Abstract
Context
Diffusion tensor imaging (DTI) studies in adults with bipolar disorder (BD) indicate altered white matter (WM) in the orbitomedial prefrontal cortex (OMPFC), potentially underlying abnormal prefrontal corticolimbic connectivity and mood dysregulation in BD.
Objective
To use tract-based spatial statistics (TBSS) to examine WM skeleton (ie, the most compact whole-brain WM) in subjects with BD vs healthy control subjects.
Design
Cross-sectional, case-control, whole-brain DTI using TBSS.
Setting
University research institute.
Participants
Fifty-six individuals, 31 having a DSM-IV diagnosis of BD type I (mean age, 35.9 years [age range, 24-52 years]) and 25 controls (mean age, 29.5 years [age range, 19-52 years]).
Main Outcome Measures
Fractional anisotropy (FA) longitudinal and radial diffusivities in subjects with BD vs controls (covarying for age) and their relationships with clinical and demographic variables.
Results
Subjects with BD vs controls had significantly greater FA (t>3.0, P≤.05 corrected) in the left uncinate fasciculus (reduced radial diffusivity distally and increased longitudinal diffusivity centrally), left optic radiation (increased longitudinal diffusivity), and right anterothalamic radiation (no significant diffusivity change). Subjects with BD vs controls had significantly reduced FA (t>3.0, P≤.05 corrected) in the right uncinate fasciculus (greater radial diffusivity). Among subjects with BD, significant negative correlations (P<.01) were found between age and FA in bilateral uncinate fasciculi and in the right anterothalamic radiation, as well as between medication load and FA in the left optic radiation. Decreased FA (P<.01) was observed in the left optic radiation and in the right anterothalamic radiation among subjects with BD taking vs those not taking mood stabilizers, as well as in the left optic radiation among depressed vs remitted subjects with BD. Subjects having BD with vs without lifetime alcohol or other drug abuse had significantly decreased FA in the left uncinate fasciculus.
Conclusions
To our knowledge, this is the first study to use TBSS to examine WM in subjects with BD. Subjects with BD vs controls showed greater WM FA in the left OMPFC that diminished with age and with alcohol or other drug abuse, as well as reduced WM FA in the right OMPFC. Mood stabilizers and depressed episode reduced WM FA in left-sided sensory visual processing regions among subjects with BD. Abnormal right vs left asymmetry in FA in OMPFC WM among subjects with BD, likely reflecting increased proportions of left-sided longitudinally aligned and right-sided obliquely aligned myelinated fibers, may represent a biologic mechanism for mood dysregulation in BD.
Bipolar disorder (BD) remains 1 of the 10 most debilitating illnesses world-wide. 1 Neuroimaging investigations have aimed to identify functional and structural neural circuitry abnormalities that may reflect pathogenetic processes of the disorder,2 particularly neural abnormalities implicated in mood dysregulation, a key symptom of BD. Although few, these studies2-4 provide some evidence for functional abnormalities in neural systems that may be associated with the following key symptoms: (1) abnormally increased activity in a subcortical limbic-centered system underlying emotion processing and (2) abnormally decreased activity in a system that includes ventral and dorsal prefrontal cortices underlying cognitive control processes. These findings require replication, but they provide a basic framework to guide examination of brain structure in subjects with BD.
Examination of gray matter (GM) within and white matter (WM) of fibers in tracts that may mediate functional relationships between these neural systems is an important focus for neuroimaging studies of BD. Attention has concentrated on the orbitomedial prefrontal cortex (OMPFC) because of its direct connections with subcortical limbic regions.5 For example, structural neuroimaging studies6-14 have demonstrated GM volume and density reductions in the OMPFC in adults with BD vs age-matched healthy control subjects, although there are discrepant findings.15,16 Microstructural abnormalities in the OMPFC in postmortem investigations of BD17 have also been reported, as well as WM volume and density abnormalities in the OMPFC18 and the corpus callosum19 in subjects with BD relative to controls. Because age-related changes in fractional anisotropy (FA) occur in the prefrontal cortex,20-22 it is important to consider age as a factor in studies of WM among subjects with BD and controls.
Diffusion tensor imaging (DTI) is a noninvasive method that detects subtle changes in tissue microstructural organization. It uses FA as an index of the ratio of diffusional anisotropy in longitudinal vs oblique directions of WM tracts. Diffusion tensor imaging has an advantage over structural neuroimaging measures of WM volume or density because it examines specific WM tracts and can be used to study connectivity between neural regions of interest in BD. However, the few DTI studies in BD have used a region-of-interest approach that limits examination to WM tracts in specific regions of the prefrontal cortex. These studies demonstrated decreased FA in dorsal prefrontal WM tracts in adolescents with BD23 and in a few subjects with BD24 relative to age-matched controls. Other studies using DTI and traditional diffusion-weighted imaging, have reported increased diffusivity in WM in bilateral OMPFC tracts25 and in bilateral dorsal prefrontal tracts in subjects with BD.26 However, one study27 reported greater rather than reduced FA among subjects with BD relative to controls in WM in the right anterofrontal cortex, and another study28 reported significantly greater FA in the midline of the genu of the corpus callosum among subjects with BD relative to controls. A third study29 reported significantly more reconstructed fibers in the left uncinate fasciculus (connecting the OMPFC, amygdala, and hippocampus) among subjects with BD relative to controls.
A problem with DTI is the interpretation of changes, particularly increases in FA in individuals with pathologic conditions (eg, subjects with BD) vs controls. Greater FA may reflect greater myelination of WM fibers, greater number of myelinated fibers, or greater longitudinal vs oblique directional alignment of myelinated fibers in WM tracts. Alternatively, changes in FA may result from excessive partial volume averaging from differently oriented fibers.30-32 Reduced FA has been associated with local edema, cerebrospinal fluid,33 compromised myelin structure, changes in axonal morphologic structure, and altered interaxonal spacing of fiber bundles.30,34-36 Therefore, eigenvalues representing measures of longitudinal and radial diffusivities have been derived and validated37,38 as more specific measures of diffusivity in the principal longitudinal diffusion direction and transverse direction, respectively. These measures can help interpret FA changes in WM tracts in pathologic groups by providing information regarding likely alterations in the proportion of longitudinally vs obliquely aligned myelinated fibers. Increased longitudinal diffusivity suggests greater numbers of longitudinally aligned fibers, and decreased radial diffusivity suggests reduced numbers of obliquely aligned fibers. Another problem with DTI is that analysis is compromised by the use of standard registration algorithms such that there is no satisfactory solution to the question of how to align FA images from multiple subjects in voxelwise analyses.39 A recent advance is the development of tract-based spatial statistics (TBSS), an automated observer-independent method of aligning FA images from multiple subjects to allow groupwise comparisons of DTI data.39,40 Tract-based spatial statistics TBSS also focus on the WM skeleton (ie, the most compact whole-brain WM). To our knowledge, this technique has been used to study preterm infants and adolescent development,41,42 schizophrenia,43,44 and multiple sclerosis45 but not subjects with BD.
The goal of this study was to use TBSS (by means of FA and longitudinal and radial diffusivities) to examine alterations in alignment of myelinated fibers in WM tracts in the prefrontal cortex, and particularly the OMPFC, among subjects with BD. Existing data25 suggest greater apparent diffusion coefficient in the orbitoprefrontal cortex and reduced FA in WM in the dorsal prefrontal cortices among subjects with BD vs controls. Therefore, we hypothesized that subjects with BD relative to controls would show significantly greater FA in WM in the OMPFC but significantly reduced FA in dorsal prefrontal cortical WM. We then aimed to examine between-group differences in longitudinal and radial diffusivities in regions of altered FA among subjects with BD vs controls to aid interpretation of these FA changes in WM among subjects with BD. The recently demonstrated increased number of longitudinally aligned reconstructed fibers in the left uncinate fasciculus among subjects with BD29 allowed us to hypothesize that greater FA in the OMPFC among subjects with BD would be associated with greater longitudinal diffusivity or reduced radial diffusivity, suggestive of greater longitudinal vs oblique alignment of fibers in OMPFC WM. Together, these alterations in FA and radial and longitudinal diffusivities in the OMPFC may underlie mood dysregulation among subjects with BD. In exploratory analyses, we examined relationships between significant changes in FA and demographic and clinical variables among subjects with BD.
METHODS
PARTICIPANTS
The study was approved by the University of Pittsburgh Institutional Review Board. Thirty-one subjects with BD (mean [SD] age, 35.9 [8.9] years [age range, 24-52 years); male to female ratio, 11:20), all with BD type I diagnosed according to the criteria of the DSM-IV46 and using the Structured Clinical Interview for DSM-IV, Research Version47 participated in the study. Sixteen subjects with BD (mean [SD] age, 34.2 [8.4] years; male to female ratio, 8:8) were in remission, and 14 subjects with BD (mean [SD] age, 38.0 [9.3] years; male to female ratio, 2:12) were in depressed episodes (based on Structured Clinical Interview for DSM-IV criteria). On the imaging day, all remitted subjects with BD had a Young Mania Rating Scale48 score of 10 or less and a 25-item Hamilton Rating Scale for Depression (HRSD-25)49 score of 7 or less; 1 remitted subject with BD had mild depressive symptoms but did not meet criteria for depressed episode (HRSD-25 score, 11; age, 49 years). On the imaging day, 8 depressed subjects with BD had low depression severity (HRSD-25 score, <17) but remained in depressed episodes, not meeting criteria for remission. All had experienced at least 2 episodes of depression or mania in the past 4 years.
Ten subjects with BD had a lifetime history of alcohol or other drug abuse. Remitted subjects with BD and depressed subjects with BD did not differ significantly in age, age at illness onset, illness duration, or history of alcohol or other drug abuse, but they differed in depression severity (t[29]=−5.82, P≤.001) and in sex ratio (, P=.03). More women than men were among the depressed subjects with BD relative to remitted subjects with BD (Table 1).
Table 1.
Demographic and Clinical Variables
| Variable and Group | Age, Mean (SD), y | Statistic | 2-Tailed P Value |
|---|---|---|---|
| Age at imaging, y | |||
| BD (n=31) | 35.90 (8.88) | t[54]=2.60 | .01a |
| Control (n=25) | 29.48 (9.43) | ||
| Depressed with BD (n=14) | 38.00 (9.30) | t[29]=1.20 | .24 |
| Remitted with BD (n=17) | 34.18 (8.41) | ||
| Male to female ratio | |||
| BD (11:20) | … | .52 | |
| Control (11:14) | … | ||
| Depressed with BD (2:12) | … | .03a | |
| Remitted with BD (9:8) | … | ||
| National Adult Reading Test | |||
| BD (n=31) | 110.80 (7.47) | t[53]=−1.48 | .14 |
| Control (n=24)b | 113.62 (9.12) | ||
| Age at onset of illness, y | |||
| Remitted with BD (n=17) | 21.65 (6.96) | t[29]=−1.18 | .25 |
| Depressed with BD (n=14) | 25.43 (10.75) | ||
| Illness duration, y | |||
| Remitted with BD (n=17) | 11.82 (6.31) | t[29]=−1.59 | .88 |
| Depressed with BD (n=14) | 12.29 (9.79) | ||
| Medication load | |||
| Remitted with BD (n=17) | 2.76 (1.79) | t[29]=−1.61 | .12 |
| Depressed with BD (n=14) | 3.79 (1.72) | ||
| 25-Item Hamilton Rating Scale for Depression | |||
| Remitted with BD (n=17) | 2.06 (2.59) | t[29]=−5.82 | <.001a |
| Depressed with BD (n=14) | 15.21 (8.13) | ||
| Lithium carbonate (on/off) | |||
| Remitted with BD (6/11) | … | .98 | |
| Depressed with BD (5/9) | … | ||
| Mood stabilizer (on/off) | |||
| Remitted with BD (10/7) | … | .10 | |
| Depressed with BD (12/2) | … | ||
| Antipsychotics (on/off) | |||
| Remitted with BD (9/8) | … | .82 | |
| Depressed with BD (8/6) | … | ||
| Antidepressants (on/off) | |||
| Remitted with BD (8/9) | … | .87 | |
| Depressed with BD (7/7) | … | ||
| Benzodiazepines (on/off) | |||
| Remitted with BD (5/12) | … | .24 | |
| Depressed with BD (7/7) | … | ||
| Lifetime history of alcohol or other drug abuse (on/off) | |||
| Remitted with BD (6/10)c | … | c | .72 |
| Depressed with BD (4/8)c | … |
Abbreviations: BD, bipolar disorder; ellipses, not applicable.
Significant at P≤.05 threshold. Statistics refer to between-group differences for all subjects with BD vs control subjects or for remitted subjects vs depressed subjects with BD.
Missing for 1 control subject.
Missing for 1 remitted and 2 depressed subjects.
Twenty-five controls (mean [SD] age, 29.48 [9.43] years [age range, 19-52 years]; male to female ratio, 11:14) with no previous psychiatric history (based on Structured Clinical Interview for DSM-IV criteria) or psychiatric history in first- or second-degree relatives participated in the study (Table 1). Of 56 individuals, 31 had a DSM-IV diagnosis of BD type 1 (mean [SD] age, 35.9 [8.9] years [age range, 19-52 years]). Controls were sex ratio—matched and premorbid IQ—matched with subjects with BD but were younger than subjects with BD (t[54]=2.60, P=.01). All participants were right-handed (based on criteria by Annett50).
Exclusion criteria for all participants included a history of head injury (from medical records and self-report), the presence of Axis II borderline personality disorder, cognitive impairment (score of <24 on the Mini-Mental State Examination51), premorbid IQ estimate of less than 85 (National Adult Reading Test),52 systemic medical illness (including neurologic disorders [history of epilepsy, dementia, cardiovascular accident, loss of consciousness for 10 minutes, or a neurodevelopmental or neurodegenerative disorder]), exclusion criteria for participation in magnetic resonance imaging studies (positive pregnancy test for women, proneness to panicking in enclosed spaces, or the presence of or a questionable history of metallic objects in the body [eg, aneurysm clips or pacemakers]), and cardiovascular, respiratory, gastrointestinal, urologic, metabolic, and endocrine disorders. Lifetime history of or current alcohol or other drug abuse (determined by saliva and urine screens) was an additional exclusion criterion for controls.
The participant population reflected the demographics of Pittsburgh and the surrounding area and the patient population of the University of Pittsburgh Medical Center, recruited through the Western Psychiatric Institute and Clinic, Mood Disorder Treatment and Research Program and through local advertising. All participants were made aware of the objectives of the study and signed informed consent documents to participate in the study.
MEDICATION LOAD
A problem for neuroimaging studies of subjects with BD is the potential confounding effect of psychotropic medication load because it is difficult to recruit medication-free subjects with BD into such studies.53 We aimed to examine the potential effect of psychotropic medication load, reflecting the number and dosage of different medications, on FA in subjects with BD. We used a strategy that has been developed for measuring this.53-56 For antidepressants and mood stabilizers, we categorized each medication into low-dose or high-dose groupings as previously performed.57 We considered individuals as taking low doses if at levels 1 and 2 of these previous criteria and individuals as taking high doses if at levels 3 and 4. We added a no-dose subtype for those not taking these medications. We converted antipsychotics to chlorpromazine hydrochloride dose equivalents, coding them as 0 (no medication), 1 (equal to or below the chlorpromazine dose equivalent), or 2 (above the chlorpromazine dose equivalent) relative to the mean effective daily dose of chlorpromazine as defined previously.58 Benzodiazepine dose was coded as 0, 1, or 2 relative to the midpoint of the recommended daily dose range for each medication recommended in the Physicians’ Desk Reference. We generated a composite measure of medication load by summing all individual medication codes for each medication category for each individual participant. Remitted subjects with BD and depressed subjects with BD did not differ significantly in medication load (Table 1).
We aimed to examine the potential effects of different classes of psychotropic medications on FA in subjects with BD because specific medications, including mood stabilizers such as lithium carbonate and sodium valproate, are associated with neurotrophic effects in subjects with BD.59 Among subjects with BD, 11 subjects (5 depressed and 6 remitted) were taking lithium, 17 subjects (8 depressed and 9 remitted) were taking antipsychotics, 22 subjects (12 depressed and 10 remitted) were taking mood stabilizers, 15 subjects (7 depressed and 8 remitted) were taking antidepressants, and 12 subjects (7 depressed and 5 remitted subjects) were taking benzodiazepines. No significant difference was noted between these subgroups in proportions taking vs those not taking lithium or each psychotropic medication class (eTable 1; http://www.archgenpsychiatry.com).
DATA ACQUISITION
Magnetic resonance images were acquired at the Brain Imaging Research Center, University of Pittsburgh and Carnegie Mellon University (3-T Siemens Magnetom Allegra syngo MR-2004A; Siemens Medical Systems, Iselin, New Jersey). A standard head coil was used for radio frequency transmission and reception of the magnetic resonance signal, and restraining foam pads were used for minimizing head motion. Anatomic images covering the entire brain were acquired using a sagittal 3-dimensional MPRAGE sequence parallel to the anterior commissure—posterior commissure line (echo time, 2.48 milliseconds; repetition time, 1630 milliseconds; flip angle, 8°; field of view, 200×200 cm; 224 sagittal 0.8-mm-thick sections; matrix size, 50×250 voxels; and acquisition time, 6 minutes 7 seconds). Diffusion tensor data were acquired using a coronal diffusion-weighted single-shot spin-echoplanar imaging sequence parallel to the anterior commissure—posterior commissure line (repetition time, 4400 milliseconds; echo time, 76 milliseconds, bandwidth, 1860 Hz/pixel; flip angle, 90°; field of view, 200×200 cm; thirty-three 3-mm-thick sections; no gaps; matrix size, 80×128 voxels; echo-planar imaging factor, 128; and acquisition time, 6 minutes 16 seconds). Two b values were used; 1 image at 0 s/mm2 (no diffusion weighting) and 6 non-coplanar images at 850 s/mm2 (diffusion-weighting b value) were acquired, parameters similar to those used in recent DTI studies.41,44 Fat saturation was used to remove scalp signal, which can disrupt neural signal owing to chemical shift or ghosting artifacts. An expert radiologist screened all the MPRAGE images for visible WM and other pathologic findings as part of our institutional review board—approved protocol.
DATA ANALYSIS
Data were transferred to a Unix-based workstation. Diffusion-weighted images were analyzed using the Functional Magnetic Resonance Imaging of the Brain Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl). Data were inspected for motion artifacts, and then diffusion-weighted images were registered to the image obtained without diffusion weighting (as a reference) by affine transformations to minimize distortions due to eddy currents and to reduce simple head motion using eddy current correction. Images were extracted using the brain extraction tool60 part of the FSL package.61
A diffusion tensor model was fitted at each voxel, providing a voxelwise calculation of FA.62 Fractional anisotropy can be expressed in terms of the following 3 eigenvalues: λ1 is the principal longitudinal diffusion direction, λ2 and λ3 are directions perpendicular to the principal diffusion direction.
Whole-brain voxelwise analysis of FA data was performed by aligning each subject’s FA image into a higher-resolution FA standard space (Montreal Neurological Institute [MNI] atlas, Montréal, Québec, Canada; http://www.bic.mni.mcgill.ca/bic_welcome.html) according to a nonlinear registration algorithm implemented in TBSS).39,40 The derived mean FA image was minimized to generate a template skeleton embodying the center of all tracts derived from the whole group. An FA threshold of 0.20 or higher was set to exclude peripheral tracts that might lead to erroneous interpretations due to anatomic inter-subject variability or partial volume effects with GM. Each subject’s aligned FA data were projected onto this template skeleton. Local FA maxima were then estimated using voxelwise between-subject statistics (Randomise [a TBSS statistical tool]; http://www.fmrib.ox.ac.uk/fsl/randomise/index.html). We used nonparametric 2-sample independent t test to compare groups based on a permutation method because of the nonparametric distribution of the data. Age was entered into this analysis as a confound regressor to ensure that any observed effect of group on FA was independent of age-related changes. We then performed nonparametric permutation tests to test for between-group differences in FA as previously described.41 We used a stringent threshold (median t value [t50] within the group of voxels, ≥3.0; P≤.001 uncorrected; number of permutations, 10 000; and smoothing factor, 5). We considered a cluster as a group of contiguous voxels with P<.001 and with 5 voxels or more. We controlled for multiple voxel-level comparisons within each cluster showing between-group differences in FA determined in the already described whole-brain analyses using a small-volume correction (P≤.05) with an anatomically defined regional mask in the relevant WM tract that contained approximately 100 times the number of voxels than each cluster and using another FSL statistical tool (false discovery rate; http://www.fmrib.ox.ac.uk/fsl/randomise/fdr.html). We determined the most probable anatomic localization of each cluster by means of the FSL atlas tool (http://www.fmrib.ox.ac.uk/fsl/fslview/atlas-descriptions.html) using all anatomic templates (Talairach atlas, MNI structural atlas, Jülich histologic atlas, Oxford thalamic connectivity atlas, Harvard-Oxford cortical and subcortical structural atlases, and Johns Hopkins University DTI-based WM atlases). All reported brain images were acquired using the “tbss_fill” script from the FSL package.
WHOLE-BRAIN VOXELWISE ANALYSIS OF LONGITUDINAL AND RADIAL DIFFUSIVITIES
Longitudinal (principal diffusion direction, λ1) and radial (transverse diffusion component, [(λ2+λ3)/2] diffusivity values were computed for clusters showing a significant FA change (increase or decrease) between subjects with BD and controls at the already defined threshold. This value was t≥3.0 (P≤.001) in each cluster showing a significant (P≤.05) between-group difference in FA.
RELATIONSHIP WITH DEMOGRAPHIC AND CLINICAL VARIABLES
Fractional anisotropy values were extracted at local maxima within clusters showing significant between-group differences in FA to assess the relationship between FA in these clusters and demographic and clinical variables (age, age at illness onset, duration of illness, depression severity, and medication load) by means of parametric and nonparametric correlational analyses as appropriate using commercially available software (SPSS for Windows, version 15.0; SPSS Institute, Chicago, Illinois) and a statistical threshold of P≤.01 to control for multiple tests in each region. To examine effects of substance abuse, depressed episode (ie, depressed vs remitted), and taking vs not taking lithium, and each medication class (mood stabilizers, benzodiazepines, antipsychotics, and antidepressants) on FA in all regions that distinguished subjects with BD from controls, we used 2-way independent t tests to compare FA values (extracted at local maxima within clusters showing significant differences in FA between subjects with BD and controls) between relevant subgroups. For subjects with BD taking vs not taking lithium and each medication class, we used a statistical threshold of P=.01 (P=.05 divided by 5) to control for the 5 separate tests in each cluster.
RESULTS
Regions showing greater FA in subjects with BD vs controls included the following 3 clusters in the left uncinate fasciculus: 1 cluster along the central part of the tract in proximity to the insula (5 voxels, t50=3.0, maximum t [tmax]=3.3, P=.05 corrected, Cohen d=1.00) and 2 clusters within OMPFC WM (5 voxels, t50=4.5, tmax=5.3, P=.05 corrected, Cohen d=0.97; and 7 voxels, t50=4.2, tmax=4.6, P=.05 corrected, Cohen d=1.1) (Figure 1A and B). Greater FA was associated in the first of these clusters with greater longitudinal diffusivity among subjects with BD vs controls (t50=3.1, P≤.001) and in the other 2 clusters with reduced radial diffusivity among subjects with BD vs controls (t50=6.9, P≤.001; and t50=4.5, P≤.001; respectively) (Figure 2A and B). The following 3 clusters showing greater FA in subjects with BD vs controls were observed in the left optic radiation: 1 cluster in the temporal cortex (9 voxels, t50=3.1, tmax=3.3, P=.05 corrected, Cohen d=0.78) and 2 clusters within the cuneus (11 voxels, t50=3.5, tmax=3.9, P=.05 corrected, Cohen d=0.62; and 17 voxels, t50=3.8, tmax=4.4, P=.05 corrected, Cohen d=0.95) (Table 2). In the latter of these regions, greater FA was associated with greater longitudinal diffusivity (t50=3.0, P≤.001) and with a trend-level reduction in radial diffusivity among subjects with BD vs controls. There were trend-level increases in longitudinal diffusivity in the other 2 regions. An additional cluster showing greater FA among subjects with BD vs controls was observed in the right anterothalamic radiation (5 voxels, t50=3.2, tmax=3.4, P=.05 corrected, Cohen d=1.03) associated only with a trend-level reduction in radial diffusivity in subjects with BD vs controls.
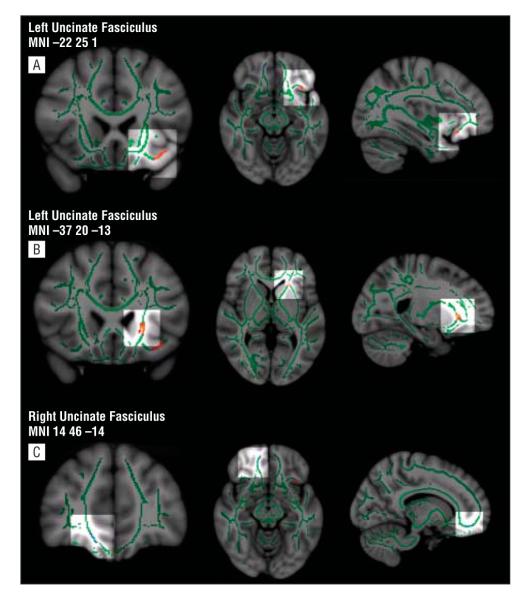
Figure 1.
Fractional anisotropy (FA) maps showing (from left to right) coronal, axial, and sagittal views. The brain and tract-based spatial statistics (TBSS) templates are the standard Montreal Neurological Institute (MNI) atlas MNI152 1-mm brain template and the FA white matter skeleton (ie, the most compact whole-brain white matter) used for TBSS analysis (shown in green). Colored voxels represent regions in which FA differs significantly in subjects with bipolar disorder (BD) vs control subjects. Red-yellow indicates greater FA in subjects with BD vs controls; light blue, decreased FA in subjects with BD vs controls (t>3.0 and P≤.05 corrected for both [scale ranging from red and/or blue to yellow and/or light blue]). All brain images were acquired using the “tbss_fill” script, which allows better visualization of the regions of interest. A, Three-dimensional views highlighting in red-yellow the central cluster in the left uncinate fasciculus in which FA was significantly increased in subjects with BD vs controls (t=3.0, P≤.05 corrected). B, Three-dimensional views highlighting in red-yellow an orbitomedial prefrontal cortex cluster in the left uncinate fasciculus in which FA was significantly increased in subjects with BD vs controls (t=4.5, P≤.05 corrected). C, Three-dimensional views highlighting in light blue a cluster in the right uncinate fasciculus in which FA was significantly reduced in subjects with BD vs controls (t=3.3, P≤.05 corrected).
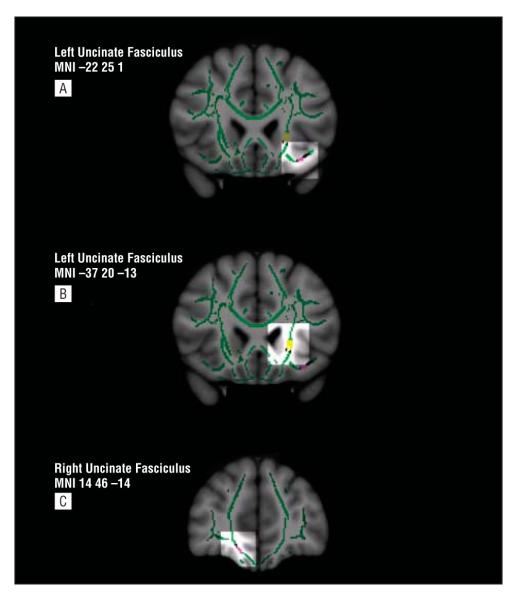
Figure 2.
Templates as in Figure 1. Here fractional anisotropy (FA) maps in black (for increased and decreased FA). Overlaid on FA maps are longitudinal diffusivity in yellow and radial diffusivity in purple (t>3.0, P≤.001 for both). A, Coronal view highlighting in yellow a central cluster in the left uncinate fasciculus in which longitudinal diffusivity was significantly increased in subjects with bipolar disorder (BD) vs controls (t=3.1, P≤.001). B, Three-dimensional views highlighting in purple an orbitomedial prefrontal cortex cluster in the left uncinate fasciculus in which radial diffusivity was significantly decreased in subjects with BD vs controls (t=6.9, P≤.001). C, Three-dimensional views highlighting in purple a cluster in the right uncinate fasciculus in which radial diffusivity was significantly increased in subjects with BD vs controls (t=3.8, P≤.001). MNI indicates Montreal Neurological Institute atlas x, y, and z coordinates.
Table 2.
Regions Showing Greater and Reduced Fractional Anisotropy (FA) in 31 Subjects With Bipolar Disorder (BD) vs in 25 Control Subjectsa
| No. of Voxels |
MNI Atlas Coordinates x, y, z, mm |
White Matter Tract |
Corresponding Cortical Area |
Group | FA Difference, Mean (SD) |
tmax Value |
Value (P Value) |
Cohen d |
||
|---|---|---|---|---|---|---|---|---|---|---|
| t50 | Longitudinal Diffusivity |
Radial Diffusivity |
||||||||
| ↑ FA in Subjects With BD vs Control Subjects | ||||||||||
| 5 | −37, 20, −13 | Left uncinate fasciculus |
OMPFC | BD | 3785.2 (1508.1) | 5.3 | 4.5 (<.001) | −3.5 (.91) | 6.9 (.001) | 0.97 |
| Control | 2605.8 (821.2) | |||||||||
| 7 | −33, 21, −17 | Left uncinate fasciculus |
OMPFC | BD | 4331.2 (1576.0) | 4.6 | 4.2 (<.001) | −4.0 (.91) | 4.5 (.002) | 1.10 |
| Control | 2922.7 (871.1) | |||||||||
| 17 | −13, −80, 19 | Left optic radiation | Cuneus | BD | 4369.1 (893.1) | 4.4 | 3.8 (<.001) | 2.4 (.002) | 2.8 (.001) | 0.95 |
| Control | 3611.8 (672.3) | |||||||||
| 11 | −8, −82, 27 | Left optic radiation | Cuneus | BD | 4166.5 (999.5) | 3.9 | 3.5 (<.001) | 3.5 (.001) | 1.5 (.03) | 0.62 |
| Control | 3605.2 (812.3) | |||||||||
| 5 | 3, −7, −2 | Right anterothalamic radiation |
Thalamus | BD | 4223.7 (471.0) | 3.4 | 3.2 (<.001) | −0.2 (.68) | 1.5 (<.001) | 1.03 |
| Control | 3779.9 (383.9) | |||||||||
| 9 | −39, −40, −41, 0 | Left optic radiation | Temporal cortex |
BD | 6286.9 (533.4) | 3.3 | 3.1 (.001) | 1.6 (.03) | 2.8 (.005) | 0.78 |
| Control | 5893.9 (476.2) | |||||||||
| 7 | −22, 25, 1 | Left uncinate fasciculus |
OMPFC or insula |
BD | 6352.6 (579.5) | 3.3 | 3.0 (.001) | 3.1 (.001) | 1.0 (.008) | 1.00 |
| Control | 5819.0 (483.6) | |||||||||
| ↓ FA in Subjects With BD vs Control Subjects | ||||||||||
| 5 | 14, 46, −14 | Right uncinate fasciculus |
OMPFC | BD | 5064.6 (695.8) | 3.9 | 3.3 (<.001) | 0.2 (.46) | 3.8 (.001) | 1.10 |
| Control | 5869.2 (756.8) | |||||||||
Abbreviations: MNI, Montreal Neurological Institute; OMPFC, orbitomedial prefrontal cortex.
Boldfaced FA indexes for clusters with t≥3.0 (P≥.001 uncorrected) and longitudinal and radial diffusivity indexes with t≥3.0 (P≤.001 uncorrected). All of these regions survived small-volume corrections (false discovery rate, P≤.05) to control for multiple tests. Boldface also indicates significant differences in longitudinal and radial diffusivities between subjects with BD vs control subjects in these clusters; longitudinal diffusivity varies in line with FA, while radial diffusivity varies inversely to FA. For increased FA, longitudinal diffusivity is increased in subjects with BD vs controls, while radial diffusivity is decreased in subjects with BD vs controls. For decreased FA, longitudinal diffusivity is decreased in subjects with BD vs controls, while radial diffusivity is increased in subjects with BD vs controls.
One region showed reduced FA in subjects with BD vs controls. The right uncinate fasciculus (5 voxels, t50=3.3, tmax=3.9, P=.05 corrected, Cohen d=1.10) (Figure 1C) was associated with greater radial diffusivity (t=3.8, P=.001) in subjects with BD vs controls (Table 2 and Figure 2C).
RELATIONSHIP BETWEEN REGIONS SHOWING ABNORMAL FA AND DEMOGRAPHIC AND CLINICAL VARIABLES IN SUBJECTS WITH BD
Regions Showing Greater FA in Subjects With BD vs Controls
There were significant negative correlations between age at imaging and FA in a cluster in the left uncinate fasciculus (MNI x, y, z coordinates, −33, 21, −17; Spearman rank correlation, −0.53; P=.002) and FA in the right anterothalamic radiation (r=−0.48, P=.006), as well as between medication load and FA in a cluster in the left optic radiation (MNI x, y, z coordinates, −39, −40, 1; r=−0.50; P=.004) (Table 3). Trending negative correlations between demographic and clinical variables among subjects with BD in these and other regions are also given in Table 3.
Table 3.
Relationship Between Fractional Anisotropy (FA) and Clinical and Demographic Variables in 31 Subjects With Bipolar Disorder (BD) vs in 25 Control Subjects
| Cluster (Montreal Neurological Institute Atlas x, y, and z Coordinates) |
Group | Statistic | Age at Imaging |
Age at Onset of Illness |
Illness Duration |
Medication Load |
25-Item Hamilton Rating Scale for Depression |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlation |
P Value |
Correlation |
P Value |
Correlation |
P Value |
Correlation |
P Value |
Correlation |
P Value |
|||
| Left uncinate fasciculus (−37, 20, −13) |
BD | ρ | −0.03 | .86 | −0.10 | .61 | 0.08 | .68 | −0.40 | .03 | −0.02 | .93 |
| Control | ρ | −0.17 | .43 | |||||||||
| BD + control | r | −0.19 | .17 | |||||||||
| Left uncinate fasciculus (−33, 21, −17) |
BD | ρ | −0.53a | .002 | −0.35 | .05 | −0.20 | .29 | −0.20 | .28 | −0.42 | .02 |
| Control | ρ | −0.29 | .17 | |||||||||
| BD + control | r | 0.04 | .78 | |||||||||
| Left optic radiation (−13, −80, 19) |
BD | r | −0.27 | .15 | −0.31 | .10 | 0.03 | .88 | −0.07 | .72 | −0.17 | .37 |
| Control | r | −0.21 | .31 | |||||||||
| BD + control | ρ | 0.01 | .97 | |||||||||
| Left optic radiation (−8, −82, 27) |
BD | r | −0.28 | .13 | −0.05 | .81 | −0.25 | .18 | −0.16 | .39 | −0.28 | .13 |
| Control | r | −0.33 | .11 | |||||||||
| BD + control | ρ | −0.20 | .14 | |||||||||
| Right anterothalamic radiation (3, −7, −2) |
BD | r | −0.48a | .006 | −0.29 | .11 | −0.23 | .21 | −0.33 | .07 | −0.29 | .11 |
| Control | r | −0.28 | .18 | |||||||||
| BD + control | ρ | 0.01 | .92 | |||||||||
| Left optic radiation (−39, −40, 1) |
BD | r | −0.40 | .03 | −0.19 | .31 | −0.26 | .16 | −0.50a | .004 | −0.04 | .82 |
| Control | r | −0.30 | .15 | |||||||||
| BD + control | ρ | −0.24 | .08 | |||||||||
| Left uncinate fasciculus (−22, 25, 1) |
BD | r | −0.34 | .06 | 0.00 | .98 | −0.42 | .02 | −0.41 | .02 | −0.08 | .68 |
| Control | r | 0.20 | .33 | |||||||||
| BD + control | ρ | 0.07 | .61 | |||||||||
| Right uncinate fasciculus (14, 46, −14) |
BD | r | −0.46a | .009 | −0.31 | .09 | −0.16 | .40 | −0.27 | .14 | −0.03 | .86 |
| Control | r | 0.26 | .21 | |||||||||
| BD + control | ρ | −0.27 | .048 | |||||||||
Significant at P<.01 (2-tailed) threshold.
Regions Showing Reduced FA in Subjects With BD vs Controls
A significant negative correlation was noted between age at imaging and right uncinate fasciculus FA (r=−0.46, P=.009) in subjects with BD. These findings are summarized in Table 3.
RELATIONSHIP BETWEEN DEPRESSED EPISODE AND REGIONS SHOWING ABNORMAL FA IN SUBJECTS WITH BD
Regions Showing Greater FA in Subjects With BD vs Controls
Depressed subjects with BD vs remitted subjects with BD showed decreased FA in the left optic radiation (MNI x, y, z coordinates,−8, −82, 27; t[29]=3.1; P=.004) (Table 3). Because depressed subjects with BD and remitted subjects with BD differed in sex ratio, we examined the relationship between sex (male vs female) and FA in this region among all subjects with BD. Neither a sex difference nor a sex×mood interaction (depressed subjects with BD vs remitted subjects with BD) was significant on left optic radiation FA in subjects with BD (eTable 2 and eTable 3).
Regions Showing Reduced FA in Subjects With BD vs Controls
Depressed subjects with BD vs remitted subjects with BD did not differ in right uncinate fasciculus FA. These results are summarized in eTable 2).
RELATIONSHIP BETWEEN PSYCHOTROPIC MEDICATION CLASS AND FA IN REGIONS SHOWING ABNORMAL FA IN SUBJECTS WITH BD
Regions Showing Greater FA in Subjects With BD vs Controls
Subjects with BD taking vs not taking mood stabilizers showed significantly decreased FA in the left optic radiation (MNI x, y, z coordinates, −39, −40, 1, −41, 0; t[29]=−4.28; P<.001) and in the right anterothalamic radiation (t[29]=−2.60, P=.01). There were no significant effects of other medication use in any regions showing between-group differences in FA among subjects with BD (Table 4).
Table 4.
Relationship Between Fractional Anisotropy (FA) and Classes of Medication in Subjects With Bipolar Disorder (BD)
| Cluster (Montreal Neurological Institute Atlas x, y, and z Coordinates) |
Class of Medication and Lifetime History of Alcohol or Other Drug Abuse (On/Off) |
Statistic | 2-Tailed P Value |
Mean FA Difference (On Minus Off) |
|---|---|---|---|---|
| Left uncinate fasciculus (−37, 20, −13) | Mood stabilizers (22/9) | t[29]=−3.23a | .003 | −1682.16 |
| Lithium carbonate (11/20) | t[29]=1.10 | .28 | 521.55 | |
| Antipsychotics (17/14) | t[29]=0.73 | .47 | 399.70 | |
| Antidepressants (15/16) | t[29]=−0.66 | .51 | −363.54 | |
| Benzodiazepine (12/19) | t[29]=−0.91 | .37 | −507.91 | |
| Alcohol or other drug abuse (10/18) | t[26]=0.96b | .35 | 548.55 | |
| Left uncinate fasciculus (−33, 21, −17) | Mood stabilizers (22/9) | t[29]=−0.67 | .51 | −420.08 |
| Lithium (11/20) | t[29]=0.91 | .37 | 540.09 | |
| Antipsychotics (17/14) | t[29]=0.25 | .81 | 142.21 | |
| Antidepressants (15/16) | t[29]=0.04 | .97 | 23.73 | |
| Benzodiazepine (12/19) | t[29]=−1.71 | .10 | −963.21 | |
| Alcohol or other drug abuse (10/18) | t[26]=0.79b | .44 | 459.82 | |
| Left optic radiation (−13, −80, 19) | Mood stabilizers (22/9) | t[27]=−0.89 | .38 | −249.58 |
| Lithium (11/20) | t[29]=0.79 | .44 | 266.77 | |
| Antipsychotics (17/14) | t[29]=0.49 | .63 | 160.54 | |
| Antidepressants (15/16) | t[29]=−2.05 | .05 | −624.12 | |
| Benzodiazepine (12/19) | t[29]=0.44 | .66 | 147.36 | |
| Alcohol or other drug abuse (10/18) | t[26]=0.14b | .89 | 50.68 | |
| Left optic radiation (−8, −82, 27) | Mood stabilizers (22/9) | t[29]=−1.92 | .07 | −726.10 |
| Lithium (11/20) | t[29]=0.33 | .74 | 126.71 | |
| Antipsychotics (17/14) | t[29]=0.94 | .35 | 341.19 | |
| Antidepressants (15/16) | t[29]=−2.52 | .02 | −834.20 | |
| Benzodiazepine (12/19) | t[29]=−0.17 | .86 | −65.34 | |
| Alcohol or other drug abuse (10/18) | t[26]=0.10b | .92 | 40.27 | |
| Right anterothalamic radiation (3, −7, −2) | Mood stabilizers (22/9) | t[29]=−2.60a | .01 | −444.18 |
| Lithium (11/20) | t[29]=1.24 | .22 | 217.90 | |
| Antipsychotics (17/14) | t[29]=−0.30 | .77 | −51.56 | |
| Antidepressants (15/16) | t[29]=0.45 | .66 | 76.95 | |
| Benzodiazepine (12/19) | t[29]=−2.42 | .02 | −389.77 | |
| Alcohol or other drug abuse (10/18) | t[26]=0.59b | .56 | 105.50 | |
| Left optic radiation (−39, −40, 1) | Mood stabilizers (22/9) | t[29]=−4.28a | <.001 | −719.51 |
| Lithium (11/20) | t[29]=−0.01 | .99 | −1.52 | |
| Antipsychotics (17/14) | t[29]=1.66 | .11 | 310.03 | |
| Antidepressants (15/16) | t[29]=−0.73 | .47 | −140.91 | |
| Benzodiazepine (12/19) | t[29]=−1.56 | .13 | −299.64 | |
| Alcohol or other drug abuse (10/18) | t[26]=1.61b | .12 | 322.11 | |
| Left uncinate fasciculus (−22, 25, 1) | Mood stabilizers (22/9) | t[29]=−2.19 | .04 | −473.31 |
| Lithium (11/20) | t[29]=1.06 | .30 | 230.80 | |
| Antipsychotics (17/14) | t[29]=−0.58 | .56 | −123.33 | |
| Antidepressants (15/16) | t[29]=−0.16 | .88 | −33.19 | |
| Benzodiazepine (12/19) | t[29]=−2.46 | .02 | −485.50 | |
| Alcohol or other drug abuse (10/18) | t[26]=3.89a,b | .001 | 711.01 | |
| Right uncinate (14, 46, −14) | Mood stabilizers (22/9) | t[29]=−1.85 | .08 | −488.73 |
| Lithium (11/20) | t[29]=2.04 | .05 | 506.56 | |
| Antipsychotics (17/14) | t[29]=−0.80 | .43 | −202.22 | |
| Antidepressants (15/16) | t[29]=−0.19 | .85 | −48.31 | |
| Benzodiazepine (12/19) | t[29]=−0.73 | .47 | −187.68 | |
| Alcohol or other drug abuse (10/18) | t[26]=1.68b | .11 | 439.08 |
Significant at P≤.01 threshold.
Data missing for 1 remitted and 2 depressed subjects.
Regions Showing Reduced FA in Subjects With BD vs Controls
Subjects with BD taking vs not taking any type of medication did not differ in right uncinate fasciculus FA. These results are summarized in Table 4.
RELATIONSHIP BETWEEN SUBSTANCE ABUSE AND REGIONS SHOWING ABNORMAL FA IN SUBJECTS WITH BD
Regions Showing Greater FA in Subjects With BD vs Controls
Subjects having BD with vs without a lifetime history of alcohol or other drug abuse had significantly decreased left uncinate fasciculus FA. The MNI x, y, and z coordinates were −22, 25, 1, and 1 (t[26]=3.89, P<.001).
Regions Showing Reduced FA in Subjects With BD vs Controls
Subjects having BD with vs without a lifetime history of alcohol or other drug abuse did not differ in right uncinate fasciculus FA. These results are summarized in Table 4.
RELATIONSHIP BETWEEN FA AND AGE IN CONTROLS AND ALL PARTICIPANTS
There were no significant correlations with age at imaging in any regions showing between-group differences in FA among controls or among the entire sample of subjects with BD and controls. These results are summarized in Table 3.
COMMENT
The main goal of the present study was to examine FA in WM tracts connecting the OMPFC with subcortical limbic regions and with dorsal prefrontal cortical regions in subjects with BD. We used TBSS, which is a new technique. Consistent with our hypotheses was our major finding of greater FA in the left uncinate fasciculus among subjects with BD vs controls that concurs with a recent study29 of more reconstructed fibers in the left uncinate fasciculus among subjects with BD vs controls. Contrary to our hypotheses, we found reduced FA in the right uncinate fasciculus and greater FA in non—a priori regions, including the right anterothalamic radiation and the left optic radiation among subjects with BD vs controls.
The uncinate fasciculus is the major fiber tract connecting the inferofrontal and anterotemporal cortices,63 and it runs upward over the lateral nuclei of the amygdala, terminating in the OMPFC in the rectal gyrus (Brodmann area 11), retrolateral orbital cortex (Brodmann area 12), and subcallosal area (Brodmann area 25). The uncinate fasciculus connects the subcortical limbic regions and the regions within the OMPFC that are important for mood regulation. Therefore, abnormal FA in bilateral uncinate fasciculi may contribute to mood dysregulation among subjects with BD.
To help interpret our FA findings in subjects with BD, we used measures of longitudinal and radial diffusivities. In a previous study,27 greater FA in subjects with BD was interpreted as a change in the directional alignment of fibers; however, longitudinal and radial diffusivities were not measured. In another study,28 significantly greater FA in the midline of the genu of the corpus callosum in subjects with BD relative to controls was interpreted as one of several possibilities, including fewer obliquely oriented fibers, changes in axonal integrity, tighter packing of axons, less permeable myelin sheaths, or an abnormality of a set of fibers in a large group of intersecting fiber pathways, but again the study excluded measures of longitudinal and radial diffusivities to distinguish among these possibilities. We showed that greater FA among subjects with BD vs controls in 2 clusters in the left uncinate fasciculus in the OMPFC was associated with reduced radial diffusivity, consistent with fewer obliquely oriented fibers, and in 1 cluster centrally in the tract was associated with greater longitudinal diffusivity, consistent with more longitudinally aligned fibers. Conversely, the association between greater radial diffusivity and reduced FA in subjects with BD vs controls in the right uncinate fasciculus probably reflected more obliquely oriented fibers, although it could also reflect local inflammatory changes in subjects with BD.
Our findings make a significant contribution to existing data regarding structural abnormalities in the OMPFC among subjects with BD6-17 by indicating abnormal asymmetry between proportions of obliquely aligned vs longitudinally aligned fibers in the left uncinate fasciculus and the right uncinate fasciculus, which may relate to abnormal right vs left hemispheric processing of emotion, previously demonstrated in unipolar depression.64 We speculate that an abnormally reduced proportion of obliquely aligned fibers in the left uncinate fasciculus in the OMPFC, together with an abnormally increased proportion of longitudinally aligned fibers in the central part of the tract nearer to the insula and subcortical limbic regions, may be associated with an imbalance between “feed forward” OMPFC-subcortical and “feed back” OMPFC-subcortical connectivity. Conversely, an increased proportion of obliquely aligned fibers, with or without local inflammatory changes, in the right uncinate fasciculus may be associated with aberrant bidirectional OMPFC-subcortical connectivity. This putative right vs left difference in OMPFC-subcortical connectivity may represent a potential biologic mechanism for mood dysregulation in subjects with BD and should be a focus of future studies.
Regarding our findings in non—a priori regions, we demonstrated greater left optic radiation FA associated with greater longitudinal diffusivity, suggesting more longitudinally aligned fibers in this tract among subjects with BD vs controls. We also demonstrated in subjects with BD vs controls greater right anterothalamic radiation FA connecting the medial and anterior thalamic nuclei with the pre-frontal cortex but unassociated with any significant change in longitudinal or radial diffusivity in subjects with BD. Although difficult to interpret, these findings may be linked with previously observed patterns of abnormally elevated right thalamic activity in response to emotional stimuli,65 abnormally elevated left medio-occipital cortical activity during attention and executive function tasks,24,66,67 and attentional bias to salient material68 in subjects with BD. They require further examination in future studies.
Our exploratory analyses revealed significant negative correlations between bilateral uncinate fasciculi and right anterothalamic radiation FA and age at imaging in subjects with BD but not controls. These findings were not a confound of the older age of subjects with BD because we covaried for age in our between-group analyses of whole-brain FA. Indeed, our use of age as a covariate may have obscured any reductions in FA in WM in dorsal prefrontal cortical regions among subjects with BD that we had hypothesized based on previous findings in BD among younger adults and adolescents. Several studies69-75 report reduced FA in different cortical regions among individuals with late-life unipolar depression vs age-matched controls. Together with our findings of negative associations between age and FA in several WM tracts among subjects with BD but not controls, these data suggest that reductions in WM FA with increasing age may characterize individuals with mood disorders.
We found a significant negative correlation between FA and psychotropic medication load among subjects with BD in a cluster in the left optic radiation, as well as significantly decreased FA among subjects with BD taking mood stabilizers in the same left optic radiation cluster and in the right anterothalamic radiation. Together, these findings suggest an ameliorative effect of psychotropic medication and particularly mood stabilizers in reducing abnormally increased FA among subjects with BD. They parallel previous evidence among subjects with BD showing neurotrophic effects of mood stabilizers on GM in left-sided regions,76 as well as associations between psychotropic medication use and increased GM in left13 and bilateral6 OMPFC among subjects with BD. We did not find a significant difference in FA between subjects with BD taking vs not taking lithium, suggesting that mood stabilizers other than lithium may contribute to changes in FA among subjects with BD.
Depressed subjects with BD relative to remitted subjects with BD demonstrated significantly reduced FA in the left optic radiation that was not confounded by the greater number of female than male depressed subjects with BD, as there was neither a sex difference in FA nor a sex×mood interaction on FA in this cluster among subjects with BD. Our findings indicate similar negative relationships between depressed episode and FA and between mood stabilizer use and FA in subjects with BD, particularly within the left optic radiation. Although depressed subjects with BD and remitted subjects with BD did not differ in illness duration, total medication load, or proportion of individuals taking each psychotropic medication class, it is possible that depressed subjects with BD may have been prescribed more mood stabilizers previously in their illness history. Longitudinal within-group studies are required to examine relationships between change in depression severity, medication use, and FA over time in subjects with BD.
A limitation of our study is that all subjects with BD were medicated, although we demonstrated ameliorative rather than confounding effects of total medication load and mood stabilizer use in reducing rather than increasing left uncinate fasciculus FA among subjects with BD. Future studies could attempt to recruit nonmedicated subjects with BD to examine differences in FA between medicated and nonmedicated subjects with BD. Several subjects with BD were also using illicit substances. Because many subjects with BD use illicit substances,77 it remains difficult and is probably unrepresentative of the bipolar population overall to exclude subjects with BD and current substance abuse from studies. Subjects with BD having a lifetime history of alcohol or other drug abuse showed decreased left uncinate fasciculus FA, suggesting a potential ameliorative rather than confounding effect of alcohol or other drug abuse on FA in this region. We did not include measures of tobacco use in our analyses; this could be included in future studies of subjects with BD.
To our knowledge, this is the first study to use TBSS to examine WM in subjects with BD. Our main finding of greater left and reduced right uncinate fasciculus FA, which was unrelated to medication use, likely reflects an abnormal right vs left asymmetry in alignment of fibers in OMPFC WM that may be associated with mood dysregulation among subjects with BD. Our findings make a significant contribution to the emerging literature high-lighting a role of the OMPFC in the pathogenesis of BD and demonstrate the usefulness of TBSS as a tool for examination of WM in subjects with BD.
Acknowledgments
Funding/Support: This study was supported in part by grant 1 R01 MH076971-01 from National Institutes of Health (Dr Phillips) and by a NARSAD (National Alliance for Research on Schizophrenia and Depression) Independent Investigator Award (Nellie Blumenthal Investigator) (Dr Phillips).
Footnotes
Financial Disclosure: Dr Kupfer is a consultant for Servier Amerique and serves on advisory boards for Pfizer, Inc, Eli Lilly & Company, Forest Pharmaceuticals, Inc, F. Hoffman—La Roche Ltd, and Solvay/Wyeth Pharmaceuticals.
Additional Information: eTables 1 through 3 are available at http://www.archgenpsychiatry.com.
REFERENCES
- 1.Murray CJL, Lopez AD. Evidence-based health policy: lessons from the Global Burden of Disease Study. Science. 1996;274(5288):740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- 2.Phillips ML, Vieta E. Identifying functional neuroimaging biomarkers of bipolar disorder: toward DSM-V. Schizophr Bull. 2007;33(4):893–904. doi: 10.1093/schbul/sbm060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowden CL, Singh V. Valproate in bipolar disorder: 2000 onwards. Acta Psychiatr Scand Suppl. 2005;426(426):13–20. doi: 10.1111/j.1600-0447.2005.00522.x. [DOI] [PubMed] [Google Scholar]
- 4.Yurgelun-Todd DA, Ross AJ. Functional magnetic resonance imaging studies in bipolar disorder. CNS Spectr. 2006;11(4):287–297. doi: 10.1017/s1092852900020782. [DOI] [PubMed] [Google Scholar]
- 5.Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg HP, Krystal JH, Bansal R, Martin A, Dziura J, Durkin K, Martin L, Gerard E, Charney DS, Peterson BS. Age, rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: a cross-sectional study. Biol Psychiatry. 2006;59(7):611–618. doi: 10.1016/j.biopsych.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 7.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 8.Frangou S. The Maudsley Bipolar Disorder Project. Epilepsia. 2005;46(suppl 4):19–25. doi: 10.1111/j.1528-1167.2005.463005.x. [DOI] [PubMed] [Google Scholar]
- 9.Hirayasu Y, Shenton ME, Salisbury DF, Kwon JS, Wible CG, Fischer IA, Yurgelun-Todd D, Zarate C, Kikinis R, Jolesz FA, McCarley RW. Subgenual cingulate cortex volume in first-episode psychosis. Am J Psychiatry. 1999;156(7):1091–1093. doi: 10.1176/ajp.156.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lochhead RA, Parsey RV, Oquendo MA, Mann JJ. Regional brain gray matter volume differences in patients with bipolar disorder as assessed by optimized voxel-based morphometry. Biol Psychiatry. 2004;55(12):1154–1162. doi: 10.1016/j.biopsych.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 11.López-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry. 2002;52(2):93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- 12.Lyoo IK, Kim MJ, Stoll AL, Demopulos CM, Parow AM, Dager SR, Friedman SD, Dunner DL, Renshaw PF. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55(6):648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Nugent AC, Milham MP, Bain EE, Mah L, Cannon DM, Marrett S, Zarate CA, Pine DS, Price JL, Drevets WC. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. Neuroimage. 2006;30(2):485–497. doi: 10.1016/j.neuroimage.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 14.Sassi RB, Brambilla P, Hatch JP, Nicoletti MA, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Reduced left anterior cingulate volumes in untreated bipolar patients. Biol Psychiatry. 2004;56(7):467–475. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Adler CM, Levine AD, DelBello MP, Strakowski SM. Changes in gray matter volume in patients with bipolar disorder. Biol Psychiatry. 2005;58(2):151–157. doi: 10.1016/j.biopsych.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Farrow TF, Whitford TJ, Williams LM, Gomes L, Harris AWF. Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. Biol Psychiatry. 2005;58(9):713–723. doi: 10.1016/j.biopsych.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 17.Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95(22):13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruno SD, Barker GJ, Cercignani M, Symms M, Ron MA. A study of bipolar disorder using magnetization transfer imaging and voxel-based morphometry. Brain. 2004;127(pt 11):2433–2440. doi: 10.1093/brain/awh274. [DOI] [PubMed] [Google Scholar]
- 19.Brambilla P, Nicoletti M, Sassi RB, Mallinger AG, Frank E, Keshavan MS, Soares JC. Corpus callosum signal intensity in patients with bipolar and unipolar disorder. J Neurol Neurosurg Psychiatry. 2004;75(2):221–225. [PMC free article] [PubMed] [Google Scholar]
- 20.Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- 21.Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study [published correction appears in Arch Gen Psychiatry. 2001;58(8):774] Arch Gen Psychiatry. 2001;58(5):461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- 22.Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44(2):259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Adler CM, Adams J, DelBello MP, Holland SK, Schmithorst V, Levine A, Jarvis K, Strakowski SM. Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: a diffusion tensor imaging study. Am J Psychiatry. 2006;163(2):322–324. doi: 10.1176/appi.ajp.163.2.322. [DOI] [PubMed] [Google Scholar]
- 24.Adler CM, Holland SK, Schmithorst V, Wilke M, Weiss KL, Pan H, Strakowski SM. Abnormal frontal white matter tracts in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2004;6(3):197–203. doi: 10.1111/j.1399-5618.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- 25.Beyer JL, Taylor WD, MacFall JR, Kuchibhatla M, Payne ME, Provenzale JM, Cassidy F, Krishnan KR. Cortical white matter microstructural abnormalities in bipolar disorder. Neuropsychopharmacology. 2005;30(12):2225–2229. doi: 10.1038/sj.npp.1300802. [DOI] [PubMed] [Google Scholar]
- 26.Regenold WT, D’Agostino CA, Ramesh N, Hasnain M, Roys S, Gullapalli RP. Diffusion-weighted magnetic resonance imaging of white matter in bipolar disorder: a pilot study [published correction appears in Bipolar Disord. 2006;8(6):754] Bipolar Disord. 2006;8(2):188–195. doi: 10.1111/j.1399-5618.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- 27.Haznedar MM, Roversi F, Pallanti S, Baldini-Rossi N, Schnur DB, Licalzi EM, Tang C, Hof PR, Hollander E, Buchsbaum MS. Fronto-thalamo-striatal gray and white matter volumes and anisotropy of their connections in bipolar spectrum illnesses. Biol Psychiatry. 2005;57(7):733–742. doi: 10.1016/j.biopsych.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Yurgelun-Todd DA, Silveri MM, Gruber SA, Rohan ML, Pimentel PJ. White matter abnormalities observed in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2007;9(5):504–512. doi: 10.1111/j.1399-5618.2007.00395.x. [DOI] [PubMed] [Google Scholar]
- 29.Houenou J, Wessa M, Douaud G, Leboyer M, Chanraud S, Perrin M, Poupon C, Martinot JL, Paillere-Martinot ML. Increased white matter connectivity in euthymic bipolar patients: diffusion tensor tractography between the subgenual cingulate and the amygdalo-hippocampal complex. Mol Psychiatry. 2007;12(11):1001–1010. doi: 10.1038/sj.mp.4002010. [DOI] [PubMed] [Google Scholar]
- 30.Beaulieu C. The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed. 2002;15(78):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 31.Le Bihan D. Diffusion, perfusion and functional magnetic resonance imaging [in French] J Mal Vasc. 1995;20(3):203–214. [PubMed] [Google Scholar]
- 32.Schwartz ED, Hackney DB. Diffusion-weighted MRI and the evaluation of spinal cord axonal integrity following injury and treatment. Exp Neurol. 2003;184(2):570–589. doi: 10.1016/S0014-4886(03)00295-4. [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee P, Miller JH, Shimony JS, Philip JV, Nehra D, Snyder AZ, Conturo TE, Neil JJ, McKinstry RC. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol. 2002;23(9):1445–1456. [PMC free article] [PubMed] [Google Scholar]
- 34.Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23(5):794–802. [PMC free article] [PubMed] [Google Scholar]
- 35.Concha L, Beaulieu C, Gross DW. Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Ann Neurol. 2005;57(2):188–196. doi: 10.1002/ana.20334. [DOI] [PubMed] [Google Scholar]
- 36.Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Röther J. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage. 2004;22(4):1767–1774. doi: 10.1016/j.neuroimage.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 37.Hasan KM, Narayana PA. Retrospective measurement of the diffusion tensor eigenvalues from diffusion anisotropy and mean diffusivity in DTI. Magn Reson Med. 2006;56(1):130–137. doi: 10.1002/mrm.20935. [DOI] [PubMed] [Google Scholar]
- 38.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 39.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 40.Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, Robson MD, Jones DK, Klein JC, Bartsch AJ, Behrens TE. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protocols. 2007;2(3):499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- 41.Anjari M, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edwards AD, Counsell SJ. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. Neuroimage. 2007;35(3):1021–1027. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 42.Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39(1):52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 43.Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM, James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130(pt 9):2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- 44.Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry. 2008;63(5):512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 45.Cader S, Johansen-Berg H, Wylezinska M, Palace J, Behrens TE, Smith S, Matthews PM. Discordant white matter N-acetylasparate and diffusion MRI measures suggest that chronic metabolic dysfunction contributes to axonal pathology in multiple sclerosis. Neuroimage. 2007;36(1):19–27. doi: 10.1016/j.neuroimage.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 46.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 47.First MB, Spitzer RL, Gibbon ML, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders—Research Version, Patient Edition (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- 48.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 49.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970;61(3):303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- 51.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 52.Nelson HE, Willison JR. The Revised National Adult Reading Test—Test Manual. NFER-Nelson; Windsor, Berkshire, England: 1991. [Google Scholar]
- 53.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165(3):313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilbert AR, Mataix-Cols D, Almeida JR, Lawrence N, Nutche J, Diwadkar V, Keshavan MS, Phillips ML. Brain structure and symptom dimension relationships in obsessive-compulsive disorder: a voxel-based morphometry study. J Affect Disord. 2008;109(12):117–126. doi: 10.1016/j.jad.2007.12.223. [DOI] [PubMed] [Google Scholar]
- 55.Almeida JRC, Akkal D, Hassel S, Travis MJ, Banihashemi L, Kerr N, Kupfer DJ, Phillips ML. Reduced gray matter volume in ventral prefrontal cortex but not amygdala in bipolar disorder: significant effects of gender and trait anxiety. Psychiatry Res Neuroimaging. doi: 10.1016/j.pscychresns.2008.02.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hassel S, Almeida JRC, Kerr N, Nau S, Ladouceur CD, Fissell K, Kupfer DJ, Phillips ML. Elevated striatal and decreased dorsolateral prefrontal cortical activity to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord. doi: 10.1111/j.1399-5618.2008.00641.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):10–17. [PubMed] [Google Scholar]
- 58.Davis JM, Chen N. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol. 2004;24(2):192–208. doi: 10.1097/01.jcp.0000117422.05703.ae. [DOI] [PubMed] [Google Scholar]
- 59.Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK. Lithium-induced increase in human brain grey matter [published correction appears in Lancet. 2000;356(9247):2104] Lancet. 2000;356(9237):1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- 60.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 62.Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 63.Highley JR, Walker MA, Esiri MM, Crow TJ, Harrison PJ. Asymmetry of the uncinate fasciculus: a post-mortem study of normal subjects and patients with schizophrenia. Cereb Cortex. 2002;12(11):1218–1224. doi: 10.1093/cercor/12.11.1218. [DOI] [PubMed] [Google Scholar]
- 64.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 65.Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55(6):578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 66.Strakowski SM, Adler CM, Holland SK, Mills N, DelBello MP. A preliminary fMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology. 2004;29(9):1734–1740. doi: 10.1038/sj.npp.1300492. [DOI] [PubMed] [Google Scholar]
- 67.Strakowski SM, Adler CM, Holland SK, Mills NP, DelBello MP, Eliassen JC. Abnormal FMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. Am J Psychiatry. 2005;162(9):1697–1705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- 68.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception, II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 69.Alexopoulos GS, Kiosses DN, Choi SJ, Murphy CF, Lim KO. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry. 2002;159(11):1929–1932. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- 70.Bae JN, MacFall JR, Krishnan KR, Payne ME, Steffens DC, Taylor WD. Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biol Psychiatry. 2006;60(12):1356–1363. doi: 10.1016/j.biopsych.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 71.Li L, Ma N, Li Z, Tan L, Liu J, Gong G, Shu N, He Z, Jiang T, Xu L. Prefrontal white matter abnormalities in young adult with major depressive disorder: a diffusion tensor imaging study. Brain Res. 2007;1168:124–128. doi: 10.1016/j.brainres.2007.06.094. [DOI] [PubMed] [Google Scholar]
- 72.Murphy CF, Gunning-Dixon FM, Hoptman MJ, Lim KO, Ardekani B, Shields JK, Hrabe J, Kanellopoulos D, Shanmugham BR, Alexopoulos GS. White-matter integrity predicts Stroop performance in patients with geriatric depression. Biol Psychiatry. 2007;61(8):1007–1010. doi: 10.1016/j.biopsych.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nobuhara K, Okugawa G, Sugimoto T, Minami T, Tamagaki C, Takase K, Saito Y, Sawada S, Kinoshita T. Frontal white matter anisotropy and symptom severity of late-life depression: a magnetic resonance diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2006;77(1):120–122. doi: 10.1136/jnnp.2004.055129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang Q, Huang XB, Hong N, Yu X. White matter microstructural abnormalities in late-life depression. Int Psychogeriatr. 2007;19(4):757–766. doi: 10.1017/S1041610207004875. [DOI] [PubMed] [Google Scholar]
- 75.Yuan Y, Zhang Z, Bai F, Yu H, Shi Y, Qian Y, Zang Y, Zhu C, Liu W, You J. White matter integrity of the whole brain is disrupted in first-episode remitted geriatric depression. Neuroreport. 2007;18(17):1845–1849. doi: 10.1097/WNR.0b013e3282f1939f. [DOI] [PubMed] [Google Scholar]
- 76.Atmaca M, Ozdemir H, Cetinkaya S, Parmaksiz S, Belli H, Poyraz AK, Tezcan E, Ogur E. Cingulate gyrus volumetry in drug free bipolar patients and patients treated with valproate or valproate and quetiapine. J Psychiatr Res. 2007;41(10):821–827. doi: 10.1016/j.jpsychires.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 77.Goodwin FK, Jamison KR, Ghaemi SN. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. Oxford University Press; New York, NY: 2007. [Google Scholar]