Summary
The removal of apoptotic cells by phagocytic neighbors is essential for metazoan development but remains poorly characterized. Here we report the discovery of a novel Drosophila phagocytosis receptor, Six-microns-under (SIMU), which is expressed in highly phagocytic cell types during development and required for efficient apoptotic cell clearance by glia in the nervous system and by macrophages elsewhere. SIMU is part of a conserved family of proteins that includes CED-1 and Draper (DRPR). Phenotypic analysis reveals that simu acts upstream of drpr in the same pathway and affects the recognition and engulfment of apoptotic cells, while drpr affects their subsequent degradation. SIMU strongly binds to apoptotic cells, presumably through its EMILIN-like domain, but requires no membrane anchoring, suggesting that it can function as a bridging molecule. Our study introduces an important new factor in tissue-resident apoptotic clearance and underscores the prominent role of glia as ‘semi-professional’ phagocytes in the nervous system.
Introduction
The elimination of superfluous or damaged cells through programmed cell death plays an essential role in metazoan development and tissue homeostasis; its critical final stage is the clearance of the apoptotic cells through phagocytosis. The proper recognition, uptake, and degradation of dying cells is accomplished either by ‘professional’ phagocytes, such as macrophages and immature dendritic cells, or by ‘non-professional’ tissue-resident neighboring cells. While professional phagocytes have been studied extensively, relatively little is known about the biological significance and the molecular underpinnings of tissue-resident phagocytosis (Henson and Hume, 2006).
Apoptotic cell clearance is a complex process, involving recognition, engulfment, and phagosome formation and maturation as distinct steps (Figure 1E) (for review see Grimsley and Ravichandran, 2003; Stuart and Ezekowitz, 2005): The apoptotic cell displays distress (‘eat me’) signals that are recognized by the phagocyte, either directly by phagocytic receptors or indirectly through bridging molecules (opsonins) supplied systemically through the serum or secreted locally by the phagocyte. Two types of phagocytic receptors have been implicated in this recognition process: tethering receptors without a significant intracellular domain, such as CD36 (Franc et al., 1999; Savill et al., 1992) or SRA (Platt and Gordon, 1998), and docking receptors with non-catalytic intracellular domains permitting interaction with other proteins, such as CED1 and its homolog DRPR (Freeman et al., 2003; Zhou et al., 2001) or LRP (Ogden et al., 2001). The clustering of both types of receptors is thought to be required for the recruitment of the downstream machinery to the docking sites, which leads to cytoskeletal reorganization and engulfment of the apoptotic cell. The phagocytosis process is completed by the formation of a phagosome and its maturation to a phagolysosome, effecting the degradation of the apoptotic particle.
Figure 1.

Apoptosis and glial phagocytosis in late nervous system development. (A) lateral view of a CM1 stained stage 16 embryo, showing that at this developmental stage apoptosis is largely restricted to the CNS (arrows). (B) ventral and (C) transverse view (from dashed area in B) of CNS (ELAV, pink), showing that macrophages (CRQ, green) hover around it without entering. (D) Schematic of transverse section through CNS, showing the positions of astrocytic cell body glia and macrophages; grey box indicates position of confocal sections shown in panels F–H. (E) Schematic depicting the different stages of phagocytosis – apopotic cell recognition, engulfment, phagosome formation and maturation. (F)–(H) Ventral view of CNS in confocal stacks, showing membrane-labeled glia (repo::CD8GFP, green) and different apoptosis/phagocytosis markers (red): (F) AnnexinV as an early, (G) activated Caspase 3 (CM1) as a later apoptosis marker, (H) Lysotracker as a phagosome maturation marker. Circles in (F) highlight unengulfed cells with high levels of AnnexinV staining.
Insight into the molecular mechanisms by which ‘non-professional’ tissue-resident cells effect apoptotic clearance came initially from the worm, which does not have professional phagocytes. Genetic screens identified several phagocytosis genes that fall into two partially redundant pathways, one consisting of a phagocytic docking receptor (CED-1), its adaptor (CED-6), an ABC transporter (CED-7) and dynamin, and the other of an actin-regulating protein complex (CED-2/5/10/12) that presumably acts downstream of an unknown phagocytic receptor (for review see Mangahas and Zhou, 2005; Reddien and Horvitz, 2004; Yu et al., 2006). Recently, the phosphatidylserine receptor BAI1 was found to act upstream of the homologous complex in mouse (Park et al., 2007). In Drosophila, to date only three factors have been implicated in apoptotic cell clearance: the macrophage-specific CD36 homolog Croquemort (CRQ; Franc et al., 1999), the F Box protein Pallbearer (Silva et al., 2007), and the broadly expressed CED-1 homolog DRPR, which plays a role in glial phagocytosis of apoptotic neurons (Freeman et al., 2003). A recent study suggests that the apoptotic clearance function of CED-1/DRPR is conserved in vertebrates (Hamon et al., 2006).
Thus, most of the players involved in tissue-resident clearance are still unknown, in particular the phagocytic receptors and their cognate ligands on the apoptotic cell. More generally, a better understanding of the cellular and molecular underpinnings of apoptotic clearance in vivo is highly desirable. In the work presented here, we demonstrate that glia are the main phagocytes in the late developing nervous system of the fly. We describe a novel tethering receptor named Six-microns-under (SIMU), which functions in apoptotic clearance by both glia and macrophages, and find that it acts upstream of DRPR in the same pathway. Our study reveals an evolutionary expansion and concomitant specialization of the repertoire of CED1-like receptors, with SIMU required for the recognition and engulfment, and DRPR for the degradation of corpses.
Results
Glia clear apoptotic neurons in late embryogenesis
In Drosophila development, the elimination of superfluous cells through apoptosis occurs in three main phases, first in mid to late embryogenesis, then in mid pupa, and again in the early adult. Much of the cell death occurs in the nervous system, where almost half the cells are removed (Abrams et al., 1993; Rogulja-Ortmann et al., 2007). Apoptotic cell clearance in embryogenesis was originally thought to be performed primarily by ‘professional’ phagocytes, the macrophages (Franc et al., 1999; Sonnenfeld and Jacobs, 1995), but more recent work (Freeman et al., 2003; Mergliano and Minden, 2003), including ours presented here, shows that both the ectoderm and the glia are avid phagocytes in their own right.
When tracking apoptosis in the embryonic nervous system with activated caspase antibodies (CM1) we observe a high and steady amount of neuronal cell death between 13 and 16 hrs of development (stage 16; Figure 1A; Rogulja-Ortmann et al., 2007). By this stage, the ventral nerve cord is ensheathed by an epithelium formed by surface glia (Schwabe et al., 2005), and macrophages are found hovering around the nerve cord without entering it (Figure 1B, C). When we examine the neural cortex, the portion of the nerve cord that harbors the neuronal cell bodies and where most apoptosis takes place (Figure 1D), we find that nearly all CM1-positive apoptotic particles are either engulfed or at least touched by neighboring glia, which are visualized using a transgenic glial membrane marker (repo::CD8GFP) (Figure 1G, cf. Sonnenfeld and Jacobs, 1995); this suggests that in normal tissue the activation of effector caspase is concurrent with phagocytic uptake and thus relatively late. Based on their morphology and position, the CNS glia fall into three main categories – glia that ensheath the axon tracts (‘neuropile glia’) or the nerve cord as a whole (‘surface glia’), and astrocyte-like glia residing in the neural cortex (‘cell body glia’) (Ito, 1995). These cell body glia appear to be the principal phagocytes in the nervous system at later stages of embryonic development. Unlike vertebrates, the Drosophila nervous system does not contain microglia, which are blood-derived but tissue-resident ‘professional’ macrophages.
The phagocytic role of glia is corroborated by live observation with additional death and phagocytosis markers. Phosphatidylserine, a phospholipid that is restricted to the inner leaflet of the plasma membrane in healthy cells, accumulates on the outer surface during apoptosis (van den Eijnde et al., 1998) and is recognized with high affinity by AnnexinV. When fluorescent AnnexinV probe is injected into embryos that express the glial membrane marker ( repo::CD8GFP ), we find most of the strongly AnnexinV-positive particles touched or engulfed by the glia, but a significant fraction remains untouched (Figure 1F), suggesting that AnnexinV is an earlier or more sensitive marker for apoptosis than activated caspase CM1 antibody (Figure 1G). By contrast, when lysosomal activity is monitored in vivo by injecting fluorescent Lysotracker, we find that nearly all lysosomal activity in the nerve cord coincides with the glia (Figure 1H). In embryos lacking apoptosis, all three markers show accordant changes in their distribution (Figure S1). Multiple independent markers thus indicate that the removal of apoptotic cells in the nerve cord is entirely carried out by the tissue-resident glial cells. This finding is consistent with results of a genome-wide transcription profiling of FACS sorted embryonic glia, in which phagocytosis genes are highly represented, including homologs of vertebrate phagocytic receptors, DRPR, and other orthologs of the two worm phagocytosis pathways (H. Courvoisier, D. L., J. Fak, N. Rajewsky, and U. G., unpublished data).
The simu gene is expressed in all three phagocytic cell populations
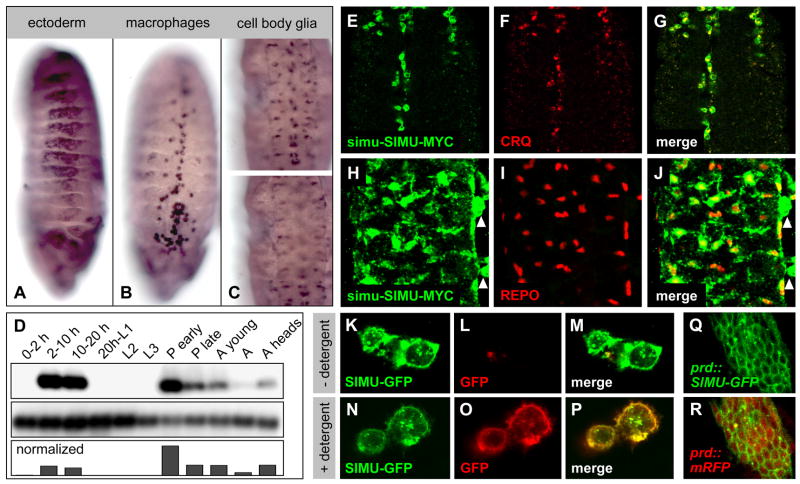
To begin our molecular and genetic investigation of glial phagocytosis, we focused on a novel gene we named six-microns-under (simu, =CG16876), which ranked near the top in our glial transcription profile and whose expression and molecular features made it a particularly promising candidate. Northern analysis shows a dynamic expression of simu, which parallels the main epochs of developmental apoptosis: mid to late embryogenesis, pupae and young adult. It is also expressed in the adult nervous system (Figure 2D). RNA in situ hybridization reveals that simu is expressed specifically in all three phagocytic cell populations: at mid embryogenesis (stages 10–15), it is found in macrophages and the ectoderm (Figure 2A, B), and later on (stage 16) also in the glia, with the strongest expression in the astrocytic cell body glia (Figure 2C). This intriguing expression pattern suggested an involvement of simu in the phagocytosis of apoptotic cells.
Figure 2.
Expression of simu transcript and protein fusions. (A)–(C) RNA in situ hybridization (simu ORF as probe) of whole mount embryos showing simu transcript accumulation in the ectoderm (A; lateral view stage 13), the macrophages (B, ventral view, stage 14) and the astrocytic cell body glia (C, ventral views, stage 16). (E)–(J) This expression is confirmed using MYC-tagged SIMU (green) under simu promoter control (see Figure 5A) and celltype-specific markers (red): CRQ for macrophages (F, G), and REPO for glia (I, J). Arrowheads in (H, J) indicate SIMU-positive but REPO-negative macrophages outside the CNS. (D) Northern analysis (simu ORF as probe), showing a single 1.3 kb simu transcript with strong expression in mid and late embryogenesis, no expression in larvae, strong expression in midpupae, and weak expression in adults, particularly heads. (K)–(P) C-terminally GFP-tagged SIMU expressed in S2 cells localizes to the plasma membrane as visualized by live GFP fluorescence (K, N), but the GFP tag is recognized by antibody only after detergent treatment (compare L, O). (Q, R) Expression of SIMU-GFP in ectodermal stripes in the embryo (prd-Gal4 driver) also shows cell surface expression; overlay with prd::mRFP in (R).
We identified a 2kb genomic fragment upstream of the simu translation start site which, when fused to a cytoplasmic GFP reporter (simu-cytGFP), recapitulates the endogenous expression pattern of the locus, namely expression in ectoderm, macrophages and glia. We use this simu-cytGFP reporter in some of our descriptive and genetic analysis, in particular our time lapse recordings of phagocytosis in vivo (see below). Since we have not been able to generate SIMU antibodies (the protein is very difficult to express in bacteria), we also used this 2 kb simu promoter to drive a SIMU-MYC protein fusion (simu-SIMU-MYC) which serves as our immun-histochemical marker for SIMU protein expression in vivo (Figure 2E–J). simu-SIMU-MYC shows strong expression on the cell surface of macrophages and glia, but loses ectodermal expression earlier than the simu-cytGFP reporter, suggesting a difference in protein turnover between SIMU and GFP.
The simu genomic null mutant shows impaired recognition and engulfment of apoptotic particles
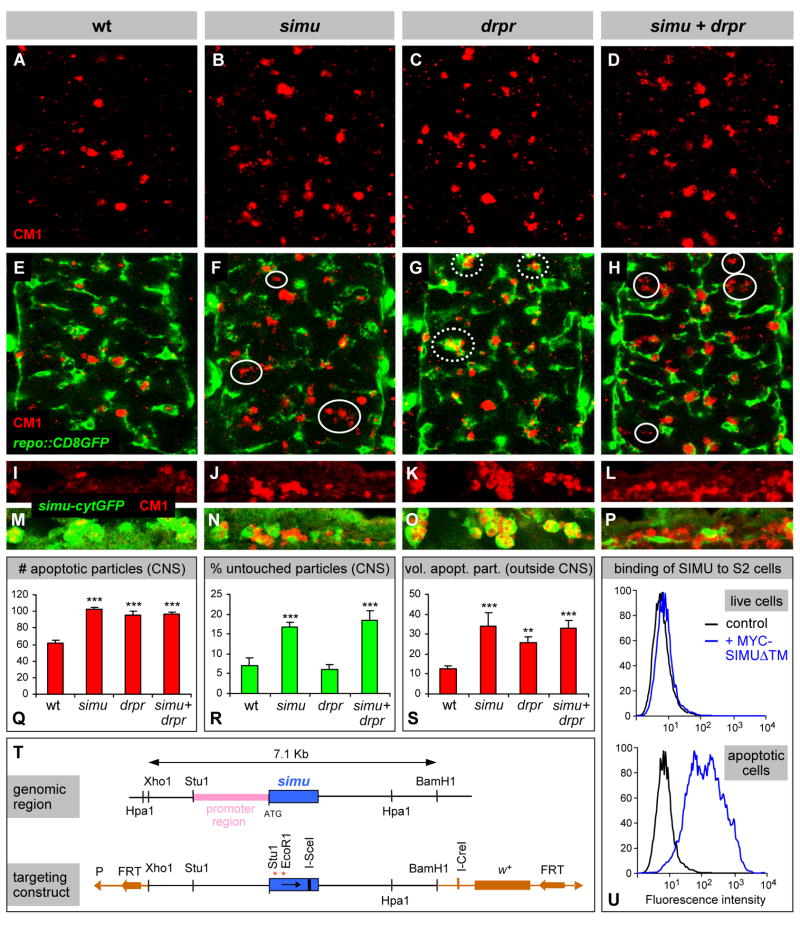
To examine the biological function of simu, we sought to generate a genomic knockout. Since there are no P insertions in the vicinity of the simu locus (the nearest P element was 40 kb away), targeted homologous recombination was the only viable strategy. We engineered a simu null allele in which both in-frame ATGs of the coding region were replaced by stop codons (Figure 3T) and swapped the mutant version into the endogenous locus (see Experimental Procedures). The success of the genomic knockout was confirmed both at the molecular level, by PCR and Southern blot analysis, and genetically: a deficiency uncovering the simu locus (Df(2L)Sco7) fails to complement our simu null mutant. A genomic fragment containing the entire transcribed region and 2 kb upstream completely rescues the simu phenotype (Figure 5B, see below).
Figure 3.
Genetic and phenotypic analysis of simu and drpr. (A)–(H) Projections from confocal stacks of neural cortex layer of the CNS at embryonic stage 16; ventral view; apoptotic cells in red (CM1) and glia in green (repo::CD8GFP). In simu (B, F) and drpr (C, G) null mutants, the number of apoptotic particles almost doubles compared to wildtype (A, E), but does not increase further in the simu, drpr double mutant (D, H); for quantitation see (Q). In simu mutants the apoptotic cells accumulate outside (circles), in drpr mutants inside the glia (dotted circles), in the double mutant they accumulate outside (circles); for quantitation see (R). (I)–(P) Macrophages (green, simu-cytGFP) show the same phenotypic defects as glia in all genotypes. (Q)–(S) Quantification of phenotypic defects: (Q) total number of apoptotic particles within CNS sections; (R) fraction of apoptotic particles that are completely untouched by glia; (S) volume of apoptotic particles in the embryo outside the CNS (stage 14). Columns represent mean values ± SEM, n=7–12, asterisks indicate statistical significance vs. wild type, as determined by one-way ANOVA, with Student-Newmann-Keuls post-hoc test, *** p<0.001, ** p<0.01. (T) Schematic of the simu genomic region and of the simu knockout targeting construct. (U) Flow cytometric analysis of MYC-SIMUΔTM protein binding to S2 cells. Apoptotic S2 cells (bottom) are strongly bound by SIMU, live cells (top) are not.
Figure 5.
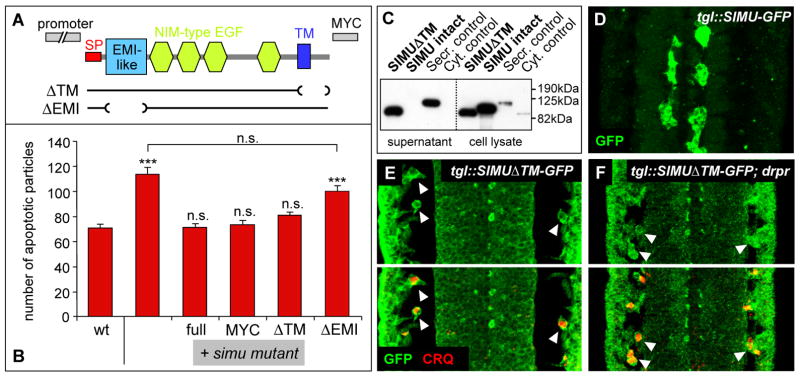
Structure-function analysis of SIMU protein. (A) Schematic showing the domain structure of the SIMU protein, the general design of the transgenes (direct fusion to simu promoter; C-terminal MYC-tag) and the specific deletions made. (B) Quantification of phenotypic rescue of simu null mutants by the different simu transgenes. Columns represent mean total number of apoptotic particles within confocal stacks from the neural cortex layer of the CNS, ± SEM, n=7–12, asterisks indicate statistical significance of comparison with wild type, as determined by one-way ANOVA with Student-Newmann-Keuls post-hoc test, *** p<0.001, n.s. p>0.05. (C) Western blot analysis of transfected S2 cells showing that SIMU lacking the transmembrane domain (SIMUΔTM) is secreted, while fullength SIMU is retained in the cells. (D)–(F) In vivo distribution of GFP-tagged SIMU (green) expressed under a longitudinal glial-specific driver (tgl-Gal4). Fullength SIMU is found only on the surface of expressing cells (D), while the secreted SIMUΔTM is found throughout the embryo (E), with accumulation on the surface of CRQ-positive (red) macrophages (arrowheads) and the ectoderm. This accumulation is also found in drpr null embryos (F); both single label and merged images are shown.
simu mutant animals are homozygous viable and show no gross morphologic abnormalities. To test for phagocytosis defects in vivo, we transgenically labeled the phagocytic cell populations (repo::CD8GFP for glia; simu-cytGFP for macrophages); apoptotic particles were labeled with activated caspase antibodies (CM1). In simu mutants the number, position and overall morphology of CNS glia in general and of the highly phagocytic cell body glia in particular appear normal (average glial cell number 37.2 ±5.6, compared to 37.1 ±3.8 in wildtype, measured in representative stacks of confocal images, see Experimental Procedures). However, the clearance of apoptotic cells is markedly impaired. The number and total volume of apoptotic particles is increased about twofold over wildtype (Figure 3A, B, Q), and the apoptotic particles are much less likely to be engulfed: the fraction of particles that are completely untouched by glial cells is increased threefold in simu mutants over wildtype (Figure 3E, F, R), indicating a significant impairment in the recognition and/or engulfment of apoptotic cells. Similar phenotypes are observed in macrophage phagocytosis: Again, the number (44.8 ±5.0, compared to 46.3 ±4.3 in wildtype, see Experimental Procedures), migration pattern and morphology of the macrophages appear normal; however, the number and volume of apoptotic particles in the embryo outside the nervous system is strongly increased (Figure 3S) and many particles remain unengulfed, often forming large clusters near the macrophages (Figure 3I, J, M, N). These findings point to the same corpse recognition and engulfment defect as in the glia.
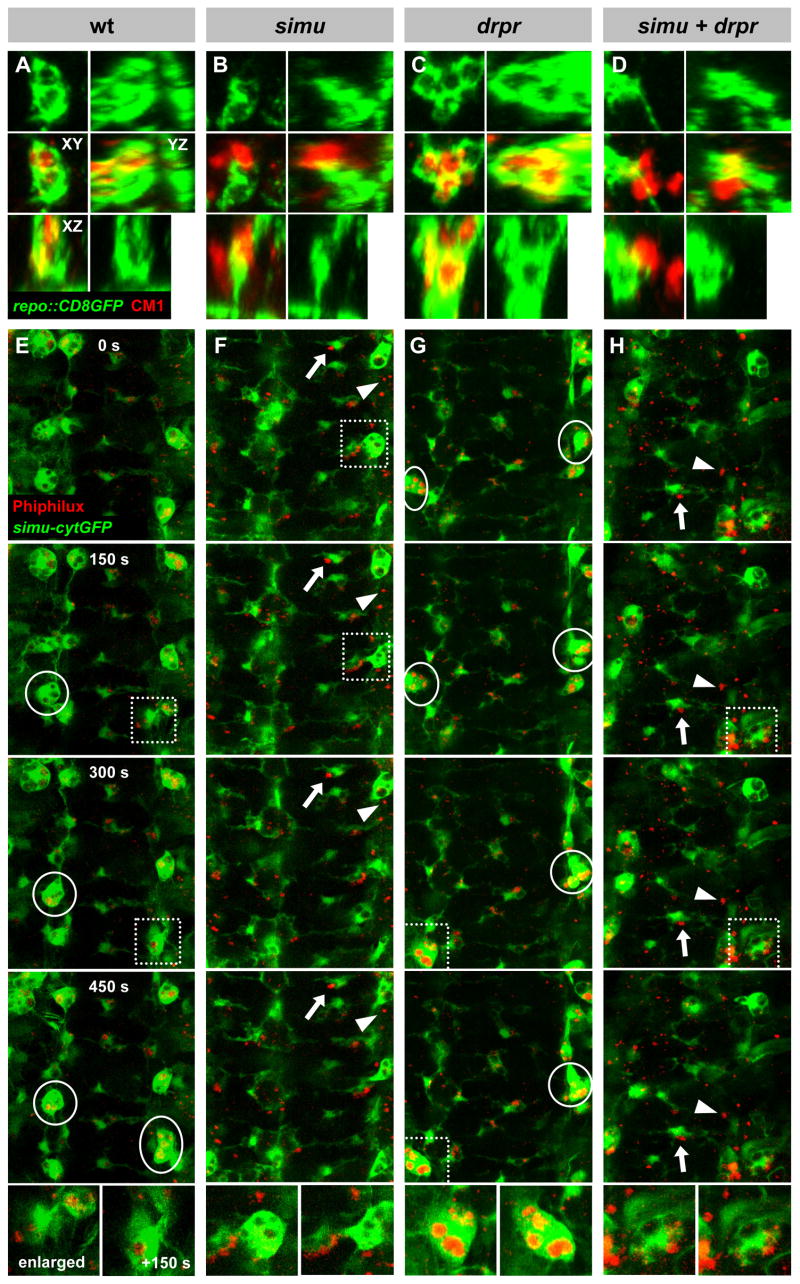
To gain insight into the cellular dynamics, we carried out time lapse recordings in live embryos, with simu-cytGFP labeling glia and macrophages, and injected fluorogenic Caspase-3 substrate Phiphilux labeling apoptotic cells (see Experimental Procedures). In wildtype, almost all Caspase-3 positive particles are engulfed or at least touched by a phagocyte, as observed before in fixed material. Both glia and macrophages are constantly sending out processes to probe their environment and quickly engulf apoptotic cells; the macrophages move around at high speed (~1 μm/min), while the glia remain stationary (Figure 4E, Movie S1). In the simu mutant, both search behavior and motility of the phagocytes are normal, but many apoptotic particles remain completely untouched for extended periods of time or are briefly touched without being tethered. In addition, many particles attach to the phagocyte cell surface without being engulfed; occasionally, we observe the formation of incomplete phagocytic cups (Figure 4F, Movie S2). These findings clearly indicate that simu does not influence the general behavior and morphology of phagocytes, but rather affects specifically their ability to recognize and engulf apoptotic particles.
Figure 4.
3D and dynamic analysis of simu and drpr phenotypes. (A)–(D) 3D analysis of engulfment of apoptotic particles (CM1, red) by glia (repo::CD8GFP, green) in the CNS. Shown are single label (glia) and merged images of local projections of 4–5 μm depth centered around representative individual glia/apoptotic particles, in three orthogonal planes (XY, YZ, XZ) as indicated; see Experimental Procedures. Note complete engulfment of apoptotic particles in wt and drpr, while in simu and in the simu; drpr double mutant particles are attached but unengulfed, or completely untouched. For additional examples, see Figure S3. (E)–(H) Time-lapse recordings of phagocytosis in stage 16 embryos. Glia and macrophages are labeled with simu-cytGFP (green), apoptotic cells with the fluorogenic Caspase 3 substrate Phiphilux (red); selected consecutive frames are shown (movies are available in the Supplement); enlarged view of individual macrophages over two consecutive frames is shown at right, with origin indicated by dotted boxes. In wildtype (E), nearly all apoptotic particles are touched or engulfed (circles); in simu mutants (F), many apoptotic particles are untouched (arrowheads) or remain on the phagocyte cell surface (arrows); in drpr mutants (G), apoptotic particles accumulate inside the phagocytes (circles); in the simu; drpr double mutant (H), particles accumulate outside the phagocytes, similar to the simu single mutant.
Simu protein structure and function
The simu gene is predicted to encode a 377 aa transmembrane protein, comprising an N-terminal signal peptide, a large extracellular portion, a single putative transmembrane (TM) domain and a short cytoplasmic tail at the C-terminus (Figure 5A). The extracellular portion of the protein consists of an N-terminal EMILIN (EMI)-like domain (Callebaut et al., 2003) and four EGF domains with highly stereotyped spacing of Cys residues, recently named Nimrod (NIM) repeats (Kurucz et al., 2007). Intriguingly, the combination of an EMI(-like) domain, with a highly conserved CCxGY motif at the C-terminal end of the domain, directly followed by a NIM repeat (Figure 6E) is found across the vertebrate/invertebrate divide in a small number of secreted as well as transmembrane factors, some of which have been shown to function in phagocytosis. They include the CED-1 homologs in all species (DRPR, human MEGF10, mouse Jedi), the fly bacterial phagocytosis factor Eater (Kocks et al., 2005) and the large Nimrod gene cluster located at 34E (Kurucz et al., 2007), the most proximal member of which is simu (=NimC4), as well as human SREC (see Figure S2 for a detailed comparison).
Figure 6.
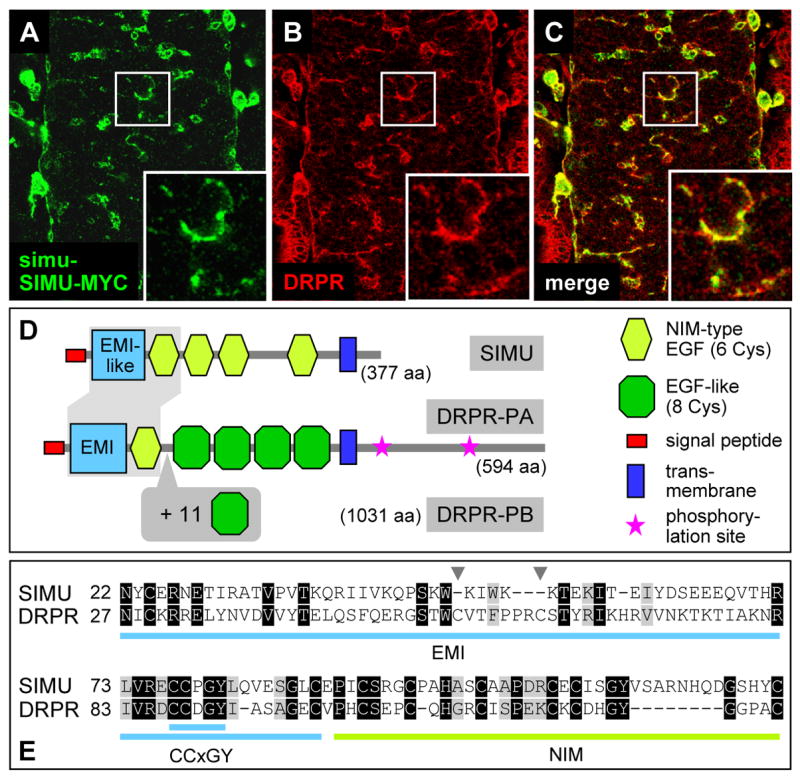
Comparison of the SIMU and DRPR proteins. (A)–(C) In vivo expression patterns, showing strong overlap between SIMU (visualized by simu-SIMU-MYC; green) and DRPR (anti-DRPR antibody; red) in glia and macrophages; magnification of boxed areas at right bottom, showing strong co-localization of the two proteins at the cell surface. (D) Schematic representation of domain organization of SIMU and of the two isoforms of DRPR. (E) Sequence alignment of the N-terminal EMI(-like)+NIM domain of DRPR and SIMU, identical residues are boxed in black, similar residues in grey. The EMI-like domain in SIMU lacks two internal Cysteines that are present in DRPR (arrowheads), but has both the first Cysteine and the highly conserved CCxGY motif. See also Figure S2.
To confirm the overall protein organization of SIMU and determine its orientation within the plasma membrane, we C-terminally tagged SIMU with GFP and expressed the protein fusion in Drosophila tissue culture (S2) cells. Live observation detects SIMU-GFP at the plasma membrane (Figure 2K, N); after fixation, GFP antibodies recognize the fusion protein only when cells are permeabilized with detergent (Figure 2L, O), indicating that the C-terminus is intracellular and thus identifying SIMU as a type I transmembrane protein. Consistent with these results, both SIMU-GFP and SIMU-MYC localize to the plasma membrane in the embryo (Figure 2Q, R).
Given that the simu phenotype reveals a function in the recognition and engulfment of apoptotic particles, we sought to test whether the SIMU protein is able bind to apoptotic cells in vitro. To this end, we produced a secreted tagged version of SIMU, comprising the entire extracellular domain of the protein, in human HEKc18 cells. Strikingly, while affinity-purified HIS-MYC-SIMUΔTM protein shows very little binding to live S2 cells, consistent with the small percentage of dying cells within the culture, we observe very strong binding to apoptotic S2 cells (Figure 3U).
Interestingly, SIMU is also sufficient to increase uptake of apoptotic cells in a heterologous system: We overexpressed the protein in COS7 cells using transient transfection (see Experimental Procedures) with GFP as a negative and the known tethering receptor CRQ as a positive control (Franc et al., 1996). Compared to GFP-transfected cells, SIMU-transfected cells showed a modest increase in the uptake of apoptotic cells (1.3 fold), very similar to that of CRQ (1.4 fold). Taken together, these data complement the results of our in vivo analysis and provide further strong evidence for a role of SIMU in the recognition and uptake of apoptotic particles.
In vivo analysis of SIMU protein truncations
To gain further insight into the molecular function of SIMU, we removed portions of the protein and examined whether the truncations affect its function in vivo. The truncated forms were fused directly to the simu promoter (Figure 5A); we also created MYC-tagged versions of the truncated forms to directly monitor their expression level and localization. Both untagged and tagged truncated versions were placed in the simu mutant background and assayed for their ability to rescue the phagocytosis defect; for each transgene two different genomic insertions were tested. As a baseline, we established that the fullength version of the SIMU protein completely rescues the simu mutant phenotype, both when untagged and in its MYC-tagged form (Figure 5B).
First, we removed the EMI-like domain, whose biological function has not been established, from SIMU’s N-terminus (SIMUΔEMI). SIMUΔEMI is properly expressed and localized to the plasma membrane (data not shown), but does not rescue the simu mutant phenotype (Figure 5B). This indicates that the EMI-like domain is essential for SIMU function and for the first time implicates this type of protein domain in apoptotic clearance. Second, we removed the transmembrane domain and small intracellular portion from SIMU’s C-terminus (SIMUΔTM). We confirmed that SIMUΔTM is indeed secreted by S2 cell transfection (Figure 5C) and in vivo expression, the latter resulting in abundant distribution throughout the embryo, but with particular enrichment on macrophage surfaces (Figure 5D, E). Surprisingly, SIMUΔTM rescues the simu mutant phenotype almost completely, indicating that membrane anchoring is dispensable for SIMU function (Figure 5B). This intriguing result suggests that the SIMU protein is able to act in two different modes – as a tethering transmembrane receptor as well as a secreted bridging molecule.
simu function is epistatic to drpr in the phagocytosis of apoptotic cells
SIMU’s extracellular domain structure is akin to that of DRPR, the Drosophila homolog of CED-1 (Figure 6D, E), but unlike SIMU, DRPR is a docking receptor with a long cytoplasmic domain that contains two conserved tyrosine phosphorylation sites known to be required for the recruitment of cytoplasmic factors (Zhou et al., 2001). To further explore the similarity between SIMU and DRPR, we compared their expression in the embryo. Similar to SIMU, DRPR is strongly expressed in the ectoderm, macrophages and glia (Figure 6A–C; Freeman et al., 2003). Indeed, double labeling of simu-SIMU-MYC and DRPR antibodies reveals strongly overlapping expression of the two proteins on the cell surface of macrophages and glia. Interestingly, the DRPR protein distribution on the plasma membrane is fairly uniform, while SIMU’s appears more patchy (Figure 6C).
Given the similarities in molecular structure and expression pattern, we sought to compare the simu and drpr mutant phenotypes. Similar to simu, drpr null mutants are also adult viable. In our fixed material phagocytosis assay, they show the same roughly twofold increase in the number and volume of apoptotic particles in the nerve cord as simu mutants (Figure 3C, Q; Freeman et al., 2003) and the same increase in total particle volume outside the CNS (Figure 3S). Unlike in simu, however, there is no significant defect in engulfment: the fraction of untouched apoptotic particles is the same in drpr mutants as in wildtype (Figure 3R). Instead, the excess apoptotic particles accumulate inside the phagocytes (Figure 3G, O); this topology is confirmed by 3D reconstructions of deep confocal series with appropriate cross sections (Figure 4C, compare with Figure 4A, B; also Figure S3). Time lapse recordings of drpr mutants further corroborate these results: The general searching behavior and motility of the phagocytes appear normal, and unlike in simu but similar to wildtype, few apoptotic particles remain unengulfed or untouched. However, apoptotic particles accumulate within the phagocytes: while in wildtype the majority of phagosomes are Caspase 3-negative and thus appear unlabeled, almost all are Caspase 3-positive in drpr mutants (Figure 4G; Movie S3). These findings indicate that drpr and simu have distinct functions, with simu involved in recognition and uptake, and drpr primarily required for the degradation of cell corpses.
To test for interaction between the two genes, we generated simu; drpr double mutants. We find the same increase in the total number and volume of apoptotic particles as in either single mutant (Figure 3D, L, Q, S); this lack of an additive effect indicates that simu and drpr act in the same genetic pathway. Moreover, both fixed material analysis and time lapse recordings show that the simu phenotype is epistatic over that of drpr: In the simu; drpr double mutant, apoptotic particles accumulate outside the phagocytes, and the fraction of completely untouched apoptotic particles is the same as in the simu single mutant (Figure 3H, P, R; Figure 4D, H; Movie S4). The simu; drpr double mutant thus closely resembles the simu single mutant, indicating that simu acts upstream of drpr in the same phagocytic pathway. These results are rather surprising, since in the worm CED-1 is required for the recognition and uptake as well as for the degradation of apoptotic particles (Yu et al., 2006; Zhou et al., 2001).
Discussion
In this study, we have demonstrated that tissue-resident clearance by neighboring glia plays a major role in the removal of apoptotic neurons during nervous system development and characterized the function of the novel tethering receptor SIMU in this important process.
Using several different markers, we show that, once the nerve chord is ensheathed and macrophages no longer have access, astrocytic glia avidly phagocytose apoptotic cells in their vicinity. This pronounced phagocytic activity is reflected on a molecular level by a strong differential expression of phagocytosis genes in the glia. Our in vivo time-lapse recordings indicate that the stationary glia are as efficient and fast in corpse uptake as the highly motile macrophages, and that differences in clearance are largely a reflection of differential motility. Notably, glia also phagocytose axon branches during neuronal remodeling and injury-induced Wallerian degeneration (Awasaki and Ito, 2004; Hoopfer et al., 2006; MacDonald et al., 2006). Collectively, these findings indicate that glia are potent ’semi-professional’ phagocytes and that, at least during development, tissue-resident apoptotic clearance equals macrophage-mediated clearance in importance. In vertebrates, tissue-resident phagocytosis by neighbors has been demonstrated in vivo in some contexts including glia (Hanayama and Nagata, 2005; Nakanishi and Shiratsuchi, 2004; Parnaik et al., 2000; Wood et al., 2000), but its overall contribution to apoptotic clearance and the underlying molecular mechanisms have not been established (Henson and Hume, 2006).
Our investigation of the novel gene simu was originally motivated by two intriguing features - its expression during all major epochs of developmental apoptosis and in the major phagocytic cell types, in particular the glia, and a protein domain structure that is similar to, but distinct from that of the CED-1 homolog DRPR. The two proteins are expressed in strongly overlapping patterns throughout embryogenesis and largely co-localize at the plasma membrane of glia and macrophages. Our genetic analysis now reveals that they are components of the same phagocytic pathway, with simu acting upstream of drpr. Observations both in fixed material and in time lapse recordings show simu affecting the early steps of recognition and engulfment of apoptotic particles, while drpr affects their subsequent degradation; the double mutant phenotype closely resembles that of simu alone, indicating epistasy of simu over drpr. Unlike DRPR, SIMU lacks a large cytoplasmic domain with docking sites for downstream effectors, and our results suggest that its molecular function is to recognize the apoptotic cell and tether it to the phagocyte. Purified SIMU protein strongly binds to apoptotic cells in vitro; the in vivo analysis indicates that the N-terminal EMI-like domain, which is essential for SIMU function, is likely involved in the recognition and binding. To date, EMI domains have only been implicated in protein-protein interaction, suggesting that SIMU may recognize a protein rather than a lipid or carbohydrate component on the apoptotic cell. The lack of a cytoplasmic domain and the dispensability of membrane-anchoring for in vivo function imply that SIMU must interact - directly or indirectly - with docking receptors on the phagocyte surface to effect engulfment and subsequent degradation. Given its phenotype and action downstream of simu, drpr is the prime candidate for mediating degradation. However, the difference in the simu and drpr phenotypes and, in particular, the essentially unimpaired engulfment of apoptotic particles we observe in drpr mutants strongly argues for the existence of an intermediary factor that controls the cytoskeletal reorganization necessary for engulfment downstream of simu. The existence of a SIMU binding partner other than DRPR is supported by our finding that, in drpr null mutants, secreted SIMUΔTM still accumulates on macrophage cell surfaces (Figure 5F), and by the fact that we observe no physical interaction between SIMU and DRPR in co-immunoprecipitation experiments (data not shown). Thus, the following model seems most likely: As part of a linear pathway, SIMU interacts with a factor X, leading to the recruitment of cytoskeletal components for engulfment and phagosome formation, followed by X interacting with DRPR, which leads to the recruitment of degradation components for phagosome maturation. The identification of factor X and its extra- and intracellular binding partners will be the subject of future investigation, and understanding the mechanisms by which the different phagocytic receptors cluster and coordinate their function will be of particular interest.
The existence of the SIMU protein was unexpected both genomically and genetically: The C. elegans genome does not contain a SIMU homolog and in fact no other EMI(-like)+NIM domain protein. Moreover, in contrast to drpr, CED-1 is crucial for recognition, engulfment and the degradation of corpses in the worm (Yu et al., 2006; Zhou et al., 2001), suggesting that biological functions that are performed by a single factor in the worm become distributed among more specialized proteins in the fly. Intriguingly, when the worm CED-1 mutant is rescued by a transgene that lacks the intracellular docking domain, recognition and engulfment, but not degradation, are restored, resulting in a drpr-like phenotype (Zhou et al., 2001). Several recent papers have suggested that DRPR acts as an engulfment receptor in different developmental contexts in the fly (Awasaki et al., 2006; Freeman et al., 2003; Hoopfer et al., 2006; Li and Baker, 2007; MacDonald et al., 2006). Most observed an increase in the number of apoptotic cells or axon fragments in drpr mutants, which is consistent with our findings. Unlike our analysis, however, none of these studies established whether the excess apoptotic particles accumulate outside or inside the respective phagocytes. It is also noteworthy that apoptotic clearance is much faster (minutes vs. hours or days) than axon pruning/Wallerian degeneration, and possibly involves distinct signals from the degrading cells (caspase vs non-caspase-mediated mechanism; Awasaki and Ito, 2004; Finn et al., 2000; Watts et al., 2003), making it likely that differences exist at the level of the recognition factors.
The comparison of apoptotic clearance in worm, fly and vertebrate provides interesting evolutionary perspectives. Between worm and fly, the demand for apoptotic clearance increases both in quantitative and qualitative terms: During worm development, only 10% of cells are fated to die and they are removed shortly after their birth through phagocytosis by immediate neighbors, apparently without need for dedicated phagocytes (Lettre and Hengartner, 2006). During fly development, a much larger proportion of cells die through apoptosis (>30%, Abrams et al., 1993; Rogulja-Ortmann et al., 2007) and their death is spread out over a wide range of stages in their life cycle – immediately after their birth, during fating and differentiation, and even after long-term function (Truman et al., 1992). To meet this challenge, macrophages emerge as professional phagocytes, and in addition, several cell types differentially increase their phagocytic capacity and become ‘semi-professional’ phagocytes, including the ectoderm and the glia. In vertebrates, the apoptotic load is presumably even greater (Henson and Hume, 2006; Jacobson et al., 1997), and the cellular complexity of the innate immune system is of course greatly increased. Strikingly, these changes are accompanied by an increase in complexity at the molecular level, both in terms of a greater structural diversity of phagocytic receptors generally, and of CED-1 like factors in particular: While C. elegans seems to have only one CED-1 gene encoding a single protein form, Drosophila has two different DRPR isoforms with distinct extracellular domains, and the entire Nimrod gene family, including SIMU and Eater (Kurucz et al., 2007). This family contains both secreted and transmembrane proteins, and receptors for the clearance of apoptotic cells (SIMU) and pathogens (Eater, NIM C1), but the function of most familiy members is not known. The in vivo function of the vertebrate CED-1 like factors is also unknown, but cell culture experiments suggest that MEGF10 is a phagocytic receptor for apoptotic cells and SREC for (oxidized) LDL (Adachi et al., 1997; Hamon et al., 2006). The molecular comparisons indicate that these phagocytic factors all share an N-terminal EMI(-like)+NIM core, presumably for recognition, and that invertebrates and vertebrates amplified different EGF-type repeats present in the ancestral CED-1 (Figure S2), either to achieve appropriate spacing or to facilitate homotypic cis-interaction in factor clustering.
This molecular expansion is likely accompanied by an increase in functional redundancy between phagocytic pathways: Already in the worm, at least two (CED-1/6/7 and CED-2/5/10/12) and likely a third, currently unknown, pathway participate in the phagocytosis process (Mangahas and Zhou, 2005; Reddien and Horvitz, 2004); as shown here for fly, joint removal of simu and drpr results in viable adults whose apoptotic clearance is impaired but not abrogated, indicating the existence of additional as yet unidentified pathways; finally, in vertebrates, a large number of different types of macrophage receptors have been identified, which under single mutant conditions either only reduce apoptotic clearance or have no effect at all (Grimsley and Ravichandran, 2003; Platt and Gordon, 2001; Scott et al., 2001; Stuart and Ezekowitz, 2005)
Overall, our study has shown that the differences between ‘professional’ and ‘non-professional’ phagocytes are perhaps more fluid than originally thought: Not only can tissue-resident phagocytic cells differentially upregulate a similar molecular repertoire of phagocytic pathways, but they can use it as quickly and efficiently as the macrophages when confronted with apoptotic neighbors. This suggests a simple division of labor: in freely accessible spaces, actively patrolling macrophages clear the corpses, while in sequestered spaces, such as the late nervous system, the task falls to resident neighbors like the glia. In addition to its role in development, the efficient clearance of apoptotic cells is essential for avoiding secondary necrosis and exposure of cytoplasmic components to the immune system, acutely causing inflammation and chronically auto-immune disease. While apoptotic clearance by tissue-resident cells has received relatively little attention in vertebrate research, its comprehensive analysis in genetic models such as the fly will continue to provide interesting and novel insight into the underlying cellular and molecular mechanisms.
Experimental procedures
Fly strains and molecular biology
The following fly strains were obtained from published sources: repo-Gal4 (V. Auld); crq::cytGFP (P. Martin; gift from H. Agaisse and N. Perrimon); UAS-CD8GFP (L.Luo); Df(2L)Sco7 (#6069; Bloomington); prd-Gal4 (#1947; Bloomington); drpr 5 (M. Freeman); Df(3L)H99 (H. Steller). A detailed description of all constructs generated for this study is available upon request, including UAS-mRFP, UAS-SIMU-GFP, simu-cytGFP, simu-SIMUΔEMI-(4xMyc), simu-SIMUΔTM-(4xMyc), HIS-MYC-SIMUΔTM, and the simu null pTV2 targeting construct.
For the genomic knockout, we assembled a contiguous 7.1 kb piece of genomic DNA containing the simu coding region, in which stop codon mutations and restriction sites (EcoRI, StuI, I-SceI) had been introduced by PCR (Rong and Golic, 2000; Rong et al., 2002) and placed into pTV2. Using two different genomic insertions of the donor construct (X, III), we recovered 3 and 14 targeted events, respectively, from 500,000 screened flies; from both screens, single-copy reduction events carrying the engineered mutations were recovered and confirmed by PCR and Southern blot analysis. Northern and Southern analysis were carried out using standard protocols, RNA in situ hybridization according to (www.fruitfly.org/about/methods/RNAinsitu.html).
Immunohistochemistry and imaging
Drosophila Schneider (S2) cells were transfected using cellfectin (Invitrogen) and plated on poly-L-lysine (Sigma) coated slide chambers. Immunohistochemistry followed standard procedures using mouse anti-GFP (Molecular Probes; 1:50), mouse anti-MYC (Santa-Cruz, 1:200), rat polyclonal anti-CRQ (Nakanishi; 1:100); mouse anti-REPO (Developmental Studies Hybridoma Bank; 1:5); rabbit anti-DRPR (Freeman; 1:500), rabbit anti-activated Caspase3 (CM1; Cell signaling Technology; 1:25). Fluorescent secondary antibodies were diluted 1:200 (Cy3 Jackson ImmunoResearch; Alexa Fluor 488 Molecular Probes). Live imaging was carried out by dechorionating embryos (stage 16), mounting them under halocarbon oil, injecting 2–3% egg volume AnnexinV (Molecular Probes) or Lysotracker ( Molecular Probes; 1:10) and imaging after 30–60′ incubation. All confocal images were acquired using a Zeiss LSM 510 system. Image analysis was performed using Zeiss LSM 510 and Imaris (Bitplane) software.
Phagocytosis assays
To quantitate the number of apoptotic particles and their engulfment by glia, confocal stacks (6 sections; total 3 μm) were acquired from the neural cortex of stage 16 ventral nerve cords, where most apoptosis occurs and where cell body glia reside. To quantitate the number of apoptotic particles outside the CNS, stage 14 embryos were imaged from lateral to medial, excluding the nerve cord (confocal stacks with 50 sections; total 43 μm). 3D reconstructions were built and the number and volume of CM1-positive particles measured with an appropriate isosurfacing threshold using Imaris software. The percentage of completely untouched particles was determined by analyzing the relative position of glia to apoptotic cells. All data were collected with identical microscope and software parameter settings. Statistical significance of differences between experiments was assessed by one-way ANOVA with Student-Newman-Keuls post hoc test, with n=7–12 for the CNS analysis and n=5–9 for the whole embryo analysis. The number of glial cells and macrophages was assessed using confocal stacks of similar origin and size as described above (glia labeled with anti-REPO, macrophages labeled with crq::cytGFP) and manual or computer-assisted (Imaris) cell counting; results are reported as mean ± SD, n=6). The high-resolution 3D analysis of the engulfment of CM1-positive particles by glia was performed using deep confocal stacks of stage 16 ventral nerve cords (~30 sections, total ~15 μm) and the Imaris section tool.
For time lapse recordings, simu-cytGFP embryos were injected with 1% egg volume of 10 μM Phiphilux (OncoImmunin) and imaged on a Zeiss LSM 510 system using an Apochromat 40X water immersion objective, taking confocal stacks (8 sections, total 7 μm) at 90–150 second intervals. NIH ImageJ software was used to process the images and generate maximum-intensity projections of the stacks.
To test for heterologous induction of phagocytosis, COS7 cells were transiently co-transfected with pCDNA-Gal4 and UAS-cytGFP (negative control; Clontech) or UAS-CRQ (positive control; K. White) or UAS-SIMU. To obtain apoptotic cells, COS7 cells were treated 16h with 75 μM etoposide and labeled with DiI (Molecular Probes). 70 hours after transfection, the transfected COS7 cells were incubated with apoptotic COS7 cells (1:5 ratio; 1.5h), washed (3x cold PBS), trypsinized and analyzed by flow cytometry. Transfected and apoptotic cells were distinguished by different FSC and SSC. For each experiment, 10,000 events were collected and the data were analyzed using Cell Quest and FlowJo software; experiments were repeated 4 times, with similar results.
Simu protein purification and binding to apoptotic cells
The extracellular portion of SIMU (residues 19–322) was cloned into a HIS-MYC-tag containing vector (pCEP-Pu; Thur et al., 2001), stably transfected into HEKc18 cells using Fugene 6 reagent (Roche) and puromycin selection and the tagged protein affinity-purified from supernatant using TALON metal affinity beads (Clontech) according to manufacturer’s specifications. To induce apoptosis, S2 cells were treated with 75 μM etoposide for 16h. 6 μg of purified HIS-MYC-SIMU-ΔTM were incubated with live and apoptotic S2 cells (PBS+1%NHS; 1.5h). Cells were then incubated with anti-MYC Ab (Santa Cruz Biotechnology; 1:100; 1h) and fluorescent secondary Ab (Alexa Fluor 488 – anti-mouse; Molecular Probes; 1:400; 40 min) and analyzed by flow cytometry. For each experiment, 10,000 events were collected and the data were analyzed using Cell Quest and FlowJo software; experiments were repeated 3 times, with similar results.
Supplementary Material
Acknowledgments
We would like to thank V. Auld, M. Freeman, L. Luo, P. Martin, Y. Nakanishi, H. Steller, K. White, the Bloomington Stock Center, the Drosophila Genomics Resource Center, and the Developmental Studies Hybridoma Bank for fly strains, cDNAs, and antibodies. We also thank A. North and her staff at the Rockefeller Bioimaging Facility for superb technical support, E. Arama and H. Steller for sharing their insights on apoptosis, and M. Boutros, M. Raff, and H. Steller for comments on the manuscript. We are very much indebted to all members of the Gaul lab for their continuing support and constructive criticism throughout the project, in particular T. Das for advice on the design of the simu knockout, J. Fak for Northern analysis and transgene injections, M. Schroeder for assistance with sequence analysis and alignments, and U. Unnerstall for his help with image analysis and figure design. We gratefully acknowledge financial support by a Rockefeller University Women & Science Fellowship (E. K.) and from the NIH (U.G, grant no. EY011560).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams JM, White K, Fessler LI, Steller H. Programmed cell death during Drosophila embryogenesis. Development. 1993;117:29–43. doi: 10.1242/dev.117.1.29. [DOI] [PubMed] [Google Scholar]
- Adachi H, Tsujimoto M, Arai H, Inoue K. Expression cloning of a novel scavenger receptor from human endothelial cells. J Biol Chem. 1997;272:31217–31220. doi: 10.1074/jbc.272.50.31217. [DOI] [PubMed] [Google Scholar]
- Awasaki T, Ito K. Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr Biol. 2004;14:668–677. doi: 10.1016/j.cub.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, Ito K. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron. 2006;50:855–867. doi: 10.1016/j.neuron.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Callebaut I, Mignotte V, Souchet M, Mornon JP. EMI domains are widespread and reveal the probable orthologs of the Caenorhabditis elegans CED-1 protein. Biochem Biophys Res Commun. 2003;300:619–623. doi: 10.1016/s0006-291x(02)02904-2. [DOI] [PubMed] [Google Scholar]
- Finn JT, Weil M, Archer F, Siman R, Srinivasan A, Raff MC. Evidence that Wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. J Neurosci. 2000;20:1333–1341. doi: 10.1523/JNEUROSCI.20-04-01333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franc NC, Dimarcq JL, Lagueux M, Hoffmann J, Ezekowitz RA. Croquemort, a novel Drosophila hemocyte/macrophage receptor that recognizes apoptotic cells. Immunity. 1996;4:431–443. doi: 10.1016/s1074-7613(00)80410-0. [DOI] [PubMed] [Google Scholar]
- Franc NC, Heitzler P, Ezekowitz RA, White K. Requirement for croquemort in phagocytosis of apoptotic cells in Drosophila. Science. 1999;284:1991–1994. doi: 10.1126/science.284.5422.1991. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38:567–580. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- Grimsley C, Ravichandran KS. Cues for apoptotic cell engulfment: eat-me, don’t eat-me and come-get-me signals. Trends Cell Biol. 2003;13:648–656. doi: 10.1016/j.tcb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Hamon Y, Trompier D, Ma Z, Venegas V, Pophillat M, Mignotte V, Zhou Z, Chimini G. Cooperation between Engulfment Receptors: The Case of ABCA1 and MEGF10. PLoS ONE. 2006;1:e120. doi: 10.1371/journal.pone.0000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanayama R, Nagata S. Impaired involution of mammary glands in the absence of milk fat globule EGF factor 8. Proc Natl Acad Sci U S A. 2005;102:16886–16891. doi: 10.1073/pnas.0508599102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol. 2006;27:244–250. doi: 10.1016/j.it.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Hoopfer ED, McLaughlin T, Watts RJ, Schuldiner O, O’Leary DD, Luo L. Wld(s) Protection Distinguishes Axon Degeneration following Injury from Naturally Occurring Developmental Pruning. Neuron. 2006;50:883–895. doi: 10.1016/j.neuron.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Ito K, Urban J, Technau GM. Distribution, classification, and development of Drosophila glial cells in the late embryonic and early larval ventral nerve chord. Rouxs Arch Dev Biol. 1995;204:284–307. doi: 10.1007/BF02179499. [DOI] [PubMed] [Google Scholar]
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- Kocks C, Cho JH, Nehme N, Ulvila J, Pearson AM, Meister M, Strom C, Conto SL, Hetru C, Stuart LM, et al. Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in Drosophila. Cell. 2005;123:335–346. doi: 10.1016/j.cell.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Kurucz E, Markus R, Zsamboki J, Folkl-Medzihradszky K, Darula Z, Vilmos P, Udvardy A, Krausz I, Lukacsovich T, Gateff E, et al. Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr Biol. 2007;17:649–654. doi: 10.1016/j.cub.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Lettre G, Hengartner MO. Developmental apoptosis in C. elegans: a complex CEDnario. Nat Rev Mol Cell Biol. 2006;7:97–108. doi: 10.1038/nrm1836. [DOI] [PubMed] [Google Scholar]
- Li W, Baker NE. Engulfment is required for cell competition. Cell. 2007;129:1215–1225. doi: 10.1016/j.cell.2007.03.054. [DOI] [PubMed] [Google Scholar]
- MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Mangahas PM, Zhou Z. Clearance of apoptotic cells in Caenorhabditis elegans. Semin Cell Dev Biol. 2005;16:295–306. doi: 10.1016/j.semcdb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Mergliano J, Minden JS. Caspase-independent cell engulfment mirrors cell death pattern in Drosophila embryos. Development. 2003;130:5779–5789. doi: 10.1242/dev.00824. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Shiratsuchi A. Phagocytic removal of apoptotic spermatogenic cells by Sertoli cells: mechanisms and consequences. Biol Pharm Bull. 2004;27:13–16. doi: 10.1248/bpb.27.13. [DOI] [PubMed] [Google Scholar]
- Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- Parnaik R, Raff MC, Scholes J. Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr Biol. 2000;10:857–860. doi: 10.1016/s0960-9822(00)00598-4. [DOI] [PubMed] [Google Scholar]
- Platt N, Gordon S. Scavenger receptors: diverse activities and promiscuous binding of polyanionic ligands. Chem Biol. 1998;5:R193–203. doi: 10.1016/s1074-5521(98)90156-9. [DOI] [PubMed] [Google Scholar]
- Platt N, Gordon S. Is the class A macrophage scavenger receptor (SR-A) multifunctional? - The mouse’s tale. J Clin Invest. 2001;108:649–654. doi: 10.1172/JCI13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien PW, Horvitz HR. The engulfment process of programmed cell death in caenorhabditis elegans. Annu Rev Cell Dev Biol. 2004;20:193–221. doi: 10.1146/annurev.cellbio.20.022003.114619. [DOI] [PubMed] [Google Scholar]
- Rogulja-Ortmann A, Luer K, Seibert J, Rickert C, Technau GM. Programmed cell death in the embryonic central nervous system of Drosophila melanogaster. Development. 2007;134:105–116. doi: 10.1242/dev.02707. [DOI] [PubMed] [Google Scholar]
- Rong YS, Golic KG. Gene targeting by homologous recombination in Drosophila. Science. 2000;288:2013–2018. doi: 10.1126/science.288.5473.2013. [DOI] [PubMed] [Google Scholar]
- Rong YS, Titen SW, Xie HB, Golic MM, Bastiani M, Bandyopadhyay P, Olivera BM, Brodsky M, Rubin GM, Golic KG. Targeted mutagenesis by homologous recombination in D. melanogaster. Genes Dev. 2002;16:1568–1581. doi: 10.1101/gad.986602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe T, Bainton RJ, Fetter RD, Heberlein U, Gaul U. GPCR signaling is required for blood-brain barrier formation in drosophila. Cell. 2005;123:133–144. doi: 10.1016/j.cell.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- Silva E, Au-Yeung HW, Van Goethem E, Burden J, Franc NC. Requirement for a Drosophila E3-ubiquitin ligase in phagocytosis of apoptotic cells. Immunity. 2007;27:585–596. doi: 10.1016/j.immuni.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld MJ, Jacobs JR. Macrophages and glia participate in the removal of apoptotic neurons from the Drosophila embryonic nervous system. J Comp Neurol. 1995;359:644–652. doi: 10.1002/cne.903590410. [DOI] [PubMed] [Google Scholar]
- Stuart LM, Ezekowitz RA. Phagocytosis: elegant complexity. Immunity. 2005;22:539–550. doi: 10.1016/j.immuni.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Thur J, Rosenberg K, Nitsche DP, Pihlajamaa T, Ala-Kokko L, Heinegard D, Paulsson M, Maurer P. Mutations in cartilage oligomeric matrix protein causing pseudoachondroplasia and multiple epiphyseal dysplasia affect binding of calcium and collagen I, II, and IX. J Biol Chem. 2001;276:6083–6092. doi: 10.1074/jbc.M009512200. [DOI] [PubMed] [Google Scholar]
- Truman JW, Thorn RS, Robinow S. Programmed neuronal death in insect development. J Neurobiol. 1992;23:1295–1311. doi: 10.1002/neu.480230917. [DOI] [PubMed] [Google Scholar]
- van den Eijnde SM, Boshart L, Baehrecke EH, De Zeeuw CI, Reutelingsperger CP, Vermeij-Keers C. Cell surface exposure of phosphatidylserine during apoptosis is phylogenetically conserved. Apoptosis. 1998;3:9–16. doi: 10.1023/a:1009650917818. [DOI] [PubMed] [Google Scholar]
- Watts RJ, Hoopfer ED, Luo L. Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron. 2003;38:871–885. doi: 10.1016/s0896-6273(03)00295-2. [DOI] [PubMed] [Google Scholar]
- Wood W, Turmaine M, Weber R, Camp V, Maki RA, McKercher SR, Martin P. Mesenchymal cells engulf and clear apoptotic footplate cells in macrophageless PU.1 null mouse embryos. Development. 2000;127:5245–5252. doi: 10.1242/dev.127.24.5245. [DOI] [PubMed] [Google Scholar]
- Yu X, Odera S, Chuang CH, Lu N, Zhou Z. C. elegans Dynamin mediates the signaling of phagocytic receptor CED-1 for the engulfment and degradation of apoptotic cells. Dev Cell. 2006;10:743–757. doi: 10.1016/j.devcel.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Hartwieg E, Horvitz HR. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell. 2001;104:43–56. doi: 10.1016/s0092-8674(01)00190-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.