mainly sensory, although in most cases it is reversible and can be managed by dose reductions and delays in treatment. Therapy should be interrupted at the first signs of neuropathy and should be restarted when neuropathy has returned to the baseline level or when severity has diminished to grade 1.
Dose modification of ixabepilone, capecitabine, or both, is the most effective approach to managing the hematological and other nonhematological toxicities associated with this combination.18,44 Several doses and schedules for ixabepilone have been investigated, and alternative daily and weekly schedules continue to be studied in ongoing efforts to optimize the agent’s therapeutic ratio.
DRUG INTERACTIONS
In vitro studies have identified CYP 3A4 as the main route of oxidative metabolism of ixabepilone.18 Inhibition of this enzyme may decrease metabolism and increase plasma concentrations of ixabepilone. Ixabepilone doses should be reduced if the drug is being administered concomitantly with a strong inhib itor of CYP 3A4, such as ketoconazole (Nizoral, Janssen), itraconazole (Sporanox, Janssen), ritonavir (Norvir, Abbott), amprenavir (Agenerase, Glaxo-SmithKline), indinavir (Crixivan, Merck), nelfinavir (Viracept, Agouron), delavirdine (Rescriptor, Pfizer) or voriconazole (Vfend, Pfizer). In vitro data are consistent with available clinical data (e.g., with ketoconazole).64 Caution should be used when mild or moderate CYP 3A4 inhibitors such as erythromycin, fluconazole (Diflucan, Pfizer), and verapamil (e.g., Calan, Pfizer) are given.18
The use of strong CYP 3A4 inducers, such as dexamethasone, phenytoin (Dilantin, Pfizer), carbamazepine (Tegretol, Novartis), rifampin, and phenobarbital, may lead to subtherapeutic levels of ixabepilone; caution is advised if concomitant enzyme inducers must be administered. Ixabepilone does not induce or inhibit CYP 3A4 or other liver microsomal enzymes by clinically relevant amounts; therefore, plasma levels of other drugs that are substrates for CYP enzymes should not be affected by ixabepilone.18
COST
As of November 2007, the average wholesale price (AWP) of ixabepilone, according to the manufacturer, was $921.96 for the 15-mg kit and $2,765.89 for the 45-mg kit.67 The AWP for a typical 75-mg dose of ixabepilone would be $4,609.81. These prices do not take into account other pharmacoeconomic parameters, such as specialist equipment or nurse time, that may influence the overall cost of administration of ixabepilone. For example, because of the presence of Cremophor EL, non-DEHP bags and tubing are required to prevent the plasticizer from leaching into the solution. Currently, lactated Ringer’s solution in non-DEHP IV bags is available only through a single manufacturer.
CONCLUSION
Ixabepilone is minimally susceptible to resistance mechanisms that have a detrimental impact on the efficacy of taxanes and anthracyclines. Ixabepilone thus represents a clinically useful addition to the armamentarium of therapeutic agents available for patients with metastatic breast cancer when resistance to active agents has developed through earlier lines of therapy. For this purpose, ixabepilone has shown clinical benefits for patients across a wide spectrum of this disease, including those with extensive, aggressive, and heavily pretreated tumors. It has also demonstrated synergistic activity when given with capecitabine, an antineoplastic agent that is commonly used for refractory disease.
On the basis of positive phase 2 and 3 clinical trial data, ixabepilone has now received regulatory approval for use with capecitabine for patients with metastatic or locally advanced breast cancer after failure of an anthracycline and a taxane and as monotherapy in the event of failure with an anthracycline, a taxane, and capecitabine.
In phase 2 studies, ixabepilone alone has demonstrated encouraging activity against advanced and metastatic disease across several therapy lines, suggesting that it may have the potential to replace taxanes as initial therapy in selected patients in the metastatic setting and may warrant investigation as an adjuvant therapy for early breast cancer.65
The efficacy of ixabepilone may be enhanced through its use in combination with other antineoplastic agents; several studies in which ixabepilone is combined with targeted therapies are ongoing or planned.66 Most notably, a phase 2 randomized trial (NCT00370552) is now under way to compare two schedules of ixabepilone plus bevacizumab (Avastin, Genentech), with paclitaxel plus bevacizumab given as a first-line therapy for locally recurrent or metastatic breast cancer.66
Ixabepilone has a manageable safety profile. The prescribing information contains four warnings and precautions, including those regarding hypersensitivity (typically related to the diluent; treated with premedication with histamine antagonists) and fetal harm when administered to pregnant women. Additional warnings relate to neuropathy (primarily sensory) and myelosuppression. Neuropathy is cumulative, is generally reversible, and can be managed by dose adjustment and treatment delays. Myelosuppression (typically neutropenia) can be managed with dose adjustments.18
Ixabepilone represents a promising addition to the therapeutic options available for advanced breast cancer. Overall survival data from phase 3 clinical trials are expected to be available in the near future. Through an extensive clinical development program, ixabepilone has demonstrated antitumor activity for a wide range of solid tumors.
Footnotes
Disclosure: Dr. Egerton has no conflict of interest to disclose regarding this manuscript and has no relationship to any of the companies that market epothilone derivatives. Editorial assistance from L. C. Shepherd, a medical writer, was funded by Bristol-Myers Squibb, but Dr. Egerton received no financial incentive for the writing of this article.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Gennari A, Conte P, Rosso R, et al. Survival of metastatic breast carcinoma patients over a 20-year period: A retrospective analysis based on individual patient data from six consecutive studies. Cancer. 2005;104:1742–1750. doi: 10.1002/cncr.21359. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton A, Hortobagyi G. Chemotherapy: What progress in the last 5 years? J Clin Oncol. 2005;23:1760–1775. doi: 10.1200/JCO.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 4.Colozza M, de Azambuja E, Personeni N, et al. Achievements in systemic therapies in the pregenomic era in metastatic breast cancer. Oncologist. 2007;12:253–270. doi: 10.1634/theoncologist.12-3-253. [DOI] [PubMed] [Google Scholar]
- 5.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 6.Hortobagyi GN. Treatment of breast cancer. N Engl J Med. 1998;339:974–984. doi: 10.1056/NEJM199810013391407. [DOI] [PubMed] [Google Scholar]
- 7.Valero V, Hortobagyi GN. Are anthracycline–taxane regimens the new standard of care in the treatment of metastatic breast cancer? J Clin Oncol. 2003;21:959–962. doi: 10.1200/JCO.2003.11.071. [DOI] [PubMed] [Google Scholar]
- 8.Bernard-Marty C, Cardoso F, Piccart MJ. Facts and controversies in systemic treatment of metastatic breast cancer. Oncologist. 2004;9:617–632. doi: 10.1634/theoncologist.9-6-617. [DOI] [PubMed] [Google Scholar]
- 9.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 10.Lee JJ, Swain SM. Development of novel chemotherapeutic agents to evade the mechanisms of multidrug resistance (MDR) Semin Oncol. 2005;32(Suppl 7):S22–S26. doi: 10.1053/j.seminoncol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Clarke R, Leonessa F, Trock B. Multidrug resistance/P-glycoprotein and breast cancer: Review and meta-analysis. Semin Oncol. 2005;32(Suppl 7):S9–S15. doi: 10.1053/j.seminoncol.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Fornier M. Epothilones in breast cancer: Review of clinical experience. Ann Oncol. 2007;18(Suppl 5):S16–S21. doi: 10.1093/annonc/mdm174. [DOI] [PubMed] [Google Scholar]
- 13.Fojo AT, Menefee M. Microtubule targeting agents: Basic mechanisms of multidrug resistance (MDR) Semin Oncol. 2005;32(Suppl 7):S3–S8. doi: 10.1053/j.seminoncol.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Faneyte IF, Kristel PM, van de Vijver MJ. Multidrug resistance associated genes MRP1, MRP2 and MRP3 in primary and anthracycline exposed breast cancer. Anticancer Res. 2004;24:2931–2939. [PubMed] [Google Scholar]
- 15.Burger H, Foekens JA, Look MP, et al. RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer: Correlation with chemotherapeutic response. Clin Cancer Res. 2003;9:827–836. [PubMed] [Google Scholar]
- 16.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 17.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 18.Princeton, NJ: Bristol-Myers Squibb; Oct, 2007. Ixempra (ixabepilone), package insert. [Google Scholar]
- 19.Pivot X, Dufresne A, Villanueva C. Efficacy and safety of ixabepilone, a novel epothilone analogue. Clin Breast Cancer. 2007;7:543–549. doi: 10.3816/CBC.2007.n.009. [DOI] [PubMed] [Google Scholar]
- 20.Bollag DM, McQueney PA, Zhu J, et al. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 21.Cortes J, Baselga J. Targeting the microtubules in breast cancer beyond taxanes: The epothilones. Oncologist. 2007;12:271–280. doi: 10.1634/theoncologist.12-3-271. [DOI] [PubMed] [Google Scholar]
- 22.Nettles JH, Li H, Cornett B, Krahn JM, Snyder JP, Downing KH. The binding mode of epothilone A on α,β-tubulin by electron crystallography. Science. 2004;305:866–869. doi: 10.1126/science.1099190. [DOI] [PubMed] [Google Scholar]
- 23.Lee FY, Borzilleri R, Fairchild CR, et al. BMS-247550: A novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res. 2001;7:1429–1437. [PubMed] [Google Scholar]
- 24.Bode CJ, Gupta ML, Jr, Reiff EA, et al. Epothilone and paclitaxel: Unexpected differences in promoting the assembly and stabilization of yeast microtubules. Biochemistry. 2002;41:3870–3874. doi: 10.1021/bi0121611. [DOI] [PubMed] [Google Scholar]
- 25.Brooks TA, Minderman H, O’Loughlin KL, et al. Taxane-based reversal agents modulate drug resistance mediated by P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Mol Cancer Ther. 2003;2:1195–1205. [PubMed] [Google Scholar]
- 26.Rowinsky EK, Calvo E. Novel agents that target tublin and related elements. Semin Oncol. 2006;33:421–435. doi: 10.1053/j.seminoncol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Goodin S, Kane MP, Rubin EH. Epothilones: Mechanism of action and biologic activity. J Clin Oncol. 2004;22:2015–2025. doi: 10.1200/JCO.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Abraham J, Agrawal M, Bakke S, et al. Phase I trial and pharmacokinetic study of BMS-247550, an epothilone B analog, administered intravenously on a daily schedule for five days. J Clin Oncol. 2003;21:1866–1873. doi: 10.1200/JCO.2003.03.063. [DOI] [PubMed] [Google Scholar]
- 29.Mani S, McDaid H, Hamilton A, et al. Phase I clinical and pharmacokinetic study of BMS-247550, a novel derivative of epothilone B, in solid tumors. Clin Cancer Res. 2004;10:1289–1298. doi: 10.1158/1078-0432.ccr-0919-03. [DOI] [PubMed] [Google Scholar]
- 30.Spriggs DR, Soignet S, Bievenu B, et al. Phase 1 first-in-man study of epothilone B analog BMS-247550 in patients with advanced cancer (American Society of Clinical Oncology meeting, Abstract 428a) Proc Am Soc Clin Oncol. 2001;20 [Google Scholar]
- 31.Gadgeel SM, Wozniak A, Boinpally RR, et al. Phase I clinical trial of BMS-247550, a derivative of epothilone B, using accelerated titration 2B design. Clin Cancer Res. 2005;11:6233–6239. doi: 10.1158/1078-0432.CCR-05-0127. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang SH, Agrawal M, Edgerly M, et al. A phase I clinical trial of ixabepilone (BMS-247550), an epothilone B analog, administered intravenously on a daily schedule for 3 days. Cancer. 2005;103:1932–1938. doi: 10.1002/cncr.20977. [DOI] [PubMed] [Google Scholar]
- 33.Awada A, Bleiberg H, de Valeriola D, et al. Phase I clinical and pharmacology study of the epothilone analog BMS-247550 given weekly in patients with advanced solid tumors (American Society of Clinical Oncology meeting, Abstract 427) Proc Am Soc Clin Oncol. 2001;20 [Google Scholar]
- 34.Hao D, Hammond LA, deBono JS, et al. Continuous weekly administration of the epothilone-B derivative, BMS-247550 (NSC710428): A phase I and pharmacokinetic (PK) study (American Society of Clinical Oncology meeting, Abstract 411) Proc Am Soc Clin Oncol. 2002;21 [Google Scholar]
- 35.Burris HA, Awada A, Jones S, et al. Phase I study of the novel epothilone BMS-247550 administered weekly in patients with advanced malignancies (American Society of Clinical Oncology meeting, Abstract 412) Proc Am Soc Clin Oncol. 2002;21 [Google Scholar]
- 36.Perez EA, Lerzo G, Pivot X, et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2007;25:3407–3414. doi: 10.1200/JCO.2006.09.3849. [DOI] [PubMed] [Google Scholar]
- 37.Thomas E, Tabernero J, Fornier M, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancer. J Clin Oncol. 2007;25:3399–3406. doi: 10.1200/JCO.2006.08.9102. [DOI] [PubMed] [Google Scholar]
- 38.Low JA, Wedam SB, Lee JJ, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in metastatic and locally advanced breast cancer. J Clin Oncol. 2005;23:2726–2734. doi: 10.1200/JCO.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 39.Roché H, Yelle L, Cognetti F, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, as first-line therapy in patients with metastatic breast cancer previously treated with anthracycline chemotherapy. J Clin Oncol. 2007;25:3415–3420. doi: 10.1200/JCO.2006.09.7535. [DOI] [PubMed] [Google Scholar]
- 40.Denduluri N, Low JA, Lee JJ, et al. Phase II trial of ixabepilone, an epothilone B analog, in patients with metastatic breast cancer previously untreated with taxanes. J Clin Oncol. 2007;25:3421–3427. doi: 10.1200/JCO.2006.10.0784. [DOI] [PubMed] [Google Scholar]
- 41.Baselga J, Gianni L, Llombart A, et al. Predicting response to ixabepilone: Genomics study in patients receiving single agent ixabepilone as neoadjuvant treatment for breast cancer (San Antonio Breast Cancer Symposium, Abstract 305) Breast Cancer Res Treat. 2005;94(Suppl 1) [Google Scholar]
- 42.Denduluri N, Lee JJ, Walshe J, et al. Phase II trial of ixabepilone, an epothilone B analog, given daily for three days every three weeks, in metastatic breast cancer. Invest New Drugs. 2007;25:63–67. doi: 10.1007/s10637-006-9006-7. [DOI] [PubMed] [Google Scholar]
- 43.Lee FY, Camuso A, Castenada S, et al. Preclinical efficacy evaluation of ixabepilone (BMS-247550) in combination with cetuximab or capecitabine in human colon and lung carcinoma xenografts (American Society of Clinical Oncology meeting, Abstract 12017) J Clin Oncol. 2006;24(Suppl 18S) [Google Scholar]
- 44.Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25:5210–5217. doi: 10.1200/JCO.2007.12.6557. [DOI] [PubMed] [Google Scholar]
- 45.Vahdat L, Klimovsky J, Bunnell C. Phase I/II trial in patients with metastatic breast cancer (MBC) previously treated with a taxane and an anthracycline: Final safety data (American Society of Clinical Oncology meeting, Abstract 1006) J Clin Oncol. 2006;24(Suppl 18S) [Google Scholar]
- 46.Bunnell C, Klimovsky J, Thomas E. Final efficacy results of a phase I/II trial of ixabepilone in combination with capecitabine in patients with metastatic breast cancer (MBC) previously treated with a taxane and an anthracycline (American Society of Clinical Oncology meeting, Abstract 10511) J Clin Oncol. 2006;24(Suppl 18S) [Google Scholar]
- 47.Vansteenkiste J, Lara PN, Jr, Le Chevalier T, et al. Phase II clinical trial of the epothilone B analog, ixabepilone, in patients with non–small-cell lung cancer whose tumors have failed first-line platinum-based chemotherapy. J Clin Oncol. 2007;25:3448–3455. doi: 10.1200/JCO.2006.09.7097. [DOI] [PubMed] [Google Scholar]
- 48.Burtness B, Goldwasser MA, Axelrod R, et al. A randomized phase II study of BMS-247550 (ixabepilone) given daily × 5 days ever y 3 weeks or weekly in patients with metastatic or recurrent squamous cell cancer of the head and neck (American Society of Clinical Oncology meeting, Abstract 5532) J Clin Oncol. 2006;24(Suppl 18S) [Google Scholar]
- 49.Rosenberg JE, Weinberg VK, Kelly WK, et al. Activity of second-line chemotherapy in docetaxel-refractory hormone-refractory prostate cancer patients: Randomized phase 2 study of ixabepilone or mitoxantrone and prednisone. Cancer. 2007;110:556–563. doi: 10.1002/cncr.22811. [DOI] [PubMed] [Google Scholar]
- 50.Liu G, Wang W, Dipaola R, et al. A phase II study of a weekly schedule of BMS-247550 for patients with hormone-refractory prostate cancer: A trial of the Eastern Cooperative Oncology Group (E3803) (American Society of Clinical Oncology meeting, Abstract 4618) J Clin Oncol. 2007;24(Suppl 18S) [Google Scholar]
- 51.Hussain M, Tangen CM, Lara PN, Jr, et al. Ixabepilone (epothilone B analogue BMS-247550) is active in chemotherapynaive patients with hormone-refractory prostate cancer: A Southwest Oncology Group trial S0111. J Clin Oncol. 2005;23:8724–8729. doi: 10.1200/JCO.2005.02.4448. [DOI] [PubMed] [Google Scholar]
- 52.Dreicer R, Li S, Manola J, et al. Phase 2 trial of epothilone B analog BMS-247550 (ixabepilone) in advanced carcinoma of the urothelium (E3800): A trial of the Eastern Cooperative Oncology Group. Cancer. 2007;110:759–763. doi: 10.1002/cncr.22839. [DOI] [PubMed] [Google Scholar]
- 53.Zhuang SH, Menefee M, Kotz H, et al. A phase II clinical trial of BMS-247550 (ixabepilone), a microtubule-stabilizing agent in renal cell cancer (American Society of Clinical Oncology meeting, Abstract 4550) J Clin Oncol. 2004;22(Suppl 14S) [Google Scholar]
- 54.Feldman DR, Kondagunta GV, Ginsberg MS, et al. Phase II trial of ixabepilone in patients with cisplatin-refractory germ cell tumors. Invest New Drugs. 2007;25:487–490. doi: 10.1007/s10637-007-9059-2. [DOI] [PubMed] [Google Scholar]
- 55.Whitehead RP, McCoy S, Rivkin SE, et al. A phase II trial of epothilone B analogue BMS-247550 (NSC #710428) ixabepilone in patients with advanced pancreas cancer: A Southwest Oncology Group study. Invest New Drugs. 2006;24:515–520. doi: 10.1007/s10637-006-8440-x. [DOI] [PubMed] [Google Scholar]
- 56.Ajani JA, Safran H, Bokemeyer C, et al. A multicenter phase II study of BMS-247550 (ixabepilone) by two schedules in patients with metastatic gastric adenocarcinoma previously treated with a taxane. Invest New Drugs. 2006;24:441–446. doi: 10.1007/s10637-006-7304-8. [DOI] [PubMed] [Google Scholar]
- 57.Singh DA, Taber D, Ansari R, et al. A phase II trial of the epothilone B analog BMS-247550 in patients with hepatobiliary cancer (HBC): An updated analysis (American Society of Clinical Oncology meeting, Abstract 14050) J Clin Oncol. 2006;24(Suppl 18S) [Google Scholar]
- 58.Smith SM, Pro B, van Besien K, et al. A phase II study of epothilone B analog BMS-247550 (NSC 710428) in patients with relapsed aggressive non-Hodgkin’s lymphomas (American Society of Clinical Oncology meeting, Abstract 6625) J Clin Oncol. 2005;23(Suppl 16S) [Google Scholar]
- 59.O’Connor O, Straus D, Moskowitz C, et al. Targeting the microtubule apparatus in indolent and mantle cell lymphoma with the novel epothilone analog BMS 247550 induces major and durable remissions in ver y drug resistant disease (American Society of Clinical Oncology meeting, Abstract 6569) J Clin Oncol. 2005;23(Suppl 16S) [Google Scholar]
- 60.Aghajanian C, Burris HA, III, Jones S, et al. Phase I study of the novel epothilone analog ixabepilone (BMS-247550) in patients with advanced solid tumors and lymphomas. J Clin Oncol. 2007;25:1082–1088. doi: 10.1200/JCO.2006.08.7304. [DOI] [PubMed] [Google Scholar]
- 61.Takimoto CH, Liu PY, Lenz H, et al. A phase I pharmacokinetic (PK) study of the epothilone B analogue, ixabepilone (BMS-247550), in patients with advanced malignancies and varying degrees of hepatic impairment: A Southwest Oncology Group Early Therapeutics Committee and NCI Organ Dysfunction Working Group trial (American Society of Clinical Oncology meeting, Abstract 2004) J Clin Oncol. 2006;24(Suppl 18S) [Google Scholar]
- 62.Markman M. Management of toxicities associated with the administration of taxanes. Expert Opin Drug Saf. 2003;2:141–146. doi: 10.1517/14740338.2.2.141. [DOI] [PubMed] [Google Scholar]
- 63.Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12:601–609. doi: 10.1634/theoncologist.12-5-601. [DOI] [PubMed] [Google Scholar]
- 64.Goel S, Goldberg G, Iacono LC, et al. Effect of ketoconazole on the pharmaco-kinetics and pharmacodynamics of ixabepilone (American Society of Clinical Oncology meeting, Abstract 2005) J Clin Oncol. 2006;24(Suppl 18S) [Google Scholar]
- 65.Gianni L. Ixabepilone and the narrow path to developing new cytotoxic drugs. J Clin Oncol. 2007;25:3389–3391. doi: 10.1200/JCO.2007.10.9504. [DOI] [PubMed] [Google Scholar]
- 66.ClinicalTrialsgov: a service of the U.S. National Institutes of Health. Available at: www.clinicaltrials.gov Accessed October 2007.
- 67.Academy of Managed Care Pharmacy (AMCP) Formulary Dossier for Ixempra (ixabepilone) Nov, 2007.