Abstract
The hallmarks of human malignant gliomas are their marked invasiveness and vascularity. Because angiogenesis and tumor invasion have been associated with extracellular matrix degradation and intercellular tight junctions, the involvement of zonulin in glioma biology is in the focus. We selected for histological examination five cases of glioblastoma WHO IV (nomenclature of the World Health Organization) and one case each from astrocytoma WHO III, meningioma WHO III, and meningioma WHO I as control samples. The meningioma WHO I is regarded as benign, whereas the meningioma WHO III is recognized as the transition form of malignant tumors in humans. The visualization of a newly designed antibody against human zonulin was studied in triple-labeling studies using fluorescence immunocytochemistry and compared with the expression of c-kit and glial fibrillary acidic protein in differently developed human gliomas. We found that increasing the expression of c-kit is accompanied by an increase of zonulin expression. Both are correlated to the degree of malignancy of human brain tumors. The expression of zonulin is correlated to the degradation of the blood-brain barrier as revealed by Griffonia simplicifolia lectin. In differently graded tumors, we found differently graded involvement of blood vessels in the tumor development, explaining patients' survival.
Introduction
The regulation of intercellular tight junctions in the intestinal epithelium by zonulin is well known. It is conceivable that zonulin participates under physiological conditions not only in the small intestine [1,2] but also throughout a wide range of extraintestinal epithelia as well as the ubiquitous vascular endothelium, including the blood-brain barrier [3]. Dysregulation of this hypothetical zonulin model may contribute to disease states that involve disordered intercellular communication, including malignant transformation, which leads to new therapy options in oncology. Down-regulation of zonulin may inhibit tumor-mediated angiogenesis and glioma growth in vivo. That scenario had been proven for c-kit expression. Both zonulin and the c-kit pathway play important roles in tumor-induced angiogenesis. Primary human gliomas express c-kit in a grade-dependent manner and induce normal neurons to express c-kit in brain regions infiltrated by glioma cells, areas that colocalize with prominent angiogenesis. Overexpression of c-kit is associated with a shorter survival in patients with malignant gliomas [4]. Thus, the c-kit pathway plays an important role in tumor-induced angiogenesis within the brain. The inhibition of c-kit by synthetic small molecules has become a promising new therapy option in oncology [4]. The authors reported about therapy responders and nonresponders. Statistical analysis did not reveal any correlation between expression of tyrosine kinase receptors (e.g., c-kit) and patients' survival. Maybe the relevant link in the therapy chain is the tight junction of the blood-brain barrier.
Here, we try to provide evidence for the graded expression of both zonulin and c-kit in differently graded tumors. Glial fibrillary acidic protein (GFAP) was used as another tumor and degeneration marker. Blood vessels are revealed by Griffonia simplicifolia lectin (GSI).
Materials and Methods
Samples
All procedures used in the present study were approved by the Ethics Committee of the University of Leipzig (no. 086-2008). The rules of the Declaration of Helsinki from 1975 (revised in 1983) were followed. All procedures were carried out with the understanding and written consent of the subjects. A total of eight human biopsies were used. Five cases of glioblastoma WHO IV and one case each of astrocytoma WHO III, meningioma WHO III, and meningioma WHO I as control samples were selected for histological evaluation. The meningioma WHO I is regarded as benign, whereas the meningioma WHO III is reckoned as the transition form of malignant tumors in humans. The most aggressive tumor of the sample is the glioblastoma WHO IV.
Native frozen sections (8 µm) were cut on a cryotome. A Cy3-conjugated antibody against GFAP was used to reveal the tumor extension, lectin histochemistry for the visualization of blood vessels, and immunohistochemistry for c-kit and zonulin was performed on serially cut sections as detailed later. Certain sections were double-stained with GSI and antibodies directed against GFAP, c-kit, and zonulin, respectively, to reveal a correlation of those markers. Most of the sections were counterstained with 4′,6-diamidino-2-phenylindol (Serva) for nuclei labeling.
GSI Histochemistry
After two rinses with PBS followed by two rinses with 0.05 M Tris-HCl + 0.9% NaCl (TBS), the sections were incubated with biotinylated GSI (b-GSI, L-1766; Sigma, Munich, Germany) at a concentration of 10 µg of b-GSI/ml TBS containing 2% bovine serum albumin (TBS-BSA) overnight at 4°C. Sections were rinsed four times (15 minutes each) with TBS and further incubated for 1 hour in streptavidin-Cy3 or streptavidin-Cy2 (20 µg/ml; Dianova, Hamburg, Germany), rinsed, and double-stained immunohistochemically or dried and coverslipped.
Immunohistochemistry
After blocking with goat normal serum, the sections were treated with the primary antibody against c-kit (developed in mouse) and zonulin (newly designed by Immundiagnostik AGBensheim, Germany; developed in rabbit). Fluorescence immunoreactivities were visualized by means of Cy3-conjugated goat-antimouse antibody (diluted 1:150; Dianova) and/or Cy3-, Cy2-conjugated donkey-antirabbit or goat-antirabbit, respectively.
Control sections were treated similarly but using a nonspecific mouse or rabbit IgG1 (DAKO, Cologne, Germany) instead of primary antibodies. Fluorescence of sections was studied microscopically with a photomicroscope “Axiophot” (Zeiss, Jena, Germany) equipped with epifluorescence.
For double or triple stainings, we combined the secondary antibodies avoiding the use of two identical color conjugations and same host species.
Results
All stagings of tumors that were revealed by the expression of c-kit (Figure 1, A–C) were also revealed by zonulin expression. Nevertheless, there are some differences:
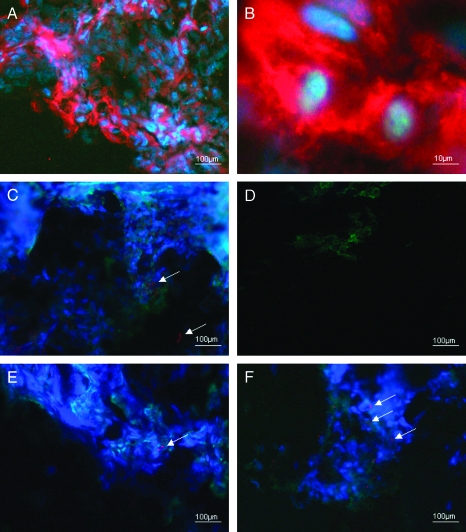
Figure 1.
Lower (A) and higher magnifications (B) of the expression of c-kit (red) in the cytoplasm of glioblastoma WHO IV, DAPI counterstainings of cell nuclei (blue) display signs of proliferation. Triple stainings of astocytoma WHO III: red indicates c-kit expression (arrows), green at the periphery reveals zonulin expression, and DAPI labels the cell nuclei blue (C). The meningioma WHO I was used as control: some green-colored zonulin expression is displayed only; double staining for both c-kit and zonulin expression (D). Comparison of the meningioma WHO I (E) and WHO III (F) by the expression of zonulin and binding sites of GSI agglutinin indicating degraded and normal blood-brain barrier: the benign meningioma shows normally developed endothelium (green) and extremely few degradations of that (revealed by the expression of zonulin, red), whereas the normal feature of the endothelium of the blood-brain barrier disappears in meningioma WHO III and some more red-colored zonulin expression is visible (arrows).
We did not find any expression of c-kit, but some expression of zonulin in meningioma WHO I (Figure 1D). In the sample of meningioma WHO III, both c-kit and zonulin were expressed. To some extent, we further found a colocalization of those.
Comparison of meningioma WHO I and WHO III by the expression of zonulin and binding sites of GSI agglutinin indicated normal and degraded blood-brain barrier. Whereas the benign meningioma showed normally developed endothelium and extremely few degradation, the normal feature of the endothelium of the blood-brain barrier disappeared in meningioma WHO III (Figure 1, E and F).
The expression of GFAP displayed no colocalization with c-kit and zonulin (Figure 2, A and B) with the exception of GFAP localization at the walls of the blood vessels (Figure 2B).
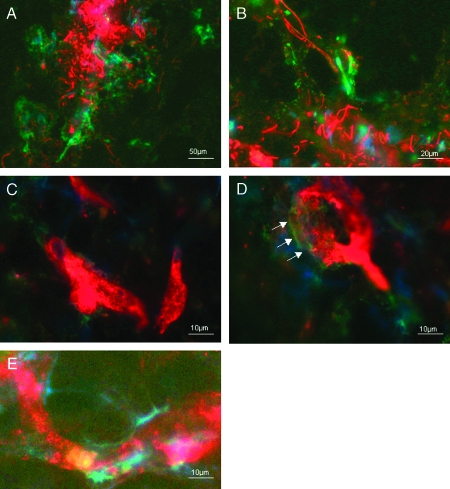
Figure 2.
Lower (A) and higher magnifications (B) of the expression of GFAP (red) and expression of zonulin (green) in glioblastoma WHO IV: no colocalization. Features for starting of degradation (green) of blood vessels (indicated by binding site of GSI agglutinin, red) in astocytoma WHO III: no colocalization (C, arrows in D). (E) Alterations of the blood-brain barrier in glioblastoma WHO IV. White-yellow: colocalization of binding sites of GSI agglutinin and zonulin.
All features of the starting degradation of blood vessels are revealed by binding sites of GSI agglutinin in astrocytoma WHO III. The development of disturbance of blood vessels was shown in Figure 2, C and D, displaying nearly all stages of alterations of the blood-brain barrier in glioblastoma WHO IV. Here, we found colocalization of zonulin and binding sites of GSI agglutinin (indicated by white-yellow color, which was the result of both red and green fluorescence). There is some evidence from our immunocytochemical studies that the expression of zonulin correlated with the aggressivity of the tumor in a more specialized way than the expression of c-kit. Zonulin expression could clearly indicate the degree of the disturbance of the blood-brain barrier and the walls of the blood vessels, respectively (Figure 2, D and E).
Discussion
Zonulin was firstly described as zonula occludens toxin (zot). It is produced by Vibrio cholerae and has the ability to increase mucosal permeability by reversibly affecting the structure of tight junctions [5–9]. Even Fasano [7] suggested that this characteristic of zonulin could work also throughout a wide range of extraintestinal epithelia as well as the ubiquitous vascular endothelium, including the blood-brain barrier. Dysregulation (both over and under expression) may contribute to disease states that involve disordered intercellular communication [10] including malignant transformation and metastasis. Here, we tested that hypothesis by comparing the zonulin expression with established markers of human glial tumors such as c-kit and GFAP [11,12]. The correlation to blood vessels or rather the blood-brain barrier was studied by the binding sites of GSI agglutinin.
Glial fibrillary acidic protein is a common marker for glial cells regardless of their origin and developmental status. We used this marker to identify possible necrosis when expressed without any vascularity in the vicinity.
The gene product of c-kit has been shown to be expressed in cells of glial tumors with higher malignancy [13]. The authors found a preferred occurrence in both cells of pericyte or fibroblast morphology and in few neoplastic cells of overt astrocyte morphology. Cetin et al. [14] investigated 52 glial tumors of various histologic types and grades and found that 75% of the specimens were positive for the c-kit product. The proportion of high-grade tumors was statistically significantly greater than low-grade tumors; glioblastoma revealed the highest degree of c-kit expression. These results were supported by our findings. The new suggested perspective on these results is strongly supported by the zonulin expression in glial tumors, firstly reported here. If the aggressivity of the tumor depends on an increased expression of c-kit and its activated form, which is supported by the fact that both are increased frequently in glioblastoma [15], then the intercellular communication plays the key role [16]. The intercellular communication is under the control of zonulin. The different responsiveness of glioblastomas to specific tyrosine kinase inhibitors as targets for therapy could be explained by the somewhat different expression of zonulin compared with the expression of c-kit in glioblastomas. In conclusion, the estimation of zonulin expression could be helpful for therapy decision and success. By studying these parameters simultaneously in a large number of the same tumor samples, we hope to clarify their respective contributions to the aggressiveness of human gliomas.
Acknowledgments
The authors thank Ralf Schober (Independent Department of Neuropathology) for the histological grading.
References
- 1.Clemente MG, De Virgiliis S, Kang JS, Macatagney R, Musu MP, Di Pierro MR, Drago S, Congia M, Fasano A. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. 2003;52:218–223. doi: 10.1136/gut.52.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Pierro M, Lu R, Uzzau S, Wang W, Margaretten K, Pazzani C, Maimone F, Fasano A. Zonula occludens toxin structure-function analysis. Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J Biol Chem. 2001;276:19160–19165. doi: 10.1074/jbc.M009674200. [DOI] [PubMed] [Google Scholar]
- 3.Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83:724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haberler C, Gelpi E, Marosi C, Rössler K, Birner P, Budka H, Hainfellner JA. Immunohistochemical analysis of platelet-derived growth factor receptor-alpha, -beta, c-kit, c-abl, and arg proteins in glioblastoma: possible implications for patient selection for imatinib mesylate therapy. J Neurooncol. 2006;76:105–109. doi: 10.1007/s11060-005-4570-9. [DOI] [PubMed] [Google Scholar]
- 5.Drago S, El Asmar R, Di Pierro M, Grazia Clemente M, Tripathi A, Sapone A, Thakar M, Iacono G, Carroccio A, D'Agate C, et al. Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. 2006;41:408–419. doi: 10.1080/00365520500235334. [DOI] [PubMed] [Google Scholar]
- 6.El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, Catassi C, Fasano A. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002;123:1607–1615. doi: 10.1053/gast.2002.36578. [DOI] [PubMed] [Google Scholar]
- 7.Fasano A. Regulation of intercellular tight junctions by zonula occludens toxin and its eukaryotic analogue zonulin. Ann N Y Acad Sci. 2000;915:214–222. doi: 10.1111/j.1749-6632.2000.tb05244.x. [DOI] [PubMed] [Google Scholar]
- 8.Marinaro M, Fasano A, Magistris MT. Zonula occludens toxin acts as an adjuvant through different mucosal routes and induces protective immune responses. Infect Immun. 2003;71:1897–1902. doi: 10.1128/IAI.71.4.1897-1902.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci. 2000;113:4435–4440. doi: 10.1242/jcs.113.24.4435. [DOI] [PubMed] [Google Scholar]
- 10.Catassi C, Fasano A. Celiac disease. Curr Opin Gastroenterol. 2008;24:687–691. doi: 10.1097/MOG.0b013e32830edc1e. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura H, Takeshima H, Makino K, Kuratsu J. C-kit expression in germinoma: an immunohistochemistry-based study. J Neurooncol. 2005;75:163–167. doi: 10.1007/s11060-005-1593-1. [DOI] [PubMed] [Google Scholar]
- 12.Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S, Passaniti A, Menon J, Walling J, Bailey R, et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9:287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Mennel HD, Hallier-Neelsen M, Hagner S, Benes L. Two novel cell specific receptor proteins, CRLR and CD 117 in human glial tumors. Clin Neuropathol. 2006;25:107–114. [PubMed] [Google Scholar]
- 14.Cetin N, Dienel G, Gokden M. CD117 expression in glial tumors. J Neurooncol. 2005;75:195–202. doi: 10.1007/s11060-005-2318-1. [DOI] [PubMed] [Google Scholar]
- 15.Sihto H, Tynninen O, Bützow R, Saarialho-Kere U, Joensuu U. Endothelial cell KIT expression in human tumors. J Pathol. 2007;211:481–488. doi: 10.1002/path.2125. [DOI] [PubMed] [Google Scholar]
- 16.Madara JL. Loosening tight junctions. J Clin Invest. 1989;83:1089–1094. doi: 10.1172/JCI113987. [DOI] [PMC free article] [PubMed] [Google Scholar]