Abstract
Galectins, soluble intracellular and extracellular β-galactoside-binding proteins, are known to be involved in the progression and metastasis of various cancers, including prostate adenocarcinoma, but the detailed mechanism of their biological roles remains elusive. In the prostate cancer cell lines PC-3 and DU-145, galectin 3 (gal3) is present at normal levels, whereas in LNCaP, its expression is silenced. In LNCaP, the gal3 promoter was heavily methylated, whereas PC-3 or DU-145 cells showed negligible or no methylation in the gal3 promoter indicating a negative correlation between gal3 promoter methylation and its expression. On immunohistochemical analysis of normal and tumor prostate tissues, gal3 was found expressed both in nucleus and cytoplasm of benign prostatic hyperplasia, high-grade prostatic intraepithelial neoplasia, and stage I. The expression of the gal3 was found drastically downregulated in advanced stages and, interestingly, mostly in the cytoplasm. On methylation analysis, the gal3 promoter in stage II prostate adenocarcinoma (PCa) was found heavily methylated, whereas in stages III and IV, it was only lightly methylated. However, in stage I PCa, both heavy and light methylations were observed in the gal3 promoter. In normal and benign prostatic hyperplasia tissues, the gal3 promoter was almost unmethylated. The differential cytosine methylation in the gal3 promoter in stages I to IV PCa enabled us to develop and validate a methylation-specific polymerase chain reaction-based sensitive assay specific for stages I and II PCa. These stages are considered the critical stages for successful intervention, thus underscoring the significance of this diagnostic assay.
Introduction
Cell surface proteins such as integrins, osteopontin, and lectins play important roles in cell-cell and cell-extracellular matrix interactions and in signal transduction, which, in turn, modulate several biological processes including apoptosis, angiogenesis, and cancer metastasis [1–3]. Galectins (gal), a family of β-galactoside-binding proteins, are involved in growth development as well as cancer progression and metastasis [4,5]. However, the detailed mechanisms of these functions remain largely unknown. Of the 15 members of the galectin family identified so far, gal1, gal2, gal5, gal7, gal10, gal11, gal13, gal14, and gal15 are examples of the “proto” type galectins (one carbohydrate-recognition domain [CRD] per subunit), whereas gal4, gal6, gal8, gal9, and gal12 are “tandem-repeat” type galectins, which contain two CRDs [6]. Gal3 is the only representative of the “chimera” galectin type, containing one CRD connected to a collagen-like sequence rich in proline and glycine [7]. Galectins may exert their multiple biological roles intracellularly within the nucleus or the cytoplasm, or after their secretion, at the cell surface and/or the extracellular space, mediating interactions between cells and the extracellular matrix [2,5,6].
Although most galectins are ubiquitously expressed in various human tissues, in most cancers, galectins were either upregulated or downregulated relative to the normal parental tissue [8]. For example, gal1 is upregulated in thyroid cancer and uterine sarcoma [9,10], but its expression is decreased in head and neck cancer relative to the normal tissue [11]. Similarly, expression of galectin 3 (gal3) is found upregulated in gastric cancer [12], liver cancer [13], and thyroid cancer [9], but its expression is downregulated in head and neck cancer [11] and uterine sarcoma [10] compared with normal tissues. In prostate adenocarcinoma (PCa), the expression of gal3 is found decreased compared with normal prostate tissue [14,15]. In contrast, the expression patterns of gal8 in prostate tumors are more complex [16,17]. The expression of some proto type isoforms of gal8, such as gal8e and gal8g, is increased in the malignant prostate epithelial cells compared with that in normal and benign cells [18]. However, some tandem-repeat isoforms of gal8, such as gal8a and gal8b, are equally expressed in normal, benign, and malignant prostate epithelial cells [18].
Prostate adenocarcinoma is the second most common cancer in men, and the second leading cause of cancer death (Cancer Facts, National Cancer Institute; http://cis.nci.nih.gov/fact/5_29.htm). Hormone therapy is the treatment of choice in men with metastatic PCa, although eventually most patients relapse to a fatal hormone-insensitive state. However, if PCa is diagnosed in its early stages such as stages I and II when the tumor is still confined to the prostate gland, it can be effectively treated and cured. Combined with the digital rectal examination, elevated levels of the prostate-specific antigen (PSA) have been widely used to diagnose PCa (Cancer Facts, National Cancer Institute; http://cis.nci.nih.gov/fact/5_29.htm). Various conditions such as enlargement or inflammation of the prostate, however, can also lead to elevated levels of PSA. Conversely, PSA levels may be normal despite the presence of PCa. Thus, PSA screening for early detection of PCa is not suitable owing to the highly prevalent false-positive and -negative PSA test results (sensitivity, 90%; specificity, 10%–31%) [19]. Actually, only 25% to 30% of men who underwent biopsy owing to elevated PSA levels had their specimens diagnosed with PCa [20]. Most recently, assays based on the detection of the specific serum marker EPCA-2 (sensitivity, 94%; specificity, 92%) [21] and overexpression of telomerase (sensitivity, 58%; specificity, 100%) or the DD3 gene (sensitivity, 67%; specificity, 83%) [19] have been established, which bear great promise for more accurate PCa diagnosis, thus reducing the number of unnecessary biopsies.
Epigenetic alterations, including hypermethylation of gene promoters, are also early events in neoplastic progression [22]. Such alterations are believed to contribute to the neoplastic process by transcriptional silencing of tumor suppressor gene expression [23]. Thus, methylated genes can serve as biomarkers for early detection of cancer [24]. In the past years, several qualitative and quantitative polymerase chain reaction (PCR) methods based on the methylation of single gene (such as glutathione S-transferase P1 [GSTP1]; specificity, 79% [25]) or multiple-gene cohort (such as P16/ARF/MGMT/GSTP1; theoretical sensitivity, 73%; theoretical specificity, 98% [26]), and recently, a multiplexed urine assay consisting of three methylation markers, GSTP1, RARB, and APC (sensitivity, 55%; specificity, 80%) have been developed [27]. However, these detection methods are yet to be improved in both sensitivity and specificity, and most importantly, they are ineffective for the detection of early stages of prostate cancer [27,28]. Therefore, a reliable marker for early detection of PCa is urgently needed.
We have previously demonstrated that in the LNCaP cell line, the expression of gal3 is negatively regulated by hypermethylation of its promoter [18]. In this study, we immunohistochemically analyzed the expression of gal3 in normal and PCa tissues. Gal3 was detected in both the nucleus and the cytoplasm of normal, benign prostatic hyperplasia (BPH), high-grade prostatic intraepithelial neoplasia (HGPIN), and stage I tissues. However, the expression of gal3 was dramatically decreased in more advanced stages and, interestingly, mostly in the cytoplasm. Moreover, we have demonstrated heavy cytosine methylation in the promoter of gal3 in stage II PCa, light methylation of gal3 promoter in stages III and IV, and both heavy and light methylations of gal3 promoter in stage I PCa. The gal3 promoter in normal and BPH tissues is almost unmethylated. On the basis of the methylation results, we developed a methylation-specific (MS) PCR for the detection of early stages of prostate cancer.
Materials and Methods
Source of Prostate Cancer Cell Lines and Tissues
The human prostate cancer cell lines LNCaP (gal3-negative), PC-3 (gal3-positive), and DU-145 (gal3-positive) were purchased from American Type Culture Collection (ATCC, Manassas, VA). The tissues from both white and African (aged 48–78 years) were procured from various sources such as Ambion, National Disease Research Interchange (NDRI, Philadelphia, PA), Cooperative Human Tissue Network (CHTN) of the National Cancer Institute (see Table 1 for pathological details). Each tissue specimen was annotated with the American Joint Committee on Cancer (AJCC) staging and TNM classification and Gleason score. Fresh frozen sera from patients with normal and tumor prostate were obtained from Asterand (asterand.com) and NDRI.
Table 1.
Pathological Details of Tissue (T) and Serum (S) Specimens and Summary of MS-PCR Results.
| Sample T/S | Tissue Type | AJCC Stage | AJCC TNM Classification | Gleason Score | Age (years) | Race | Tumor Content | Specimen Source | Specimen ID | Gal3 MS-PCR | GSTP1 MS-PCR |
| N-1 (T) | Normal | NA | NA | NA | 66 | White | NA | Ambion | 0505-006 | - | - |
| N-2 (T) | Normal | NA | NA | NA | 79 | White | NA | Ambiom | 0505-007-1 | - | - |
| N-3 (T) | Normal | NA | NA | NA | 66 | White | NA | Ambion | 0505-007-2 | - | - |
| N-4 (T) | Normal | NA | NA | NA | 59 | White | NA | NDRI | OD23155 | - | - |
| N-5 (T) | Normal | NA | NA | NA | 73 | U | NA | NDRI | OD23791 | - | + |
| B-1 (T) | BPH | NA | NA | NA | 78 | U | NA | NDRI | 83-1107 | - | - |
| B-2 (T) | BPH | NA | NA | NA | U | U | NA | NDRI | 0056582-01 | - | - |
| I-1 (T) | Tumor | I | T1 Nx Mx | U | 60 | U | U | Collaborat | 244850 | + | - |
| I-2 (T) | Tumor | I | T1 Nx Mx | 3 + 3 = 6 | 81 | U | U | Collaborat | 239521 | + | - |
| I-3 (T) | Tumor | I | T1 Nx Mx | 3 + 3 = 6 | 74 | U | U | Collaborat | 238708 | + | + |
| I-4 (T) | Tumor | I | T1 Nx Mx | 3 + 3 = 6 | 76 | U | U | Collaborat | 233011 | + | + |
| I-5 (T) | Tumor | I | T1 Nx Mx | 2 + 3 = 5 | 85 | U | U | Collaborat | 218496 | + | + |
| I-6 (T) | Tumor | I | T1 Nx Mx | 4 + 3 = 7 | 78 | U | U | Collaborat | 218163 | + | + |
| I-7 (T) | Tumor | I | T1 Nx Mx | 3 + 3 = 6 | 67 | U | U | Collaborat | 211841 | + | + |
| I-8 (T) | Tumor | I | T1 Nx Mx | 4 + 5 = 9 | 66 | U | U | Collaborat | 209668 | + | + |
| I-9 (T) | Tumor | I | T1 Nx Mx | 3 + 2 = 5 | 77 | U | U | Collaborat | 209667 | + | + |
| I-10 (T) | Tumor | I | T1 Nx Mx | 3 + 4 = 7 | 64 | U | U | Collaborat | 207087 | + | + |
| I-11 (T) | Tumor | I | T1 Nx Mx | 3 + 4 = 7 | 70 | U | U | Collaborat | 204782 | + | + |
| II-1 (T) | Tumor | II | T2c Nx Mx | 3 + 4 = 7 | 63 | African | 15% | Ambion | 0505-023 | + | + |
| II-2 (T) | Tumor | II | T2c Nx Mx | 3 + 4 = 7 | 69 | White | 20% | CHTN | MAD06-00234 | + | + |
| II-3 (T) | Tumor | II | T2c Nx Mx | 3 + 3 = 6 | 48 | White | 15% | CHTN | MAD06-00550 | + | + |
| II-4 (T) | Tumor | II | T2c Nx Mx | 3 + 4 = 7 | 61 | White | 10% | CHTN | MAD07-00014 | + | + |
| II-5 (T) | Tumor | II | T2 Nx Mx | 3 + 4 = 7 | 73 | U | 2–6% | NDRI | OD23791 | + | - |
| II-6 (T) | Tumor | II | T2c Nx Mx | 3 + 3 = 6 | 63 | White | 10% | CHTN | MAD06-00581 | + | + |
| II-7 (T) | Tumor | II | T2c Nx Mx | 3 + 3 = 6 | 50 | African | U | CHTN | MAD06-00504 | + | + |
| III-1 (T) | Tumor | III | T3 N0 Mx | 3 + 4 = 7 | 62 | White | U | Ambion | 0405-037 | - | + |
| III-2 (T) | Tumor | III | T3b N0 Mx | 4 + 3 = 7 | 48 | White | 90% | CHTN | MAD03-01617 | + | + |
| III-3 (T) | Tumor | III | T3a Nx Mx | 3 + 3 = 6 | 58 | White | 20% | CHTN | MAD05-00155 | - | + |
| III-4 (T) | Tumor | III | T3a Nx Mx | 4 + 3 = 7 | 66 | White | 10% | CHTN | MAD06-00118 | - | + |
| III-5 (T) | Tumor | III | T3a Nx Mx | 4 + 3 = 7 | 66 | White | 25% | CHTN | MAD06-00536 | - | + |
| III-6 (T) | Tumor | III | T3a Nx Mx | 3 + 3 = 6 | 44 | White | 30% | CHTN | MAD06-00681 | - | + |
| III-7 (T) | Tumor | III | T3 Nx Mx | 4 + 3 = 7 | 45 | U | U | NDRI | OD20866 | - | - |
| IV-1 (T) | Tumor | IV | T3b N1 Mx | 5 + 5 = 10 | 59 | White | 80% | NDRI | OD23155 | - | + |
| IV-2 (T) | Tumor | IV | T3b N1 Mx | U | 66 | U | U | CHTN | MAD05-00059 | - | + |
| B-1 (S) | BPH | NA | NA | NA | U | U | NA | NDRI | 0056582-02 | - | - |
| II-1 (S) | Tumor | II | T2b N0 Mx | 4 + 3 = 7 | 63 | White | U | Asterand | 55916A2 | + | + |
| II-2 (S) | Tumor | II | T2c N0 Mx | 3 + 3 = 6 | U | White | U | Asterand | 47256A1 | + | + |
| III-1 (S) | Tumor | III | T3a N0 Mx | 3 + 4 = 7 | 66 | White | U | Asterand | 42629A1 | - | + |
| IV-1 (S) | Tumor | IV | T3a N1 Mx | 4 + 4 = 8 | 59 | White | U | Asterand | 53962A1 | - | + |
Collaborat indicates collaborator (Dr. Francesco Cappello, University of Palermo, Italy); NA, not applicable; ND, not determined; Negl, negligible; U, unknown.
Cell Culture
LNCaP cells were cultured in phenol red-free RPMI 1640 medium (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (Quality Biologicals, Gaithersburg, MD), 100 units/ml penicillin G sodium, and 100 µg/ml streptomycin sulfate (Sigma). The cell lines PC-3 and DU-145 were cultured in a mixture of Dulbecco's modified Eagle medium and F-12 (1:1 dilution; both from Sigma) supplemented with 10% fetal bovine serum, penicillin, and streptomycin. All cells were cultured in the presence of 5% CO2 at 37°C.
Expression of Galectins in PCa Cell Lines by Reverse Transcription-PCR and Western Blot
For RT PCR analysis, total RNA extraction, first strand cDNA synthesis, and PCR amplification were performed as previously described [18]. The nonoverlapping gal1- and gal3-specific primers were reported earlier [18]. For Western blot, total cell extracts were subjected to sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blot probed with rabbit polyclonal anti-gal3 antibodies [29]. For the development of antibodies, recombinant gal3 was expressed into Escherichia coli from a construct made into the pET 30 Ek/Lic vector (Novagen, Madison, WI) and purified on a lactosyl-Sepharose column as previously described [29]. Antibodies were raised in rabbits using the purified gal3, affinity-purified on a Protein A-Sepharose column, and their specificities were validated as previously described [29]. Preimmune serum was also subjected to Protein A-Sepharose column to obtain normal IgG. To construct the gal3 expression plasmid, NdeI and EcoRI restriction sites were produced by PCR at the 5′- and 3′-end, respectively, of the gal3 cDNA and the product was ligated into the cohesively NdeI/EcoRI-digested expression vector pET 30 Ek/Lic vector according to the manufacturer's instructions.
Histochemical Analyses of Normal and Tumor Prostate Tissues
Immunohistochemical detection of gal3 using specific anti-gal3 antibody was performed on 5-µm-thick paraffin-embedded sections containing the most representative tumor areas. In brief, sections were deparaffinized in xylene, hydrated through graded concentrations of ethanol and then with distilled water. Samples were heated in a microwave oven in 1x Target Retrieval solution (DAKO, Carpinteria, CA) and then washed with PBS for 5 minutes. All sections were incubated in 3% hydrogen peroxide to inhibit endogenous peroxidase. Anti-gal3 antibody (10 µg/ml) was applied to the slides and incubated for 30 minutes at room temperature in a humidified chamber. Protein A-Sepharose-purified preimmune rabbit serum was used as control. Sections were incubated with post primary block for 15 minutes and polymer for 15 minutes (NovoLink Polymer Kit; Novocastra, Vision BioSystems, Newcastle upon Tyne, United Kingdom). Staining was visualized with the diaminobenzidine chromogen and counterstained with Mayer's hematoxylin.
Evaluation of Immunohistochemical Staining
Ten high-power field (HPF) (magnification, x400) of each slide of tumor has been evaluated by two independent observers (F.C. and V.R.) to semiquantify the percentage of gal3 immunopositivity following the protocols previously described [30], and the results have been used for statistical analyses (analysis of variance, P < .05). When present in the sections, normal and hypertrophic prostate glands as well as prostatic intraepithelial neoplasia adjacent to the tumors have also been evaluated.
Methylation Analyses: Bisulfite Modification of Genomic DNA Followed by PCR, Cloning, and Sequencing
Genomic DNA from normal, BPH, and tumor tissues were prepared using the NucleoSpin Genomic DNA Extraction Kit (Macherey-Nagel, Inc., Bethlehem, PA) following the manufacturer's instructions. Occasionally, genomic DNA was extracted using DNAEasy kit (Qiagen, Inc., Valencia, CA) following the manufacturer's protocol. To identify cytosine methylation in the gal3 promoter, DNA (500 ng) from the cell lines or tissues was treated with sodium bisulfite by EZ Gold Methylation Kit (Zymo Research, Orange, CA) according to the manufacturer's instructions and subsequently amplified by PCR [31] with primer pairs located outside the CpG sites, using the Multiplex PCR kit (Qiagen, Inc.). This method allows precise analysis of methylation in a selected region by converting all non-methylated cytosines (C) into uracil (U) while methylated cytosines remain unchanged. The primers for gal3 after taking into account the bisulfite conversion reaction were as follows: (a) forward primer (HuG3BPF3), 5′-GGAGAGGGTGGGGGATAG-3′ derived from the wild-type sequence 5′-GGAGAGGGCGGGGGACAG-3′ (ranging from -277 to -260 nt of the promoter sequence [32]); and (b) reverse primer (HuG3BPR3), 5′-ACACCCTCTCCCCTACCC-3′ derived from the wild-type sequence 5′-GCGCCCTCTCCCTGCCC-3′ (ranging from +90 to +107 nt of the promoter sequence). The PCR product was cloned into a pGEM-T vector (Promega, Madison, WI) and sequenced [18].
Methylation-Specific and Unmethylation-Specific PCR
The difference between the CD-PCR described in the previous paragraph and the MS-PCR is the location of primer site. In CD-PCR, primers are designed based on the regions outside the CpG islands, and the methylation status of the CpG islands is investigated by cloning and sequencing of the PCR product. In MS-PCR, the primers are designed on the CpG sites to enable discrimination between methylated and unmethylated alleles after bisulfite treatment, and thus, the methylation status of the CpG islands is assessed directly from the presence or absence of a PCR product. To identify any unmethylated DNA allele, PCR was also performed using “unmethylated” primers. The PCR product obtained with the unmethylated primers serves also as a positive control for the presence of DNA in the PCR reaction, which is particularly relevant when methylation-specific polymerase chain reaction-based sensitive assay (MS-PCR) with the methylated primers yield a negative result. Taking into account the bisulfite conversion reaction, the “methylated” primers were designed as follows: forward primer HuG3BPMF1, 5′-CGTTTCGTCGGCGTTCG-3′ (ranging from .9 to +8 of the promoter sequence [32]); and reverse primer HuG3BPMR1, 5′-CACGCAACTCACCGCTCG-3′ (ranging from +47 to +64 of the promoter sequence). The unmethylated primers, designed by taking into account the bisulfite conversion reaction, were as follows: forward primer HuG3BPUF1, 5′-GAGGTTTGGAGTTATTGTTTTGTTGGTG-3′ (ranging from -24 to +4 of the promoter sequence); and reverse primer HuG3BPUR1, 5′-CCCCACACAACTCACCACTCA-3′ (ranging from +47 to +67 of the promoter sequence). The PCR product is expected only in tubes where methylated primers interact with the methylated DNA and where unmethylated primers interact with the unmethylated DNA. The specificities of the methylated and unmethylated primers were tested using known methylated and unmethylated DNA under the same PCR conditions. An equal amount of bisulfite-treated DNA (75 ng) was used for each PCR reaction, and the product was subjected to agarose gel electrophoresis on a 5% TopVision LE GQ agarose (Fermentas, Glen Burnie, MD).
For GSTP1 unmethylation-specific PCR and MS-PCR assays, the unmethylated and methylated primers were designed on the basis of results previously reported [33], after taking into account the bisulfite conversion reaction. The primers were as follows: unmethylated forward primer GSTP1UF1, 5′-GGTTAGTTGTGTGGTGATTTTGGG-3′ (ranging -196 to -173 nt of the promoter sequence), unmethylated reverse primer GSTP1UR1, 5′-AACCTCACAACCTCCAAACC-3′ (ranging from -26 to -7); methylated forward primer GSTP1MF1, 5′-TAGTTGCGCGGCGATTTC-3′ (ranging from -193 to -176), and methylated reverse primer GSTP1MR1, 5′ AAAACCTCGCGACCTCCG 3′ (ranging from -22 to -5). A second set of methylated primers (forward GSTP1MF2, 5′-CGGGGTGTAGCGGTCGTC-3′ ranging from -141 to -124 nt of the promoter sequence, and reverse primer GSTP1MR2, 5′-GCCCCAATACTAAATCACGAC-3′ ranging from -74 to -53 nt) were designed based on the study by Cairns et al. [25]. The corresponding unmethylated primers were as follows: forward GSTP1UF2, 5′-GATGTTTGGGGTGTAGTGGTTGTTG-3′ ranging from -147 to -123 nt, and reverse GSTP1UR2, 5′-CCACCCCAATACTAAATCACAACACC-3′, ranging from -76 to -51 nt of the promoter sequence.
Serum samples from healthy individuals and PCa patients were subjected to gal3 and GSTP1 MS-PCR as described previously. For this purpose, genomic DNA from each serum sample was isolated using the NucleoSpin Genomic DNA extraction kit (Macherey-Nagel, Inc.) and bisulfite treated using EZ Gold Methylation Kit as described previously.
Results
Expression of Gal3 in PCa Cell Lines and Prostate (Normal and Tumor) Tissues
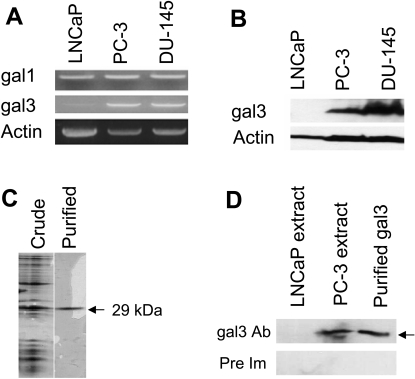
Expression of gal3 in PCa cells was examined by RT-PCR and Western blot. Gal3 was highly expressed in PC-3 and DU-145 cells, but negligible or very weak gal3 expression was observed in LNCaP cells either by RT-PCR (Figure 1A) or by Western blot (Figure 1B). The gal3 antibodies used in the Western blot were prepared from the affinity-purified gal3 (purity assessed by SDS-PAGE; Figure 1C), and their specificity for gal3 was validated, as the PC-3, but not LNCaP, and cell extract yielded a band corresponding to 29 kDa (Figure 1D, upper panel, arrow). Preimmune IgG did not result in any band (Figure 1D, lower panel).
Figure 1.
Expression of gal3 (A, B). Expression of gal3 in PCa cell lines as determined by RT-PCR (A) and Western blot (B). Purification of human recombinant gal3 (C). 15% SDS-PAGE of the crude extract and the lactosyl-Sepharose-purified gal3 (indicated by single band). Specificity of anti-gal3 antibodies (D). Crude cell extracts from LNCaP (gal3-negative) and PC-3 (gal3-positive) along with the purified recombinant gal3 (for positive control) were tested with the purified anti-gal3 antibodies (upper panel) and the purified IgG from pre-immune serum (lower panel) on Western blot.
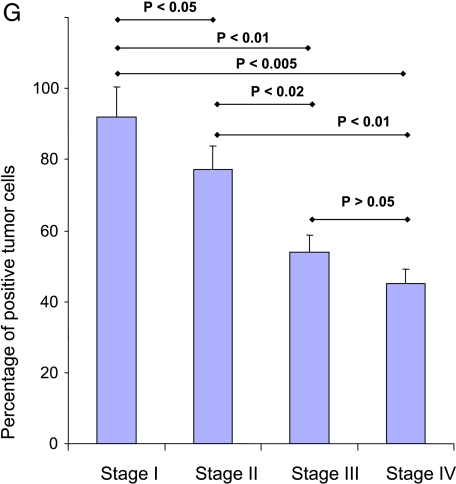
We investigated gal3 expression in normal, BPH, and various stages of PCa (Figure 2). Gal3 was found strongly expressed both in nucleus and cytoplasm in normal (not shown), BPH (Figure 2A), and HGPIN, a precursor lesion to development of invasive prostatic adenocarcinoma (Figure 2B), tissues. Gal3 showed decreasing immunopositivity during stage evolution. The data are statistically significant among groups, except for stages III to IV comparison (Figure 2G). Moreover, localization of gal3 is interesting during stage evolution. In particular, stage I tumors showed a strong immunopositivity in both the nucleus and the cytoplasm (Figure 2C), whereas in more advanced stages, immunostaining was less intense and localized mainly in cytoplasm, with rare, occasional nucleus positivity (Figure 2, D–F). However, 2 of 10 stage I specimens showed little or no gal3 immunopositivity (not shown).
Figure 2.
(A–F) Expression of gal3 as determined by immunohistochemical staining using affinity purified specific rabbit anti-gal3 antibodies. Representative immunohistochemistry results. Original magnification, x400. (A) BPH. (B) HGPIN (aged 76 years). (C) Stage I (T1 N0 Mx, aged 78 years). (D) Stage II (T2 N0 Mx, aged 67 years). (E) Stage III (T3 N0 Mx, aged 78 years). (F) Stage IV (T4 N0 Mx, aged 76 years). In panels A to C, intense gal3 expression is observed in both the nucleus and the cytoplasm. Insets of panels A to C show gal3 positivity in nuclei. In D, a hypertrophic gland in the middle right, with a very intensive positivity mainly in the cytoplasm, surrounded by tumoral tissue with a lower positivity mainly in the cytoplasm. (G) Statistical analyses of immunohistochemical results. Analysis of variance showing a significant decrease of gal3 positivity among groups, except when compared with stages III and IV.
Expression of Gal3 in PCa Cell Lines Is Negatively Correlated with Methylation of Its Promoter
As silencing of the gal3 in LNCaP was shown to be regulated by methylation of its promoter [18], we analyzed promoter methylation in the gal3 DNA from PC-3 and DU-145 cells after bisulfite treatment of DNA followed by CD-PCR using the HuG3BPF3 and HuG3BPR3 primers. No methylation was observed in the promoter of gal3 DNA from either PC-3 or DU-145 cell lines (Figure 3), indicating a negative correlation between gal3 promoter methylation and gal3 expression.
Figure 3.
Methylation profile of gal3 promoter region (384 bp) from PCa cell lines and normal and tumor prostate tissues. Each row represents a single cloned allele, and each oval represents a single CpG site (open oval, unmethylated; closed oval, methylated). The numbering in the schematic diagram at the top represents the position relative to the published transcription site (+1, indicated by the arrow). For each sample, at least 20 clones were sequenced. Two representative sequences for each of BPH, tumor stage I and stage IV, and four representative sequences for each of stages II and III were shown.
Expression of Gal3 in Normal and Tumor Prostate Tissues Is Negatively Correlated with Methylation of Its Promoter
To determine whether the differential expression of gal3 in various stages of tumor is correlated with the cytosine methylation, we examined the methylation patterns of the gal3 promoter in normal and tumor tissues after bisulfite treatment of DNA followed by CD-PCR using the same primers used for the cell line DNA. Results revealed that the gal3 promoter from multiple stage II tumor specimens is heavily methylated throughout its entire length, but that from multiple stages III and IV tumor specimens is lightly methylated. Whereas gal3 promoter in stage III showed few methylation sites, mostly between -199 and -252 nt, the gal3 promoter from stage IV tumor specimens was methylated between -112 and -227 nt (Figure 3). In stage I PCa, however, both light and heavy methylations are evident in the gal3 promoter. In multiple normal prostate and BPH samples, the gal3 promoter was almost unmethylated (Figure 3). Overall, results indicated that the decreased expression of gal3 in tumor prostate is associated with the hypermethylation of its promoter.
Gal3 MS-PCR Assay for the Detection of Stages I and II Prostate Tumor
The methylation patterns of the gal3 promoter from the stage I to IV of PCa led us to design primers specific for stages I and II PCa and develop the MS-PCR assay (patent pending). Of 34 tissues (5 normal, 2 BPH, 11 stage I, 7 stage II, 7 stage III, and 2 stage IV) tested, gal3 MS-PCR identified all stage I and II tumor samples (Figure 4). The positive PCR reaction was specific as the methylated primers yielded product only with the methylated DNA control, but not with the unmethylated DNA control. The gal3 MS-PCR was negative for normal, BPH, stage III (except one), and stage IV samples. The PCR with the unmethylated primers were positive in all samples including stage II indicating the presence of unmethylated gal3 DNA. The product obtained from stage I and II tumor with the unmethylated primers may not be specific, as unmethylated primers also yielded product with the control methylated DNA. To identify the presence of any unmethylated DNA in stage I and II tumor, the bisulfite-treated DNAs from the stage I and II tumor were subjected to CD-PCR using the HuG3BPF3 and HuG3BPR3 primers followed by cloning and sequencing. Sequencing results revealed not only fully methylated DNA (Figure 3) but also unmethylated DNA (not shown). The presence of unmethylated DNA allele in the PCa specimens may be because the tissue sample is heterogeneous in tumor content.
Figure 4.
Polymerase chain reaction of bisulfite-treated DNA isolated from normal, BPH, and tumor prostate tissues and unmethylated control and methylated control plasmid DNA with gal3 unmethylated (U) and methylated (M) primer pairs. The products 92 and 73 bp obtained from unmethylated and methylated primer pairs, respectively, are shown by arrows. An equal amount of bisulfite-treated DNA (75 ng) was used for each PCR reaction, and the product was subjected to agarose gel electrophoresis on a 5% TopVision LE GQ agarose. For unmethylated and methylated controls, 25 ng of plasmid DNA was used.
GSTP1 MS-PCR Assay for the Detection of Later Stages of Prostate Cancer
Because the gal3 MS-PCR assay described previously reliably identifies all stages I and II PCa tested, negative results from this assay may indicate that the test specimen either is normal or contains a tumor at a stage other than stages I and II. Thus, the gal3 MS-PCR assay is unable to distinguish specimens from individuals with normal tissue and those patients with stage III or IV PCa (metastatic stage) tumors. To address this assay limitation, an MS-PCR assay based on the methylation pattern of the GSTP1 gene was introduced to identify PCa of stages III and IV. Methylation of GSTP1 correlates positively with tumor grade and stage (i.e., low methylation in early stages and high methylation in late stages [34]). Therefore, a method based on GSTP1 promoter methylation may not be suitable to identify prostate cancer at early stages, especially at stage II (sensitivity, 50%; specificity, almost 100%) [26]. Our results revealed that the GSTP1 MS-PCR detected all stage IV samples (2/2) but only three of seven samples of stage III (Figure 5). However, the GSTP1 MS-PCR was also positive for 9 of 11 stage I and for 6 of 7 stage II samples. The GSTP1 MS-PCR was negative for BPH (2/2) and normal samples (except 1 of 5; Figure 5). The inability of GSTP1 MS-PCR to detect all tumor samples especially higher stage was probably due to the primer location, which was based on the promoter methylation results in the LNCaP cell line [33]. Thus, a second set of methylated primers (forward primer GSTP1MF2 and reverse primer GSTP1MR2) based on the study by Cairns et al. [25] was designed. The sensitivity of the GSTP1 MS-PCR with the second set of primers previously discussed was considerably higher for stage III tumor specimens (6 positive of 7 samples; Figure 5, bottom panel) compared with the first primer set. Although further optimization of the GSTP1 MS-PCR assay to further increase the sensitivity will be required to reach the targeted assay sensitivity and specificity, the results from the combined MS-PCR assays for gal3 and GSTP1 clearly identified all but one PCa sample, including all PCa of early stages.
Figure 5.
Polymerase chain reaction of bisulfite-treated DNA isolated from normal, BPH, and tumor prostate tissues with GSTP1 unmethylated (U) and methylated (M) primer pairs. The products 190 and 189 bp obtained from unmethylated and methylated primer pairs, respectively, are shown by arrows. Bottom panels: Bisulfite-treated DNA from stages III and IV tumors were also subjected to PCR with a second pair of unmethylated (expected product size, 97 bp) and methylated (expected product size, 89 bp) primers from GSTP1 promoter.
Gal3 and GSTP1 MS-PCR Assays from Normal and Tumor Patient Serum Samples
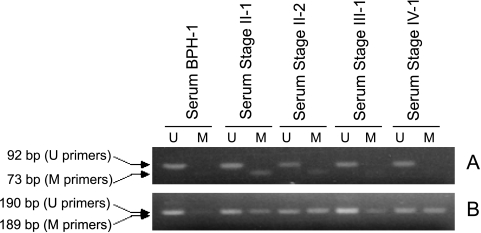
To examine if methylated gal3 and GSTP1 DNA from early stages of PCa can be detected in biological fluids such as serum and urine, the bisulfite-treated DNA from the serum samples were subjected to gal3- and GSTP1-MS-PCR assays. Identification of methylated DNA in urine would be a critical aspect in the development of noninvasive diagnostic of early stages of prostate cancer. Serum and urine are known to have significant amount of soluble circulating small DNA [34,35]. Moreover, tumor-specific sequences including GSTP1 were detected in DNA isolated from serum and urine [26,36–39]. Of a very limited set of serum samples tested, the gal3 MS-PCR assay was positive for all stage II samples (2/2) and, as expected, negative for BPH (1/1), stage III (1/1), and stage IV (1/1) samples (Figure 6A). Further, the GSTP1 MS-PCR assay using the primer pair GSTP1MF1 and GSTP1MR1 identified all tumor serum samples (Figure 6B). Our results indicate that the combined MS-PCR assay (gal3 and GSTP1) may represent a powerful tool to reliably identify both early and advanced PCa, including 100% sensitivity for stage II PCa, which is considered the critical stage for successful intervention.
Figure 6.
Polymerase chain reaction of bisulfite-treated DNA isolated from BPH and tumor serum samples with gal3 (A) and GSTP1 (B) unmethylated (U) and methylated (M) primer pairs. An equal amount of bisulfite-treated DNA (75 ng) was used for each PCR reaction, and the product was subjected to agarose gel electrophoresis on either 5% (A) or 3% (B) TopVision LE GQ agarose.
Discussion
It has become clear that epigenetic events such as DNA methylation and histone modification play a crucial role in cancer, including prostate cancer [40–42]. DNA methylation, catalyzed by DNA methyltransferases, refers to the covalent bonding of a methyl group specifically to the dinucleotide CpG. DNA methylation is believed to alter chromosome structure and define regions for transcriptional regulation. Clusters of CpG sites (known as CpG islands) are found in the promoter region of approximately 60% of genes, in exons and introns, and in repetitive elements [43]. In normal cells, most CpG islands in the promoter regions are unmethylated whereas CpG islands in intronic regions and repetitive sequences are heavily methylated, perhaps to help the cell identify regions for gene transcription. In cancer cells, including prostate cancer, two types of DNA methylation alterations have been demonstrated. The first is a global hypomethylation, in which the genomes of cancer cells show decreased methylation compared with normal cells [44]. This hypomethylation is primarily due to the loss of methylation in repetitive elements and other nontranscribed regions of the genome, which results in genomic instability. The second type of methylation alteration in cancer cells, known as gene hypermethylation, is the methylation of CpG islands that lie in promoter regions of tumor suppressor and other regulatory genes that are normally unmethylated. The promoter regions of these genes are inactivated by methylation, and their gene expression is silenced [41,42,45,46].
In prostate cancer, a large number of genes such as GSTP1, MGMT, RASSF1A, APC, and RARβ are hypermethylated [43]. Silencing of these genes is thought to contribute to initiation and progression of the disease [43]. In this study, we have shown that the gal3 promoter shows almost complete methylation in all CpG sites in early stages of prostate cancer, such as stage II, but is lightly methylated in later stages (III and IV). The gal3 promoter in stage I PCa, however, showed both light and heavy methylation. The methylation pattern of the gal3 promoter in the various stages of PCa, and in particular its complete methylation in early stages, is unique because in other genes such as GSTP1, CpG methylation correlates positively with tumor grade and stage (i.e., low methylation in early stages and high methylation in late stages) [34]. Moreover, heavy methylation of gal3 throughout the promoter region is distinct only in stages I and II PCa, but light methylation in the region far away from the transcription site is evident in later stages of PCa.
It is unknown if or how gal3 participates in PCa progression and metastasis by decreasing its expression in early stages and maintaining the low expression in later stages. In normal, BPH, HGPIN, and most stage I prostate tissues, gal3 is localized in both the nucleus and the cytoplasm, but interestingly, in the later stages (II, III, and IV) of PCa, it was mostly found in the cytoplasmic compartment as evidenced from this study and others [47]. van den Brûle et al. [47] suggested that the nuclear gal3 might play antitumor activities, whereas cytoplasmic gal3 could favor tumor progression. This notion was corroborated from a study where the LNCaP cell line was transfected to generate gal3 expression either in the nucleus or in the cytosol [48]. In that study, nuclear gal3 was shown to suppress tumor, but cytoplasmic gal3 promoted tumor [48].
The finding that the gal3 promoter is completely methylated in stages I and II PCa makes the gal3 gene an ideal candidate for developing a MS-PCR assay for early diagnosis of PCa. Because in stages I and II the tumor is still confined to the prostate gland, identification of these stages is very important for effective intervention and cure. From previous studies on methylated markers using a single gene (e.g., GSTP1 [25]) or multiple-gene cohort (e.g., P16/ARF/MGMT/GSTP1 and GSTP1/RARB/APC), it is known that these markers are not effective in detecting early stages of prostate cancer [24,27,28]. The assay developed here clearly identified all stages I and II specimens tested (both tissue and serum), although more specimens should be tested for achieving a statistically significant data set.
Because the gal3 MS-PCR is designed to target only stages I and II PCa (negative for normal, BPH, and tumors of higher stages), a complementing assay is required to distinguish all PCa stages from individuals with normal tissues and patients with BPH. Our results show that GSTP1 MS-PCR assay is useful in this regard, as methylation of GSTP1 correlates positively with tumor grade and stage [34]. The GSTP1 MS-PCR assay described here identified all but one PCa of higher stages, and thus, optimization to further increase the sensitivity will be required. Overall, the results from the combined MS-PCR assays for gal3 and GSTP1 clearly identified most PCa samples, including all stages I and II, which are the critical stages for cancer prevention and cure.
Footnotes
This study was supported by the UMBI Presidential Proof of Concept Award and in part by the US Army Medical Research and Materiel Command under W81XWH-07-1-0565 to H.A. and in part by the National Institutes of Health grant RO1 GM070589-01 to G.R.V.
References
- 1.Jain S, Chakraborty G, Bulbule A, Kaur R, Kundu GC. Osteopontin: an emerging therapeutic target for anticancer therapy. Expert Opin Ther Targets. 2007;11:81–90. doi: 10.1517/14728222.11.1.81. [DOI] [PubMed] [Google Scholar]
- 2.Nakahara S, Raz A. Biological modulation by lectins and their ligands in tumor progression and metastasis. Anticancer Agents Med Chem. 2008;8:22–36. doi: 10.2174/187152008783330833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsay AG, Marshall JF, Hart IR. Integrin trafficking and its role in cancer metastasis. Cancer Metastasis Rev. 2007;26:567–578. doi: 10.1007/s10555-007-9078-7. [DOI] [PubMed] [Google Scholar]
- 4.Nakahara S, Raz A. Regulation of cancer-related gene expression by galectin-3 and the molecular mechanism of its nuclear import pathway. Cancer Metastasis Rev. 2007;26:605–610. doi: 10.1007/s10555-007-9095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabinovich GA, Liu FT, Hirashima M, Anderson A. An emerging role for galectins in tuning the immune response: lessons from experimental models of inflammatory disease, autoimmunity and cancer. Scand J Immunol. 2007;66:143–158. doi: 10.1111/j.1365-3083.2007.01986.x. [DOI] [PubMed] [Google Scholar]
- 6.Elola MT, Wolfenstein-Todel C, Troncoso MF, Vasta GR, Rabinovich GA. Galectins: matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell Mol Life Sci. 2007;64:1679–1700. doi: 10.1007/s00018-007-7044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong HC, Honjo Y, Nangia-Makker P, Hogan V, Mazurak N, Bresalier RS, Raz A. The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res. 1999;59:6239–6245. [PubMed] [Google Scholar]
- 8.van den Brûle F, Califice S, Castronovo V. Expression of galectins in cancer: a critical review. Glycoconj J. 2004;19:537–542. doi: 10.1023/B:GLYC.0000014083.48508.6a. [DOI] [PubMed] [Google Scholar]
- 9.Xu XC, el-Naggar AK, Lotan R. Differential expression of galectin-1 and galectin-3 in thyroid tumors. Potential diagnostic implications. Am J Pathol. 1995;147:815–822. [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz G, Jr, Remmelink M, Decaestecker C, Gielen I, Budel V, Burchert M, Darro F, Danguy A, Gabius HJ, Salmon I, et al. Galectin fingerprinting in tumor diagnosis. Differential expression of galectin-3 and galectin-3 binding sites, but not galectin-1, in benign vs malignant uterine smooth muscle tumors. Am J Clin Pathol. 1999;111:623–631. doi: 10.1093/ajcp/111.5.623. [DOI] [PubMed] [Google Scholar]
- 11.Choufani G, Nagy N, Saussez S, Marchant H, Bisschop P, Burchert M, Danguy A, Louryan S, Salmon I, Gabius HJ, et al. The levels of expression of galectin-1, galectin-3, and the Thomsen-Friedenreich antigen and their binding sites decrease as clinical aggressiveness increases in head and neck cancers. Cancer. 1999;86:2353–2363. doi: 10.1002/(sici)1097-0142(19991201)86:11<2353::aid-cncr25>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 12.Lotan R, Ito H, Yasui W, Yokozaki H, Lotan D, Tahara E. Expression of a 31-kDa lactoside-binding lectin in normal human gastric mucosa and in primary and metastatic gastric carcinomas. Int J Cancer. 1994;56:474–480. doi: 10.1002/ijc.2910560404. [DOI] [PubMed] [Google Scholar]
- 13.Hsu DK, Dowling CA, Jeng KC, Chen JT, Yang RY, Liu FT. Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int J Cancer. 1999;81:519–526. doi: 10.1002/(sici)1097-0215(19990517)81:4<519::aid-ijc3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Ellerhorst J, Troncoso P, Xu XC, Lee J, Lotan R. Galectin-1 and galectin-3 expression in human prostate tissue and prostate cancer. Urol Res. 1999;27:362–367. doi: 10.1007/s002400050164. [DOI] [PubMed] [Google Scholar]
- 15.Pacis RA, Pilat MJ, Pienta KJ, Wojno K, Raz A, Hogan V, Cooper CR. Decreased galectin-3 expression in prostate cancer. Prostate. 2000;44:118–123. doi: 10.1002/1097-0045(20000701)44:2<118::aid-pros4>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Su ZZ, Lin J, Shen R, Fisher PE, Goldstein NI, Fisher PB. Surface-epitope masking and expression cloning identifies the human prostate carcinoma tumor antigen gene PCTA-1, a member of the galectin gene family. Proc Natl Acad Sci USA. 1996;93:7252–7257. doi: 10.1073/pnas.93.14.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danguy A, Rorive S, Decaestecker C, Bronckart Y, Kaltner H, Hadari YR, Goren R, Zich Y, Petein M, Salmon I, et al. Immunohistochemical profile of galectin-8 expression in benign and malignant tumors of epithelial, mesenchymatous and adipous origins, and of the nervous system. Histol Histopathol. 2001;16:861–868. doi: 10.14670/HH-16.861. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed H, Banerjee PB, Vasta GR. Differential expression of galectins in normal, benign and malignant prostate epithelial cells: silencing of galectin-3 expression in prostate cancer by its promoter methylation. Biochem Biophys Res Commun. 2007;358:241–246. doi: 10.1016/j.bbrc.2007.04.114. [DOI] [PubMed] [Google Scholar]
- 19.Hessels D, Verhaegh GW, Schalken JA, Witjes JA. Applicability of biomarkers in the early diagnosis of prostate cancer. Expert Rev Mol Diagn. 2004;4:513–526. doi: 10.1586/14737159.4.4.513. [DOI] [PubMed] [Google Scholar]
- 20.Keetch DW, Catalona WJ, Smith DS. Serial prostatic biopsies in men with persistently elevated serum prostate specific antigen values. J Urol. 1994;151:1571–1574. doi: 10.1016/s0022-5347(17)35304-1. [DOI] [PubMed] [Google Scholar]
- 21.Leman ES, Cannon GW, Trock BJ, Sokoll LJ, Chan DW, Mangold L, Partin AW, Getzenberg RH. EPCA-2: a highly specific serum marker for prostate cancer. Urology. 2007;69:714–720. doi: 10.1016/j.urology.2007.01.097. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 23.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 24.Fackler MJ, McVeigh M, Mehrotra J, Blum MA, Lange J, Lapides A, Garrett E, Argani P, Sukumar S. Quantitative multiplex methylation-specific PCR assay for the detection of promoter hypermethylation in multiple genes in breast cancer. Cancer Res. 2004;64:4442–4452. doi: 10.1158/0008-5472.CAN-03-3341. [DOI] [PubMed] [Google Scholar]
- 25.Cairns P, Esteller M, Herman JG, Schoenberg M, Jeronimo C, Sanchez-Cespedes M, Chow NH, Grasso M, Wu L, Westra WB, et al. Molecular detection of prostate cancer in urine by GSTP1 hypermethylation. Clin Cancer Res. 2001;7:2727–2730. [PubMed] [Google Scholar]
- 26.Hoque MO, Topaloglu O, Begum S, Henrique R, Rosenbaum E, Van Criekinge W, Westra WH, Sidransky D. Quantitative methylation-specific polymerase chain reaction gene patterns in urine sediment distinguish prostate cancer patients from control subjects. J Clin Oncol. 2005;23:6569–6575. doi: 10.1200/JCO.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Vener T, Derecho C, Baden J, Wang H, Rajpurohit Y, Skelton J, Mehrotra J, Varde S, Chowdary D, Stallings W, et al. Development of a multiplexed urine assay for prostate cancer diagnosis. Clin Chem. 2008;54:874–882. doi: 10.1373/clinchem.2007.094912. [DOI] [PubMed] [Google Scholar]
- 28.Tokumaru Y, Harden SV, Sun DI, Yamashita K, Epstein JI, Sidransky D. Optimal use of a panel of methylation markers with GSTP1 hypermethylation in the diagnosis of prostate adenocarcinoma. Clin Cancer Res. 2004;10:5518–5522. doi: 10.1158/1078-0432.CCR-04-0108. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed H, Du SJ, O'Leary N, Vasta GR. Biochemical and molecular characterization of galectins from zebrafish (Danio rerio). Notochord-specific expression of a proto type galectin (Drgal1-L2) during early embryogenesis. Glycobiology. 2004;14:219–232. doi: 10.1093/glycob/cwh032. [DOI] [PubMed] [Google Scholar]
- 30.Waltregny D, Bellahcene A, Van Riet I, Fisher LW, Young M, Fernandez P, Dewe W, De Leval J, Castronovo V. Prognostic value of bone sialo-protein expression in clinically localized human prostate cancer. J Nat Cancer Inst. 1998;90:1000–1008. doi: 10.1093/jnci/90.13.1000. [DOI] [PubMed] [Google Scholar]
- 31.Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, Grigg GW, Molloy PL, Paul CL. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci USA. 1992;89:1827–1831. doi: 10.1073/pnas.89.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kadrofske MM, Openo KP, Wang JL. The human LGALS3 (galectin-3) gene: determination of the gene structure and functional characterization of the promoter. Arch Biochem Biophys. 1998;349:7–20. doi: 10.1006/abbi.1997.0447. [DOI] [PubMed] [Google Scholar]
- 33.Singal R, Wert J, Bashambu M. Cytosine methylation represses glutathione S-transferase P1 (GSTP1) gene expression in human prostate cancer cells. Cancer Res. 2001;61:4820–4826. [PubMed] [Google Scholar]
- 34.Jerónimo C, Henrique R, Hoque MO, Mambo E, Ribeiro FR, Varzim G, Oliveira J, Teixeira MR, Lopes C, Sidransky D. A quantitative promoter methylation profile of prostate cancer. Clin Cancer Res. 2004;10:8472–8478. doi: 10.1158/1078-0432.CCR-04-0894. [DOI] [PubMed] [Google Scholar]
- 35.Botezatu I, Serdyuk O, Potapova G, Shelepov V, Alechina R, Molyaka Y, Ananév V, Bazin I, Garin A, Narimanov M, et al. Genetic analysis of DNA excreted in urine: a new approach for detecting specific genomic DNA sequences from cells dying in an organism. Clin Chem. 2000;46:1078–1084. [PubMed] [Google Scholar]
- 36.Anker P, Lyautey J, Lederrey C, Stroun M. Circulating nucleic acids in plasma or serum. Clin Chim Acta. 2001;313:143–146. doi: 10.1016/s0009-8981(01)00666-0. [DOI] [PubMed] [Google Scholar]
- 37.Goessl C, Krause H, Muller M, Heicappell R, Schrader M, Sachsinger J, Miller K. Fluorescent methylation-specific polymerase chain reaction for DNA-based detection of prostate cancer in bodily fluids. Cancer Res. 2000;60:5941–5945. [PubMed] [Google Scholar]
- 38.Ziegler A, Zangemeister-Wittke U, Stahel RA. Circulating DNA: a new diagnostic gold mine? Cancer Treat Rev. 2002;28:255–271. doi: 10.1016/s0305-7372(02)00077-4. [DOI] [PubMed] [Google Scholar]
- 39.Su YH, Wang M, Brenner DE, Ng A, Melkonyan H, Umansky S, Syngal S, Block TM. Human urine contains small, 150 to 250 nucleotide-sized, soluble DNA derived from the circulation and may be useful in the detection of colorectal cancer. J Mol Diagn. 2004;6:101–107. doi: 10.1016/S1525-1578(10)60497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe M, Takagi A, Matsiuzaki T, Kami D, Toyota M, Hirokawa Y, Shiraishi T. Knowledge of epigenetic influence for prostate cancer therapy. Curr Cancer Drug Targets. 2006;6:533–551. doi: 10.2174/156800906778194568. [DOI] [PubMed] [Google Scholar]
- 42.Li LC, Dahiya R. Epigenetics of prostate cancer. Front Biosci. 2007;12:3377–3397. doi: 10.2741/2320. [DOI] [PubMed] [Google Scholar]
- 43.Cross SH, Bird AP. CpG islands and genes. Curr Opin Genet Dev. 1995;5:309–314. doi: 10.1016/0959-437x(95)80044-1. [DOI] [PubMed] [Google Scholar]
- 44.Ehrlich M. DNA methylation in cancer: too much, but also too little. Oncogene. 2002;21:5400–5413. doi: 10.1038/sj.onc.1205651. [DOI] [PubMed] [Google Scholar]
- 45.Attwood JT, Yung RL, Richardson BC. DNA methylation and the regulation of gene transcription. Cell Mol Life Sci. 2002;59:241–257. doi: 10.1007/s00018-002-8420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diaw L, Woodson K, Gillespie JW. Prostate cancer epigenetics: a review on gene regulation. Gene Regul Syst Bio. 2007;1:313–325. doi: 10.4137/grsb.s398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Brûle FA, Waltregny D, Liu FT, Castronovo V. Alteration of the cytoplasmic/nuclear expression pattern of galectin-3 correlates with prostate carcinoma progression. Int J Cancer. 2000;89:361–367. doi: 10.1002/1097-0215(20000720)89:4<361::aid-ijc8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 48.Califice S, Castronovo V, Bracke M, van den Brûle F. Dual activities of galectin-3 in human prostate cancer: tumor suppression of nuclear galectin-3 vs tumor promotion of cytoplasmic galectin-3. Oncogene. 2004;23:7527–7536. doi: 10.1038/sj.onc.1207997. [DOI] [PubMed] [Google Scholar]