Abstract
Notch receptor signaling is required for T cell development, but its role in NK cell development is poorly understood. We compared the ability of the five mammalian Notch ligands (Jagged1, Jagged2, Delta1, Delta3, or Delta4) to induce NK cell development from human hematopoietic progenitor cells (HPCs). CD34+ HPCs were cultured with OP9 stromal cell lines transduced with one of the Notch ligands or with OP9 stromal cells alone, in the presence of IL-7, Flt3L, and IL-15. Differentiation and expansion of CD56+CD3− cells was greatly accelerated in the presence of Jagged2, Delta-1, or Delta-4, versus culture in the absence of ligand or in the presence of Jagged1 or Delta3. At four weeks, cultures containing Jagged2, Delta1, or Delta4 contained 80–90% NK cells, with the remaining cells being CD33+ myeloid cells. Notch-induced NK (N-NK) cells resembled CD56bright NK cells in that they were CD16-, CD94−, CD117+, and KIR-. They also expressed NKp30, NKp44, NKp46, 2B4, and DNAM-1, with partial expression of NKG2D. The N-NK cells displayed cytotoxic activity against the K562 and RPMI-8226 cell lines, at levels similar to activated peripheral blood NK cells, although killing of Daudi cells was not present. N-NK cells were also capable of IFN-γ secretion. Thus, Notch ligands have differential ability to induce and expand immature but functional NK cells from CD34+ HPCs. The use of Notch ligands to generate functional NK cells in vitro may be significant for cellular therapy purposes.
Introduction
Natural killer (NK) cells are lymphocytes which arise from a common lymphoid progenitor cell during hematopoiesis. Unlike T and B lymphocytes, however, NK cells do not express rearranged antigen receptors and are therefore considered part of the innate immune system. NK cells have the unique ability to kill tumor or virally-infected cells without prior immunization. Another major function of NK cells is to secrete cytokines, such as IFN-γ and TNF-α, which augment inflammatory immune responses and also influence adaptive immunity. Human NK cells are defined as CD56+CD3− lymphocytes, which morphologically are indistinguishable from CD8+ granular T cells. The circulating pool of NK cells in humans consists of two populations: CD56dimCD16bright and CD56brightCD16dim. The former population is predominant (>95% of circulating NK cells), has more cytotoxic than cytokine-producing activity, and may represent a mature NK cell population which evolves from CD56brightCD16dim cells.(1) Accordingly, CD56bright NK cells express CD117, higher levels of CD94 (which heterodimerizes with NKG2A (Natural Killer Group 2, member A) or NKG2C to form HLA-E recognition receptors), and CCR7, which may allow for NK cell trafficking to lymph nodes.(2) In contrast, CD56dim NK cells express higher levels of killer immunoglobulin-like receptors (KIRs) and the natural cytotoxicity receptors (NCRs), which include NKp30, NKp44, and NKp46, important for tumor cell recognition and lysis. CD56dim NK cells down-regulate expression of CCR7 and express CXCR1, which binds IL-8 and is postulated to aid in the CD56dim NK cell response to inflammation.(2) Members of the KIR family are critical for transmission of inhibitory signals to NK cells upon binding of self-MHC class I molecules, thus mediating self-tolerance of CD56dim NK cells. Expression of CD16, also known as the low-affinity IgG Fc receptor, also allows CD56dim NK cells to mediate antibody-dependent cell-mediated cytotoxicity in the presence of antibody specific to target cells. Both types of NK cells express NKG2D, a homodimeric activating receptor which binds stress-inducible ligands on tumor cells.
It has been traditionally postulated that human NK cells develop in the bone marrow, but recent data suggest that NK cell precursors from the bone marrow may migrate to secondary lymphoid organs, where maturation of NK precursors into CD56bright NK cells can occur. The NK cell precursors identified in lymph nodes have been divided into subsets based on expression of CD34, CD94, and CD117.(3–5) In line with this hypothesis, CD56bright NK cells are found preferentially in lymph nodes and can express CD16, KIRs, and NCRs, as well as acquire cytotoxic activity, upon activation.(6, 7)
In vitro, both mouse and human NK cells can differentiate from bone marrow- or cord blood-derived hematopoietic progenitor cells (HPCs) in the presence of cytokines alone; these NK cells appear phenotypically immature and resemble CD56bright cells.(8) Culture of HPCs in the presence of both cytokines and stromal cells allows for further maturation of NK cells, including acquisition of KIRs or the murine equivalent, the Ly49 receptor family.(9, 10) The signals mediated by stromal cells to induce NK cell maturation are unknown, but one potential signal may be from Notch ligands expressed by stromal cells.
The evolutionarily conserved Notch receptor pathway plays important roles in cell-fate decisions and is essential for T cell development.(11) In vertebrates, 4 different Notch receptors, Notch 1–4, may bind up to 5 known ligands, Jagged1, Jagged2, Delta1, Delta3, and Delta4. Binding of different Notch ligands to distinct Notch receptors can variably influence T cell development.(12–15) In particular, the Notch1:Delta4 interaction has been shown to have greater capacity to induce murine T cell development versus Notch binding of Delta1.(12) Although T and NK cells are postulated to derive from a common T/NK precursor, the Notch1 receptor is not absolutely required for NK cell development in vivo, as an inducible Notch1-knockout mouse has normal levels of NK1.1+ cells.(16) The importance of signaling via other Notch receptors in NK cell development is unclear. In vitro, Notch signaling mediated by binding of Jagged2, in the presence of cytokines important for NK cell differentiation (IL-15 or IL-2), induces murine NK cell development from Lin-Sca1+c-kit+ HPCs,(17) while Delta1 has recently been shown to cause NK cell differentiation from human CD34+ HPCs.(18) Thus, there is in vitro evidence that Notch signaling can drive NK cell differentiation from the early hematopoietic precursors.
To further define the capacity of Notch ligands to modulate development of human NK cells, we determined the differential ability of the 5 different Notch ligands found in vertebrates to induce NK cell differentiation and expansion from CD34+ HPCs isolated from umbilical cord blood (UCB). In addition, we analyzed the cell surface phenotype and function of Notch-induced NK (N-NK) cells and compared these characteristics to NK cells generated in vitro in the absence of Notch signaling, as well as to NK cells found in normal adult peripheral blood. N-NK cells display a predominantly immature CD56bright surface phenotype, with no expression of CD16, and the capacity to secrete IFN-γ. Unlike physiologic CD56bright NK cells, however, they do not express CD94 and have only moderate levels of NKG2D expression. In addition, N-NK cells lack the inhibitory receptors NKG2A and KIRs, yet they do express all three of the NCRs, NK-30, NKp44, and NKp46, and are able to lyse hematopoietic tumor cell lines in vitro. Thus, the receptor profile of N-NK cells is biased toward activating NK cell receptors. Finally, the presence of Notch ligand stimulates marked expansion of NK cells from HPCs, resulting in average yields of 700 to 1100 NK cells per input cell.
The use of Notch ligands to generate large numbers of functional, inhibitory receptor-negative NK cells may be useful for producing human NK cells for cell therapy purposes, as infusion of allogeneic NK cells is one strategy for tumor immunotherapy.(19, 20) The absence on N-NK cells of inhibitory receptors (KIR and NKG2A) which recognize MHC class I antigens would be advantageous in an allogeneic therapy setting, circumventing the need for KIR:HLA ligand mismatch between donor and recipient. Elucidation of the ability of different Notch ligands to induce NK cell differentiation and expansion, as well as evidence for the functionality of N-NK cells, allow for more optimal design of in vitro NK cell culture strategies for cell therapy.
Methods
OP9 cell lines
The murine bone marrow stromal cell line OP9(21) was transduced with one of five different Notch ligands in a bicistronic message with green fluorescent protein (GFP) in the vector Ret-10, to generate the lines OP9-Jagged1, OP9-Jagged2, OP9-Delta1, OP9-Delta3, and OP9-Delta4, as previously described.(22) A control cell line, OP9-Ret-10, was transduced with vector containing GFP alone. The OP9 cell lines were maintained in α-MEM with 20% FBS and antibiotics.
Human cells and OP9 co-culture
Peripheral blood was obtained from healthy volunteers via the Stem Cell Core Facility of the Case Comprehensive Cancer Center via IRB-approved protocols, and NK cells were isolated from the mononuclear cell (MNC) fraction using a negative selection kit from Miltenyi Biotec (Bergisch Gladbach, Germany). For isolation of CD34+ HPCs, UCB units were obtained from the labor and delivery unit at University Hospitals Case Medical Center under an IRB-approved protocol. After ficoll gradient, CD34+ cells were isolated from UCB MNCs using the CD34 MACS system from Miltenyi Biotec. Flow cytometric analysis of purified CD34+ cells routinely demonstrated ≥97% purity. CD34+ cells were used fresh or cryopreserved for later use.
For co-culture, 2 × 104 OP9 cells were seeded per well in 12-well plates and allowed to adhere at least 4 hours at 37°C, after which time 2–2.5 × 104 CD34+ UCB HPCs were plated onto the OP9 cells in RPMI supplemented with 10% FBS and antibiotics. Cells were cultured in the presence of recombinant cytokines: hIL-7 (10 ng/ml), hFlt3 ligand (10 ng/ml), and hIL-15 (10 ng/ml). In some initial experiments, hIL-2 (25 ng/ml) was used in place of hIL-15. When indicated, cultures were further stimulated with hIL-12 and hIL-18, both used at100 ng/ml. All cytokines were purchased from R&D Systems (Minneapolis, MN). Half of the media in the co-culture wells was changed twice weekly, with addition of fresh cytokines. To prevent OP9 overgrowth, HPCs were transferred to fresh OP9 monolayers weekly. After two weeks of co-culture, the hematopoietic cells were split in half onto new OP9 monolayers at least weekly, to prevent overcrowding. Some co-cultures were plated and cultured in the presence of 10 μm of the γ-secretase inhibitor, L-685,458 (Sigma Aldrich, St. Louis, MO) dissolved in DMSO.(22)
Flow cytometry
Cells were harvested from co-culture at indicated time points and were treated with human FcR blocking reagent (Miltenyi Biotec) prior to antibody staining. The following anti-human antibodies were used for analysis: CD1a-PE, CD2-FITC, CD3-FITC, CD7-FITC, CD11b (MAC-1)-FITC, CD11c-FITC, CD16-FITC or –PE, CD18-FITC, CD19-FITC, CD33-PE, CD56-FITC or –PE or –APC, CD62L (L-selectin)-PE, CD94-FITC, CD117-PE, CD181 (CXCR1)-FITC, CD244 (2B4)-PE, CD226 (DNAM-1)-FITC, CD314 (NKG2D)-FITC or –PE, ICAM-1-PE, and perforin (eBioscience, San Diego, CA); CD335 (NKp46)-PE, CD336 (NKp44)-PE, and CD337 (NKp30)-PE (Miltenyi Biotec); CD11a (LFA-1)-PE, NKG2A-PE, and NKG2C-PE (R&D Systems); CD158a-PE and CD158b-PE (Immunotech Beckman Coulter, Marseille, France); DX9 (BD Pharmingen, San Jose, CA). KIR expression was determined by combining the CD158a, CD158b, and DX9 antibodies, which combination will detect KIR2DL1/2/3, KIR2DS1/2/4, and KIR3DL1.(23) For analysis of perforin expression, cells were fixed and permeabilized using FIX & PERM® (Invitrogen, Carlsbad, CA) according to manufacturer’s protocol and stained with perforin and CD56 antibodies. Three color (FITC, PE, propidium iodide [PI]) analysis was performed on a FACScan machine (Becton Dickinson, Franklin Lakes, NJ) and analyzed with the manufacturer’s CellQuest software. Alternatively, four color (FITC, PE, PI, APC) analysis was performed using a FACSAria (Becton Dickinson) and analyzed using BD’s FACScalibur software. All analysis used forward and side scatter gating to exclude OP9 cells and PI exclusion to gate on live cells.
Cytotoxicity assays and inhibition of perforin
The Cytotox 96® non-radioactive cytotoxicity assay (Promega, Madison WI) measures LDH release by colorimetric methods. After 3 or 4 weeks of co-culture, harvested cells were analyzed for percent CD56+CD3− (effector, E) cells by flow cytometry. For cells derived in the absence of Notch ligand, due to the low level of CD56+CD3− cells, NK cell enrichment was performed via negative selection using anti-CD13 antibody (eBioscience) and goat-anti mouse IgG magnetic beads (Miltenyi Biotec). Effector cells were plated in triplicate in 96-well round-bottom plates with 1 or 1.5 × 104 target (T) cells at indicated E:T ratios. After 4 hours at 37°C, plates were centrifuged and supernatant was collected and assayed for LDH release according to manufacturer protocol. Percent specific lysis was calculated using averaged absorbance values: [(experimental − E spontaneous − T spontaneous)/(T maximum − T spontaneous)] × 100. The following human hematopoietic cell lines were used as target cells: Daudi, Jurkat, RPMI-8226, and U266 (American Type Culture Collection, Manassas, VA); Jurkat, HL-60 (kind gifts of Dr. Stanton Gerson); K562 (kind gift of Dr. Clifford Harding).
For inhibition of perforin, E cells were pre-treated with either concanamycin-A (Sigma Aldrich) dissolved in DMSO at indicated concentrations or DMSO for 1 hour at 37°C, then washed twice before use in the cytoxicity assay.
IFN-γ ELISA
Supernatants were collected from co-cultures and analyzed for the presence of IFN-γ using a standard sandwich ELISA containing capture and detection antibodies from eBioscience.
Statistical analysis
Significant differences between groups were detected by two-tailed Student’s t test.
Results
The Notch ligands Jagged2, Delta1, and Delta4 induce CD56+CD3− cells from human HPCs
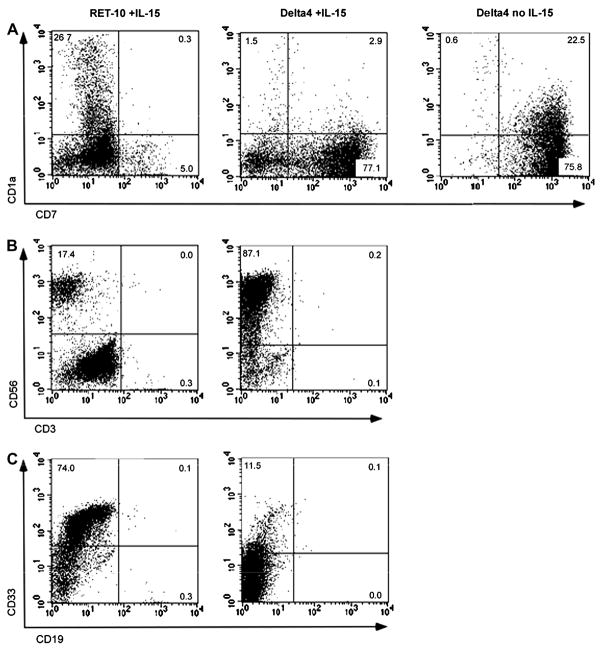
To determine whether Notch ligands are able to generate NK cells from human HPCs, we used a bone marrow stromal cell line, OP9, transduced with each of the individual Notch ligands (Jagged1, J1; Jagged2, J2; Delta1, D1; Delta3, D3; Delta4, D4) in a bicistronic message with GFP, resulting in the lines OP9-J1, OP9-J2, OP9-D1, OP9-D3, and OP9-D4.(22) A control cell line, OP9-Ret-10, contained vector having only the GFP sequence. Expression of transduced Notch ligand by each OP9 line was proportional to the expression of GFP, and flow cytometric analysis demonstrated a range of green fluorescence intensity from 0.9- to 2.5-fold versus OP9-Ret-10. CD34+ cells isolated from umbilical cord blood were cultured with each cell line, in the presence of IL-7, Flt3L, and IL-15. After 21 days in culture, the Notch ligands Jagged2, Delta1, and Delta4 demonstrated the greatest capacity for inducing NK cell development, as measured by percent CD56+CD3− cells in the culture (Fig. 1A), with Jagged2 producing an average of 76% NK cells, Delta1, 67%, and Delta4, 70% NK cells in the presence of IL-15. The non-NK cells in the cultures containing Jagged2, Delta1, and Delta4 consisted of CD33+ myeloid cells and CD7+CD1a- lymphoid precursors, presumed to be NK cell precursors due to the lack of CD1a expression (Fig. 2). In contrast, in the absence of IL-15, Notch signaling via Jagged2, Delta1, and Delta4 drove the development of T cell precursors, defined as being positive for both CD7 and CD1a (Fig. 2A and data not shown), in agreement with prior observations(24) and with the established role for Notch signaling in T cell development. Cultures containing Jagged1 or Delta3 or without Notch ligand in the presence of IL-15 consistently produced less than 25% CD56+CD3− cells at day 21 of culture; these cultures consisted predominantly of CD33+ cells (Fig. 2 and data not shown). Similar results for each Notch ligand were seen when IL-2 was used in culture instead of IL-15 (Fig. 1 and data not shown). For the remainder of the experiments, IL-15 was used to drive NK cell differentiation in the co-cultures.
Fig. 1.
The Notch ligands Jagged2, Delta1, and Delta4 accelerate NK cell differentiation from human CD34+ HPCs. (A) The development of NK cells (defined as CD56+CD3−) from CD34+ HPCs is enhanced by co-culture with OP9 cells expressing Jagged2, Delta1, or Delta4, versus OP9 cells expressing Jagged1 or Delta3 or containing vector (Ret-10) alone. All cultures contain IL-7 and Flt3-L plus IL-15 (black bars) or IL-2 (gray bars). Data shown is from day 21 of co-culture and represents the mean +/− SD of at least 3 experiments. (B) The percentage of Notch-induced NK (N-NK) cells in IL-15+ cultures increases over time and peaks after 4 weeks. Data shown represent the mean +/− SD of at least 3 experiments. (C) NK cell differentiation is inhibited by the presence of a gamma-secretase inhibitor (GSI), L-685,458, at day 21 of culture. Data shown represents the mean of 2 experiments.
Fig. 2.
Culture of CD34+ HPCs in the presence of IL-15 and Notch ligand drives NK cell differentiation, without T or B cell maturation. Flow cytometric analysis of 4 week-old cultures containing the Notch ligand Delta4 and IL-15 demonstrate abundance of CD7+CD56+ NK cells and no T cell precursors (A), mature T cells (CD3+; B), or B cells (CD19+; C), while culture without IL-15 in the presence of Delta4 demonstrates predicted T cell precursors (CD1a+CD7+; A). Culture without Notch ligand (RET-10) in the presence of IL-15 demonstrates few CD7+ NK cell precursors and CD56+ NK cells (A, B), with mostly CD33+ myeloid cells and no development of T or B cells (A–C). All cultures contained IL-7 and FltL. Data from cultures containing the Notch ligands Jagged2 or Delta1 showed similar results to the Delta4 culture. Data shown is representative of at least 4 experiments.
The appearance of NK cells could be seen as early as day 14 in culture (Fig. 1B) and continued to rise until day 28, at which time NK cells became 80–90% of total hematopoietic cells in co-cultures containing Jagged2, Delta1, or Delta4 (Fig. 1C). The NK cells continued to dominate cultures by day 42, but proliferation decreased after day 28, and the cultures ceased to proliferate between days 35–42 (data not shown). No CD19+ or CD3+ cells were seen in any culture condition at any time point (Fig. 2). The induction of CD56+CD3− by Jagged2, Delta1, or Delta4 was dependent on signaling via the Notch receptor, as the level of NK cell differentiation was greatly reduced in the presence of a gamma-secretase inhibitor (GSI), an inhibitor of Notch signaling (Fig. 1B). The presence of the GSI, however, failed to abrogate NK cell differentiation in cultures containing OP9-Ret-10, indicating that the development of NK cells in these cultures was not dependent on Notch signaling.
In addition to inducing NK cell differentiation, Notch signaling via ligation with Jagged2, Delta1, or Delta4 also caused marked expansion of total NK cell number, resulting in average yields of 700 (Delta1), 800 (Jagged2), or 1100 (Delta4) NK cells per input cell on day 28 of culture, versus an average yield of 200 NK cells per input cell in the absence of Notch ligand (Fig. 3).
Fig. 3.

The Notch ligands Jagged2, Delta1, and Delta4 induce NK cell expansion from human CD34+ HPCs. The number of NK cells (defined as CD56+CD3−) derived per single CD34+ input cell at day 28 of culture in the presence of IL-15 is shown; each symbol represents an individual experiment with the horizontal bar indicating the mean (n = 5–7 independent experiments). P values generated by student’s t-test are shown.
The surface phenotype of N-NK cells resembles, but is distinct from, that of immature (CD56bright) NK cells
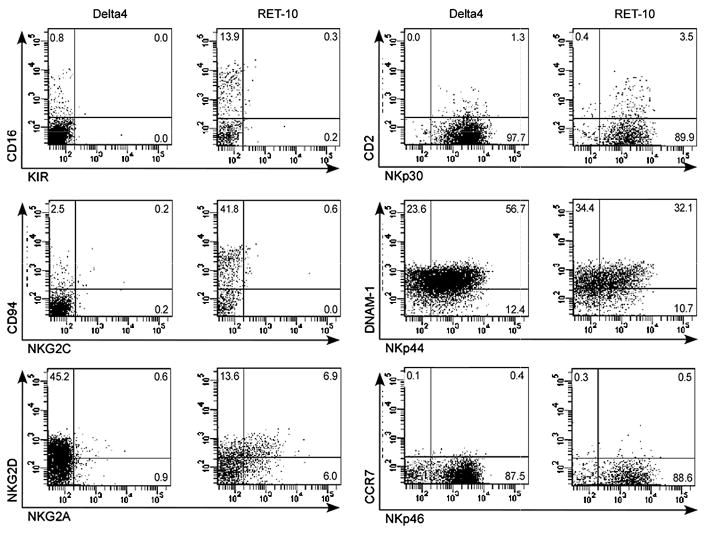
We next determined the maturation stage of N-NK cells by surface receptor expression. Flow cytometric immunophenotyping was performed on the NK cells in cultures containing Jagged2, Delta1, or Delta4, and compared to that of NK cells developed in the absence of Notch signaling or normal peripheral blood NK cells (Table 1). N-NK cells had a predominantly immature NK cell phenotype, with bright expression of CD56, expression of CD117 and CD7, and very little, if any, expression of CD16, CD94, and KIRs (Fig. 2 and 4). The lack of CD94 expression corresponded to an absence of detectable NKG2A and NKG2C expression (Fig. 4), both of which form heterodimers with CD94 to form receptors that mediate inhibitor signals upon binding to HLA-E expressed by target cells. Interestingly, N-NK cells expressed moderate levels of the activating receptor NKG2D and high levels of the NCRs NKp30, NKp44, and NKp46 (Fig. 4). This was in contrast to NK cells found in peripheral blood, which expressed only NKp30 and NKp46, and NK cells developed in vitro in the absence of Notch ligand, which expressed low levels of NKp44 (Table 1). NKp44 is an activation-induced receptor,(7, 25) and the high expression of all three NCRs on N-NK cells may indicate an activated state of these cells. Interestingly, CCR7 was not expressed by N-NK cells, although the adhesion ligands CD62L, CD11a, and CD18 were (Table 1). N-NK cells also failed to express high levels of CXCR1 and CD11b (Mac-1), markers of mature, CD56dim peripheral blood NK cells.(1, 4, 5)
Table 1.
Surface phenotype of Notch-induced NK cells (day 28 of culture), as compared to NK cells developed in the absence of Notch and normal peripheral blood NK cells.*
| OP9-J2 | OP9-D1 | OP9-D4 | OP9-Ret-10 | PB NK | |
|---|---|---|---|---|---|
| Markers of NK cell maturity/lineage | |||||
| CD16 | 3.3 ± 3.6 | 3.4 ± 1.7 | 3.8 ± 2.0 | 30.1 ± 9.8 | 97.1 ± 2.3 |
| CD94 | 8.4 ± 3.7 | 15.6 ± 8.3 | 10.1 ± 10.2 | 51.6 ± 10.0 | 76.1 ± 12.0 |
| CD117 | 94.8 ± 9.5 | 90.1 ± 11.2 | 91.2 ± 15.0 | 21.3 ± 25.1 | 5.9 ± 6.2 |
| CD11b | 21.9 ± 13.0 | 18.7 ± 10.7 | 20.4 ± 9.5 | 68.2 ± 10.7 | 97.2 ± 1.5 |
| CD2 | 4.3 ± 6.0 | 4.3 ± 4.0 | 5.5 ± 5.7 | 8.8 ± 4.2 | 80.8 ± 0.9 |
| CD7 | 56.5 ± 12.8 | 57.1 ± 8.9 | 74.2 ± 9.5 | 25.6 ± 6.2 | 98.8 ± 1.1 |
| DNAM-1 | 55.9 ± 18.6 | 39.6 ± 24.0 | 67.0 ± 15.0 | 73.8 ± 12.1 | 84.2 ± 14.7 |
| 2B4 | 99.3 ± 1.3 | 97.9 ± 2.6 | 99.4 ± 0.8 | 91.4 ± 7.0 | 99.9 ± 0.2 |
| Inhibitory NK receptors | |||||
| NKG2A | 1.6 ± 0.8 | 1.9 ± 1.2 | 2.4 ± 2.2 | 14.5 ± 6.4 | 23.2 ± 23.6 |
| KIR§ | 0.1 ± 0.1 | 0.2 ± 0.4 | 0.0 ± 0.0 | 0.4 ± 0.2 | 68.7 ± 15.4 |
| Activating NK receptors | |||||
| NKG2C | 0.2 ± 0.3 | 0.3 ± 0.2 | 0.1 ± 0.1 | 1.4 ± 1.8 | 19.2 ± 26.6 |
| NKG2D | 31.2 ± 17.8 | 17.9 ± 15.0 | 33.6 ± 26.5 | 45.1 ± 11.6 | 54.1 ± 1.8 |
| NKp30 | 80.8 ± 21.4 | 76.3 ± 23.9 | 65.2 ± 24.5 | 81.4 ± 7.2 | 91.5 ± 13.9 |
| NKp44 | 47.8 ± 11.5 | 40.2 ± 11.9 | 56.1 ± 15.0 | 21.9 ± 10.4 | 0.8 ± 1.1 |
| NKp46 | 94.5 ± 5.1 | 90.4 ± 3.8 | 93.3 ± 7.5 | 92.2 ± 2.2 | 82.4 ± 22.6 |
| Adhesion molecules | |||||
| CCR7 | 3.7 ± 2.9 | 2.5 ± 1.6 | 2.7 ± 1.2 | 3.2 ± 0.7 | 0.1 ± 0.1 |
| CXCR1 | 1.9 ± 2.8 | 1.7 ± 1.4 | 1.3 ± 1.0 | 2.5 ± 1.0 | 71.9 ± 5.5 |
| CD62L | 52.7 ± 13.1 | 61.6 ± 11.4 | 67.2 ± 19.8 | 75.0 ± 6.5 | 60.1 ± 23.5 |
| ICAM-1 | 97.0 ± 3.1 | 96.1 ± 3.8 | 95.1 ± 3.6 | 97.7 ± 1.3 | 97.2 ± 0.1 |
| CD11a | 55.1 ± 23.5 | 52.2 ± 23.8 | 65.3 ± 21.7 | 71.2 ± 9.6 | 73.6 ± 17.8 |
| CD18 | 83.7 ± 9.1 | 77.1 ± 8.4 | 78.8 ± 13.6 | 98.1 ± 0.8 | 100.0 ± 0.0 |
Numbers indicate percentage of CD56+CD3− cells positive for that antigen and are expressed as the mean of 3 experiments using OP9 cells, +/− standard deviation. Data for PB NK is derived from 3 normal donors.
Antibodies used also detect the activating receptors KIR2DS1, KIR2DS2, and KIR2DS4.
Fig. 4.
N-NK cells have a predominantly immature NK cell surface phenotype but do express NKG2D and the natural cytotoxicity receptors, NKp30, NKp44, and NKp46. Expression of NK cell markers was examined on gated CD56+CD3− cells from 4 week-old co-cultures containing Delta4 or no Notch ligand (RET-10). KIR expression was examined by an antibody cocktail (DX9 + CD158a + CD158b). Data from cultures containing the Notch ligands Jagged2 or Delta1 showed similar results to the Delta4 culture. Data shown is representative of at least 4 independent experiments.
Both N-NK cells and NK cells derived in the absence of Notch ligand have bright CD56 expression and lack expression of more mature NK cell markers (such as CD16, CD94, KIR, and Mac-1). However, the NK cells derived in the absence of Notch ligand differed from N-NK cells, having higher levels of CD94 and NKG2A and lower levels of CD11. These results are consistent with a more mature phenotype, similar to that described in published in vitro models of NK cell development using CD34+ cord blood HPCs cultured with stromal cells.(4, 23, 26) Thus, the overall phenotype of N-NK cells is distinct from any previously described in vitro or in vivo NK cell subset.
N-NK cells have cytotoxic capacity and can secrete IFN-γ
Among normal human NK cell subsets, the CD56dim population has more cytotoxic activity than the CD56bright population, while CD56bright cells play an important role in secretion of IFN-γ and other inflammatory cytokines. In contrast to the immature surface phenotype observed for N-NK cells, we consistently noticed that N-NK cells began to lyse the OP9 stromal cells during the third week of co-culture, indicating the presence of cytolytic capability. To directly demonstrate the functional capacity of N-NK cells, cytotoxicity was measured by in vitro assays using the human hematopoietic tumor cell lines K562 (erythroleukemia), RPMI-8226 (multiple myeloma), and Daudi (Burkitt lymphoma; Fig. 5A). N-NK cells derived from Notch signaling via Jagged2, Delta1, and Delta4 demonstrated similar levels of cytotoxicity. RPMI-8226 cells express high levels of surface MHC class I molecules, while K562 and Daudi do not (data not shown), indicating that the cytotoxic activity of N-NK cells is not inhibited by tumor cell expression of MHC class I. This is not surprising, given the absence of KIR and CD94/NKG2A and CD94/NKG2D expression by N-NK cells. The cytotoxic ability of N-NK cells was dependent on perforin, as the addition of the perforin inhibitor concanamycin-A abrogated cytotoxic activity against K562 cells (Fig. 5B). N-NK cells displayed moderate perforin expression by flow cytometry (Fig. 5C) and morphologic assessment of N-NK cells by light microscopy demonstrated the presence of cytoplasmic granules, consistent with a large granular lymphocyte morphology (not shown). We also tested the cytotoxic function of NK cells from control co-cultures without Notch ligand, after enriching these NK cells to percentages greater than 50% by negative cell selection. The cytotoxic function of NK cells developed in the absence of Notch ligand was similar to that of N-NK cells: when compared side by side, the average percent specific lysis for K562 cells was 45.7% for NK cells grown in the presence of Delta4 vs. 50.1% for NK cells grown in the absence of Notch ligand (E:T ratio of 7:1), and 34.2% vs. 28.5%, respectively, for killing of RPMI-8226 cells (E:T ratio of 7:1).
Fig. 5.
N-NK cells have perforin-dependent cytotoxic activity. (A) Cytotoxicity assays demonstrate activity against the human leukemia cell lines K562 and RPMI-8226 but not Daudi. N-NK cells from week 3 or 4 of co-culture were used as effector cells. (B) N-NK cell cytotoxicity assay against K562 cells is abrogated in the presence of the perforin inhibitor concanamycin-A (CMA). The Effector:Target ratio is 10:1. (C) N-NK cells from week 4 of Delta4 co-culture express perforin by flow cytometry; N-NK cells from Jagged2 and Delta1 cultures have similar levels of perforin expression (not shown). (D) The level of cytotoxic activity of Delta4-derived N-NK cells is similar, but not identical, to activated PB NK cells (PB Stim), and is greater than unstimulated PB NK cells (PB). PB NK cells were activated by overnight incubation with IL-15 (10 ng/ml). Averages of 3 independent experiments are shown.
We next compared the cytotoxic function of N-NK cells with peripheral blood (PB) NK cells, either unstimulated or activated overnight with IL-15 (10 ng/ml). Interestingly, the killing activity of N-NK cells against K562 and RPMI-8226 was similar to activated PB NK cells (Fig. 5E), while only activated PB NK had activity against Daudi cells. This was in contrast to unstimulated PB NK (cultured overnight without IL-15), which had the lowest capacity of the three types of NK cells for killing of K562, RPMI-8226, or Daudi. Thus, although N-NK cells lack cytotoxic function against Daudi cells, the killing activity of two other cell lines was more similar to that of stimulated PB NK cells than unstimulated.
To evaluate the ability of N-NK cells to secrete cytokines, IFN-γ in co-culture supernatants was measured by standard ELISA. In the absence of IL-12 and IL-18, neither NK cells developed in the absence or presence of Notch signaling secreted IFN-γ, similar to most PB NK cells cultured in IL-15 alone (Fig. 6). However, overnight incubation with IL-12 and IL-18 resulted in secretion of IFN-γ by both N-NK cells and NK cells developed in the absence of Notch ligand (Fig 6), albeit at lower levels than IL-15-activated PB NK cells further stimulated with IL-12 and IL-18.
Fig. 6.
Both N-NK cells and NK cells developed in the absence of Notch ligand can secrete IFN-γ only after overnight incubation with IL-12 and IL-18 (100 ng/ml each). Levels of IFN-γ secreted by PB NK cells from three different healthy donors are shown for comparison. PB NK cells were cultured for two days in the presence of IL-15, with or without additional IL-12 and IL-18 on day 2.
Overall, these data indicate that although the surface phenotype of N-NK cells resembles that of an immature NK cell precursor (CD56bright, CD117+, CD16−, CD94−, KIR−, CD11b−), the N-NK cells have the functional characteristics of both CD56bright and CD56dim NK cells, i.e. cytokine secretion and cytotoxic ability, respectively, with the cytotoxic capacity of N-NK cells being similar but not identical to activated PB NK cells.
Discussion
Our data demonstrate that Notch receptor signaling via specific Notch ligands (i.e. Jagged2, Delta1, or Delta4) in human CD34+ HPCs accelerates the development of functional NK cells in vitro. Although the data we show here uses CD34+ HPCs isolated from UCB, Notch-induced NK cell differentiation was also seen in co-cultures using bone marrow-derived CD34+ HPCs (data not shown). This study is the first to delineate the differential ability of individual Notch ligands to promote human NK cell differentiation, while at the same time demonstrating a cytotoxic capacity of N-NK cells which is similar, although not identical, to that of activated NK cells from peripheral blood. Thus, our data raise the interesting possibility that Notch signaling could be used to generate large numbers of functional NK cells for cell therapy purposes, especially since N-NK cells lack both KIR and NKG2A and therefore would not be expected to be inhibited by patient class I MHC. Overcoming the inhibitory signals transmitted by MHC-recognizing receptors is an important stratagem in the use of cytotoxic NK cells as therapy against different types of malignancy.(19)
The comparison of individual Notch ligands in hematopoietic stem cell development has important clinical applications, as purified recombinant Notch ligand (specifically Delta1) is currently being investigated as a means for cell expansion in the context of hematopoietic stem cell transplantation.(27–29) Our data demonstrate that in addition to Delta1, both Jagged2 and Delta4 are able to promote human NK cell differentiation, with Jagged2 and Delta4 acting at a greater capacity than Delta1 with respect to NK cell expansion. These findings suggest that further investigation into the clinical use of recombinant Jagged2 or Delta4 in hematopoietic cell culture/expansion systems is warranted.
Our findings are in agreement with recent studies showing Jagged2 induction of NK cells from murine HPCs,(17) and Delta1 induction of cytoplasmic CD3+ NK cells from human HPCs.(18) All these studies corroborate the finding that Notch signaling via Delta ligands promotes development of a common T/NK precursor from human HPCs, at the expense of B cell development,(14) while our data are also consistent with the preferential interaction of Delta4 with Notch1, versus Delta1.(12) Interestingly, in the present system, the addition of IL-15 in the co-culture completely abrogated the development of T cells and subverted the common T/NK precursor to an exclusively NK cell fate. Thus, the regulation of lymphocyte development by Notch signaling in vitro is highly dependent upon the cell culture environment.
Compared to the development of B and T cells, the development of NK cells is only marginally understood. The growth factor that is essential for NK cell differentiation is IL-15, although FLT3 ligand and c-KIT ligand may also contribute.(8) NK cells can develop in vitro from human or mouse HPCs without contact with bone marrow stromal cells, in the presence of cytokines alone.(31, 32) Culture with stromal cells, however, allows for further maturation of NK cells in vitro, indicated by acquisition of Ly49 receptors (in mouse cells)(10) or KIRs (human).(9) NK cell development in vivo was traditionally postulated to occur in the bone marrow, since both murine and human marrow-derived HPCs differentiate into NK cells in vitro.(23, 31, 32) However, recent evidence indicates that NK cells may develop in secondary lymphoid tissue, such as lymph nodes, to which CD34+ HPCs migrate from the bone marrow.(3) Nodal CD34+ HPCs have the capacity to differentiate into CD56bright NK cells, which are more abundant in lymph nodes than the CD56dim subset.(3, 7) The development of NK cells in secondary lymphoid tissue has recently been divided into four stages: CD34+CD117−CD94− (stage I), CD34+CD117+CD94− (stage II), CD34−CD117+CD94− (stage III), and CD34−CD117+/−CD94+ (stage IV).(4) Subsequent maturation of CD56bright NK cells to CD56dim NK cells (stage V) is postulated to occur in the periphery.
A defined role for Notch signaling in NK cell development has not been elucidated. The existence of normal numbers of NK1.1+ cells in mice having an inducible knock-out of Notch1 indicates that this particular receptor is not required for NK cell development,(16) although signaling via other Notch receptors may have compensated for the lack of Notch1 in this model. The ability to generate NK cells from HPCs in stromal-free culture(31, 32) indicates that Notch signaling is not an absolute requirement for HPC commitment to NK cells. It is interesting to speculate that the additional NK cell maturation seen with co-cultured stromal cells(9, 10) may involve signaling via Notch ligands expressed by stromal cells, especially since constitutively activated Notch signaling could replace the need for stromal cells in supporting NK cell differentiation in a recent study.(30) Preliminary data from our lab also indicate that Notch signaling accelerates NK cell differentiation from CD34+ UCB cells in the absence of a supporting stroma (not shown).
Notch signaling may also be important for mature NK cell function. Gene profiling studies indicate that both CD56dim and CD56bright NK cells express both Notch1 and Notch2. Expression of both receptors is 2–3-fold higher in CD56bright cells,(25) suggesting that Notch may play a role in NK cell subset maturation and/or function. Interestingly, murine NK cells exhibit enhanced cytokine expression and cytotoxic function in response to signaling from tumor cells or dendritic cells transduced with Jagged2, indicating an ability of Notch signaling to influence mature murine NK cell function.(33) NK cell precursors and subsets would be expected to encounter Notch signaling in secondary lymphoid organs, as human Delta4 expression is detectable in lymph node tissue(34) and Delta1 is expressed in spleen, where it is essential for marginal zone B cell development.(35) Thus, Notch signaling in vivo may have distinct effects on NK cells at different stages of development.
The data from our co-culture system indicate that Notch is capable of inducing NK cell differentiation from human HPCs, with maturation to the level of the stage III NK precursor postulated by Freud et al.(4) The phenotype of N-NK cells differs slightly, however, from the observed phenotype of stage III NK precursors, which do not express NKp30, NKp46, or NKG2D.(5) In addition, the cytotoxic and cytokine-secreting capabilities of N-NK cells are not characteristic of stage III NK precursors, which do not express perforin, exhibit cytotoxic activity, or secrete IFN-γ.(4) Instead, the functionality of the N-NK cells in conjunction with their immature surface phenotype resembles the “pseudomature” NK cell that develops in vitro in the presence of IL-15; the “pseudomature” term being a reference to the observation that these NK cells have cytotoxic activity yet are CD56bright and negative for KIR.(8)
Interestingly, in comparison to N-NK cells, NK cells that developed after 4 weeks of culture in the absence of Notch ligand (OP9-Ret-10 culture) expressed higher levels of CD16, CD94, and CD11b (Mac-1) and lower levels of CD117, a phenotype more consistent with transition to a stage IV NK cell. This implies that while Notch signaling in the co-culture system accelerates differentiation of CD34+ HPCS along an NK cell path, the resulting NK cell may be fated to a developmentally immature state. We did not observe significantly increasing levels of CD94 or CD16 expression in N-NK cells between weeks 4–6 of culture, at which time the N-NK cells ceased to proliferate and overall cell numbers declined (data not shown), indicating that further maturation of N-NK cells did not occur after week 4 in this system.
A recent study using constitutively activated Notch1 signaling in CD34+ UCB cells also demonstrated immature development of NK cells which was dependent on Notch signaling.(30) However, in this study the NK cells appeared hypo-functional compared to NK cells developed in vitro in the absence of Notch signaling, with decreased production of IFN-γ and decreased cytotoxicity against K562 cells. This in contrast to our results, which indicate similar cytotoxic activity and levels of IFN-γ production between NK cells developed in the presence or absence of Notch signaling. These differences are likely due to variable levels of Notch signaling between the two systems, and suggest that in our system, Notch signaling may be down-regulated during the co-culture period, thus producing NK cells distinct from those developed in the presence of continuous Notch activation. Continuous Notch activation may thus prevent full maturation of developing NK cells.
Although Jagged2 and Delta4 were more robust than Delta1 at inducing expansion of NK cells from CD34+ HPCs, the surface phenotype and purity of the N-NK cells derived from each ligand-culture system were comparable. These observations suggest that Notch signals contributing to hematopoietic cell expansion may be distinct from signals which cause NK cell differentiation and/or maturation. Notch-induced cell expansion in our cultures may occur at the level of the CD34+ HPCs, as several studies have demonstrated the ability of Notch signaling to expand human UCB HPCs.(28, 34, 36) Indeed, data from one study suggest that Delta4 is more effective in expanding CD34+CD38-Lin- HPCs as compared to Delta1,(34) consistent with our observations with respect to NK cell expansion.
While a definitive role for in vivo Notch signaling in NK cell differentiation and maturation remains unclear, the observed phenotype and cytotoxic capacity of in vitro-derived N-NK cells suggests their potential use as cell therapeutic agents. The infusion of allogeneic NK cells as cell therapy in both solid tumor and hematologic malignancy is an active area of investigation. A Phase I trial examining the safety of allogeneic NK cell infusion demonstrated the feasibility of this procedure as well as a distinct lack of donor NK cell-mediated graft-versus-host disease (GVHD).(20) The lack of GVHD in this trial is in sharp contrast to the incidence of GVHD following the current clinical practice of donor lymphocyte infusion after hematopoietic stem cell transplantation (HSCT), where significant acute and/or chronic GVHD develops in up to half of treated patients.(37) In addition, data from haploidentical HSCT patients indicate that donor “alloreactive” NK cells, which lack MHC class I inhibitory signals due to KIR:MHC mismatch with recipient cells, may prolong survival in leukemia patients.(38–40) The prolonged survival in HSCT patients having donor KIR:recipient MHC mismatch is likely due in part to a direct anti-leukemic effect of alloreactive NK cells. Mouse models of HSCT demonstrated that alloreactive NK cells may also act as bone marrow conditioning agents and may protect against GVHD by the killing of host dendritic cells.(38)
The cytotoxic activity of N-NK cells against tumor cell lines, which is comparable (though not identical) to activated peripheral blood NK cells, indicates that these cells may act as an anti-tumor agent if infused into patients. NK cell recognition and killing of hematologic malignancy, carcinomas, and neural tumors is dependent in part upon the activating receptors NKG2D, DNAM-1, and/or the natural cytotoxicity receptors,(41–46) all of which are expressed by N-NK cells. However, N-NK cells would not be capable of antibody-dependent cell-mediated cytotoxicity, which is important in the activity of therapeutic antibodies, since these cells do not express notable levels of CD16. Interestingly, the lack of expression of KIR and NKG2A by N-NK cells predicts that these cells should be universally alloreactive, i.e. not inhibited by recipient MHC, regardless of HLA type. This would result in an ease of administration of such cells, if matching (or, more precisely, mismatching) of donor and recipient HLA could be avoided. In addition, because N-NK cells can be derived from CD34+ UCB cells, donor availability and risk are minimal issues, versus the collection of peripheral blood NK cells from adult donors via apheresis. We anticipate that further studies will elucidate the clinical potential for NK cells generated and expanded in vitro using Notch signaling.
Acknowledgments
Thanks to Yun-Fang Man for guidance concerning the OP9 co-culture system. J.B.L. is now at the Department of Research Pathology, Genentech, San Francisco. M.P. is now at the Department of Cellular and Molecular Pharmacology, Rosalind Franklin University of Medicine and Science, North Chicago. This work was supported in part by a Stem Cell Pilot Grant to R.C.B from the Center for Stem Cell and Regenerative Medicine in Cleveland, Ohio.
Footnotes
Financial Disclosure Statement
The authors have no relevant financial relationships to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 2.Campbell JJ, Qin S, Unutmaz D, et al. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001:166. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 3.Freud AG, Becknell B, Roychowdhury S, et al. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22:295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Freud AG, Yokohama A, Becknell B, et al. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203:1033–1043. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freud AG, Caligiuri MA. Human natural killer cell development. Immunological Rev. 2006;214:56–72. doi: 10.1111/j.1600-065X.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 6.Fehniger TA, Cooper MA, Nuovo G, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 7.Ferlazzo G, Thomas D, Lin S-L, et al. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172:1455–1462. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 8.Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol. 2003;3:413–425. doi: 10.1038/nri1088. [DOI] [PubMed] [Google Scholar]
- 9.Cooley S, Xiao F, Pitt M, et al. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self-MHC is developmentally immature. Blood. 2007;110:578–586. doi: 10.1182/blood-2006-07-036228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams NS, Klem J, Puzanov IJ, Sivakumar PV, Bennett M, Kumar V. Differentiation of NK1. 1+, LY49+ NK cells from flt3+ multipotent marrow progenitor cells. J Immunol. 1999;163:2648–2656. [PubMed] [Google Scholar]
- 11.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–974. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 12.Besseyrias V, Fiorini E, Strobl LJ, et al. Hierarchy of Notch-Delta interactions promoting T cell lineage commitment and maturation. J Exp Med. 2007;204:331–343. doi: 10.1084/jem.20061442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinzel K, Benz C, Martins VC, Haidl ID, Bleul CC. Bone marrow-derived hemopoietic precursors commit to the T cell lineage only after arrival in the thymic microenvironment. J Immunol. 2007;178:858–868. doi: 10.4049/jimmunol.178.2.858. [DOI] [PubMed] [Google Scholar]
- 14.Jaleco AC, Neves H, Hooijberg E, et al. Differential effects of Notch ligands Delta-1 and Jagged-1 in human lymphoid differentiation. J Exp Med. 2001;194:991–1001. doi: 10.1084/jem.194.7.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehar SM, Dooley J, Farr AG, Bevan MJ. Notch ligands Delta1 and Jagged1 transmit distinct signals to T-cell precursors. Blood. 2005;105:1440–1447. doi: 10.1182/blood-2004-08-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radtke F, Wilson A, Stark G, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 17.DeHart SL, Heikens MJ, Tsai S. Jagged2 promotes the development of natural killer cells and the establishment of functional natural killer cell lines. Blood. 2005;105:3521–3527. doi: 10.1182/blood-2004-11-4237. [DOI] [PubMed] [Google Scholar]
- 18.De Smedt M, Taghon T, Van de Walle I, De Smet G, Leclercq G, Plum J. Notch signaling induces cytoplasmic CD3 epsilon expression in human differentiating NK cells. Blood. 2007;110:2696–2703. doi: 10.1182/blood-2007-03-082206. [DOI] [PubMed] [Google Scholar]
- 19.Ljunggren H-G, Malmberg K-J. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 20.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 21.Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 22.Zhou L, Li LW, Yan Q, et al. Notch-dependent control of myelopoiesis is regulated by fucosylation. Blood. 2008;112:308–319. doi: 10.1182/blood-2007-11-115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller JS, McCullar V. Human natural killer cells with polyclonal lectin and immunoglobulinlike receptors develop from single hematopoietic stem cells with preferential expression of NKG2A and KIR2DL2/L3/S2. Blood. 2001;98:705–713. doi: 10.1182/blood.v98.3.705. [DOI] [PubMed] [Google Scholar]
- 24.La Motte-Mohs RN, Herer E, Zuniga-Pflucker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105:1431–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 25.Hanna J, Bechtel P, Zhai Y, Youssef F, McLachlan K, Mandelboim O. Novel insights on human NK cells’ immunological modalities revealed by gene expression profiling. J Immunol. 2004;173:6547–6563. doi: 10.4049/jimmunol.173.11.6547. [DOI] [PubMed] [Google Scholar]
- 26.Sivori S, Falco M, Marcenaro E, et al. Early expression of triggering receptors and regulatory role of 2B4 in human natural killer cell precursors undergoing in vitro differentiation. PNAS. 2002;99:4526–4531. doi: 10.1073/pnas.072065999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dallas MH, Varnum-Finney B, Martin PJ, Bernstein ID. Enhanced T-cell reconstitution by hematopoietic progenitors expanded ex vivo using the Notch ligand Delta1. Blood. 2007;109:3579–3587. doi: 10.1182/blood-2006-08-039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delaney C, Varnum-Finney B, Aoyama K, Brashem-Stein C, Bernstein ID. Dose-dependent effects of the Notch ligand Delta1 on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood. 2005;106:2693–2699. doi: 10.1182/blood-2005-03-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zakrzewski JL, Kochman AA, Lu SX, et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006;12:1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 30.Bachanova V, McCullar V, Lenvik T, et al. Activated Notch supports development of cytokine producing NK cells which are hyporesponsive and fail to acquire NK cell effector functions. Biol Blood Marrow Transplant. 2008;15:183–194. doi: 10.1016/j.bbmt.2008.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mrozek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632–2640. [PubMed] [Google Scholar]
- 32.Williams NS, Moore TA, Schatzle JD, et al. Generation of lytic natural killer 1. 1+, Ly-49- cells from multipotential murine bone marrow progenitors in a stroma-free culture: definition of cytokine requirements and developmental intermediates. J Exp Med. 1997;186:1609–1614. doi: 10.1084/jem.186.9.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kijima M, Yamaguchi T, Ishifune C, et al. Dendritic cell-mediated NK cell activation is controlled by Jagged-2 Notch interaction. PNAS. 2008;105:7010–7015. doi: 10.1073/pnas.0709919105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karanu FN, Murdoch B, Miyabayashi T, et al. Human homologues of Delta-1 and Delta-4 function as mitogenic regulators of primitive human hematopoietic cells. Blood. 2001;97:1960–1967. doi: 10.1182/blood.v97.7.1960. [DOI] [PubMed] [Google Scholar]
- 35.Hozumi K, Negishi N, Suzuki D, et al. Delta-like 1 is necessary for the generation of marginal zone B cells but not T cells in vivo. Nat Immunol. 2004;5:638–644. doi: 10.1038/ni1075. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki T, Yokoyama Y, Kumano K, et al. Highly efficient ex vivo expansion of human hematopoietic stem cells using Delta-1-Fc chimeric protein. Stem Cells. 2006 July 20; doi: 10.1634/stemcells.2006-0258. Epub. [DOI] [PubMed] [Google Scholar]
- 37.Loren A, Porter DL. Donor leukocyte infusions after unrelated donor hematopoietic stem cell transplantation. Curr Opin Oncol. 2006;18:107–114. doi: 10.1097/01.cco.0000208781.61452.d3. [DOI] [PubMed] [Google Scholar]
- 38.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 39.Ruggeri L, Mancusi A, Capanni M, Martelli MF, Velardi A. Exploitation of alloreactive NK cells in adoptive immunotherapy of cancer. Curr Opin Immunol. 2005;17:211–217. doi: 10.1016/j.coi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Leung W, Iyengar R, Turner V, et al. Determinants of antileukemic effects of allogeneic NK cells. J Immunol. 2004;172:644–650. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- 41.Carlsten M, Bjorkstrom NK, Norell H, et al. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res. 2007;67:1317–1325. doi: 10.1158/0008-5472.CAN-06-2264. [DOI] [PubMed] [Google Scholar]
- 42.Castriconi R, Dondero A, Corrias MV, et al. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res. 2004;64:9180–9184. doi: 10.1158/0008-5472.CAN-04-2682. [DOI] [PubMed] [Google Scholar]
- 43.Pende D, Spaggiari GM, Marcenaro S, et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112) Blood. 2005;105:2066–2073. doi: 10.1182/blood-2004-09-3548. [DOI] [PubMed] [Google Scholar]
- 44.Carbone E, Neri P, Mesuraca M, et al. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood. 2005;105:251–258. doi: 10.1182/blood-2004-04-1422. [DOI] [PubMed] [Google Scholar]
- 45.Pende D, Cantoni C, Rivera P, et al. Role of NKG2D in tumor cell lysis mediated by human NK cells: cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. Eur J Immunol. 2001;31:1076–1086. doi: 10.1002/1521-4141(200104)31:4<1076::aid-immu1076>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 46.Sivori S, Parolini S, Marcenaro E, et al. Involvement of natural cytotoxicity receptors in human natural killer cell-mediated lysis of neuroblastoma and glioblastoma cell lones. J Neuroimmunol. 2000;107:220–225. doi: 10.1016/s0165-5728(00)00221-6. [DOI] [PubMed] [Google Scholar]