Abstract
We evaluated in a population-based setting the postdiagnosis parenthood among survivors compared with the fertility patterns of siblings. Cancer patients aged 0–34 years at diagnosis were identified from the Finnish Cancer Registry (N = 25,784), and their siblings (N = 44,611) by registry linkage. Further linkage identified the offspring of the patient and sibling cohorts. The relative probabilities of parenthood for first and second births separately were estimated for male and female survivors in different diagnostic age-groups and subsites using a Cox proportional hazards model, with age as the time variable and adjusting for the birth cohort of parents. In addition, estimates were calculated for 5 diagnostic eras in all subsites combined. Compared to siblings, both female and male cancer survivors were less likely to parent at least 1 child (RR 0.46, 95% CI 0.44–0.48 and RR 0.57, 95% CI 0.54–0.60, respectively). The relative probability of parenthood was especially low in male childhood cancer survivors and female young adult cancer survivors. However, cancer patients were only slightly less likely than siblings to parent a second child, with RR 0.91, 95% CI 0.86–0.97 and RR 0.95, 95% CI 0.89–1.01 for females and males, respectively. The relative probability of parenthood increased over calendar time among young adult cancer patients. The relative probability of parenthood following early onset cancer was overall significantly reduced by ∼50%. Parenting a second child, however, was not reduced among pediatric and adolescent survivors, and only slightly reduced among early adulthood cancer survivors compared to siblings.
Keywords: cancer, late effects, parenthood, survivor
The improvement of chemo and radiotherapy regimens in the past few decades has decreased mortality1 and increased cure rates of childhood2 and early onset3 malignancy. As cure rates of childhood cancer continue to increase, more young patients will survive to reach reproductive ages.
With this expanding population of cancer patients retaining reproductive potential, research efforts are being made to investigate fertility,4-7 pregnancy outcomes,8-10 as well as health effects among offspring.11,12 However, most studies have addressed possible adverse outcomes following treatment regimens that are currently outdated.11 Several investigations focus on a small subgroup of patients with a particular cancer diagnosis,13-16 and few population-based studies have been conducted.17-19 Despite extensive research in the field of genetic late-effects, the question of whether or not therapies for cancer can cause adverse effects in the next generation remains unanswered.20,21
As parenthood after cancer diagnosis has become a reality for a growing population of cancer survivors, reproductive potential is becoming a major quality-of-life factor. Two surveys showed that cancer diagnosis increases the value placed on family and the importance of parenthood for survivors.22,23 Cancer survivors face anxiety and fears relating to reduction in overall fertility, possible pregnancy risks, as well as health effects in offspring.23,24 There is, thus, a growing awareness of the need for adequate family planning and counseling of cancer survivors.25
The aim here is to present the design and cohort characteristics of a large-scale population-based, registry linkage study and to investigate postdiagnosis parenthood for a wide range of cancer survivors, diagnosed in childhood, adolescence and early adulthood.
Material and methods
Cancer diagnoses have been reported to the Finnish Cancer Registry (FCR) by physicians, hospitals and pathological laboratories since 1953. The register is population-based, nation-wide and almost complete (99% for solid tumors, 92% for hematological malignancies and 100% for childhood cancers).26 The FCR data include person data (date of birth, gender, name, residence), cancer data (date of diagnosis, primary site, histology, malignancy, stage, basis of diagnosis) and initial treatment data as well as possible date and cause of death.
The cancer patient cohort was identified from the files of the FCR.26 Patients were included if they were (i) aged less than 35 years at diagnosis; (ii) diagnosed with a malignant neoplasm (including benign central nervous system (CNS) tumors and those of uncertain malignancy); (iii) diagnosed between January 1, 1953 and December 31, 2004; and (iv) alive in 1967 or born thereafter. In our study, a further eligibility criterion was that the cancer patient reached reproductive age, defined as 16 years. We were interested in postdiagnosis parenthood defined as having 1 or more offspring more than 9 months (270 days) after diagnosis. For this exercise, only those patients parenting their first child after diagnosis were included.
Identification of siblings, spouses and offspring
Since 1967, every resident of Finland has been given a unique personal identification number (PIN), which can be used for linkage of records from different registries and databases. The Population Register Centre hosts a nation-wide central population register (CPR) and it includes the name and former names, PIN, municipality of birth and residence (with residence history), date of emigration or date of death of each individual living in Finland and alive in 1967 or born thereafter. Further, links to parents, children and siblings are reliably available for family members born after 1955.
Siblings of cancer patients were identified by linking the PIN of the patient to his or her mother and father and listing all other children of the patient's parents. By further linkage within the CPR, we identified the spouses and offspring of cancer patients and their siblings. A father was defined as the legal parent of a child.
Survivor and sibling cohorts
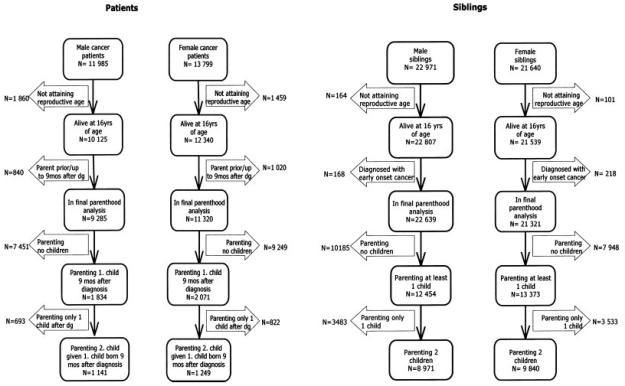
A total of 25,784 cancer patients fulfilled the inclusion criteria. Of them, 22,465 (87%) attained fertile age (16 years) and 3,905 (15%) (1,834 male and 2,071 female survivors) parented at least 1 child 9 months or more after diagnosis (Fig. 1).
Figure 1.
The early onset cancer survivor and sibling cohorts. Number of cancer patients, patients with offspring (parents), their siblings and those siblings parenting children.
In total, 44,611 siblings of cancer patients were identified from the CPR of whom 44,346 (99%) attained reproductive age (16 years). After excluding the 386 siblings with cancer, 25,827 siblings (58%) (12,454 brothers and 13,373 sisters) parented at least 1 child (Fig. 1).
Statistical analysis
Probability of parenthood among patients was compared to that of siblings. In parenthood analyses, patients with at least 1 child born already before diagnosis and up to 9 months after diagnosis were excluded (N = 1,860) (Fig. 1).
Separate analyses were performed for the first live-born child (at least 9 months after cancer diagnosis for patients) and the second live-born child. The follow-up started at the 16th birthday for siblings and for patients either at the 16th birthday or 9 months after the date of cancer diagnosis for those diagnosed with cancer after the age of 15 years and 3 months. The end of follow-up was defined as the date of birth of a child, the date of death, permanent emigration or the end of December 2006, whichever came first. In the analyses for the second live-born child, follow-up began at the time of the delivery of the first live-born child and ended at the delivery of the second live-born child.
Cox proportional hazards models with age as the time variable were used in assessing the effects of various variables.27 The relative risks obtained expressed the relative probabilities of parenthood adjusted for birth cohort. Statistical significance was obtained by comparing appropriate hierarchical models. The final results are presented by comparing each set of cancer patients defined by gender, age at diagnosis and site category (including all sites combined) with the total group of siblings of same gender. These models included the calendar time of birth (birth cohort) as a categorical covariate classified as follows: (i) before 1951, (ii) 1951–1959, (iii) 1960–1969, (iv) 1970–1979 and (v) 1980 or after. An additional analysis using the abovementioned model and variables was used to asses the effect of diagnostic era on the relative probability of parenthood. Diagnostic era was defined as follows: (i) 1953–1962, (ii) 1963–1972, (iii) 1973–1982, (iv) 1983–1992 and (v) 1993–2004.
Model-based, unconditional and conditional, cumulative probabilities of parenthood were also calculated for all subsites combined by gender and diagnostic age-groups.
All statistical analyses were performed with STATA software, release 9.2.
Ethical issues
The study protocol was approved by the ethical committee of South-West Finland Hospital District Review Board, and permits for registry linkage were obtained from the Finnish Ministry of Social Affairs and Health and the Population Register Center.
Results
Cancer survivors were divided into 3 diagnostic age-groups by age at diagnosis, pediatric (0–14 years at diagnosis), adolescent (15–19 years) and young adult (20–34 years) groups. Of all cancer survivors that fulfilled the eligibility criteria, 6,071 (24%) were pediatric patients, 2,654 (10%) adolescents and 17,059 (66%) young adults (Table I). The cancer site grouping used for pediatric and adolescent patients is based on the International Classification of Childhood Cancer.28 The carcinomas and other malignant epithelial neoplasm group was further divided into major subsites to account for different malignancy patterns among young adults.
TABLE I.
CHARACTERISTICS OF CANCER PATIENTS BY CANCER SITE AND DIAGNOSTIC AGE-GROUP
| Cancer site1 | Pediatric (0–14 yrs)2 |
Adolescent (15–19 yrs) |
Young adult (20–34 yrs) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males |
Females |
Males |
Females |
Males |
Females |
|||||||
| Patients | Proportion (%) | Patients | Proportion (%) | Patients | Proportion (%) | Patients | Proportion (%) | Patients | Proportion (%) | Patients | Proportion (%) | |
| Leukemia | 963 | 29.1 | 867 | 31.3 | 205 | 15.0 | 132 | 10.3 | 510 | 7.0 | 351 | 3.6 |
| Lymphomas | 397 | 12.0 | 218 | 7.9 | 327 | 23.9 | 273 | 21.2 | 1,465 | 20.0 | 1,013 | 10.4 |
| Hodgkin lymphoma | 120 | 3.6 | 86 | 3.1 | 212 | 15.5 | 211 | 16.4 | 877 | 12.0 | 645 | 6.6 |
| Non-Hodgkin lymphoma | 277 | 8.4 | 132 | 4.8 | 115 | 8.4 | 62 | 4.8 | 588 | 8.0 | 368 | 3.8 |
| Central nervous system | 829 | 25.1 | 691 | 25.0 | 243 | 17.8 | 188 | 14.6 | 933 | 12.8 | 927 | 9.5 |
| Sympathetic nervous system | 206 | 6.2 | 166 | 6.0 | 4 | 0.3 | 10 | 0.8 | 25 | 0.3 | 24 | 0.2 |
| Retinoblastoma | 106 | 3.2 | 81 | 2.9 | 0 | 0.0 | 0 | 0.0 | 1 | 0.0 | 0 | 0.0 |
| Renal tumors | 202 | 6.1 | 172 | 6.2 | 6 | 0.4 | 8 | 0.6 | 94 | 1.3 | 100 | 1.0 |
| Hepatic tumors | 35 | 1.1 | 18 | 0.7 | 11 | 0.8 | 10 | 0.8 | 40 | 0.5 | 51 | 0.5 |
| Malignant bone tumors | 124 | 3.8 | 99 | 3.6 | 136 | 9.9 | 87 | 6.8 | 231 | 3.2 | 138 | 1.4 |
| Soft-tissue sarcomas | 241 | 7.3 | 175 | 6.3 | 110 | 8.0 | 116 | 9.0 | 614 | 8.4 | 618 | 6.3 |
| Germ cell, trophoblastic and other gonadal neoplasms | 74 | 2.2 | 82 | 3.0 | 140 | 10.2 | 100 | 7.8 | 1,122 | 15.3 | 671 | 6.9 |
| Testes | 57 | 1.7 | 133 | 9.7 | 1,083 | 14.8 | ||||||
| Ovary | 46 | 1.7 | 89 | 6.9 | 578 | 5.9 | ||||||
| Other germ cell | 17 | 0.5 | 36 | 1.3 | 7 | 0.5 | 11 | 0.9 | 39 | 0.5 | 93 | 1.0 |
| Carcinomas and other malignant epithelial neoplasms3 | 100 | 3.0 | 167 | 6.0 | 164 | 12.0 | 346 | 26.9 | 2,170 | 29.7 | 5,722 | 58.7 |
| Thyroid | 15 | 0.5 | 39 | 1.4 | 27 | 2.0 | 141 | 11.0 | 296 | 4.0 | 1,206 | 12.4 |
| Cervix | 0 | 2 | 0.2 | 705 | 7.2 | |||||||
| Uterus | 0 | 1 | 0.1 | 69 | 0.7 | |||||||
| Breast | 0 | 1 | 1 | 0.1 | 6 | 0.5 | 5 | 0.1 | 1,697 | 17.4 | ||
| Stomach | 0 | 0 | 3 | 0.2 | 1 | 0.1 | 177 | 2.4 | 186 | 1.9 | ||
| Colon | 27 | 0.8 | 61 | 2.2 | 43 | 3.1 | 87 | 6.8 | 309 | 4.2 | 391 | 4.0 |
| Urinary bladder | 0 | 0.0 | 1 | 0.0 | 1 | 0.1 | 2 | 0.2 | 126 | 1.7 | 36 | 0.4 |
| Melanoma | 28 | 0.8 | 19 | 0.7 | 53 | 3.9 | 64 | 5.0 | 570 | 7.8 | 850 | 8.7 |
| Other carcinomas | 30 | 0.9 | 46 | 1.7 | 36 | 2.6 | 42 | 3.3 | 687 | 9.4 | 582 | 6.0 |
| Other and unspecified malignant neoplasms | 28 | 0.8 | 30 | 1.1 | 23 | 1.7 | 15 | 1.2 | 106 | 1.4 | 133 | 1.4 |
| Total | 3,305 | 100.0 | 2,766 | 100.0 | 1,369 | 100.0 | 1,285 | 100.0 | 7,311 | 100.0 | 9,748 | 100.0 |
The cancer site grouping used for pediatric and adolescent patients is based on the International Classification of Childhood Cancer.26
Diagnostic age-groups were defined according to age at diagnosis.
The carcinomas and other malignant neoplasms group is further divided into major subsites to account for the different malignancy patterns among young adults.
For the entire patient cohort, there was a transition from a preponderance of hematopoietic system neoplasms in children to carcinomas and other malignant epithelial neoplasms among young adults. The distribution of main diagnostic sites was similar among males and females in the pediatric and adolescent diagnostic age groups. In the group of young adult males, the most common malignancies were carcinomas and other malignant epithelial neoplasms (30%), testicular cancer (15%), CNS tumors (13%) and Hodgkin lymphoma (HL) (12%). Among young adult females the most common malignancies were breast cancer (17%), thyroid cancer (12%) and CNS tumors (10%) (Table I).
The relative probability of having their first or second child among cancer survivors was estimated only for those survivors who had neither parented children before diagnosis nor up to 9 months after diagnosis. The relative probability of having a first child was significantly reduced for all cancer sites and diagnostic age-groups combined; RR 0.57, 95% CI 0.54–0.60 for males and RR 0.46, 95% CI 0.44–0.48 for females. The relative probability of parenting a second child was, however, only slightly reduced compared to that of siblings; RR 0.95, 95% CI 0.89–1.01 among males and RR 0.91, 95% CI 0.86–0.97 among females.
Among males, the relative probability of parenthood was significantly higher among the adolescent cancer survivors compared to the adult and childhood age groups (Table II). In females, the relative probability of parenthood was similarly reduced among pediatric and adolescent cancer survivors and significantly lowers among adult cancer survivors (Table II).
TABLE II.
PARENTHOOD FOLLOWING CANCER DIAGNOSIS IN MALES AND FEMALES
| Cancer site | Pediatric (0–14 yrs) |
Adolescent (15–19 yrs) |
Young adult (20–34 yrs) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | N12 | RR1 | 95% CI | N23 | RR2 | 95%CI | N | N1 | RR1 | 95%CI | N2 | RR2 | 95%CI | N | N1 | RR1 | 95%CI | N2 | RR2 | 95%CI | |
| Males | |||||||||||||||||||||
| Leukemia | 300 | 43 | 0.48 | 0.35–0.64 | 26 | 1.32 | 0.86–2.01 | 197 | 21 | 0.60 | 0.39–0.94 | 11 | 0.72 | 0.40–1.29 | 486 | 23 | 0.29 | 0.19–0.44 | 11 | 0.62 | 0.34–1.11 |
| Lymphomas | 219 | 58 | 0.57 | 0.44–0.73 | 39 | 1.03 | 0.75–1.42 | 319 | 101 | 0.87 | 0.71–1.06 | 63 | 1.06 | 0.83–1.36 | 1,309 | 238 | 0.47 | 0.41–0.54 | 133 | 0.95 | 0.80–1.13 |
| Hodgkin lymphoma | 101 | 20 | 0.41 | 0.27–0.62 | 8 | 1.43 | 0.71–2.86 | 223 | 68 | 0.77 | 0.61–0.98 | 43 | 0.98 | 0.73–1.33 | 765 | 149 | 0.45 | 0.38–0.53 | 88 | 0.97 | 0.78–1.21 |
| Non-Hodgkin lymphoma | 118 | 38 | 0.72 | 0.53–0.99 | 31 | 0.96 | 0.67–1.37 | 96 | 33 | 1.18 | 0.84–1.67 | 20 | 1.27 | 0.82–1.98 | 544 | 89 | 0.51 | 0.41–0.63 | 45 | 0.92 | 0.69–1.23 |
| Central nervous system | 387 | 59 | 0.28 | 0.22–0.37 | 38 | 1.17 | 0.85–1.62 | 234 | 50 | 0.42 | 0.32–0.56 | 35 | 1.05 | 0.75–1.47 | 832 | 133 | 0.52 | 0.43–0.61 | 70 | 0.99 | 0.78–1.26 |
| Sympathetic nervous system | 59 | 12 | 0.48 | 0.27–0.84 | 7 | 0.78 | 0.35–1.73 | 4 | 0 | – | – | 0 | – | – | 25 | 3 | 0.76 | 0.24–2.35 | 2 | 0.59 | 0.15–2.36 |
| Retinoblastoma | 69 | 23 | 0.58 | 0.38–0.87 | 14 | 0.95 | 0.56–1.61 | 0 | 0 | – | – | 0 | – | – | 1 | 0 | – | – | 0 | – | – |
| Renal tumors | 102 | 30 | 0.65 | 0.46–0.93 | 22 | 1.24 | 0.80–1.90 | 6 | 2 | 2.51 | 0.63–10.04 | 2 | 0.83 | 0.21–3.32 | 80 | 14 | 0.72 | 0.42–1.21 | 9 | 1.08 | 0.56–2.08 |
| Hepatic tumors | 12 | 1 | 0.27 | 0.04–1.93 | 1 | 1.24 | 0.17–8.79 | 9 | 0 | – | – | 0 | 1.04 | 0.98–1.10 | 40 | 3 | 1.61 | 0.52–5.01 | 3 | 2.32 | 0.58–9.27 |
| Malignant bone tumors | 72 | 30 | 0.81 | 0.57–1.16 | 24 | 0.97 | 0.65–1.47 | 136 | 26 | 0.84 | 0.58–1.23 | 17 | 1.22 | 0.76–1.96 | 202 | 51 | 0.70 | 0.53–0.92 | 36 | 0.97 | 0.70–1.35 |
| Soft tissue sarcomas | 138 | 46 | 0.71 | 0.53–0.95 | 33 | 0.82 | 0.58–1.16 | 108 | 32 | 0.80 | 0.57–1.12 | 26 | 0.72 | 0.49–1.06 | 538 | 120 | 0.66 | 0.55–0.79 | 71 | 0.82 | 0.65–1.03 |
| Germ cell, trophoblastic and other gonadal neoplasms | 30 | 6 | 0.44 | 0.20–0.98 | 5 | 1.07 | 0.44–2.57 | 139 | 27 | 0.67 | 0.46–0.97 | 17 | 1.21 | 0.74–1.98 | 1,003 | 201 | 0.50 | 0.44–0.58 | 110 | 0.93 | 0.77–1.14 |
| Carcinomas and other malignant epithelial neoplasms | 74 | 30 | 0.69 | 0.49–0.99 | 19 | 1.16 | 0.74–1.82 | 163 | 61 | 0.86 | 0.67–1.11 | 37 | 0.89 | 0.64–1.25 | 1,859 | 361 | 0.66 | 0.60–0.74 | 240 | 0.92 | 0.81–1.05 |
| Breast | 0 | 0 | – | – | 0 | – | – | 1 | 1 | 2.10 | 0.30–14.90 | 1 | 2.41 | 0.34–17.15 | 5 | 0 | – | – | 0 | – | – |
| Thyroid | 15 | 5 | 0.75 | 0.31–1.81 | 3 | 1.42 | 0.46–4.40 | 27 | 16 | 1.04 | 0.64–1.71 | 10 | 1.29 | 0.70–2.41 | 243 | 78 | 0.71 | 0.57–0.88 | 55 | 0.96 | 0.74–1.25 |
| Other and unspecified malignant neoplasms | 14 | 8 | 0.88 | 0.44–1.75 | 5 | 0.68 | 0.26–1.82 | 23 | 7 | 0.57 | 0.27–1.21 | 5 | 1.15 | 0.48–2.76 | 96 | 14 | 0.66 | 0.39–1.11 | 10 | 1.02 | 0.55–1.90 |
| All sites | 1,476 | 346 | 0.51 | 0.46–0.57 | 233 | 1.03 | 0.90–1.18 | 1,338 | 327 | 0.70 | 0.63–0.79 | 213 | 0.97 | 0.84–1.11 | 6,471 | 1,161 | 0.56 | 0.53–0.60 | 695 | 0.92 | 0.85–0.99 |
| Females | |||||||||||||||||||||
| Leukemia | 323 | 103 | 0.74 | 0.61–0.89 | 58 | 0.89 | 0.68–1.15 | 119 | 15 | 0.53 | 0.32–0.88 | 7 | 1.12 | 0.53–2.35 | 348 | 12 | 0.14 | 0.08–0.25 | 6 | 0.5 | 0.21–1.20 |
| Lymphomas | 131 | 38 | 0.61 | 0.44–0.84 | 27 | 0.96 | 0.65–1.43 | 271 | 93 | 0.66 | 0.54–0.80 | 54 | 1.07 | 0.82–1.40 | 907 | 180 | 0.42 | 0.36–0.49 | 95 | 0.85 | 0.69–1.05 |
| Hodgkin lymphoma | 67 | 22 | 0.65 | 0.43–0.99 | 15 | 0.85 | 0.51–1.44 | 211 | 74 | 0.64 | 0.51–0.80 | 42 | 1.09 | 0.80–1.47 | 573 | 124 | 0.40 | 0.33–0.47 | 63 | 0.81 | 0.63–1.05 |
| Non-Hodgkin lymphoma | 64 | 16 | 0.56 | 0.34–0.92 | 12 | 1.16 | 0.64–2.09 | 60 | 19 | 0.76 | 0.48–1.19 | 12 | 1.02 | 0.58–1.80 | 334 | 56 | 0.47 | 0.36–0.61 | 32 | 0.94 | 0.66–1.34 |
| Central nervous system | 337 | 90 | 0.39 | 0.32–0.48 | 62 | 1.08 | 0.84–1.39 | 185 | 47 | 0.47 | 0.36–0.63 | 30 | 1.02 | 0.71–1.46 | 859 | 99 | 0.31 | 0.25–0.38 | 42 | 0.93 | 0.68–1.26 |
| Sympathetic nervous system | 52 | 25 | 0.85 | 0.58–1.26 | 19 | 0.92 | 0.59–1.44 | 10 | 0 | – | – | 0 | – | – | 18 | 3 | 0.26 | 0.09–0.82 | 1 | 0.26 | 0.04–1.88 |
| Retinoblastoma | 53 | 26 | 0.69 | 0.47–1.01 | 20 | 0.98 | 0.63–1.52 | 0 | 0 | – | – | 0 | – | – | 0 | 0 | – | – | 0 | – | – |
| Renal tumors | 90 | 36 | 0.69 | 0.50–0.96 | 23 | 0.75 | 0.49–1.14 | 8 | 2 | 1.04 | 0.34–3.23 | 1 | 2.98 | 0.42–21.1 | 87 | 10 | 0.26 | 0.13–0.49 | 3 | 0.85 | 0.21–3.40 |
| Hepatic tumors | 6 | 0 | – | – | 0 | – | – | 9 | 1 | 1.24 | 0.17–8.79 | 0 | – | – | 49 | 1 | 0.23 | 0.03–1.63 | 0 | – | – |
| Malignant bone tumors | 59 | 27 | 0.64 | 0.44–0.93 | 21 | 1.17 | 0.76–1.82 | 86 | 20 | 0.49 | 0.32–0.76 | 12 | 0.75 | 0.42–1.32 | 124 | 18 | 0.28 | 0.18–0.44 | 13 | 1.08 | 0.62–1.85 |
| Soft tissue sarcomas | 94 | 41 | 0.88 | 0.65–1.19 | 32 | 0.95 | 0.67–1.34 | 115 | 45 | 0.89 | 0.66–1.18 | 32 | 0.87 | 0.62–1.23 | 526 | 103 | 0.43 | 0.36–0.53 | 61 | 0.89 | 0.69–1.14 |
| Germ cell, trophoblastic and other gonadal neoplasms | 42 | 9 | 0.27 | 0.14–0.52 | 5 | 0.95 | 0.39–2.28 | 100 | 32 | 0.44 | 0.31–0.63 | 24 | 0.99 | 0.66–1.47 | 623 | 63 | 0.21 | 0.16–0.27 | 37 | 0.97 | 0.70–1.34 |
| Carcinomas and other malignant epithelial neoplasms | 133 | 71 | 0.86 | 0.68–1.08 | 49 | 1.21 | 0.91–1.61 | 338 | 166 | 0.75 | 0.65–0.88 | 117 | 1.04 | 0.87–1.26 | 5,075 | 665 | 0.36 | 0.34–0.40 | 380 | 0.82 | 0.74–0.91 |
| Breast | 1 | 1 | 3.73 | 0.52–26.49 | 1 | 1.72 | 0.24–12.25 | 6 | 5 | 1.27 | 0.53–3.04 | 1 | 0.84 | 0.12–6.00 | 1,591 | 83 | 0.17 | 0.14–0.21 | 39 | 0.94 | 0.68–1.31 |
| Thyroid | 36 | 16 | 0.78 | 0.48–1.28 | 9 | 1.15 | 0.60–2.22 | 136 | 72 | 0.67 | 0.53–0.85 | 48 | 1.04 | 0.78–1.39 | 974 | 229 | 0.46 | 0.40–0.53 | 137 | 0.79 | 0.66–0.94 |
| Other and unspecified malignant neoplasms | 14 | 7 | 1.27 | 0.61–2.67 | 3 | 1.14 | 0.37–3.53 | 13 | 4 | 0.76 | 0.32–1.82 | 4 | 0.88 | 0.33–2.34 | 116 | 19 | 0.40 | 0.25–0.64 | 11 | 0.92 | 0.49–1.71 |
| All sites | 1,334 | 473 | 0.62 | 0.56–0.68 | 319 | 0.99 | 0.88–1.11 | 1,254 | 425 | 0.64 | 0.58–0.70 | 281 | 1.00 | 0.89–1.13 | 8,732 | 1,173 | 0.37 | 0.34–0.39 | 649 | 0.84 | 0.77–0.91 |
Relative risks (RR) with 95% confidence intervals (CI), estimated using a Cox proportional hazards model with age as the time variable and adjusting for birth cohort for parenting 1st child (RR1) and 2nd child (RR2) at least 9 months after diagnosis.
N = total number of survivors in analysis.
N = number of survivors with 1 child after diagnosis.
N2 = number of survivors with 2 children after diagnosis.
In the pediatric diagnostic age-group, among the significant values, the lowest relative probabilities of parenthood were observed for males in the CNS tumor and HL groups and for females in the germ-cell malignancy and CNS tumor groups. The least reduced relative probabilities were in the non-Hodgkin lymphoma (NHL) and soft-tissue sarcoma groups in males, and in the renal tumor and leukemia subgroups among females.
Among adolescent survivors, the overall relative probability of parenthood did not differ significantly between males and females. In both genders, a significantly reduced relative probability of parenthood was seen in the leukemia, HL, CNS and germ-cell malignancy group as well as in the malignant bone tumor and carcinoma groups, in females.
In the young adult age-group, the relative probability of parenthood following cancer treatment was significantly lower in females compared to males. This difference was most pronounced in the malignant bone tumor, carcinomas and germ-cell malignancy groups. Among adult male survivors, the relative probability of parenthood was lowest among leukemia and HL patients. In female survivors, reductions were most pronounced among leukemia, germ-cell malignancy and breast cancer survivors. Relative probabilities of parenthood were highest among male thyroid cancer, malignant bone tumor and soft tissue sarcoma survivors. Among females the probabilities were highest among thyroid cancer, NHL and soft tissue sarcoma survivors.
There was a significant trend of an increasing relative probability of parenthood over calendar periods of diagnosis among young adult male and female survivors (Table III). In the pediatric and adolescent groups, no clear trends could be observed over time.
TABLE III.
PARENTHOOD FOLLOWING CANCER TREATMENT IN DIFFERENT DIAGNOSTIC ERAS
| Diagnostic era | Males |
Females |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–14 yrs1 |
15–19 yrs2 |
20–34 yrs3 |
0–14 yrs |
15–19 yrs |
20–34 yrs |
|||||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| 1953–1962 | 0.61 | 0.49–0.76 | 0.82 | 0.59–1.14 | 0.62 | 0.50–0.78 | 0.70 | 0.56–0.87 | 0.70 | 0.52–0.94 | 0.22 | 0.16–0.28 |
| 1963–1972 | 0.48 | 0.38–0.59 | 0.84 | 0.66–1.08 | 0.43 | 0.36–0.52 | 0.58 | 0.48–0.70 | 0.51 | 0.40–0.64 | 0.20 | 0.17–0.25 |
| 1973–1982 | 0.47 | 0.38–0.57 | 0.66 | 0.53–0.82 | 0.48 | 0.42–0.55 | 0.58 | 0.49–0.69 | 0.59 | 0.49–0.71 | 0.28 | 0.24–0.32 |
| 1983–1992 | 0.50 | 0.40–0.64 | 0.75 | 0.61–0.91 | 0.56 | 0.50–0.63 | 0.65 | 0.54–0.77 | 0.74 | 0.61–0.88 | 0.42 | 0.38–0.47 |
| 1993–2004 | 0.57 | 0.31–1.04 | 0.52 | 0.39–0.70 | 0.70 | 0.63–0.78 | 0.67 | 0.43–1.05 | 0.69 | 0.56–0.86 | 0.51 | 0.46–0.57 |
Relative risks (RR) with 95% confidence intervals (CI), estimated using a Cox proportional hazards model for parenting 1st child expressed FOR all sites combined, by gender and age at diagnosis.
Pediatric.
Adolescent.
Young adult.
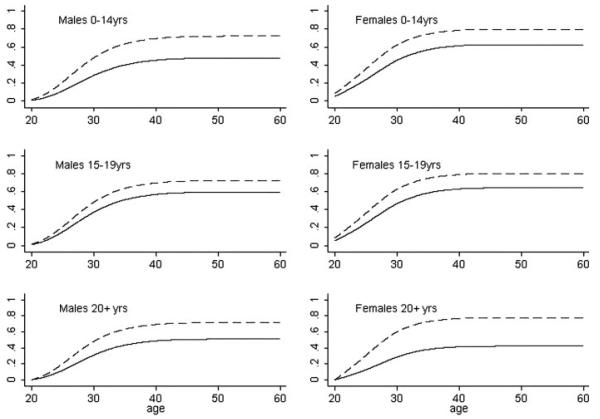
The cumulative probability of having a first child was clearly lower in cancer survivors than in siblings in all diagnostic age groups (Fig. 2). For example, the cumulative probability of parenthood by the age of 35 years was clearly lower among patients treated in early adulthood than among siblings, in males 43% vs. 63% and in females 38% vs. 73%.
Figure 2.
Model-based cumulative probabilities of first postdiagnosis parenthood. For the young adult diagnostic age group, the curves represent conditional cumulative probability of parenthood given the patient had no children at age 20. The dashed line represents siblings and the solid line represents cancer patients.
The relative probability of parenting a second child after diagnosis was also significantly reduced in the young adult diagnostic age-groups. This reduction was more pronounced among females than among males: RR 0.84, 95% CI 0.77–0.91 and RR 0.92, 95% CI 0.85–0.99, respectively. In females, the reduction was visible in the carcinoma subsite, mainly thyroid cancer, whereas in males no significant reduction by subsites could be seen. Relative probabilities in pediatric and adolescent survivors were close to 1.
Discussion
This report describes the Finnish component of the GCCT (Genetic Consequences of Therapies for Cancer), an international multi-institutional collaborative venture involving Finland (The Finnish Cancer Registry), Denmark (the Danish Cancer Society), The United Kingdom (Westlakes Research Institute and the University of Central Lancashire) and the United States (Vanderbilt University and MD Anderson Cancer Center). The distribution of cancer diagnoses in the entire patient cohort (0–34 years) followed the common incidence patterns seen in other European and Nordic countries.3,29 The probability of posttreatment parenthood was significantly reduced by ∼50% in cancer patients compared with siblings. However, the probability of having a second child after diagnosis, having parented 1 child following diagnosis, was much less reduced.
Two previous population-based studies examined parenthood following cancer. One reported a 25% reduction in parenthood among patients diagnosed with cancer between ages 17 and 44,18 while the other reported a 10-year cumulative postdiagnosis parenthood rate of only 14% among patients diagnosed at the age of 15–45.19 Both studies used the general population as a comparison group. Different methodological approaches limit direct comparison of these rates to our result, in which the probability of parenthood among cancer patients was reduced by about 50% of that among their siblings. Unlike the other 2 studies, our cohort also included pediatric cancer patients and used a sibling cohort as the comparison group. Moreover, 1 study18 reported a lowered probability of having a second child in 17–44 years old, while in our study this was also observed among survivors of young adulthood malignancies. Table IV summarizes recent studies on post-diagnosis parenthood.
TABLE IV.
SUMMARY OF RECENT STUDIES ON PARENTHOOD FOLLOWING CANCER
| Authors | Year | Patients studied | Age of patients |
Comparison group | N | Data collection | Statistical methods | Results | Limitations |
|---|---|---|---|---|---|---|---|---|---|
| Magelssen et al.19 | 2008 | All malignancies | 15–45 | Population | 13,817 | Registry linkage | Cumulative probability using Kaplan Meier with log rank test | Parenthood probability at 35yrs: 63% males, 66% females | Only adolescent and adult patients comparison group |
| Syse et al.18 | 2007 | All malignancies | 17–44 | Population | 13,452 | Registry linkage | Birth rates using discrete time hazard regression models | Birth rates reduced by 24% males ,27% females | Only adolescent and adult patients comparison group |
| Kiserud et al.16 | 2007 | Hodgkin lymphoma | 0–65 | Cancer patients | 453 | Questionnaire and medical records | Probabilities using Kaplan-Meier with log rank test | Parenthood probability: 63% males, 75% females | Limited patient group recall + selection bias comparison group |
| Brydøy et al.15 | 2005 | Testicular cancer | 20–34 | Cancer patients | 1,433 | Follow-up survey | Parenthood rate using Cox proportional hazards analyses | 15 yr paternity rate: 71% | Limited patient group recall + selection bias comparison group |
For males, the relative probability of parenthood was highest among patients treated in adolescence and similarly reduced for patients treated in childhood and adulthood. The parenthood advantage among adolescent cancer patients compared to pediatric patients can be explained by the likelihood of sperm banking increasing after puberty. Despite this equal possibility in adolescent and adult cancer patients, adolescents were more likely to parent children, which could be explained by malignancy type and treatment regimens, the distribution of primary diagnoses patterning more closely that of pediatric cancer patients.
Among females the parenthood disadvantage tilts clearly toward adulthood cancer survivors, being similar among pediatric and adolescent survivors. This disadvantage was most pronounced in the leukemia and breast cancer groups. A large proportion of young adult leukemia patients have undergone stem cell transplantation during the last decades.30 The aggressive treatments related to stem cell transplantation, such as total body irradiation, could explain the lowered parenthood rates. Psychological factors may also account for part of the difference. As following hormone dependent malignancies, here namely breast cancer, women are discouraged from further pregnancies due to the estrogen load and the related risk of recurrence, which could explain the low parenthood rates observed among breast cancer survivors in our study.
Within the male pediatric survivors group, the relative probability of parenthood was low among survivors of HL and germ cell neoplasm groups. The fertility disadvantage among males may be due to the fact that premature male gonadal tissue and spermato-genesis are known to be sensitive to the deleterious effects of radiotherapy and some chemotherapeutic agents.4-7 The probability of parenthood was also greatly reduced among males with CNS tumors and lowest in the pediatric patient group compared to the adolescent and adult groups. Alkylator-sensitivity of testicular tissue may explain a large part of the greater male disadvantage in this group. Also psychosocial factors such as finding a partner may be partly responsible in patients receiving treatment to the CNS. A recent study by Frobisher showed the lowest proportions of ever married to be among male CNS neoplasm survivors.31 The more pronounced parenthood disadvantage in the pediatric group may also be explained by a greater risk of social marginalization as a result of cognitive effects of treatment or due to fragility of social development at this phase.
In adults, the parenthood disadvantage is clearly more pronounced in females. This, conceivably, could be partly explained by differences in mothering and fathering a child from a biologic point of view. Mechanical limitations to taking a pregnancy to full-term result from radiotherapy and high-dose chemotherapy induced abdominopelvic tissue fibrosis and reduced vascular supply to reproductive organs.32-34 Premature menopause has been reported in females following cancer treatment,35 whereas an equivalent time-window of fertility does not apply to males. As female gametes are present from birth, effects on the female reproductive function are more likely to be permanent.
Among young adult survivors, a significant trend of increasing relative parenthood probability with diagnostic era was visible. This may reflect the expanding use of chemotherapy among adults, which has replaced radiotherapy as the main treatment option during the last 2 decades. Thus, the possibility of having children after cancer treatment has increased as chemotherapy is more fertility preserving than radiotherapy treatment used to be. Among pediatric patients, however, the use of chemotherapy has been a common practice for a longer period already, and there was no clear improvement to be seen by diagnostic era. Among adults the development of IVF technologies in the last 2 decades may also explain part of the increase.
Probability of having a second child was significantly reduced only among young adult survivors. Female gametes are present from birth and thus ovum harvesting is possible for pediatric and adolescent patients alike, however rarely in use at the moment. The lower relative probability of parenting both a first and second child after adulthood cancer might be explained partly by premature menopause which has been attributable largely to the deleterious effects of chemotherapy and abdominopelvic irradiation.35,36
Our study methodology offers reliable nation-wide register-based data on the long-term implications of cancer therapy. Furthermore, as siblings were used as the comparison group, confounding due to possible familial factors, e.g. certain hereditary infertility states and cultural and geographical differences were, at least partially, considered. By adjusting for birth cohort, possible trends over time were also, in part, accounted for. In previous population studies on postdiagnosis parenthood, the general population has been used as a comparison group which is not necessarily optimal in controlling for important potential confounders.18,19 Other studies that rely on questionnaire-based self-reporting could be influenced by both selection and recall bias. By excluding those who had parented a child prior to cancer, our goal was to minimize the effect of psychological factors, namely motivation to conceive, on the possibility of parenting a child after diagnosis. In this way, we attempted to make parenthood as an accurate measure of fertility as possible. Also this allowed a more just comparison of patients to siblings as by the abovementioned exclusion, the definition of 1st child was made identical in both groups.
A recent publication from the Childhood Cancer Survivor Study group reported long-term effects of childhood cancer patients treated between 1970 and 1986.37 As our diagnostic era extends to 2004, it also allows us to address the effects of more recent cancer treatments. It is evident that there is a need for further research to provide adequate and tailored family planning counseling for this growing population of cancer survivors maintaining their fertile potential.
As it is known that cancer survivors face fear and anxiety relating to fertility after diagnosis and health of offspring,23,24 we wished to address the fertility patterns of patients following treatment for early onset cancer. Although parenthood after early onset cancer diagnosis was possible among nearly half the patients, it was significantly reduced in both genders compared with siblings. However, the probability of having a second child was only slightly reduced and only among young adult survivors.
Acknowledgments
Grant sponsors: National Institutes of Health, National Cancer Institute; Grant number: CA 104666 (with Vanderbilt University); Grant sponsor: Cancer Society of Finland.
References
- 1.Moller TR, Garwicz S, Perfekt R, Barlow L, Winther JF, Glattre E, Olafsdottir G, Olsen JH, Ritvanen A, Sankila R. Late mortality among five-year survivors of cancer in childhood and adolescence. Acta Oncol (Stockholm, Sweden) 2004;43:711–18. doi: 10.1080/02841860410002860. [DOI] [PubMed] [Google Scholar]
- 2.Pritchard-Jones K, Kaatsch P, Steliarova-Foucher E, Stiller CA, Coebergh JW. Cancer in children and adolescents in Europe: developments over 20 years and future challenges. Eur J Cancer. 2006;42:2183–90. doi: 10.1016/j.ejca.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Stiller CA, Desandes E, Danon SE, Izarzugaza I, Ratiu A, Vassileva-Valerianova Z, Steliarova-Foucher E. Cancer incidence and survival in European adolescents (1978-1997). Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2006–18. doi: 10.1016/j.ejca.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Howell SJ, Shalet SM. Testicular function following chemotherapy. Hum Reprod Update. 2001;7:363–9. doi: 10.1093/humupd/7.4.363. [DOI] [PubMed] [Google Scholar]
- 5.Tomao F, Miele E, Spinelli GP, Tomao S. Anticancer treatment and fertility effects. Literature review. J Exp Clin Cancer Res. 2006;25:475–81. [PubMed] [Google Scholar]
- 6.Nieman CL, Kazer R, Brannigan RE, Zoloth LS, Chase-Lansdale PL, Kinahan K, Dilley KJ, Roberts D, Shea LD, Woodruff TK. Cancer survivors and infertility: a review of a new problem and novel answers. J Support Oncol. 2006;4:171–8. [PubMed] [Google Scholar]
- 7.Nicholson HS, Byrne J. Fertility and pregnancy after treatment for cancer during childhood or adolescence. Cancer. 1993;71:3392–9. doi: 10.1002/1097-0142(19930515)71:10+<3392::aid-cncr2820711743>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 8.Nagarajan R, Robison LL. Pregnancy outcomes in survivors of childhood cancer. J Natl Cancer Inst Monogr. 2005;34:72–6. doi: 10.1093/jncimonographs/lgi020. [DOI] [PubMed] [Google Scholar]
- 9.Signorello LB, Cohen SS, Bosetti C, Stovall M, Kasper CE, Weathers RE, Whitton JA, Green DM, Donaldson SS, Mertens AC, Robison LL, Boice JD., Jr Female survivors of childhood cancer: preterm birth and low birth weight among their children. J Natl Cancer Inst. 2006;98:1453–61. doi: 10.1093/jnci/djj394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulvihill JJ, McKeen EA, Rosner F, Zarrabi MH. Pregnancy outcome in cancer patients. Experience in a large cooperative group. Cancer. 1987;60:1143–50. doi: 10.1002/1097-0142(19870901)60:5<1143::aid-cncr2820600537>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 11.Byrne J, Rasmussen SA, Steinhorn SC, Connelly RR, Myers MH, Lynch CF, Flannery J, Austin DF, Holmes FF, Holmes GE, Strong LC, Mulvihill JJ. Genetic disease in offspring of long-term survivors of childhood and adolescent cancer. Am J Hum Genet. 1998;62:45–52. doi: 10.1086/301677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sankila R, Olsen JH, Anderson H, Garwicz S, Glattre E, Hertz H, Langmark F, Lanning M, Moller T, Tulinius H. Risk of cancer among offspring of childhood-cancer survivors. Association of the Nordic Cancer Registries and the Nordic Society of Paediatric Haematology and Oncology. N Engl J Med. 1998;338:1339–44. doi: 10.1056/NEJM199805073381902. [DOI] [PubMed] [Google Scholar]
- 13.Green DM, Fine WE, Li FP. Offspring of patients treated for unilateral Wilms' tumor in childhood. Cancer. 1982;49:2285–8. doi: 10.1002/1097-0142(19820601)49:11<2285::aid-cncr2820491114>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 14.Li FP, Gimbrere K, Gelber RD, Sallan SE, Flamant F, Green DM, Heyn RM, Meadows AT. Outcome of pregnancy in survivors of Wilms' tumor. JAMA. 1987;257:216–19. [PubMed] [Google Scholar]
- 15.Brydoy M, Fossa SD, Klepp O, Bremnes RM, Wist EA, Wentzel-Larsen T, Dahl O. Paternity following treatment for testicular cancer. J Natl Cancer Inst. 2005;97:1580–8. doi: 10.1093/jnci/dji339. [DOI] [PubMed] [Google Scholar]
- 16.Kiserud CE, Fossa A, Holte H, Fossa SD. Post-treatment parenthood in Hodgkin's lymphoma survivors. Br J Cancer. 2007;96:1442–9. doi: 10.1038/sj.bjc.6603711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boice JD, Jr, Tawn EJ, Winther JF, Donaldson SS, Green DM, Mertens AC, Mulvihill JJ, Olsen JH, Robison LL, Stovall M. Genetic effects of radiotherapy for childhood cancer. Health Phys. 2003;85:65–80. doi: 10.1097/00004032-200307000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Syse A, Kravdal O, Tretli S. Parenthood after cancer—a population-based study. Psychooncology. 2007;16:920–7. doi: 10.1002/pon.1154. [DOI] [PubMed] [Google Scholar]
- 19.Magelssen H, Melve KK, Skjaerven R, Fossa SD. Parenthood probability and pregnancy outcome in patients with a cancer diagnosis during adolescence and young adulthood. Hum Reprod. 2008;23:178–86. doi: 10.1093/humrep/dem362. [DOI] [PubMed] [Google Scholar]
- 20.Verger P. Down syndrome and ionizing radiation. Health Phys. 1997;73:882–93. doi: 10.1097/00004032-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins MM, Smith RA, Curtice LJ. Childhood cancer survivors and their offspring studied through a postal survey of general practitioners: preliminary results. J R Coll Gen Pract. 1988;38:102–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Schover LR, Brey K, Lichtin A, Lipshultz LI, Jeha S. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol. 2002;20:1880–9. doi: 10.1200/JCO.2002.07.175. [DOI] [PubMed] [Google Scholar]
- 23.Langeveld NE, Stam H, Grootenhuis MA, Last BF. Quality of life in young adult survivors of childhood cancer. Support Care Cancer. 2002;10:579–600. doi: 10.1007/s00520-002-0388-6. [DOI] [PubMed] [Google Scholar]
- 24.Schover LR. Motivation for parenthood after cancer: a review. J Natl Cancer Inst Monogr. 2005;34:2–5. doi: 10.1093/jncimonographs/lgi010. [DOI] [PubMed] [Google Scholar]
- 25.Mulvihill JJ, Byrne J. Genetic counseling of the cancer survivor. Semin Oncol Nurs. 1989;5:29–35. doi: 10.1016/0749-2081(89)90020-x. [DOI] [PubMed] [Google Scholar]
- 26.Teppo L, Pukkala E, Lehtonen M. Data quality and quality control of a population-based cancer registry. Experience in Finland. Acta Oncol. 1994;33:365–9. doi: 10.3109/02841869409098430. [DOI] [PubMed] [Google Scholar]
- 27.Cox DR. Regression models and life-tables. J R Stat Soc Ser B (Methodological) 1972;34:187–220. [Google Scholar]
- 28.International Agency for Research on Cancer . International classification of childhood cancer 1996. International Agency for Research on Cancer; Lyon, France: 1996. [Google Scholar]
- 29.van der Horst M, Winther JF, Olsen JH. Cancer incidence in the age range 0-34 years: historical and actual status in Denmark. Int J Cancer. 2006;118:2816–26. doi: 10.1002/ijc.21566. [DOI] [PubMed] [Google Scholar]
- 30.Gratwohl A, Baldomero H, Frauendorfer K, Rocha V, Apperley J, Niederwieser D. The EBMT activity survey 2006 on hematopoietic stem cell transplantation: focus on the use of cord blood products. Bone Marrow Transplant. 2008;41:687–705. doi: 10.1038/sj.bmt.1705956. [DOI] [PubMed] [Google Scholar]
- 31.Frobisher C, Lancashire ER, Winter DL, Jenkinson HC, Hawkins MM. Long-term population-based marriage rates among adult survivors of childhood cancer in Britain. Int J Cancer. 2007;121:846–55. doi: 10.1002/ijc.22742. [DOI] [PubMed] [Google Scholar]
- 32.Green DM, Peabody EM, Nan B, Peterson S, Kalapurakal JA, Breslow NE. Pregnancy outcome after treatment for Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol. 2002;20:2506–13. doi: 10.1200/JCO.2002.07.159. [DOI] [PubMed] [Google Scholar]
- 33.Sanders JE, Hawley J, Levy W, Gooley T, Buckner CD, Deeg HJ, Doney K, Storb R, Sullivan K, Witherspoon R, Appelbaum FR. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood. 1996;87:3045–52. [PubMed] [Google Scholar]
- 34.Byrne J, Mulvihill JJ, Connelly RR, Austin DA, Holmes GE, Holmes FF, Latourette HB, Meigs JW, Strong LC, Myers MH. Reproductive problems and birth defects in survivors of Wilms' tumor and their relatives. Med Pediatric Oncol. 1988;16:233–40. doi: 10.1002/mpo.2950160403. [DOI] [PubMed] [Google Scholar]
- 35.Sklar CA, Mertens AC, Mitby P, Whitton J, Stovall M, Kasper C, Mulder J, Green D, Nicholson HS, Yasui Y, Robison LL. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98:890–6. doi: 10.1093/jnci/djj243. [DOI] [PubMed] [Google Scholar]
- 36.De Bruin ML, Huisbrink J, Hauptmann M, Kuenen MA, Ouwens GM, Van't Veer MB, Aleman BM, van Leeuwen FE. Treatment-related risk factors for premature menopause following Hodgkin lymphoma. Blood. 2008;111:101–8. doi: 10.1182/blood-2007-05-090225. [DOI] [PubMed] [Google Scholar]
- 37.Robison LL, Green DM, Hudson M, Meadows AT, Mertens AC, Packer RJ, Sklar CA, Strong LC, Yasui Y, Zeltzer LK. Long-term outcomes of adult survivors of childhood cancer. Cancer. 2005;104:2557–64. doi: 10.1002/cncr.21249. [DOI] [PubMed] [Google Scholar]