Abstract
Objective
To test the hypothesis that magnetic resonance imaging (MRI)-based measurements of hippocampal volume were related to the risk of future conversion to Alzheimer's disease (AD) in elderly patients with a mild cognitive impairment (MCI)
Background
Persons who develop AD pass through a transitional state which can be characterized as a MCI. However, in some patients MCI is a more benign condition which may not progress to AD or may do so slowly.
Patients
Eighty consecutive patients who met criteria for the diagnosis of MCI were recruited from the Mayo Clinic Alzheimer's Disease Center/Alzheimer's Disease Patient Registry.
Methods
At entry into the study each patient received a MRI examination of the head from which the volumes of both hippocampi were measured. Patients were then followed longitudinally with approximately annual clinical/cognitive assessments. The primary endpoint was the crossover of individual MCI patients to the clinical diagnosis of AD during longitudinal clinical followup.
Results
Over the period of longitudinal observation, which averaged 32.6 months, 27 of the 80 MCI patients became demented. Hippocampal atrophy at baseline was associated with crossover from MCI to AD (relative risk, 0.69, p = 0.015). When hippocampal volume was entered into bivariate models with age, post menopausal estrogen replacement, standard neuropsychological tests, apolipoprotein E genotype, history of ischemic heart disease and hypertension the relative risks were not substantially different from that found univariately and the associations between hippocampal volume and crossover remained significant.
Conclusion
In elderly patients with MCI, hippocampal atrophy determined by premorbid MRI-based volume measurements is predictive of subsequent conversion to AD.
Keywords: Dementia, Alzheimer's disease, Magnetic resonance imaging, brain, Quantitative MRI, Hippocampus, Volumetric MR
INTRODUCTION
For patients who develop Alzheimer's Disease (AD) the transition from a normal cognitive state to clinically recognizable AD occurs gradually over a number of years[McKhann, 1984 #17]. It is presumed that the pathologic substrate of the cognitive decline which characterizes AD follows a similar slowly progressive course with gradual accumulation of degenerative pathology of AD ongoing for years, perhaps even decades, prior to manifestation of unequivocal clinical symptoms. Memory impairment is usually the initial manifestation of dementia in AD. The fact that the transition from normal cognition to AD is gradual however, presents clinicians with a common and difficult diagnostic problem-does evidence of a mild memory impairment in an elderly individual represent the earliest manifestation of AD, or more benign forgetfulness which may not progress to dementia? Clinical criteria for the classification of patients with a mild cognitive impairment (MCI) have been established [Petersen, 1995 #48; Smith, 1996 #629; Flicker, 1991 #636]. The rate at which MCI patients convert to AD is substantially greater than that of the general elderly population[Petersen, 1995 #48; Masur, 1994 #658; Linn, 1995 #659; Jacobs, 1995 #660; Bowen, 1997 #661] and MCI patients are the subject of several recent treatment trials.
Structural and functional imaging findings are diagnostic markers of AD[DeCarli, 1990 #51]. However, most imaging studies performed have been designed to demonstrate differences between elderly controls and patients who were already demented. These studies have not determined if an imaging test can prospectively identify patients who will subsequently manifest AD. We addressed this issue by imaging and then longitudinally following individuals who were at increased risk of AD by virtue of the diagnosis of MCI. The imaging measurement evaluated was magnetic resonance imaging (MRI)-based volume measurements of the hippocampi. We chose this measurement because: 1) medial temporal lobe limbic structures, particularly the hippocampus, play a central role in memory function and are the site of the earliest neurofibrillary pathology in AD[Braak, 1991 #217; Arriagada, 1992 #390]; 2) memory impairment is the hallmark of early AD[Welsh, 1991 #640]; and 3) MRI detects subtle medial temporal lobe damage in AD[Jack, 1992 #1; Convit, 1995 #85; Laakso, 1995 #34; de Leon, 1997 #439; Kaye, 1997 #111]. In this study we tested the hypothesis that premorbid MRI-based hippocampal volume measurements in patients with a MCI were related to the risk of subsequent conversion to AD. We also determined whether the predictive power of hippocampal volume measurements was independent of other potential predictor variables-age, apolipoprotein E (APOE) genotype, estrogen replacement, performance on selected measures of cognitive performance, hypertension and ischemic heart disease.
METHODS
Recruitment and Evaluation of Subjects
Eighty consecutive MCI patients were recruited from the Mayo Clinic AD Center and AD Patient Registry(ADC/ADPR) which are prospective, longitudinal studies of aging and dementia[Petersen, 1990 #43]. Informed consent was obtained for participation in the studies which were approved by the Mayo Institutional Review Board. Study participants were assigned to diagnostic group categories during (ADC/ADPR) consensus committee meetings consisting of a geriatrician, neurologists, neuropsychologists, psychometrists, and nurses who had seen the patient. Relevant diagnostic categories were those of MCI and AD. Criteria for the diagnosis of MCI were the following [Petersen, 1995 #48; Smith, 1996 #629]: 1) memory complaint documented by the patient or collateral source; 2) normal general cognitive function as determined by measures of general intellectual function and screening instruments; 3) normal activities of daily living as documented by history and Record of Independent Living[Weintraub, 1982 #635]; 4) dementia ruled out by Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition (DSM-III-R) criteria[Association, 1987 #16], and met no National Institute of Neurological and Communicative Disorders and Stroke-AD and Related Disorders Association (NINCDS-ADRDA) criteria for AD[McKhann, 1984 #17]; 5) objective memory impairment defined by performance at 1.5 standard deviations below age and education-matched controls on indices of memory function[Ivnik, 1992 #47]; 6) age between 60 to 89 years; 7) clinical Dementia Rating score of 0.5[Morris, 1993 #80].
Ascertainment of Endpoint
The primary endpoint or dependent variable in this study was the crossover of individual MCI patients to the AD category during longitudinal followup. Patients were enrolled throughout the study period. All study patients underwent clinical/neuropsychological reevaluations at approximately 12 month intervals. Twenty-six patients had a single serial followup assessment; 13 had two followup assessments; and 41 had three or more followup assessments. MCI patients who remained unchanged cognitively were characterized as stable and the mean followup time for these patients was 33.5 ± 17.9 months. Patients who became demented, all of whom received the diagnosis of probable AD at that time, were designated as crossover patients and the mean followup time (from enrollment to crossover) for these patients was 30.8 ± 17.3 months. The diagnosis of dementia was made according to DSM-III-R criteria[Association, 1987 #16]. The diagnosis of probable AD was made according to NINCDS-ADRDA criteria[McKhann, 1984 #17].
An MRI examination of the brain was performed within 4 months of the initial clinical assessment in all patients. These MRI studies were used in the diagnostic process only to exclude treatable causes of cognitive impairment. The hippocampal volume data were unknown to the consensus committee throughout the study.
MRI Methods
All patients were imaged at 1.5T (Signa, General Electric Medical Systems, Milwaukee, WI) using a standardized imaging protocol[Jack, 1997 #609]. Measurements of intracranial volume were derived from a T1-weighted sagittal sequence with 5 mm contiguous sections. Volume measurements of the hippocampi were derived from a T1-weighted (3D) volumetric spoiled gradient recalled echo sequence with 124 contiguous partitions, 1.6mm slice thickness, a 22 cm × 16.5 cm field of view, 192 views, and 45° flip angle.
All image processing steps in every patient were performed by the same research associate who was blinded to all clinical information (age, gender, clinical course) to insure that the volumetric data were generated in an unbiased fashion. Validation studies have shown the intra-rater test-retest coefficient of variation of hippocampal volumetric measurements to be 1.9% with this method[Jack, 1990 #41]. The 3D image dataset of each patient was realigned into an orientation perpendicular to the principal axis of the left hippocampal formation. The imaging data were then interpolated inplane to the equivalent of a 512 × 512 matrix and magnified 2x. The voxel size of the fully processed image data was 0.316mm3. The borders of the hippocampi were manually traced on the workstation screen for each image slice sequentially from posterior to anterior. Typically 40–50 imaging slices were measured for each hippocampus. Inplane hippocampal anatomic boundaries were defined to include the CA1 to CA4 sectors of the hippocampus proper, the dentate gyrus, and the subiculum[Jack, 1997 #609](Fig. 1). The posterior boundary of the hippocampus was determined by the oblique coronal anatomic section on which the crura of the fornices were defined in full profile.
Figure 1. Neuroanatomic Boundaries.
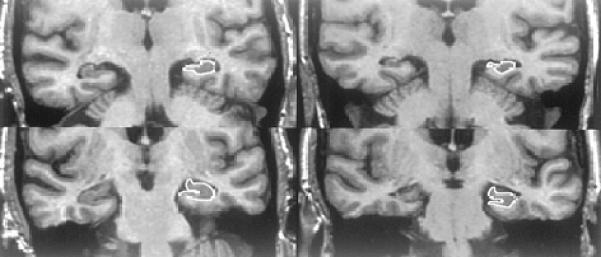
The column of images on the left are cropped oblique coronal MR images through the temporal lobes of a 75-year-old woman. The upper image is through the body of the hippocampus and lower image is through the head of the hippocampus. This MCI patient remained stable over 49 months of clinical followup. At baseline her hippocampal W score was 0.21. On the right are matched imaging sections of a 70-year-old woman, who was initially categorized as MCI, but become demented after 43.5 months of followup. Her hippocampal W score was −2.48 at entry into the study. The hippocampi of the patient who became demented (right) are visibly atrophic relative to the stable patient (left) despite the fact that the crossover patient was 5 years younger. The anatomic outlines of the left hippocampus are indicated.
Intracranial volume was determined by tracing the margin of the inner table of the skull on contiguous images from the sagittal sequence.
Apolipoprotein E Genotyping
DNA was extracted from peripheral leukocytes and amplified by polymerase chain reaction[Tsai, 1994 #102]. Polymerase chain reaction products were digested with HhaI and the fragments were separated by electrophoresis on an 8% polyacrylamide non-denaturing gel. The gel was then treated with ethidium bromide for 30 minutes, and DNA fragments were visualized by UV illumination.
Assessment of Clinical Variables
The presence or absence of hypertension and ischemic cardiac disease was assessed by review of the medical records. Patients were recorded as positive for hypertension, if hypertension or its treatment was identified at any point in time in the medical record. Patients were considered to have ischemic heart disease if any of the following were identified: angina pectorus, myocardial infarction, coronary bypass surgery, or coronary angioplasty. The time of menopause and the presence or absence of estrogen replacement therapy were also extracted from the medical records.
Statistical Methods
The hippocampal volume measurements of each patient were normalized for interindividual variation in head size by dividing hippocampal volume by the total intracranial volume of that particular patient. We have previously determined age and gender specific normal percentiles for normalized hippocampal volume in a group of 126 cognitively normal elderly controls using the MRI volumetric method described above[Jack, 1997 #609]. Age and gender specific normal percentiles for each of the 80 MCI patients were determined using this normal value data base. Each percentile was then converted to a W score. The W score is the value from a standard normal distribution corresponding to the observed percentile. For example, for a standard normal distribution, the 50th, 5th, and 2.5th percentiles are given by 0, −1.645, and −1.96, respectively. Thus, a patient with a hippocampal volume (adjusted for age and gender) at the 5th percentile in the normal value data base would receive a W score of −1.645. Similarly, a patient at the 50th percentile would receive a W score of 0. When this method of assigning W scores is applied to the normal elderly control data base, the resulting W scores precisely follow a standard normal distribution. W scores in other study populations, including our MCI cohort, can then be compared directly to this standard distribution, providing a framework for comparing hippocampal volume measurements among individual patients which were appropriately corrected for age, gender, and head size.
In addition to hippocampal volume, other predictor variables for crossover to AD which were evaluated included age, APOE genotype, Mini-Mental State Examination (MMSE)[Folstein, 1975 #73], Dementia Rating Scale (DRS)[Mattis, 1976 #72], Wechsler Memory Scale-Revised-Logical Memory II Subtest - Paragraph Retention score (WMS-R-LMRII)[Wechsler, 1987 #617], Auditory Verbal Learning Test-Percent Delayed Retention score (AVLT)[Rey, 1964 #618], the total free recall and delayed recall indices from the Free and Cued Selective Reminding Test (FCSRT)[Grober, 1988 #621], and the Controlled Oral Work Association Test total final score (COWAT). The presence of estrogen replacement, hypertension and ischemic heart disease were also modeled as potential predictor variables. (Insert DeCarli Neurology 1995 45:2077–2087 here) In the APOE ∈4 risk analysis, patients were stratified into those with genotypes known to increase the risk of AD (3/4, 4/4) and those who were ∈4 non-carriers (2/3, 3/3)[Corder, 1993 #91]. Six patients who were ∈2/4 were excluded from the APOE risk analysis because the association between AD and ∈2/4 is unclear.
While a direct comparison of the W scores of patients who did and those who did not crossover seems natural, the length of followup varied among patients. Direct comparisons between patients who crossed over versus those who did not were therefore analytically inappropriate. To accommodate variable followup periods life table methods were used to evaluate patient characteristics relating to crossover rather than discriminate function analyses or logistic regression. Each predictor variable was evaluated univariately. Due to sample size limitations, extensive multivariate modeling was not feasible. However, the possibility of confounding between hippocampal W score and other variables was assessed by fitting bivariate models evaluating hippocampal W score with each of the other predictor variables individually. APOE status, estrogen replacement, hypertension, and ischemic heart disease were entered as a dichotomous variables in all analyses. All tests were two-sided. Estimates of relative risk were obtained using usual, semi-parametric, Cox regression. For the reasons described below, a nonparametric version of Cox regression was used for hypothesis testing for quantitative variables. For these same reasons confidence intervals are not reported for estimates of risk.
Tests of hypotheses for quantitative risk factors using Cox regression are sensitive to departures from normality, in which case more accurate probability statements are obtained using logit rank tests [O'Brien, 1978 #445]. Because some of the data were skewed with the degree of skewness varying among successive risk sets, the logit rank test was used both univariately and bivariately in hypothesis testing[O'Brien, 1978 #445]. The logit rank tests were implemented computationally by treating the logit rank scores for each of the quantitative variables in each risk set as time dependent covariates in a Cox regression model.
Kaplan-Meier survival curves showing the probability of crossover for patients stratified by hippocampal W scores into three groups (W ≥ 0, 0 > W > −2.5, W ≤ −2.5) were used for display purposes only, in order to illustrate the association between hippocampal volume and crossover to AD. The W score W ≥0 was selected a priori. This is a natural cut point, indicating values in patients which are equal or greater than the mean value among controls. The cutpoint W ≤ −2.5 was selected post-hoc to optimally display the gradient of the relative risk of crossover associated with hippocampal atrophy. A W score of −2.5 corresponds to approximately the 1st percentile (more precisely the 0.6 percentile) among controls. These W score cut points were used for display purposes only (Fig. 2, Table 1). All statistical analyses (Table 2 & 3) were performed using hippocampal W score as a continuous variable.
Figure 2. Hippocampal W Score and Crossover.
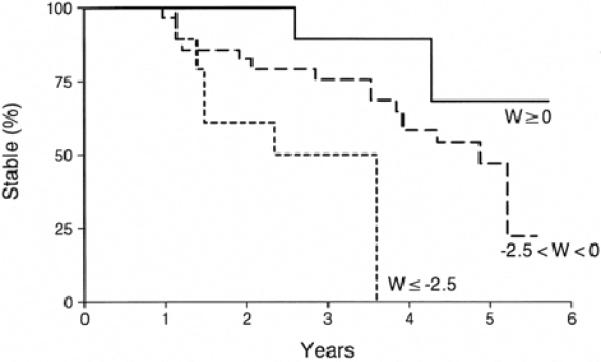
Kaplan-Meyer curves of patients whose hippocampal W score at baseline was ≥ 0 (n=13), 0 > W > −2.5 (n = 54), and ≤ −2.5 (n = 13).
Table 1.
Characterization of MCI Patients
| N | Age1 Mean ± SD | Male | MMSE2 Mean ± SD | DRS3 Mean ± SD | Education Mean ± SD | APOE∈44 | Followup Months Mean ± SD5 | Crossover to AD | |
|---|---|---|---|---|---|---|---|---|---|
| Hippocampal W Score ≥0 | 13 | 79.6 ± 6.1 | 6 (46.2%) | 26.2 ± 2.3 | 127.3 ± 6.9 | 11.7 ± 3.1 | 4 (30.8%) | 42.4 ± 15.4 | 2 (15%) |
| −2.5 < Hippocampal W Score < 0 | 54 | 78.3 ± 6.4 | 23 (42.6%) | 26.7 ± 2.3 | 127.6 ± 9.4 | 13.3 ± 3.5 | 19 (35.2%) | 32.5 ± 18.4 | 19 (35%) |
| Hippocampal W Score ≤−2.5 | 13 | 73.7 ± 8.2 | 4 (30.8%) | 25.3 ± 3.4 | 126.5 ± 8.6 | 13.7 ± 3.1 | 6 (46.2%) | 23.3 ± 10.7 | 6 (46%) |
| Total | 80 | 77.7 ± 6.8 | 33 (41.25%) | 26.4 ± 2.5 | 127.4 ± 8.8 | 13.1 ± 3.4 | 9 (36.25%) | 32.6 ± 17.6 | 27 (34%) |
Age at time of MRI study, i.e., at time patient entered into the study
MMSE - Mini-Mental Status Examination score when patient entered the study. Maximum score is 30
Dementia Rating Scale score when patient entered the study. Maximum score is 144
Numbers in this column represent the number of patients in each group who were carriers of APOE genotypes which are known to confer an increased risk of AD, ∈3/4 and 4/4. Patients (n=6) with ∈2/4 were not included.
Followup is from time of entry into study to conversion to AD in those patients who crossed over
Table 2.
Risk of Crossover: Univariate Analyses
| Variable | Relative Risk | p Value |
|---|---|---|
| Hippocampal W Score | 0.69 | 0.015 |
| Age (decades) | 0.62 | 0.042 |
| APOE ∈4 | 1.49 | 0.349 |
| MMSE1 | 0.82 | 0.089 |
| DRS2 | 0.97 | 0.026 |
| WMS-R3 | 0.96 | 0.638 |
| AVLT4 | 0.99 | 0.251 |
| FCSRT Free Recall5 | 0.97 | 0.015 |
| FCSRT Delayed Recall6 | 0.99 | 0.089 |
| COWAT7 | 1.01 | 0.316 |
| Hypertension | 1.63 | 0.272 |
| Ischemic heart disease | 0.55 | 0.272 |
| Estrogen Replacement | 1.09 | 0.864 |
Mini-Mental State Examination
Dementia Rating Scale
Wechsler Memory Scale - Revised-Logical Memory II Subtest - Delayed Paragraph Recall
Auditory Verbal Learning Test - Percent Delayed Retention
Free and Cued Selective Reminding Test - total free recall
Free and Cued Selective Reminding Test - delayed recall
Controlled Oral Word Association Test
Table 3.
Risk of Crossover: Bivariate Analyses
| Variables | Relative Risk | p value |
|---|---|---|
| Hippocampal W score | 0.71 | 0.032 |
| Age (decades) | 0.64 | 0.081 |
| Hippocampal W score | 0.70 | 0.018 |
| APOE ∈4 | 1.39 | 0.460 |
| Hippocampal W score | 0.72 | 0.011 |
| MMSE1 | 0.84 | 0.068 |
| Hippocampal W score | 0.70 | 0.024 |
| DRS2 | 0.97 | 0.043 |
| Hippocampal W score | 0.72 | 0.017 |
| WMS-R3 | 0.97 | 0.840 |
| Hippocampal W score | 0.70 | 0.023 |
| AVLT4 | 0.99 | 0.430 |
| Hippocampal W score | 0.74 | 0.026 |
| FCSRT - Free Recall5 | 0.97 | 0.026 |
| Hippocampal W score | 0.72 | 0.029 |
| FCSRT-Delayed Recall6 | 0.99 | 0.170 |
| Hippocampal W score | 0.68 | 0.026 |
| COWAT7 | 1.00 | 0.980 |
| Hippocampal W score | 0.63 | 0.011 |
| Hypertension | 1.88 | 0.220 |
| Hippocampal W score | 0.68 | 0.023 |
| Ischemic heart disease | 0.73 | 0.560 |
| Hippocampal W score | 0.68 | 0.067 |
| Estrogen replacement | 0.91 | 0.670 |
Mini-Mental State Examination
Dementia Rating Scale
Wechsler Memory Scale - Revised-Logical Memory II Subtest - Delayed Paragraph recall
Auditory Verbal Learning Test, % Delayed Retention
Free and Cued Selective Reminding Test - total free recall
Free and Cued Selective Reminding Test - delayed recall
Controlled Oral Word Association Test
RESULTS
Of the 80 patients who were classified as MCI at entry into the study three died during the followup period, two of these after they had converted to AD. The mean hippocampal W score of these 80 MCI patients was −1.24 ± 1.24 corresponding to the 11th percentile of volumes among controls after correction for age, gender, and head size. Out of the entire group of 80 MCI patients, 13 had hippocampal W scores ≥ 0 at entry into the study, indicating hippocampal volumes which were at or above the mean value expected for age and gender matched controls. Thirteen had hippocampal W scores ≤ −2.5. Fifty-four had W scores between 0 and −2.5. Patients in the three W score groups were similar to each other on most demographic, clinical, and cognitive testing variables(Table 1). Over the period of observation 27 of the 80 MCI patients converted to AD. Of the 13 MCI patients with hippocampal W scores ≥ 0 at baseline, two converted to AD; 19 of 54 with W scores between 0 and −2.5 crossed over; and, 6 of 13 with W scores ≤ −2.5 crossed over (Figure 2).
Only hippocampal volume, DRS, FCSRT Free Recall, and age were statistically significant predictor variables in univariate analyses of the risk of crossover (Table 2). The interpretation of hippocampal W score result is-for each one unit increase in the hippocampal W score (i.e., less atrophy) the relative risk (RR) for crossover declined by 31%. The risk of crossover decline with advancing decade of age. Carriers of the APO ∈4 allele were 49% more likely to crossover than non-carriers. Patients with better scores on the cognitive tests were less likely to crossover. The one exception was that of the COWAT with a relative risk of 1.01 and a non-significant p value. Patients who underwent post-menopausal estrogen replacement had a 9% greater risk of crossing over than those who did not, however this association did not approach significance (p = 0.864). Patients with a history of hypertension were more likely to crossover than those without, and individuals with a history cardiac ischemic disease were less likely to crossover than those without such a history, although neither of these associations were significant.
Separate bivariate analyses were performed with hippocampal volume together with each of the other predictor variables (Table 3). Hippocampal volume was significant in all models. The RR ratios of hippocampal volume in all bivariate models were similar to those observed univariately, suggesting the independence of hippocampal volume as a risk factor for crossover with respect to each of the other predictor variables evaluated.
DISCUSSION
Based on life table analysis, we estimate that 9% of MCI patients with hippocampal W scores ≥ 0 at baseline will convert to AD within 3 years compared to 26% of those with hippocampal W scores between 0 and −2.5, and 50% of those with W scores ≤ −2.5. There is considerable controversy whether all MCI patients will eventually progress to AD, or whether MCI represents a relatively stable condition in some. Our results indicate only that the rate of conversion is greater in MCI patients with smaller hippocampi and do not address the lifetime risk of conversion.
Old age is an established risk factor for AD, and in a cross-sectional prevalence study older age would be expected to be associated with a greater prevalence of AD[Hebert, 1995 #159]. This is not the case in a study such as ours because the rate at which individuals with MCI progress to AD does not necessarily accelerate with age. The 80 MCI patients in this cohort shared a similar cognitive and demographic profile at the point of entry into the study(Table 1). It is possible that because AD is a clinically heterogeneous disorder, patients with incipient AD who were younger could have had a more rapidly progressing form than those who were older[Jacobs, 1994 #632].
This work focused on the prediction of crossover using hippocampal volume measurements. The intent was not to conduct an exhaustive assessment of possible neuropsychological testing instruments as predictors of crossover, but to test the hypothesis that the predictive power of imaging was independent of other potential predictor variables such as standardized neuropsychological tests[Tierney, 1996 #630; Petersen, 1995 #48]. The MMSE and DRS are measures of general cognitive function while the AVLT, WMS-R-LMII, and FCSRT indices, are tests of memory[Folstein, 1975 #73; Mattis, 1976 #72; Wechsler, 1987 #617; Rey, 1964 #618; Grober, 1988 #621]. The COWAT is a test of verbal fluency measuring attention and language skills[Benton, 1983 #623]. In bivariate analyses paired with hippocampal W score the associations with risk of crossover were statistically significant for the DRS and FCSRT Free Recall.
Post menopausal estrogen replacement may decrease the risk of developing AD[Paganini-Hill, 1994 #652; Tang, 1996 #650]. However, no significant association between crossover and estrogen replacement was observed in the female members of this MCI group. The non-significant p-value (p = 0.067) observed for hippocampal W score when paired with estrogen bivariately (Table 3) may simply be the result of reduced statistical power when men were excluded from the analysis.
Polymorphisms of the apolipoprotein E gene are a significant risk factor for developing late onset AD[Corder, 1993 #91]. The ∈4 allele confers both an increased risk of developing AD and also lowers the mean age of onset in a dose dependent fashion, while the ∈2 allele is protective. A trend was present in our data indicating that the ∈3/4 or 4/4 genotypes conferred a 49% increased RR (Table 2) of crossover relative to an MCI patient with the ∈2/3 or 3/3 genotypes. APOE genotype does not influence the rate of clinical progression in patients with established AD [Growdon, 1996 #586]. However, we suspect that if the followup period were extended to increase the number of crossover events then APOE ∈4 would emerge as a significant risk factor. In an earlier study analyzing the risk of crossover as a function of several known risk factors (including age, family history, a variety of cognitive testing instruments, and APOE genotype), APOE ∈4 emerged as the most powerful predictor variable[Petersen, 1995 #48], however, imaging variables were not considered in the prior study.
Unlike genetic markers which are present at birth, imaging can only identify progression of the disease itself. This is true both for structural imaging measures and functional measures such as PET, because imaging studies become abnormal only when the disease process itself has produced deviation from normal cerebral function or anatomy[Small, 1995 #101; Reiman, 1996 #99]. Ideally the imaging findings should represent markers of incipient disease. Our data suggest that MRI-based volume measurements of the hippocampi fulfill this criteria.
These results agree with those of several recent studies. Fox et al ( ) studied seven individuals in their 40's and 50's who were members of a family with an amyloid precursor protein 717 Val-Gla pedigree. Hippocampal volume declined more rapidly in those who declined cognitively (n=3) than those who remained stable. deLeon et al ( ) employed visual assessment of the size of the perihippocampal CSF spaces on CT and found individuals with atrophy were more likely to progress to dementia. As part of a study of the oldest-old (mean age 90.4 years), Kaye et al ( ) found that the temporal lobes but not hippocampi were smaller in those who declined cognitively than those who remained stable. The unique aspects of our study were; i) the use of a rigorous quantitative MRI-based measurement; ii) fairly large longitudinal cohort (n = 80); iii) patients who were at risk for typical sporadic AD; iv) patients whose age was fairly typical rather than at the extremes of the age spectrum in which AD occurs..
There are several limitations to this study which cannot be completely addressed at this time. The criteria employed to determine the endpoint was the clinical diagnosis of AD. At our center the clinical diagnosis of probable AD is 81% accurate with respect to the pathologic diagnosis of definite AD. Thus, absolute determination of the endpoint in this study can only be ascertained in the future-at autopsy. Despite the small sample size a significant relationship between premorbid hippocampal volume and crossover to AD was demonstrated which illustrates both the strength of the association and the clinical potential of the technique. Moreover, this association was not altered when evaluated in the context of a series of bi-variate analyses which included many known risk factors for AD. Additional studies with larger sample sizes are needed, however, to more accurately delineate the rate at which risk of crossover increases with increasing hippocampal atrophy and the possibility that the relationship may be non-linear, as well as interactions with other predictor variables.
ACKNOWLEDGMENTS
Brenda Maxwell - Typist
Ruth Cha - Statistical analysis
Supported By: NIH-NIA-AG 11378, AG08031, AG06786, NINDS-NS29059, The DANA Foundation, The Alzheimer's Association.
References
- 1.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease : report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Smith GE, Ivnik RJ, Tangalos EG, et al. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory impaired individuals. JAMA. 1995;273:1274–1278. [PubMed] [Google Scholar]
- 3.Smith GE, Petersen RC, Parisi JE, et al. Definition, course, and outcome of mild cognitive impairment. Aging Neuropsychol Cogn. 1996;3:141–147. [Google Scholar]
- 4.Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly : predictors of dementia. Neurology. 1991;41:1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 5.Masur D, Sliwinski M, Lipton R, et al. Neuropsychological prediction of dementia and the absence of dementia in healthy elderly persons. Neurology. 1994;44:1427–1432. doi: 10.1212/wnl.44.8.1427. [DOI] [PubMed] [Google Scholar]
- 6.Linn R, Wolf P, Bachman D, et al. The `preclinical phase' of probable Alzheimer's disease. Arch Neurol. 1995;52:485–490. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs D, Sano M, Dooneief G, et al. Neuropsychological detection and characterization of preclinical Alzheimer's disease. Neurology. 1995;45:956–962. doi: 10.1212/wnl.45.5.957. [DOI] [PubMed] [Google Scholar]
- 8.Bowen J, Teri L, Kukull W, et al. Progression to dementia in patients with isolated memory loss. Lancet. 1997;349:763–765. doi: 10.1016/S0140-6736(96)08256-6. [DOI] [PubMed] [Google Scholar]
- 9.DeCarli C, Kaye JA, Horwitz B, Rapoport SI. Critical analysis of the use of computer-assisted transverse axial tomography to study human brain in aging and dementia of the Alzheimer type. Neurology. 1990;40:872–883. doi: 10.1212/wnl.40.6.872. [DOI] [PubMed] [Google Scholar]
- 10.Fox MC, Warrington EK, Freeborough PA. Presymptomatic hippocampal atrophy in Alzheimer's disease. A longitudinal MRI study. Brain. 1996;119:2001–2007. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- 11.Kaye JA, Swihart T, Howieson D, Dame A. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997;48:1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- 12.de Leon MJ, Golomb J, George AE, et al. The radiologic prediction of Alzheimer disease : the atrophic hippocampal formation. AJNR Am J Neuroradiol. 1993;14:897–906. [PMC free article] [PubMed] [Google Scholar]
- 13.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 14.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but no senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 15.Welsh KA, Buters N, Hughes J, et al. Detection of abnormal memory decline in cases of alzheimer's disease using CERAD neuropsychological measures. Arch Neurol. 1991;38:278–281. doi: 10.1001/archneur.1991.00530150046016. [DOI] [PubMed] [Google Scholar]
- 16.Jack CR, Jr., Petersen RC, O'Brien PC, et al. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- 17.Convit A, de Leon MH, Tarshis C, De Santi S, et al. Hippocampal volume losses in minimally impaired elderly. Lancet. 1995;345:266. doi: 10.1016/s0140-6736(95)90265-1. [DOI] [PubMed] [Google Scholar]
- 18.Laakso MP, Soininen H, Partanen K, et al. Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer's disease : correlation with memory functions. J Neural Transm. 1995;9:73–86. doi: 10.1007/BF02252964. [DOI] [PubMed] [Google Scholar]
- 19.de Leon MJ, George AE, Golomb J, et al. Frequency of hippocampal formation atrophy in normal aging and Alzheimer's disease. Neurobiol Aging. 1997;18:1–11. doi: 10.1016/s0197-4580(96)00213-8. [DOI] [PubMed] [Google Scholar]
- 20.Petersen RC, Kokmen E, Tangalos EG, et al. Mayo Clinic Alzheimer's Disease Patient Registry. Aging. 1990;2:408–415. doi: 10.1007/BF03323961. [DOI] [PubMed] [Google Scholar]
- 21.Weintraub S, Baratz R, Mesulam MM. Daily living activities in the assessment of dementia. In: Corkin S, Growdon JH, Usdin E, Wurtman RJ, editors. Alzheimer's disease: a report of progress in research. Raven Press; New York: 1982. pp. 189–192. [Google Scholar]
- 22.American Psychological Association . Diagnostic and statistical manual of mental disorders, revised. 3rd ed. American Psychological Association; Washington, DC: 1987. [Google Scholar]
- 23.Ivnik RJ, Malec JF, Smith GE, et al. Mayo's older Americans normative studies : WAIS-R, WMS-R, and AVLT norms for ages 56 through 97. Clin Neuropsychol. 1992;6:1–103. [Google Scholar]
- 24.Morris JC. The Clinical Dementia Rating (CDR) : current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 25.Jack CR, Jr., Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jack CR, Jr., Bentley M, Twomey CK, Zinsmeister AR. MR-based volume measurements of the hippocampal formation and anterior temporal lobe : validation studies. Radiology. 1990;176:205–209. doi: 10.1148/radiology.176.1.2353093. [DOI] [PubMed] [Google Scholar]
- 27.Tsai MS, Tangalos EG, Petersen RC, et al. Apolipoprotein E : risk factor for Alzheimer's disease. Am J Hum Genet. 1994;54:643–649. [PMC free article] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. `Mini-Mental State.' A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Mattis S. Mental status examination for organic mental syndromes in the elderly patient. In: Bellak KT, editor. Geriatric psychiatry. Grune & Stratton; New York: 1976. [Google Scholar]
- 30.Wechsler D. Wechsler Memory Scale-revised. Psychological Corporation; New York: 1987. [Google Scholar]
- 31.Rey A. L'examen clinique en psychologie. Presse Universitaires de France; Paris: 1964. [Google Scholar]
- 32.Grober E, Buschke H, Crystal H. Screening for dementia by memory testing. Neurology. 1988;38:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- 33.DeCarli C, Murphy DGM, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- 34.Corder EH, Saunders AM, Strittmatter WJ. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien PC. A non-parametric test for association with censored data. Biometrics. 1978;34:243–250. [PubMed] [Google Scholar]
- 36.Hebert LE, Scherr PA, Beckett LA, et al. Age-specific incidence of Alzheimer's disease in a community population. JAMA. 1995;273:1354–1359. [PubMed] [Google Scholar]
- 37.Jacobs D, Sano M, Marder KEA. Age at onset of Alzheimer's disease : relation to pattern of cognitive dysfunction and rate of decline. Neurology. 1994;44:1215–1220. doi: 10.1212/wnl.44.7.1215. [DOI] [PubMed] [Google Scholar]
- 38.Tierney MC, Szalai JP, Snow WG, et al. Prediction of probable Alzheimer's disease in memory-impaired patients : a prospective longitudinal study. Neurology. 1996;46:661–665. doi: 10.1212/wnl.46.3.661. [DOI] [PubMed] [Google Scholar]
- 39.Benton AL, Hamsher K, Varney NR, et al. Contributions to neuropsychological assessment. Oxford University Press; New York: 1983. [Google Scholar]
- 40.Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer's disease in women. Am J Epidemiol. 1994;140:256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- 41.Tang M-X, Jacobs D, Stern Y, et al. Effect of estrogen during menopause on risk and age of onset of Alzheimer's disease. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 42.Growdon J, Locasio J, Corkin S, et al. Apolipoprotein E genotype does not influence rates of cognitive decline in Alzheimer's disease. Neurology. 1996;47:444–448. doi: 10.1212/wnl.47.2.444. [DOI] [PubMed] [Google Scholar]
- 43.Small GW, Mazziotta JC, Collins MT, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA. 1995;273:942–947. [PubMed] [Google Scholar]
- 44.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the E4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]


