Summary
The synergism between red and blue light in the control of plant growth and development [1, 2] requires the co-action of the red/far-red light photoreceptor phytochrome B (phyB) and the blue/UV-A receptors, cryptochromes (cry) [3]. Here we describe the mechanism for the co-action of these photoreceptors in controlling both development and physiology. In seedlings grown under red light, a transient supplement with blue light induced persistent changes in the transcriptome and growth patterns. Blue light enhanced the expression of the transcription factors LONG HYPOCOTYL 5 (HY5) and HOMOLOG OF HY5 (HYH) [4] and of SUPPRESSOR OF PHYA 1 (SPA1) and SPA4 [5]. HY5 and HYH enhanced phyB signalling output beyond the duration of the blue-light signal and, contrary to their known role as repressors of phyA signalling [5], SPA1 and SPA4 also enhanced phyB signalling. These observations demonstrate that the mechanism of synergism involves the promotion by cry of positive regulators of phyB signalling. The persistence of the light-derived signal into the night commits the seedling to a morphogenetic and physiological program consistent with a photosynthetic lifestyle.
Results and Discussion
Synergism between phyB and cry1 generates hysteretic gene expression and growth patterns
Although several points of convergence between phy and cry signalling have been reported [6–9] none of them has been causally linked to the co-action between phy and cry. To investigate the mechanisms of phyB-cry1 co-action, we cultivated wild type (WT), cry1 and phyB seedlings of Arabidopsis thaliana for 3 days under continuous red light, a treatment that activates phyB but not cry. On the third day, the seedlings were given supplementary blue light for 3 h to activate cry, and then returned to red light (Fig. 1A). Seedlings were harvested and the processed RNA was hybridized to ATH1 Affymetrix microarrays. As a control, one set of seedlings was never subjected to the 3 h blue light treatment.
Figure 1.
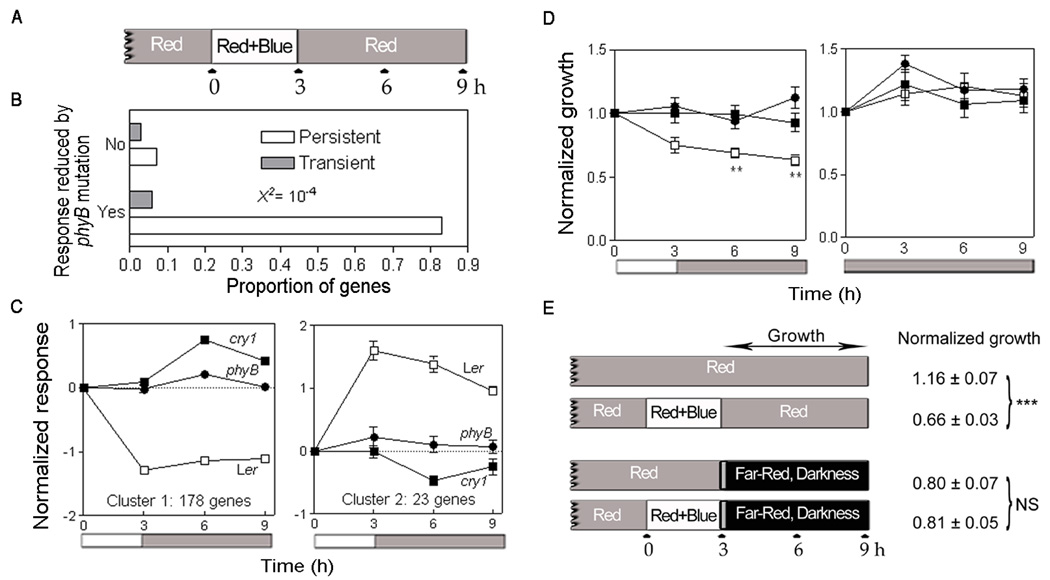
The co-action between cry1 and phyB generates hysteresis in gene expression and stem (hypocotyl) growth.
(A) Experimental protocol. Three-day-old seedlings grown under continuous red light (11 µmol . m−2 . s−1) were exposed to 3 h blue light (5 µmol . m−2 . s−1) added to the red-light background and harvested immediately after blue light (3 h), 3 h and 6 h after the end of blue light (6 h and 9 h harvest time, respectively) or without a blue light supplement as red-light controls (0 h). Light sources are described in Supplemental Experimental Procedures.
(B) Proportion of genes that show either transient or persistent responses to blue light perceived by cry1 as affected by the dependence of the blue-light response on phyB. The result of the X2 contingency test incorporating the correction of Yates is indicated.
(C) Average normalized expression of the genes corresponding to clusters containing at least 20 genes (other clusters are listed in Table S1). Both clusters correspond to the genes where the cry1-mediated response to blue light is persistent and depends on phyB.
(D) Persistent hypocotyl-growth response to blue light in the WT but not in the phyB or cry1 cry2 mutants. Three-day-old seedlings grown under continuous red light were exposed to 3 h blue light added to the red-light background (between time 0 and time 3 h). Rates are plotted at the end of the relevant 3 h-period. Data are means and SE of at least 25 (red plus blue) or 11 (red) seedlings. Data were analysed by two-way ANOVA and Bonferroni post-tests with time= 0: **= P <0.01, all the other differences with time=0 are not significant (P >0.05).
(E) The hysteresis is caused by a persistent enhancement of phyB-mediated output by transient cry1 activation. Red-light-grown seedlings were exposed to 3 h of red plus blue light or left as red-light controls and then transferred either back to red light (data from Figure 1D) or to a pulse of far-red light followed by darkness. Data were analysed by two-way ANOVA and Bonferroni post-tests. ***= P <0.001, NS not significant.
To select the genes specifically controlled by cry1 in response to blue light, we searched our database and identified 324 genes showing a response to the blue light treatment significantly reduced in the cry1 mutant (P <0.005, q <0.1 [10], Table S1). These genes were then further classified using two different criteria: a) whether the cry1-mediated response to blue light either disappears or persists beyond the duration of the blue-light treatment, and b) whether the cry1-mediated response to blue light is either reduced or not reduced in the absence of active phyB (i.e. in the phyB-mutant background). The combination of these criteria defines four groups of genes (Figure 1B). Most of the genes that show a cry1-mediated response to blue light belong to the group where the response was persistent beyond the blue light treatment and required phyB (Figure 1B). In turn, the genes of this group formed two major clusters (Figure 1C, Table S1): Cluster 1, with expression repressed by blue light, contains genes like CULLIN 4 [11], ARGONAUTE1 [12], AINTEGUMENTA [13], CYCLIN-DEPENDENT KINASE C;2 [14], SYNTAXIN 23 [15], which have known function in the regulation of development (e.g. leaf development, photomorphogenesis) and could shape plant body form and function in response to the blue light signal. Cluster 2, with expression promoted by blue light, contains several chloroplast-related genes, including two FtsH protease genes [16], suggesting that transient blue light perceived by cry1 might trigger acclimation of the photosynthetic apparatus.
In some cases, the response to a transient signal can persist well beyond the duration of the signal, a phenomenon called hysteresis [17, 18]. A brief exposure to red light, for instance, shifts phyB to its active stage that persists many hours in darkness. The hysteresis in gene expression can also have its origin in the transcriptional networks and the system design principles of these networks are under intense research given their particular importance for the transformation of short signals into developmental decisions [17, 18]. Since 3 h blue light generated changes that persisted 6 h after the termination of blue light, we conclude that the synergism between phyB and cry1 generates hysteresis in gene expression.
The inhibition of the rate of stem (hypocotyl) growth by light is a key feature of the photomorphogenic pattern of development. Prompted by the results of transcriptome studies, we used the same light protocol to investigate hypocotyl-growth responses. The experiments were done in the Columbia background for the subsequent analysis of signalling mutants and we used the cry1 cry2 double mutant because in Columbia, cry2 makes a contribution to the blue light response that is not obvious in Landsberg erecta [3] (see below). In WT seedlings exposed to continuous red light, blue light reduced hypocotyl extension growth and the rate of growth did not recover after termination of the blue-light treatment (Figure 1D). In the phyB mutant or the cry1 cry2 double mutant, blue light failed to inhibit the rate of hypocotyl elongation (Figure 1D). We conclude that the synergism between phyB and cry1-cry2 generates hysteresis in the inhibition of hypocotyl growth.
We also used a variation of the protocol shown in Fig. 1A, where at time 3 h the seedlings were given a pulse of far-red light followed by darkness to reduce the amount of active phyB to a minimum. Compared to red-light controls (never exposed to blue light), the inhibition of growth persistent beyond blue light was observed if the seedlings returned to red light at time 3 h but not if the seedlings were given far-red followed by darkness (Figure 1E). This indicates that phyB has to be active after cry activation, even if phyB had been active under red light before and during blue light. The duration of cry in its signalling stage after the termination of blue light is not established. Green light appears to return cry to its inactive state [19]. Therefore, green light given after the blue-light treatment is predicted to reduce the levels of active cry eventually present after the end of blue light. This green-light treatment did not reduce the synergism (Figure S1). We can therefore establish a sequence where cry enhances phyB-mediated signalling and not vice versa.
cry1 activity recruits new genes under phyB control
The comparison of expression in red-light controls of WT versus phyB-mutant seedlings identified 551 genes with expression affected by phyB (P <0.005, q <0.1, [10]; Table S2). However, only 6 % of the genes showing synergism between cry1 and phyB had expression levels already affected by phyB under red light alone (Table S2). This very restricted overlap indicates that activation of cry1 recruits new genes to the control by phyB, which were not affected by phyB in the absence of cry1 activity.
cry1 activity recruits new genes to phyB signalling
Consistent with previous reports, blue light promoted the expression of several genes with known function in photomorphogenesis, including SPA1, SPA4, HY5 and HYH (Figure S2 [4, 20]). SPA1, which forms complexes with E3 ligase activity [21] is a negative regulator of phyA signalling [5]. HY5 and HYH are basic leucine zipper transcription factors that mediate photomorphogenesis [4].The induction of these genes showed no synergism between phyB and cry1 because the promotion by blue light was reduced by the cry1 but not by the phyB mutation (Figure S2). The lack of hysteresis in the expression of these genes is consistent with the absence of synergism. However, the products of these genes could be involved in the generation of co-action and we therefore tested this possibility.
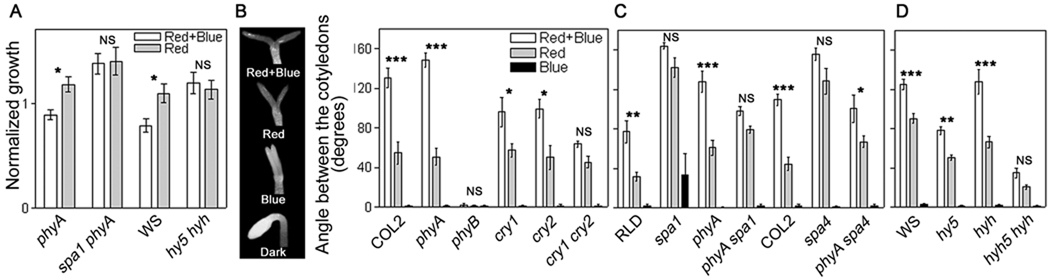
The spa1 mutation (tested in the phyA mutant background to avoid phyA-mediated effects) and the hy5 hyh mutations eliminated the inhibition of hypocotyl growth by blue light compared to the control seedlings under continuous red light (Figure 2A). Thus, SPA1, which is a negative regulator of phyA signalling [5], here appears as a positive regulator of phyB signalling. This indicates that the hysteresis in the inhibition of hypocotyl growth generated by the combined action of phyB and cry requires SPA1 and HY5/HYH. Neither SPA1 nor HY5/HYH were inhibiting hypocotyl growth immediately before blue light (absolute growth rates, means ±SE, mm h−1, phyA: 0.22 ±0.02; phyA spa1: 0.21 ±0.02; WS: 0.26 ±0.01; hy5 hyh: 0.21 ±0.01) but they are required for growth inhibition after blue light (Figure 2A). This suggests that the expression of SPA1 and HY5/HYH is below a threshold under red light and the promotion of their expression by blue light perceived by cry is necessary to trigger the persistent growth inhibition.
Figure 2.
Synergism between phyB and cryptochromes requires SPA1, SPA4, HY5 and HYH.
(A) The growth response to blue light requires SPA1 and HY5 /HYH. The average growth rate during 9 h is presented for the seedlings that received blue light plus red light during the first 3 h of this period and for red-light controls. Data are means and SE of at least 14 seedlings. The difference between red and red plus blue was analysed by two-way ANOVA and Bonferroni post-tests: NS= not significant (P >0.05), *= P <0.05.
(B) Cotyledon unfolding depends on the co-action between phyB and cry. Cotyledon unfolding under continuous red light is enhanced by a daily supplement of 3 h blue light, a treatment that is not effective if provided without the red-light background. The seedlings were grown under continuous red light daily supplemented with 3 h of blue light, continuous red light, daily blue light (3 h) without a continuous red-light background or in darkness.
(C) The co-action between phyB and cry requires SPA1, SPA4, HY5 and HYH. Data are means and SE of at least 6 replicate boxes. The difference between red and red plus blue was analysed by two-way ANOVA and Bonferroni post-tests: NS= not significant (P >0.05), *= P <0.05, **= P <0.01, ***= P <0.001.
To investigate the long-term consequences of the synergism, we used the aforementioned light protocol (Fig. 1A) repeated during three days, and included controls in darkness and controls where daily exposure to 3 h of blue light terminated with a pulse of long-wavelength far-red light followed by darkness to activate cry without activating phyB. Without the red-light background to activate phyB, blue light had no significant effects on cotyledon unfolding. However, 3 h of blue light added to a continuous red light background that activates phyB did promote cotyledon unfolding (Figure 2B). Both the phyB and the cry1 cry2 mutants failed to respond to blue light (Figure 2B). The unfolding response depends on the co-action between phyB and cry. In the spa1 and spa4 mutants, 3 h of blue light had no effect when added to red light (Figure 2C). The failure of the spa1 and spa4 mutants to respond to blue light added to red light was also evident in the phyA mutant background (Figure 2C). Therefore, SPA1 and SPA4 enhance phyB-mediated responses independently of phyA when the seedlings are exposed to blue light. The latter is a novel function of SPA genes, which could involve the degradation of negative regulators of phyB signalling. SPA proteins work in concert with COP1, and the cop1 mutants failed to show the synergism between cry and phyB (data not shown). COP1 also acts as a positive regulator of photomorphogenesis mediated by phyB [22].
The hy5 mutant showed reduced cotyledon unfolding under red light but apparently normal responses to supplementary blue light (Figure 2D). The hyh mutant showed normal unfolding under red light [4, 20] and apparently larger synergism between red and blue light than the WT (strong cotyledon unfolding in hyh has recently been reported [23]). However, the hy5 hyh double mutant failed to respond to the blue-light supplement (Figure 2C). This indicates that HY5 and HYH are redundantly required for the co-action between phyB and cry. Interestingly, 27 % of the genes promoted (cluster 2, Fig. 1C) and 11 % of the genes inhibited (cluster 1) by blue light in a phyB-dependent manner are direct targets of HY5 [24], which matches the proportion of direct targets of HY5 in microarrays comparing WT and hy5 seedlings (26 % of the genes promoted and 12 % of the genes inhibited by HY5 are their direct targets) [24]. While a significant proportion of the genes that show hysteresis in their expression patterns are direct targets of HY5, the HY5 and SPA1 promoters are not direct targets of HY5 and HYH and SPA4 are direct targets but their expression is unaffected by the hy5 mutation [4, 24]. In principle, this could account for the lack of hysteresis in the expression of HY5, HYH, SPA1 and SPA4 (Figure S2).
Light and circadian signals that control flowering and hypocotyl growth have a common model of convergence because in both cases one signal (the clock) controls mRNA levels and the other signal (light) the stability of the protein derived from this mRNA (CONSTANS in the case of flowering [25], PIF4 and PIF5 in the case of growth [26]). Since cry controls HY5 expression and light is known to down-regulate COP1-mediated degradation of HY5 protein in the proteasome [4] we investigated whether phyB was necessary to stabilize HY5 in the temporal frame when phyB is necessary for the persistent output of cry excitation. Shifting phyB to its inactive stage by far-red light abolished the persistent effect of blue light on hypocotyl growth (Figure 1E) but it had no effect on HY5 stability (Figure S3). We conclude that phyB is necessary for the response to HY5 but not to stabilize HY5. Cry could enhance the expression of HY5, HYH, SPA1 and SPA4 by inactivating COP1 and consequently stabilising transcription factors [27] acting upstream these genes. However, blue light did not enhance HY5 stability (one of the known targets of COP1 [27]) within the time frame where the synergism is observed (Figure S3), probably because red light is able per se to stabilise HY5 [27].
The synergism between phyB and cry reinforces seedling commitment to photomorphogenesis
The combined action of phyB and cry generates hysteretic responses that persist beyond the presence of the blue-light treatment required to activate cry. Since phyB remains active during the first part of the night [28, 29] we speculated that this synergism could help the plant to maintain the inhibition of hypocotyl growth during darkness. Seedlings of Arabidopsis were grown in darkness for 2 d and transferred to light (red plus blue) for 12 h followed by 12 h of darkness. In the WT, hypocotyl growth was partially reduced during light exposure and largely arrested during the subsequent night (Figure 3). The phyB or cry mutations had little effect during light exposure (their contribution can be stronger in older seedlings or at higher irradiances) but mutations affecting either phyB or cry were enough to eliminate the persistent inhibition of hypocotyl growth in subsequent darkness (Figure 3). The synergism between phyB and cry is necessary for the specification of the developmental fate of the seedling and if one of the two photoreceptor types is missing, growth returns to the values observed before the light stimulus.
Figure 3.
The synergistic action between phyB and cry1 maintains reduced growth rates during the night. Seedlings were grown against vertical agar in darkness for 2 d and transferred to light (red plus blue) for 12 h (day) followed by 12 h of darkness (night). Hypocotyl length increments were recorded during the light exposure and during the subsequent night period as well as in seedlings that remained in darkness (dark controls). Data are means and SE of at least 16 seedlings. The difference between light and darkness was analysed by two-way ANOVA and Bonferroni post-tests: NS= not significant (P >0.05), ***= P <0.001.
Conclusions
Understanding the mechanisms involved in the integration of dynamic signals is one of the challenges of modern biology [30]. The convergence between circadian cues and light typically involves the control of mRNA abundance by the clock and the control of its protein product by light [25, 26]. The convergence between phyB and cry signalling follows a model at least partially different. Blue light perceived by cry enhances the expression of SPA1, SPA4, HY5 and HYH independently of phyB. Then, SPA1, SPA4, HY5 and HYH enhance phyB-mediated signalling independently of cry (i.e. after the termination of the blue-light signal) (Figure S4). Cry recruits new genes to the control by phyB and a significant proportion of these genes are direct targets of HY5. Thanks to the action of phyB, the effects triggered by cry persist well beyond the blue light signal that activates cry, creating a hysteretic switch. Since phyB can remain active in darkness, the synergism helps to maintain the commitment to a photoautotrophic lifestyle during the night.
Experimental procedures
Microarray experiments
The cry1-1 (formerly hy4-2.23n [31]) and phyB-5 (formerly hy3 [31]) mutants and the WT used for microarray experiments are in the Landsberg erecta background. Seedlings were grown in boxes with agar as described [3]. Samples were harvested in liquid nitrogen, and total RNA was extracted using the RNEasy plant mini kit (Qiagen). For each time point, samples were pooled to obtain three (WT) or two (phyB, cry1) biologically independent replicates. cDNA and cRNA synthesis and hybridization to ATH1 Affymetrix Arabidopsis Gene Chips were performed according to Affymetrix instructions. Expression data (Table S3) were normalised, restricted by presence criteria and used for ANOVA to identify the genes showing expression responses to blue light perceived by cry1. These genes were then used to investigate the dependence of the response to blue light on phyB and the transient or persistent nature of the response to blue light by means of contrasts based on partition of the sum of squares of the ANOVA following criteria established a priori. These procedures are described in detail in Supplemental Experimental Procedures.
Measurements of hypocotyl growth and cotyledon angle
For physiological experiments we used phyB-9 [32], phyA-211 [33], cry1-304 ,cry2-1 [34], spa4-1 [35] in Columbia; hy5-KS50, hyh and hy5KS50-hyh [4] in Ws; spa1– 2 and phyA-101 [36] in RLD and phyA-101 spa4-1 in a mixed Columbia/RLD background. Seedlings were grown in boxes with agar as described [3]. Measurements of hypocotyl growth are based on the analysis of successive photographs as described in Supplemental Experimental Procedures and cotyledon angle was measured with a protractor.
Supplementary Material
Acknowledgements
We thank Dr Xing-Wang Deng for providing hy5, hyh and hy5 hyh mutant seeds and Dr Giltsu Choi for providing HY5 OX1 seeds. This work was supported by a Fogarty International Research Collaboration Award (US-NIH) 6836 to JC (NIH RO152413) with JJC as foreign collaborator and by grants from ANPCYT (Argentina), PICT 11631 and 32492 to JJC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Data
Supplemental Data include Experimental procedures, Three Tables and Four Figures.
References
- 1.Meijer G, Engelsma G. The synergistic influence of a pre-irradiation on the photoinhibition of gherkin seedlings. Photochem. Photobiol. 1965;4:251–258. [Google Scholar]
- 2.Oelmüler R, Mohr H. Mode of coaction between blue/UV light and light absorbed by phytochrome in light-mediated anthocyanin formation in the milo (Sorghum vulgare Pers.) seedling. Proc. Natl. Acad. Sci. U.S.A. 1985;82:6124–6128. doi: 10.1073/pnas.82.18.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casal JJ, Mazzella MA. Conditional synergism between cryptochrome 1 and phytochrome B Is shown by the analysis of phyA, phyB and hy4 simple, double and triple mutants in Arabidopsis. Plant Physiol. 1998;118:19–25. doi: 10.1104/pp.118.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holm M, Li-Geng M, Li-Jia Q, Deng XW. Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev. 2002;16:1247–1259. doi: 10.1101/gad.969702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laubinger S, Hoecker U. The SPA1-like proteins SPA3 and SPA4 repress photomorphogenesis in the light. Plant J. 2003;35:373–385. doi: 10.1046/j.1365-313x.2003.01813.x. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad M, Jarrillo JA, Smirnova O, Cashmore AA. The CRY1 blue light photoreceptor of Arabidopsis interacts with phytochrome A in vitro. Molecular Cell. 1998;1:939–948. doi: 10.1016/s1097-2765(00)80094-5. [DOI] [PubMed] [Google Scholar]
- 7.Más P, Devlin PF, Panda S, Kay SA. Functional interaction of phytochrome B and cryptochrome 2. Nature. 2000;408:207–211. doi: 10.1038/35041583. [DOI] [PubMed] [Google Scholar]
- 8.Guo H, Mockler T, Duong H, Lin C. SUB1, an Arabidopsis Ca2+-binding protein involved in cryptochrome and phytochrome coaction. Science. 2001;19:487–490. doi: 10.1126/science.291.5503.487. [DOI] [PubMed] [Google Scholar]
- 9.Duek PD, Fankhauser C. HFR1, a putative bHLH transcription factor, mediates both phytochrome A and cryptochrome signalling. Plant J. 2003;34:827–836. doi: 10.1046/j.1365-313x.2003.01770.x. [DOI] [PubMed] [Google Scholar]
- 10.Storey JD, Tibshirani R. Statistical significance of genomewide studies. Proc. Nat. Acad. Sci. U.S.A. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Shen Y, Tang X, Yu L, Wang J, Guo L, Zhang Y, Zhang H, Feng S, Strickland E, Zheng N, Deng XW. Arabidopsis CULLIN4 forms an E3 ubiquitin ligase with RBX1 and the CDD complex in mediating light control of development. Plant Cell. 2006;18:1991–2004. doi: 10.1105/tpc.106.043224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorin C, Bussell JD, Camus I, Ljung K, Kowalczyk M, Geiss G, McKhann H, Garcion C, Vaucheret H, Sandberg G, Bellini C. Auxin and light control of adventitious rooting in Arabidopsis require ARGONAUTE1. Plant Cell. 2005;17:1343–1359. doi: 10.1105/tpc.105.031625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nole-Wilson S, Krizek BA. AINTEGUMENTA contributes to organ polarity and regulates growth of lateral organs in combination with YABBY genes. Plant Physiol. 2006;141:977–987. doi: 10.1104/pp.106.076604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui X, Fan B, Scholz J, Chen Z. Roles of Arabidopsis cyclin-dependent kinase C complexes in cauliflower mosaic virus infection, plant growth and development. Plant Cell. 2007;19:1388–1402. doi: 10.1105/tpc.107.051375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohtomo I, Ueda H, Shimada T, Nishiyama C, Komoto Y, Hara-Nishimura I, Takahashi T. Identification of an allele of VAM3/SYP22 that confers a semi-dwarf phenotype in Arabidopsis thaliana. Plant Cell Physiol. 2005;46:1358–1365. doi: 10.1093/pcp/pci146. [DOI] [PubMed] [Google Scholar]
- 16.Zaltsman A, Ori N, Adam Z. Two types of FtsH protease subunits are required for chloroplast biogenesis and photosystem II repair in Arabidopsis. Plant Cell. 2005;17:2782–2790. doi: 10.1105/tpc.105.035071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ninfa AJ, Mayo AE. Hysteresis vs. graded responses: the connections make all the difference. STKE. 2004;232:pe20. doi: 10.1126/stke.2322004pe20. [DOI] [PubMed] [Google Scholar]
- 18.Kramer BP, Fussenegger M. Hysteresis in synthetic mammalian gene network. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9517–9522. doi: 10.1073/pnas.0500345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouly JP, Schleicher E, Dionisio-Sese M, Vandenbussche F, Van Der Straeten D, Bakrim N, Meier S, Batschauer A, Galland P, Bittl R, Ahmad M. Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states J. Biol. Chem. 2007;282:9383–9391. doi: 10.1074/jbc.M609842200. [DOI] [PubMed] [Google Scholar]
- 20.Fittinghoff K, Laubinger S, Nixdorf M, Fackendahl P, Baumgardt RL, Batschauer A, Hoecker U. Functional and expression analysis of Arabidopsis SPA genes during seedling photomorphogenesis and adult growth. Plant J. 2006;47:577–590. doi: 10.1111/j.1365-313X.2006.02812.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhu D, Maier A, Lee JH, Laubinger S, Saijo Y, Wang H, Qu LJ, Hoecker U, Deng XW. Biochemical characterization of Arabidopsis complexes containing CONSTITUTIVELY PHOTOMORPHOGENIC1 and SUPPRESSOR OF PHYA proteins in light control of plant development. Plant Cell. 2008;20:2307–2323. doi: 10.1105/tpc.107.056580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boccalandro HE, Rossi MC, Saijo Y, Deng XW, Casal JJ. Promotion of photomorphogenesis by COP1. Plant Molec. Biol. 2004;56:905–915. doi: 10.1007/s11103-004-5919-8. [DOI] [PubMed] [Google Scholar]
- 23.Alabadí D, Gallego-Bartolomé J, O L, García-Cárcel L, Rubio V, Martínez C, Frigerio M, Iglesias-Pedraz JM, Espinosa A, Deng XW, Blázquez MA. Gibberellins modulate light signaling pathways to prevent Arabidopsis seedling de-etiolation in darkness. Plant J. 2008;53:324–335. doi: 10.1111/j.1365-313X.2007.03346.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW. Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell. 2007;19:731–749. doi: 10.1105/tpc.106.047688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. Photoreceptor regulation of CONSTANS protein and the mechanism of photoperiodic flowering. Science. 2004;303:1003–1006. doi: 10.1126/science.1091761. [DOI] [PubMed] [Google Scholar]
- 26.Nozue K, Covington MF, Duek PD, Lorrain S, Fankhauser C, Harmer SL, Maloof JN. Rhythmic growth explained by coincidence between internal and external cues. Nature. 2007;448:358–361. doi: 10.1038/nature05946. [DOI] [PubMed] [Google Scholar]
- 27.Osterlund MT, Hardtke CS, Wei N, Deng XW. Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature. 2000;405:462–466. doi: 10.1038/35013076. [DOI] [PubMed] [Google Scholar]
- 28.Downs RJ, Hendricks SB, Borthwick HA. Photoreversible control of elongation of pinto beans and other plants under normal conditions of growth. Bot. Gaz. 1957;118:199–208. [Google Scholar]
- 29.Casal JJ. Phytochrome A enhances the promotion of hypocotyl growth caused by reductions of phytochrome B Pfr levels in light-grown Arabidopsis thaliana. Plant Physiol. 1996;112:965–973. doi: 10.1104/pp.112.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vivekanand P, Rebay I. Intersection of signal transduction pathways and development. Ann. Rev. Genet. 2006;40:139–157. doi: 10.1146/annurev.genet.40.110405.090555. [DOI] [PubMed] [Google Scholar]
- 31.Koornneef M, Rolf E, Spruit CJP. Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Z Pflanzenphysiol. 1980;100:147–160. [Google Scholar]
- 32.Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the Red/Far-Red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo H, Duong H, Ma N, Lin C. The Arabidopsis blue-light receptor cryptochrome 2 is a nuclear protein regulated by a blue-light dependent post-transcriptional mechanism. Plant J. 1999;19:279–289. doi: 10.1046/j.1365-313x.1999.00525.x. [DOI] [PubMed] [Google Scholar]
- 35.Laubinger S, Hoecker U. The SPA1-like proteins SPA3 and SPA4 repress photomorphogenesis in the light. Plant J. 2003;35:373–385. doi: 10.1046/j.1365-313x.2003.01813.x. [DOI] [PubMed] [Google Scholar]
- 36.Hoecker U, Tepperman JM, Quail PH. SPA1, a WD-repeat protein specific to phytochrome A signal transduction. Science. 1999;284:496–499. doi: 10.1126/science.284.5413.496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.