Abstract
Non-invasive molecular imaging of dynamic processes has benefited tremendously from the use of reporter genes. These genes encode for proteins that emit light, bind radiolabeled probes or, as covered in this review, modulate magnetic resonance (MR) contrast. Reporter genes play a pivotal role in monitoring cell trafficking, gene replacement therapy, protein-protein interactions, neuronal plasticity and embryonic development. Several strategies exist for generating MR contrast: enzyme-catalyzed chemical modification of metal-based contrast agents or (phosphorus) metabolites, using iron-binding and iron-storage proteins to accumulate iron as contrast agent, and using artificial proteins for imaging based on chemical exchange saturation transfer. MR reporter genes have the advantage that the specific signal can be co-registered with soft tissue anatomy and functional tissue information, and have therefore become an active and growing area of scientific interest.
Keywords: Molecular imaging, MR imaging, MR spectroscopy, reporter gene, ferritin
Reporter genes for molecular and cellular imaging
With more than 20,000 genes in the human genome now identified and a similar number of genes in the rat and mouse genome known (1), elucidation of their function has become the major challenge. This has been facilitated by the development of reporter genes, which are revolutionizing the way many current biomedical studies are designed and carried out. A reporter gene is a gene whose product can be readily detected and either fused to the gene of interest or replaces it. The main applications for these reporters include: (i) monitoring gene expression levels; (ii) investigating dynamic molecular interactions between proteins; (iii) studying cellular interactions (iv) tracking cells in normal and abnormal development or in cell transplantation therapy; and (v) monitoring gene replacement therapy.
Optical reporter genes are probably the most commonly used for imaging and widely developed. Throughout the years multiple genes were cloned from a variety of organisms that emit light via bioluminescence or fluorescence in multiple distinguishable wavelengths. Probably, the most recent and exciting implementation of optical reporter genes was the development of the transgenic “brainbow” mouse, in which combinatorial expression of fluorescent proteins in mouse brain resulted into the production of than 89 distinguishable colors (2). An emerging new class of reporter genes encode for proteins with affinity for radioisotopes or positron emitter probes. These receptors, transporters, or enzymes can provide quantitative images upon administration of suitable radiolabeled probes (3). MR reporter genes are unique among all reporter genes used with the various imaging modalities, since they can provide information on gene expression that can be co-registered with anatomical and functional information (4). MR reporters enable serial temporal imaging within the same subject. This is particularly useful for studying dynamic processes, for example migration of stem cells and progenitors (5), neuronal plasticity, mechanisms of development and adaptation, disease progression and response to trauma or illness, as well as processes of memory and learning.
The purpose of this review is to give a basic, brief overview of the recent developments in the field of MR reporter genes as well as to evaluate and compare the currently existing genes while suggesting future directions. A more detailed review can be found in (4, 6). For further reading, the context of MRI reporter genes is closely linked to certain MR contrast agents that can act as sensors for changes in biological or physiological conditions (7).
Basic principles of MR contrast
MR relies on measuring magnetization of magnetic nuclei subjected to radiofrequency (RF) irradiation inside a magnetic field. A variety of nuclei can be studied, with the most common one being the hydrogen nucleus (proton: 1H). For example: MRI detects properties of the water signal, mostly in terms of amount of water (so-called proton or spin density), its relaxation back to equilibrium after excitation (time constant, T1) and its signal broadening (relaxation times T2, for natural signal width, and T2*, which includes T2 effects plus magnetic field inhomogeneity contributions). Any tissue difference in terms of these properties provides contrast. Also, besides water, MR spectroscopy (MRS) can detect individual proton signals in metabolites, as well as nuclei different from protons, such as 31P, 13C, 23Na, 19F, and many more, all of which have specific frequencies. The signal acquired from different nuclei depends on their abundance in the tissue and their chemical interaction with other molecules, allowing multiple manipulations with various MR acquisition schemes in order to create different contrasts. When certain metals or organic compounds are present, endogenous contrast mechanisms may be affected. T1 relaxation can be enhanced using compounds containing paramagnetic lanthanides, with gadolinium being widely used in the clinic. Iron oxide particles, on the other hand, can drastically shorten T2 and T2*, which is manifested by darkening of the MR images. As a consequence, many iron binding proteins are potential candidates for MR reporter genes.
Chemical Exchange Saturation Transfer (CEST) is a new type of MRI contrast that has been developed, it relies on chemical exchange of protons of solutes such as contrast agents with bulk water (8). A variety of organic and organometallic compounds have a sufficient number of protons with suitable chemical exchange rates and specific MR frequencies to be excited selectively and detected sensitively. A RF pulse, called a saturation pulse, is applied at the exchangeable proton resonance frequency. This saturation, or signal loss, is then transferred via exchange to bulk water, producing a fractional reduction in the water signal (Figure 1a–d).
Figure 1. Principles of the CEST contrast mechanism.
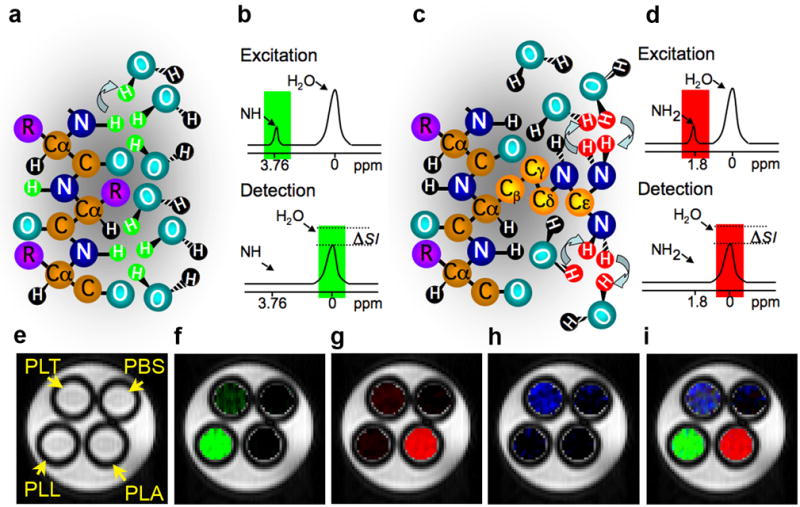
A frequency-selective saturation pulse is applied to label amide protons (A, green) or guanidyl protons (C, red) of a polypeptide contrast agent. The labeled protons exchange with water protons, which leads to a reduction in MR signal intensity (ΔSI) in a frequency-selective manner (B and D). CEST maps of MRI phantoms were acquired with frequency-selective saturation pulses; E) at ±3.7 ppm which enhances PLL, f) at ±1.8 ppm, which enhances PLA, and G) at ± 0.8 ppm which enhances PLT. The merged composition image of maps (E-G) is shown in (H). [Modified from (24) and (23)].
MR spectroscopy: enzyme-based reporter genes
Historically, the first MR reporter gene was creatine kinase (CK), an enzyme that catalyzes ATP conversion to ADP producing phosphocreatine (PCr), and can be detected by 31P MRS. Its first demonstration was in transgenic overexpression of CK in the liver (9), where high levels of PCr were observed, while PCr is absent in liver of control mice. CK was subsequently used as a marker of low-density lipoprotein receptor (LDLr) expression in LDLr deficient mice following treatment with adenovirus engineered to co-express recombinant human-LDLr and the CK-B reporter gene (10). In a recent study, MRS was used to examine different promoters in yeast cells. Genes that catalyze the formation of polyphosphate (polyP) were cloned and placed after different promoters, following which polyP levels could be monitored with 31P MR (11).
MR imaging: enzyme-based reporter genes
The overexpression of human tyrosinase initiates a chain of reactions including melanin production, which is followed by higher metal binding that may result in enhanced MR signal intensity. Using this concept, a tetracycline inducible system of tyrosinase has been developed in MCF-7 breast cancer cells (12).
Another enzyme that was exploited as MR reporter is β-galactosidase (β-gal). β-gal is an enzyme encoded by the LacZ gene of E. coli, it catalyzes hydrolysis of β-D-galactosides, and is widely used in microscopy due to its large variety of substrates. The use of β-gal in MRI was demonstrated by its effect on EgadMe (13). Once EgadMe was cleaved by β-gal and its free coordination site of Gd was exposed, it altered the relaxation time of water and created contrast. β-gal was also used in spectroscopic imaging (14).
MR imaging: iron-based reporter genes
Another possibility for creating MR reporter genes is the expression of iron related proteins. Iron, depending on its oxidation state and its structure, can act as a paramagnetic reactive metal, which is essential for body homeostasis. The manipulation of iron concentration is expected to yield a detectable change in MRI contrast, which will be able to produce a real time report on cellular events. Therefore, several iron related proteins were tested as possible MR reporters.
In the bloodstream, iron is bound to the plasma protein transferrin (Tf) and enter cells via interaction of the iron-transferrin complex with the transferrin receptor (TfR). Administration of Tf linked to MION (monocrystalline iron oxide nanocompounds) to nude mice that had hTfR transfected 9L gliosarcoma tumors, led to a significant difference in MR signal 24 hours after administration (15). TfR has also been used for monitoring therapeutic gene expression when expressed as part of a vector that carried several genes, among them a prodrug therapy gene. All three transgenes were expressed in the same cell simultaneously, and MR imaging demonstrated the ability to use it as a reporter for gene therapy (16).
In addition to its crucial role in body homeostasis, excess of iron can also cause damage to cells by catalyzing the Fenton reaction, which generates reactive oxygen species. Therefore, excess intracellular iron is stored in the ferrihydrite core of the ferritin protein. The first use of ferritin in the design of C6 glioma MR reporter cells was done with a construct that carried EGFP (enhanced green fluorescent protein) and the heavy chain of ferritin, which exhibits ferroxidase activity, under tetracycline control. Inoculation of these tumor cells in nude mice revealed a significant decrease in T2 upon tetracycline withdrawal (17). Transgenic mice that expressed the heavy chain of ferritin in a tissue specific and tetracycline dependent manner, revealed a significant change in T2 values that was tissue dependent: decreased T2 when expressed exclusively by endothelial cells as expected (Figure 2) and surprisingly increased T2 when expressed by liver hepatocytes. The reason for the opposite direction of T2 changes in the liver was found to be related to the overexpression of h-ferritin in the hepatocytes inducing mild toxicity, which resulted in water vacuole formation. These water vacuoles contributed to the elevation in T2 values due to the long T2 of free water (18).
Figure 2. MRI detection of endothelial over-expression of H-ferritin in transgenic mice.
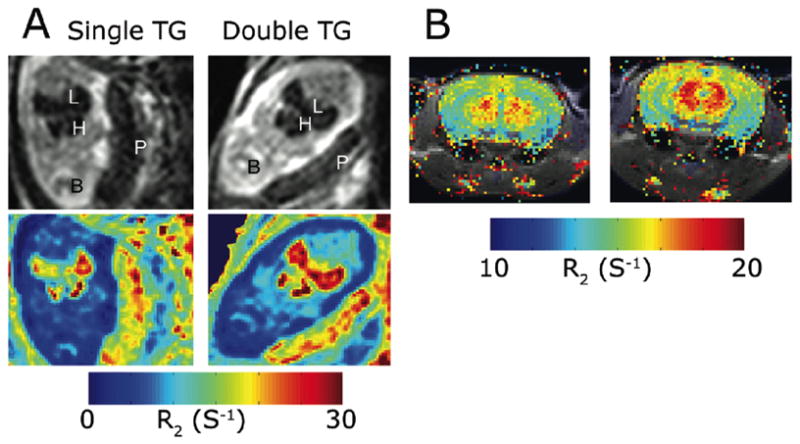
Expression of ferritin was regulated by the VE cadherin promoter in double transgenic mice, but not in single transgenic siblings. Elevated R2 was observed in the liver and heart of E13.5 embryos studied in utero (A), and in the brain of adult mice (B). Adapted from Ref (18).
Genove et al. demonstrated the use of ferritin as MR reporter by injecting adenovirus that encoded for human ferritin into brain parenchyma of mice that resulted in significant loss of signal at the site of inoculation (19). Co-expression of ferritin and TfR showed signal loss on T2- and T2*-weighted MR images of neuronal stem cells in an iron enriched environment (20). Further improvements in ferritin MR reporter gene sensitivity may be possible. For instance, Bennett et al. proposed that aggregation of ferritin could augment its efficacy. Chemical crosslinking of ferritin to actin in vitro lead to polymerization which resulted in 20% decrease in T2 (21).
Recently, Mag A, a bacterial iron transporter in A. magnetotacticum from the magnetotactic bacteria family, was expressed in 293FT cells under doxycycline regulation. Administration of doxycycline and Fe resulted significantly shorter T2, while inoculation of these cells to a mouse brain induced significant signal loss on T2*-weighted images (22).
MR imaging: CEST-based reporter genes
CEST contrast agents have two major advantages. First, they are switchable, i.e., the contrast is detectable only when a saturation pulse is applied at the specific frequency characteristic of an agent’s exchangeable protons. Otherwise, the contrast agent is MRI-invisible. The second advantage is that different contrast agents with different excitation frequencies can be used for imaging simultaneously more than one target. This property might be exploited for imaging multiple cells (e.g. for studying neuronal circuits) or the expression of multiple genes (such as in genetic circuits). This so-called “multicolor” CEST MRI concept is shown in Figure 1e-h, for the polypeptides poly-L-lysine (PLL), poly-L-arginine (PLA), and poly-L-threonine (PLT) (23). While PLL is detectable through its exchangeable amide protons (NH), PLA provides strong CEST contrast through the exchangeable guanidyl protons (NH2), while PLT has exchangeable hydroxyl protons (OH) with a unique frequency. Although each of the polypeptides provides some CEST contrast in the other polypeptides’ frequency range it is relatively easy to separate the signal derived from each polypeptide with proper image processing. Therefore, the three different polypeptides are clearly distinguishable with MRI, making them suitable candidates for labeling different targets.
As a first demonstration, a Lysine Rich Protein (LRP), containing a high density of amide protons, was cloned and expressed in 9L rat glioma cells. The LRP amino acid sequence is similar to PLL and provides MR contrast at the amide proton frequency. LRP overexpression was detectable in vitro in cell extracts and in vivo in xenografts of LRP-expressing 9L cells in mouse brain (24).
Future prospects
MR reporter genes are still in their infancy and need to be further improved to facilitate wider use. A major improvement in sensitivity for both ferritin- and CEST reporter genes is likely to occur with MR scanners moving towards ever higher fields (up to 9.4T for humans and as high as 18.8T for scanners suitable for animal research). In addition, the acquisition of more specific reporter-induced MR contrast may be achieved using optimized pulse sequences and improved ways of image processing (analogous to those used for detecting small changes in blood oxygen level dependent (BOLD) fMRI. From the molecular biology side, reporter genes may be improved through directed evolution in a fashion similar to that accomplished for the fluorescent reporter genes (25). A key challenge would be to resolve multiple targets while performing simultaneous imaging. This could include different reporters driven by different promoters or developing sophisticated data processing algorithms to distinguish gene expression temporally as well as spatially.
From the clinical point of view there are still a few significant hurdles before reporter genes can be introduced into the human body. Once this has been achieved in a safe manner, we can foresee two major clinical applications. The first one will be tracking genetically labeled immunotherapeutic or regenerative stem cells for monitoring cell survival, migration/homing, and differentiation. The second application will be monitoring gene replacement therapy; here the MR reporter genes might become the method of choice to monitor successfully induced therapeutic gene expression long before a phenotypic readout becomes available.
Table 1.
Comparison of existing MR reporter genes.
| Gene | Contrast | Substrate | Advantages | Disadvantages |
|---|---|---|---|---|
| Creatine kinase | 31P MRS | Endogenous ATP | No background signal in most tissues | Spectroscopic image (low resolution) |
| Tyrosinase | T2/T2* | Endogenous or exogenous Fe | Rapid straightforward gene product | Signal dependent on availability of iron |
| β-galactosidase | T1 | Exogenous EgadMe | Positive contrast | Exogenous agent also gives partial signal without reporter gene |
| Transferrin receptor | T2/T2* | Exogenous transferrin-MION | High sensitivity | Accessability and non-specific uptake of substrate nanoparticles |
| Ferritin | T2/T2* | Endogenous or exogenous Fe | High sensitivity | Delay of change in signal, that is dependent on Fe availability and ferritin loading factor |
| Mag A | T2/T2* | Endogenous or exogenous Fe | High sensitivity | Delay of change in signal, that is dependent on Fe availability |
| LRP | CEST | None | Multicolor detection, on-off switchable | Current low sensitivity |
Acknowledgments
The studies described here were supported, in part, by KO1 EB006394 (MTM), the Israel Science Foundation (MN), the Minerva Foundation (MN), and NIH Roadmap R21 EB005252 (JWMB).
References
- 1.Gibbs RA, Weinstock GM, Metzker ML, et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004 Apr 1;428(6982):493–521. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- 2.Livet J, Weissman TA, Kang H, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007 Nov 1;450(7166):56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- 3.Serganova I, Ponomarev V, Blasberg R. Human reporter genes: potential use in clinical studies. Nuclear medicine and biology. 2007 Oct;34(7):791–807. doi: 10.1016/j.nucmedbio.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Gilad AA, Winnard PT, Jr, van Zijl PC, Bulte JW. Developing MR reporter genes: promises and pitfalls. NMR in biomedicine. 2007 May;20(3):275–290. doi: 10.1002/nbm.1134. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Hur T, van Heeswijk RB, Einstein O, et al. Serial in vivo MR tracking of magnetically labeled neural spheres transplanted in chronic EAE mice. Magn Reson Med. 2007 Jan;57(1):164–171. doi: 10.1002/mrm.21116. [DOI] [PubMed] [Google Scholar]
- 6.Modo MMJJ, Bulte JWM. Molecular and cellular MR imaging. Boca Raton: CRC Press; 2007. [Google Scholar]
- 7.Jasanoff A. MRI contrast agents for functional molecular imaging of brain activity. Current opinion in neurobiology. 2007 Oct;17(5):593–600. doi: 10.1016/j.conb.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherry AD, Woods M. Chemical Exchange Saturation Transfer Contrast Agents for Magnetic Resonance Imaging. Annual review of biomedical engineering. 2008 Aug 15;10:391–411. doi: 10.1146/annurev.bioeng.9.060906.151929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koretsky AP, Brosnan MJ, Chen LH, Chen JD, Van Dyke T. NMR detection of creatine kinase expressed in liver of transgenic mice: determination of free ADP levels. Proceedings of the National Academy of Sciences of the United States of America. 1990 Apr;87(8):3112–3116. doi: 10.1073/pnas.87.8.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Qiao H, Lebherz C, et al. Creatine kinase, a magnetic resonance-detectable marker gene for quantification of liver-directed gene transfer. Hum Gene Ther. 2005 Dec;16(12):1429–1438. doi: 10.1089/hum.2005.16.1429. [DOI] [PubMed] [Google Scholar]
- 11.Ki S, Sugihara F, Kasahara K, et al. A novel magnetic resonance-based method to measure gene expression in living cells. Nucleic Acids Res. 2006;34(6):e51. doi: 10.1093/nar/gkl135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alfke H, Stoppler H, Nocken F, et al. In vitro MR imaging of regulated gene expression. Radiology. 2003 Aug;228(2):488–492. doi: 10.1148/radiol.2282012006. [DOI] [PubMed] [Google Scholar]
- 13.Louie AY, Huber MM, Ahrens ET, et al. In vivo visualization of gene expression using magnetic resonance imaging. Nat Biotechnol. 2000 Mar;18(3):321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 14.Kodibagkar VD, Yu J, Liu L, Hetherington HP, Mason RP. Imaging beta-galactosidase activity using (19)F chemical shift imaging of LacZ gene-reporter molecule 2-fluoro-4-nitrophenol-beta-d-galactopyranoside. Magn Reson Imaging. 2006 Sep;24(7):959–962. doi: 10.1016/j.mri.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Weissleder R, Moore A, Mahmood U, et al. In vivo magnetic resonance imaging of transgene expression. Nat Med. 2000 Mar;6(3):351–355. doi: 10.1038/73219. [DOI] [PubMed] [Google Scholar]
- 16.Ichikawa T, Hogemann D, Saeki Y, et al. MRI of transgene expression: correlation to therapeutic gene expression. Neoplasia. 2002 Nov-Dec;4(6):523–530. doi: 10.1038/sj.neo.7900266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen B, Dafni H, Meir G, Harmelin A, Neeman M. Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumors. Neoplasia. 2005 FEB;7(2):109–117. doi: 10.1593/neo.04436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen B, Ziv K, Plaks V, et al. MRI detection of transcriptional regulation of gene expression in transgenic mice. Nat Med. 2007 Apr;13(4):498–503. doi: 10.1038/nm1497. [DOI] [PubMed] [Google Scholar]
- 19.Genove G, Demarco U, Xu H, Goins WF, Ahrens ET. A new transgene reporter for in vivo magnetic resonance imaging. Nat Med. 2005 Mar 20;:450–454. doi: 10.1038/nm1208. [DOI] [PubMed] [Google Scholar]
- 20.Deans AE, Wadghiri YZ, Bernas LM, Yu X, Rutt BK, Turnbull DH. Cellular MRI contrast via coexpression of transferrin receptor and ferritin. Magn Reson Med. 2006 Jul;56(1):51–59. doi: 10.1002/mrm.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett KM, Shapiro EM, Sotak CH, Koretsky AP. Controlled aggregation of ferritin to modulate MRI relaxivity. Biophysical journal. 2008 Jul;95(1):342–351. doi: 10.1529/biophysj.107.116145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zurkiya O, Chan AW, Hu X. MagA is sufficient for producing magnetic nanoparticles in mammalian cells, making it an MRI reporter. Magn Reson Med. 2008 Jun;59(6):1225–1231. doi: 10.1002/mrm.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon MT, Gilad AA, DeLiso MA, Cromer Berman SM, Bulte JWM, van Zijl PCM. New “Multi-Color” Polypeptide DIACEST Contrast Agents for MR Imaging. Magn Reson Med. 2008;60(3):803–812. doi: 10.1002/mrm.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilad AA, McMahon MT, Walczak P, et al. Artificial reporter gene providing MRI contrast based on proton exchange. Nat Biotechnol. 2007 Feb;25(2):217–219. doi: 10.1038/nbt1277. [DOI] [PubMed] [Google Scholar]
- 25.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004 Dec;22(12):1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]


