Abstract
Purpose
To determine the effects of incorporating superparamagnetic microspheres (SPMs) into cultured human corneal endothelial cells (HCECs), and to describe preliminary experiments of HCEC transplantation, facilitated by SPMs and an external magnetic field, in a human anterior segment ex vivo model.
Methods
HCECs were cultured in monolayer and incorporated with magnetite oxide SPMs (900 nm, 300 nm, and 100 nm) at different concentrations. Cell viability, migration toward a magnetic field, and light transmittance were measured after incorporation of SPMs. HCEC transplantation to human recipients was investigated with anterior segments in organ culture subjected to an external magnetic field. Light and electron microscopy were used to assess HCEC attachment to corneal stroma.
Results
SPMs were incorporated into the cytoplasm of HCECs after overnight incubation. None of the SPMs affected the short-term viability of cultured HCECs (P>0.14, n=6) or their light transmittance (P>0.06, n=5), although there was a trend toward decreased transmittance with higher concentrations of the 900 nm SPM. Cell migration toward a magnetic field was higher for HCECs with incorporated SPMs than for HCECs without SPMs (P≤ 0.01, n=6), with dose-response relationships evident for the 300 nm and 100 nm SPMs. SPMs facilitated the attachment of HCECs to corneal stroma in the human anterior segment model with minimal change in intracameral (intraocular) pressure.
Conclusions
SPMs facilitate migration of HCECs toward a magnetic source and attachment of cells to corneal stroma without affecting cell viability or light transmittance. The human anterior segment model can be used to study HCEC transplantation.
INTRODUCTION
Human corneal endothelial cells (HCECs) have limited regenerative potential in vivo, and diseases of the corneal endothelium are treated by corneal tissue transplantation to improve vision. HCEC dysfunction (often referred to as “endothelial dysfunction”) accounted for nearly half of the 32,000 corneal transplants performed in the United States in 2003, with Fuchs’ endothelial dystrophy and pseudophakic corneal edema comprising the majority of cases.1 Since 2000, serologic screening criteria have become more stringent, limiting the donor supply, and more recently, the demand for donor corneas has increased as corneal surgeons have rapidly adopted new posterior lamellar keratoplasty techniques.2–4 Worldwide, there is a shortage of donor corneal tissue, and in fact, many countries obtain donor tissue from the United States.2
Transplantation of cultured HCECs has long been considered a method of expanding the donor pool for endothelial dysfunction,5–7 but two obstacles have hindered its development: 1) the ability to culture senescent HCECs, and 2) the delivery of HCECs to the posterior cornea in vivo. With recent improvements in culture protocols, HCECs can now be consistently cultured in vitro8, 9; however, there has been little advance in methods for delivering and establishing cultured cells to recipient corneas. Previous studies have seeded cultured endothelial cells either directly onto Descemet’s membrane of full-thickness donor tissue for subsequent penetrating keratoplasty,5–7, 10, 11 or onto collagen sheets for transplantation by using posterior lamellar keratoplasty techniques.12–15 More recently, direct cell-seeding to Descemet’s membrane has been attempted in a rabbit model,16, 17 but the well-known regenerative capacity of the rabbit endothelium in vivo18 limits interpretation of the results of these studies.
In this study, we incorporated superparamagnetic microspheres (SPMs) into HCECs and directly seeded cells to posterior corneal stroma in a human model by using an external magnetic field. We report the effects of different SPMs, one of which is an FDA-approved magnetic resonance imaging contrast agent, on cultured HCECs in vitro, and describe a human ex vivo model for studying HCEC transplantation.
METHODS
HCEC Monolayer Culture
Donor human corneas were obtained from the National Disease Research Interchange (NDRI, Philadelphia, PA). Donors were preferentially selected according to four specific criteria: 1) age 18–60 years; 2) endothelial cell density >2,500 cells/mm2; 3) death-to-preservation time <18 hours; and 4) acute cause of death, and absence of systemic disease, sepsis or ventilation time prior to death) to improve the chances of cell proliferation in vitro.19 Donor details are provided in Table 1. Corneas were received in Optisol-GS™ (Bausch & Lomb, Rochester, NY) preservation medium at 4°C, and were deemed unsuitable for clinical use.
Table 1.
Characteristics of the donors from which human corneal endothelial cells were cultured
| Donor/Eye | Donor Age (years) |
Death to Preservation Time (hours) |
Endothelial Cell Density (cells/mm2) |
Cause of Death |
|---|---|---|---|---|
| 1R | 60 | 4.5 | 2426 | Acute myocardial infarction |
| 1L | 2336 | |||
| 2R | 53 | 3 | 2888 | Drug overdose |
| 2L | 2916 | |||
| 3R | 39 | 5.5 | Not available | Acute myocardial infarction |
| 3L | ||||
| 4 | 18 | 10.5 | 3396 | Motor vehicle accident |
| 5R | 20 | 4 | 3725 | Head Trauma |
| 5L | 3534 | |||
| 6 | 49 | 8 | 3260 | Cerebrovascular accident |
| 7 | 23 | 16.5 | 3410 | Motor vehicle accident |
| 8R | 35 | 15 | 3389 | Acute myocardial infarction |
| 8L | 3184 | |||
| 9R | 18 | 1 | 3189 | Head trauma |
| 9L | 3401 | |||
| 10R | 29 | 9 | 3205 | Motor vehicle accident |
| 10L | 3210 | |||
| 11R | 60 | 4 | 2680 | Cerebrovascular accident |
| 11L | 2469 | |||
| 12 | 21 | 3 | 3048 | Motor vehicle accident |
| 13 | 59 | 7.5 | 2564 | Acute myocardial infarction |
| 14 | 28 | 11 | 3043 | Motor vehicle accident |
| 15 | 51 | 5 | 2801 | Lung cancer |
Donor endothelial cell densities were measured by the eye bank supplying the tissue.
Under an operating microscope, Descemet’s membrane (with endothelial cells) was scored with a blunt instrument within Schwalbe’s line, and the membrane was grasped gently with forceps to remove it from the corneal stroma. Descemet’s membrane, carrying endothelial cells, was incubated overnight in Opti-MEM1 (Invitrogen, Carlsbad, CA) supplemented with 8% fetal bovine serum (FBS, Gibco-Invitrogen, Carlsbad, CA) at 37°C in a humidified atmosphere containing 5% CO2. Endothelial cells were released from Descemet’s membrane by incubating in 0.02% EDTA for 1 hour with gentle agitation. The endothelial cells were pelleted by centrifugation, washed in culture medium, pelleted again, and resuspended in culture medium; our HCEC culture medium was similar to that used by Zhu & Joyce9 (Table 2). Cells were plated into a single well of a 6-well plate coated with collagen type I or collagen type IV (BD Biosciences, San Jose, CA), and incubated at 37°C in a humidified atmosphere containing 5% CO2. Initial cultures in our series were grown on collagen type IV substrate, the natural substrate for HCECs in vivo, but later cultures in our series were grown on collagen type I substrate, the predominant collagen in corneal stroma, to which we ultimately wanted to promote attachment; no morphologic or proliferation differences were noted between HCECs grown on the two substrates. Cells were grown to confluence before passaging.
Table 2.
Culture medium constituents
| Fetal Bovine Serum (Gibco-Invitrogen, Carlsbad, CA) | 8% |
| Ca++ (Calcium chloride) (Sigma, St. Louis, MO) | 200 mg/L |
| Chondroitin sulfate (Sigma, St. Louis, MO) | 0.08% |
| Ascorbic Acid (Sigma, St. Louis, MO) | 20 µg/mL |
| Pituitary extract (source of FGF) (Biomedical Technologies, Stoughton, MA) | 100 µg/mL |
| EGF(Chemicon International, Temecula, CA) | 5 ng/mL |
| NGF (Biomedical Technologies, Stoughton, MA) | 20 ng/mL |
| Insulin-Transferrin-Selenium A Supplement (100x)* (Invitrogen, Carlsbad, CA) | 10 mL/L |
| RPMI Vitamin Solution (100x) (Sigma, St. Louis, MO) | 10 mL/L |
| Antibiotic/antimycotic (100x)† (Sigma, St. Louis, MO) | 10 mL/L |
| OptiMEM-I™ (Sigma, St. Louis, MO) | Base medium |
Insulin 1 g/L, Transferrin 550 mg/L, Selenium 670 µg/L
Penicillin 10,000 U/mL, Streptomycin 10 mg/mL, Amphotericin-B 25 µg/mL
Incorporation of Superparamagnetic Microspheres (SPMs)
Three types and sizes of SPM were investigated: 900 nm (mean diameter) magnetite iron oxide (Bangs Laboratories, Inc., Fishers, IN); 300 nm (mean diameter; range, 100–390 nm) magnetite iron oxide (SPHERO™ Carboxyl Magnetic Particles, Spherotech, Inc., Lake Forest, IL); 100 nm (mean diameter; range, 80–150 nm20) ferumoxides injectable solution (Feridex I.V.®, Bayer Healthcare Pharmaceuticals, Inc., Wayne, NJ).
Different concentrations of each SPM were investigated, and compared to control HCECs without incorporated SPMs. The specific concentrations were chosen based on previous reports for the 900 nm SPM,21, 22 and to obtain a cell migration dose-response relationship for the 300 nm and 100 nm SPMs. Each SPM concentration was calculated from the initial number of cells seeded per well. For the 900 nm SPM, the number of SPMs per cell plated ranged from 0:1 (control) to 4000:1 (volume of SPM suspension added was 0 µL to 64 µL); for the 300 nm SPM, the number of SPMs per cell plated ranged from 0:1 (control) to 20000:1 (volume of SPM suspension added was 0 µL to 5 µL); for the 100 nm SPM, the volume of suspension added to the 3 ml culture medium in each well ranged from 0 µL (control) to 64 µL (the manufacturer of the 100 nm SPM was unable to calculate the concentration of their SPM in suspension). Because the number of cells increases by proliferation in vitro between plating and confluence, the true ratio of microspheres to number of cells was lower than intended, typically by 2–4 times depending on the amount of cell proliferation.
Effects of SPMs on HCECs In Vitro
SPMs were added in microliter quantities to the culture medium bathing the monolayer of confluent passage 2 HCECs. HCECs were incubated overnight with SPMs, then rinsed and left in monolayer culture for 2–8 days before examining cell viability, cell migration and cellular light transmittance in vitro. HCECs from 23 eyes of 15 donors were cultured for these experiments; passage 2 HCECs of the same eye were plated in a 6-well plate and the effects of 5 different concentrations of each SPM were compared to control (HCECs without incorporated SPMs). Experiments were repeated as many as 6 times (6 donors) for each concentration.
HCEC viability
Confluent HCECs in passage 2 were resuspended and stained with 0.4% Trypan blue to detect non-viable cells. At least 100 cells were counted by using a hemocytometer to determine the proportion of non-viable cells.
Cell Migration Toward Magnetic Field
Confluent HCECs in passage 2 were resuspended from monolayer culture and passed through a magnetic cell separation column (OctoMACS™, Miltenyi Biotech, Auburn, CA). The magnet surface field strength was 5000 Gauss (equals 0.5 Tesla). HCECs attracted to the magnet were counted in a hemocytometer and were compared to the total number of cells retrieved from the culture well.
Light Transmittance
We determined the effect of incorporating SPMs on the light transmittance of HCECs because the cornea in vivo must remain transparent to function correctly. HCECs attracted to the magnet in the cell separation column were suspended in phosphate-buffered saline (PBS) at a concentration of 300,000 cells/mL. The cell suspension was placed in a 10 mm × 10 mm cuvette and transmittance was measured across the visible spectrum (400 nm to 600 nm) by a spectrophotometer (UV-1601, Shimadzu Scientific Instruments, Columbia, MD). Transmittance was measured relative to PBS alone (without cells). Light traversing 10 mm through a suspension of 300,000 cells/mL encounters the same number of cells as light traversing a monolayer with density of 3000 cells/mm2, which would be considered a healthy endothelial cell density in normal corneas in vivo.
HCEC Transplantation to Human Anterior Segments
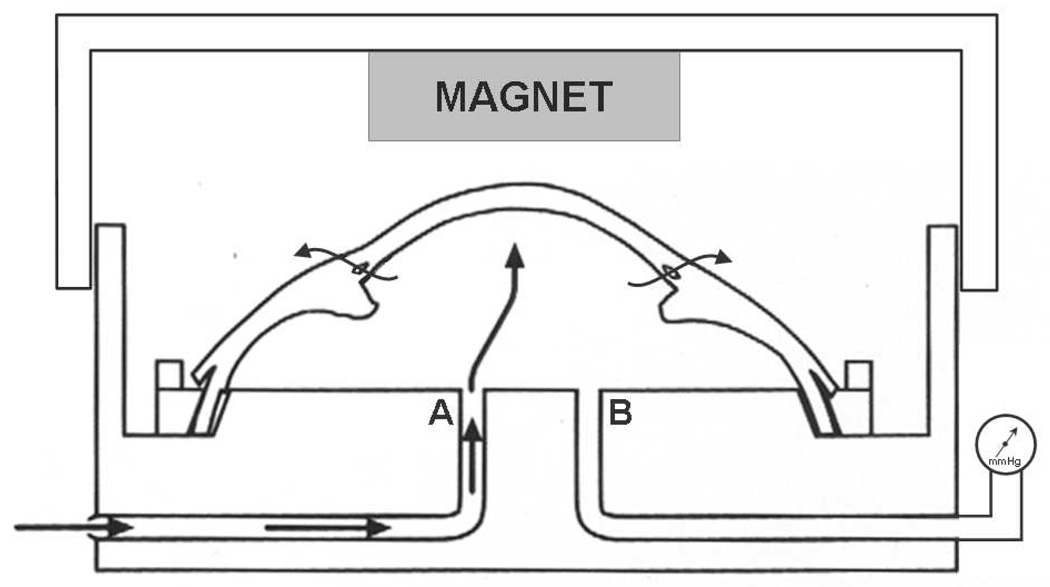
The human anterior segment perfusion organ culture model has been described in detail with respect to studying the conventional aqueous drainage pathway (Figure 1),23, 24 and the same model was used to investigate HCEC transplantation in this study. Normal human donor eyes were obtained from the Minnesota Lions Eye Bank (Minneapolis, MN) and placed in culture within 12 hours of death. Eyes were bisected at the equator, and the iris, lens and vitreous were removed; the trabecular meshwork was not removed. The remaining anterior segment was clamped in a modified Petri dish and perfused with the same culture medium used for monolayer HCEC culture (Table 2) but devoid of FBS. Culture medium was perfused at the normal human aqueous flow rate (2.5 µL/min),25 and the anterior segments were incubated at 37°C in a humidified atmosphere containing 5% CO2. Intracameral (intraocular) pressure was continuously monitored by a pressure transducer connected to an access canula in the culture dish (Figure 1), and recorded to a computer.
Figure 1. Human anterior segment model ex vivo.
Schematic of the perfusion organ culture model of human anterior segments, as described by Johnson and Tschumper.23, 24 Human anterior segment is clamped to the modified Petri dish. Culture medium is perfused via canula A, and medium exits the artificial anterior chamber via the conventional aqueous drainage pathway (arrows). Intracameral (intraocular) pressure is measured in real time via a pressure transducer attached to canula B. For human corneal endothelial cell (HCEC) transplantation studies, an external magnet was suspended from the lid of the culture dish, <3 mm above the center of the cornea. HCECs with incorporated superparamagnetic microspheres were infused through canula A and attracted toward the posterior stromal surface of the cornea by the external magnetic field.
When stable intraocular pressure was achieved in perfusion organ culture, Descemet’s membrane was removed from the cornea of the anterior segment under an operating microscope (similar to the technique used to excise Descemet’s membrane for monolayer endothelial cell culture). The anterior segment was returned to perfusion organ culture and allowed to reestablish its baseline intraocular pressure for several hours before transplanting HCECs. For transplantation, HCECs from monolayer culture, with incorporated SPMs, were labeled with a live cell stain (CM-DiI, Molecular Probes Inc., Eugene, OR) and passed through the magnetic cell separation column to select cells that migrated toward the magnet. HCECs were transferred to the anterior segment in organ culture by performing an anterior chamber culture medium exchange of 1 mL over 1–2 minutes. Anterior segments were cultured for 3–7 days after transfer of cells, and intraocular pressure was continuously recorded.
Initial cell transplantation experiments investigated HCECs incorporated with 900 nm SPMs at a concentration of 500 SPMs per cell plated, based on the optimum concentration from our in vitro data and with the goal of using the largest SPM for initial studies to increase the likelihood of successful cell transplantation. Subsequently we repeated the experiment by using the 100 nm SPMs at a concentration of 16 µL per culture well, with the long-term goal of using the lowest mass of SPM to facilitate cell transplantation. Five pairs of anterior segments were examined, and the number of cells transferred to each recipient anterior segment ranged from 300,000 to 1,000,000 (Table 3). A magnet with surface field strength of 3855 Gauss was placed <3 mm above the cornea of one anterior segment (Figure 1) for 2–7 days, whereas the fellow anterior segment of each pair was not subjected to a magnetic field (control).
Table 3.
Experimental parameters and endothelial cell density after human corneal endothelial cell transplantation in the perfusion organ culture model of human anterior segments.
| Recipient Anterior segment |
Size of superparamagnetic microsphere in donor cells (nm) |
Number of donor cells transferred |
Duration of magnetic field (days) |
Duration of organ culture after cell transfer (days) |
Donor endothelial cell density (cells/mm2) |
|---|---|---|---|---|---|
| 1 | 900 | 300,000 | 7 | 7 | 812 |
| 2 | 900 | 300,000 | 7 | 7 | 981 |
| 3 | 900 | 300,000 | 3 | 3 | 864 |
| 4 | 100 | 1,000,000 | 5 | 5 | 1050 |
| 5 | 100 | 1,000,000 | 2 | 3 | 1525 |
All experiments were performed with pairs of recipient anterior segments, with one anterior segment subjected to a magnetic field (data shown) and the fellow anterior segment acting as a control and not subjected to a magnetic field. Because few, if any, donor endothelial cells were present on corneas of control anterior segments, these data have not been included in the table.
Morphologic Methods
Fluorescence Microscopy
Fresh corneal tissue sections were mounted with medium containing DAPI (Vectashield, Vector Laboratories, Burlingame, CA) on microscope slides, covered with a 1 mm-deep adhesive chamber gasket, and examined by fluorescence microscopy.
Light Microscopy
Tissue sections of the recipient cornea were isolated from experimental and control anterior segments. Sections were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer. Histologic examination with light microscopy was performed on representative samples of anterior segments transplanted with HCECs containing 900 nm or 100 nm SPMs. Tissue was embedded in paraffin, cut 4 µm thick, and stained with hematoxylin and eosin, and PAS (Periodic Acid Schiff).
Transmission Electron Microscopy
Tissue sections were prepared for transmission electron microscopy by dehydration in ascending concentrations of ethanol and embedding in epoxy resin. Thin sections were mounted on copper grids, stained with uranyl acetate (saturated solution in 50% ethanol) and 0.1% lead citrate and examined on a JEOL 1400 transmission electron microscope (Peabody, MA).
Data Analysis
Differences in cell viability, transmittance and attraction toward a magnetic field between HCECs incorporated with SPMs and HCECs without SPMs (control) were assessed by using paired t-tests for normally distributed data; p≤ 0.05 was considered statistically significant. For non-significant differences, we calculated the minimum detectable differences assuming paired tests with α=0.05 and β=0.20. Results of the HCEC transplantation studies were qualitatively analyzed.
RESULTS
Incorporation of SPMs into HCECs
HCECs were successfully and consistently cultured in monolayer. At confluence, cell morphology in vitro was similar to the hexagonal mosaic of corneal endothelial cells in vivo (Figure 2). SPMs were located within the cytoplasm of HCECs after overnight incubation with SPMs (Figure 2 and Figure 3). The mechanism of uptake of SPMs by HCECs was not determined.
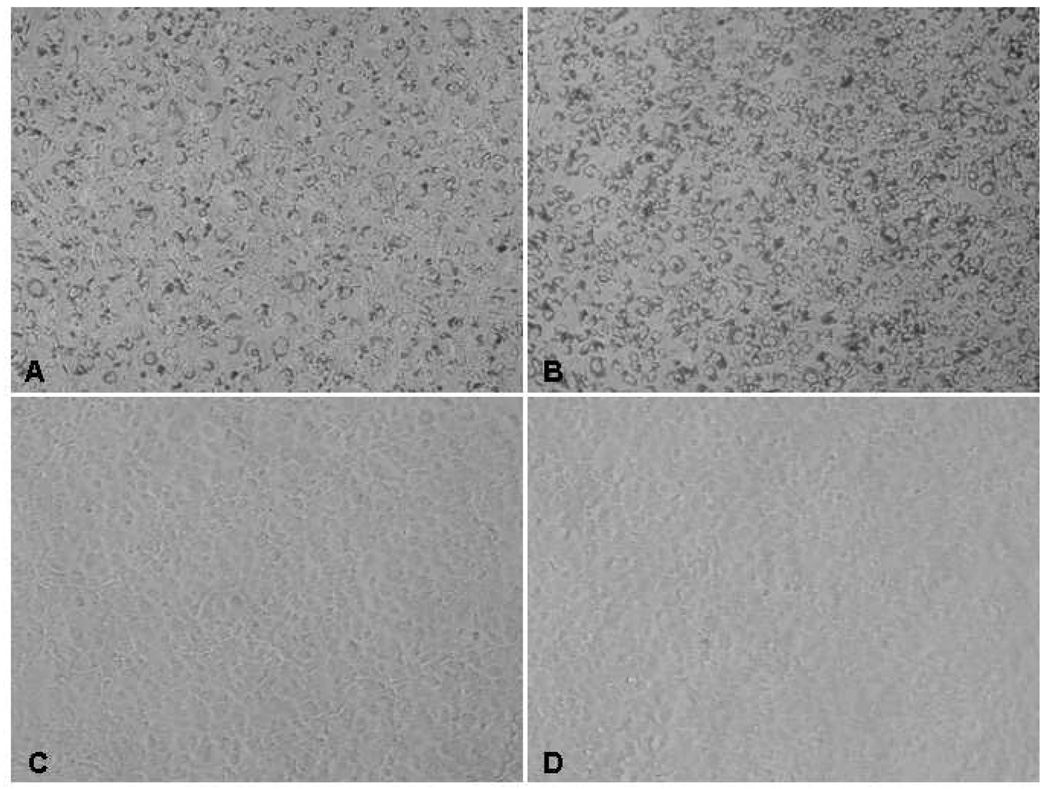
Figure 2. Phase-contrast microscopy images of human corneal endothelial cells (HCECs) in monolayer culture with and without incorporated superparamagnetic microspheres (SPMs).
After overnight incubation, confluent HCECs in monolayer were incorporated with A) 900 nm SPMs at 500 SPMs per cell plated, B) 900 nm SPMs at 500 SPMs per cell plated, and C) 100 nm SPMs at 16 µL per culture well. The 900 nm SPM was easily visible in the cells, whereas the 100 nm SPM was not, with the latter appearing similar to HCECs without SPMs (D). At confluence, HCECs assume a near hexagonal morphology similar to that of corneal endothelium in vivo. The cells shown are from one donor in passage 2; magnification, 100x.
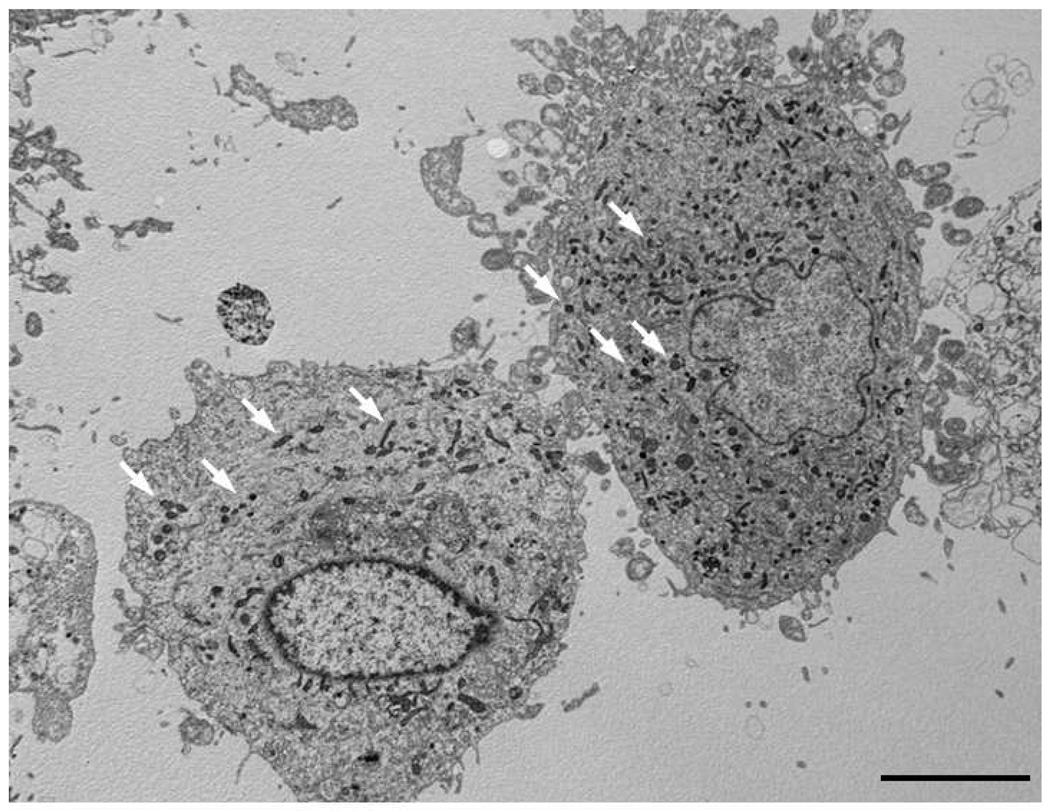
Figure 3. Human corneal endothelial cells (HCECs) with incorporated superparamagnetic microspheres (SPMs).
Transmission electron microscopy showed SPMs (black dots, some of which are denoted by white arrows) within the cytoplasm of cultured HCECs in vitro after overnight incubation. The mechanism of uptake of SPMs into HCECs is not known. Bar, 5 µm.
Effects of SPMs on HCECs in vitro
Cell Viability and Migration
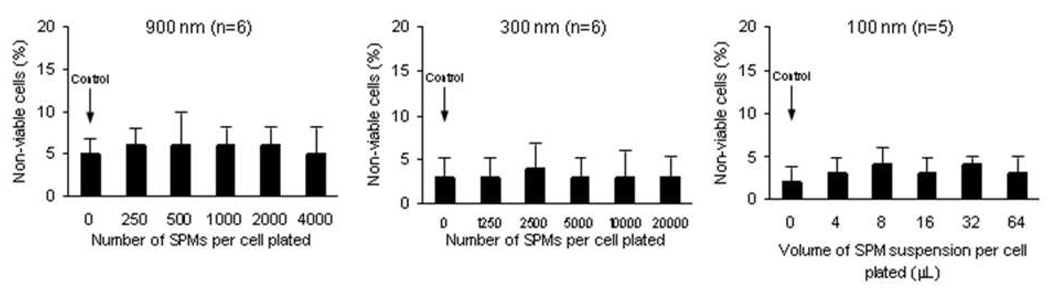
None of the SPMs incorporated by cultured HCECs at any of the concentrations tested decreased cell viability compared to control HCECs without SPMs (900 nm SPM: p> 0.71, n=6; 300 nm SPM: p> 0.33, n=6; 100 nm SPM: p> 0.14, n=5) and there were no dose-response relationships at the concentrations tested (Figure 4). The mean minimum detectable difference in cell viability between HCECs with SPMs and control HCECs without SPMs was 4.0% (α=0.05, β=0.20, paired tests).
Figure 4. Human corneal endothelial cell (HCEC) viability after incorporation of superparamagnetic microspheres (SPMs).
None of the three sizes of SPMs incorporated by cultured HCECs decreased cell viability in the short-term (900 nm SPM: p> 0.71, n=6; 300 nm SPM: p> 0.33, n=6; 100 nm SPM: p> 0.14, n=5) and there were no dose-response relationships at the concentrations tested. The mean minimum detectable difference in cell viability between HCECs with SPMs and HCECs without SPMs was 4.0% (α=0.05, β=0.20, paired tests).
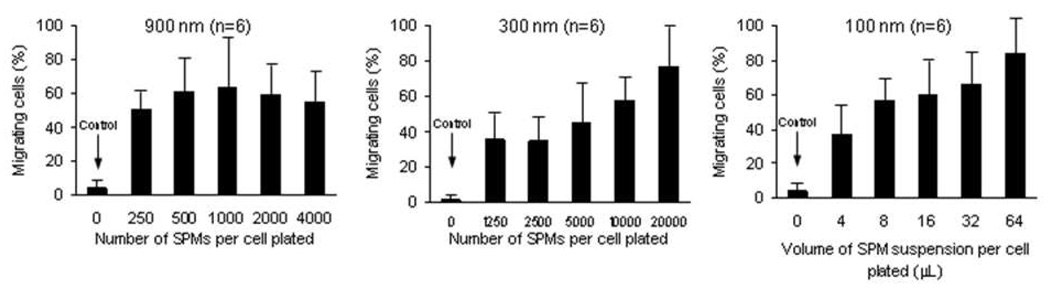
HCEC cells containing SPMs consistently migrated to a magnetic field compared to control HCECs without SPMs (900nm: p< 0.004, n=6; 300 nm: p< 0.008, n=6; 100 nm: p≤ 0.01, n=6) (Figure 5). At the concentrations of SPM tested, increased migration towards a magnetic field was observed in a dose-dependent manner for HCECs incorporated with 300 nm and 100 nm SPMs. A dose-response migration was not evident for HCECs incorporated with 900 nm SPMs..
Figure 5. Human corneal endothelial cell (HCEC) migration toward a magnetic field after incorporation of superparamagnetic microspheres (SPMs).
For the 900 nm, 300nm, and 100 nm SPMs, all concentrations tested resulted in significant HCEC migration toward a magnetic field when compared to control HCECs without SPMs ((900 nm: p< 0.004, n=6; 300 nm: p< 0.008, n=6; 100 nm: p≤ 0.01, n=6). No dose-response relationship was apparent for the 900 nm SPM. For the 300 nm and 100 nm SPMs, a dose-response relationship was evident with higher cell migration at higher SPM concentrations.
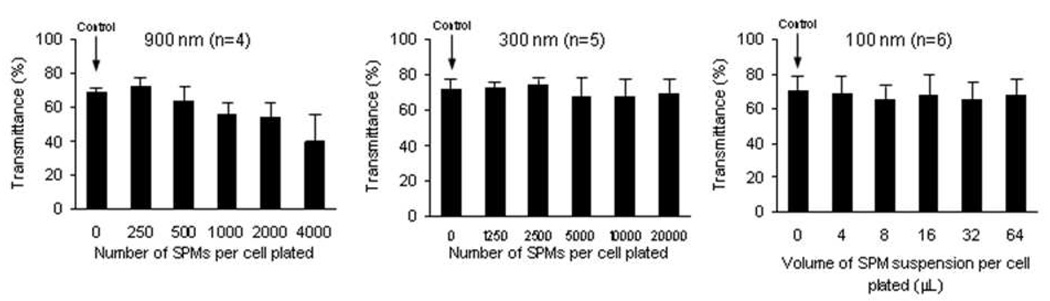
Light Transmittance In Vitro
For HCECs incorporated with the 900 nm SPM, transmittance (at a wavelength of 560 nm) did not differ from control for any concentration (p> 0.06, n=4), although there was a trend toward decreased transmittance at the highest concentrations tested (minimum detectable difference between 4000 SPMs per cell plated and control was 47.1% (α=0.05, β=0.20, n=4, paired test; Figure 6). For HCECs incorporated with the 300 nm and 100 nm SPMs, transmittance did not differ from control HCECs without SPMs for any concentration (300 nm SPM, p> 0.07, n=5; 100 nm SPM, p> 0.06, n=6) and there were no dose-response relationships. The mean minimum detectable difference in transmittance between HCECs with SPMs and control HCECs without SPMs was 15.0% (α=0.05, β=0.20, paired tests).
Figure 6. Light transmittance in vitro of human corneal endothelial cells (HCECs) incorporated with superparamagnetic microspheres (SPMs).
For HCECs incorporated with the 900 nm SPM, transmittance did not differ from control for any concentration (p> 0.06, n=4), although there was a trend toward decreased transmittance at the highest concentrations tested (minimum detectable difference between 4000 SPMs per cell plated and control was 47.1% (α=0.05, β=0.20, n=4, paired test). For HCECs incorporated with the 300 nm and 100 nm SPMs, transmittance did not differ from control for any concentration (300 nm SPM: p> 0.07, n=5; 100 nm SPM: p> 0.06, n=6) and there were no dose-response relationships. The mean minimum detectable difference in transmittance between HCECs with SPMs and HCECs without SPMs was 15.0% (α=0.05, β=0.20, paired tests).
HCEC Transplantation in Organ Culture Model
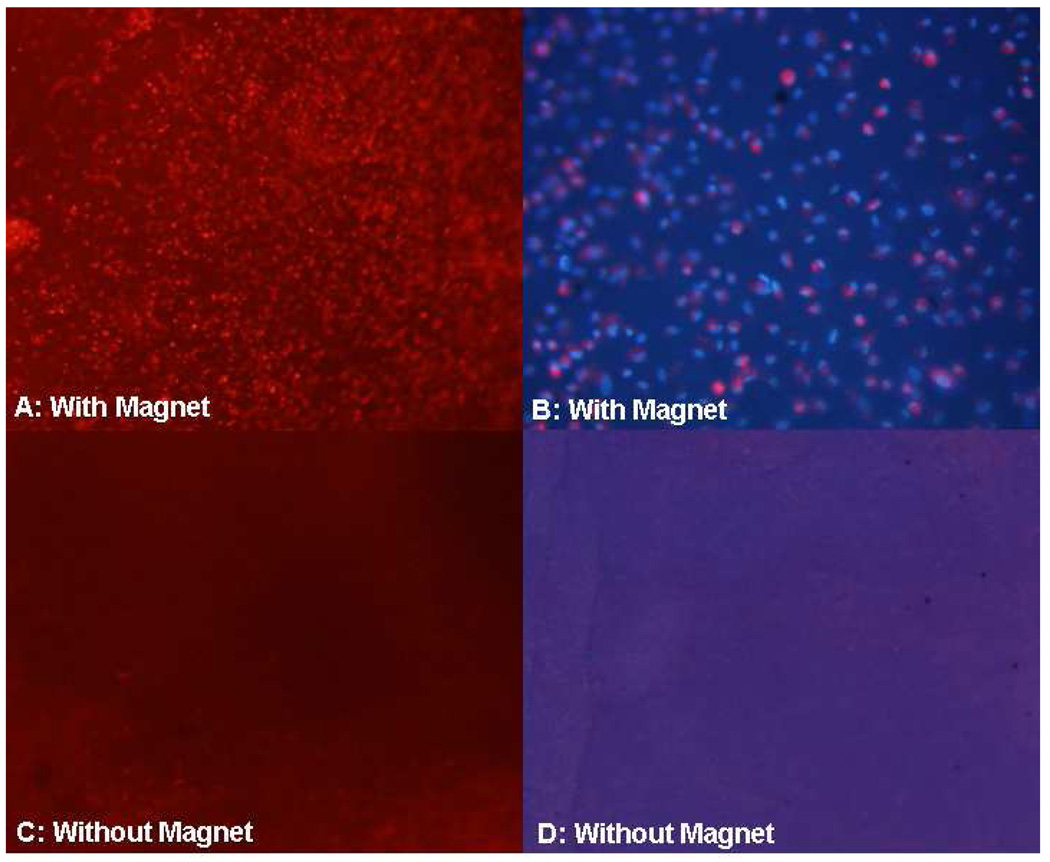
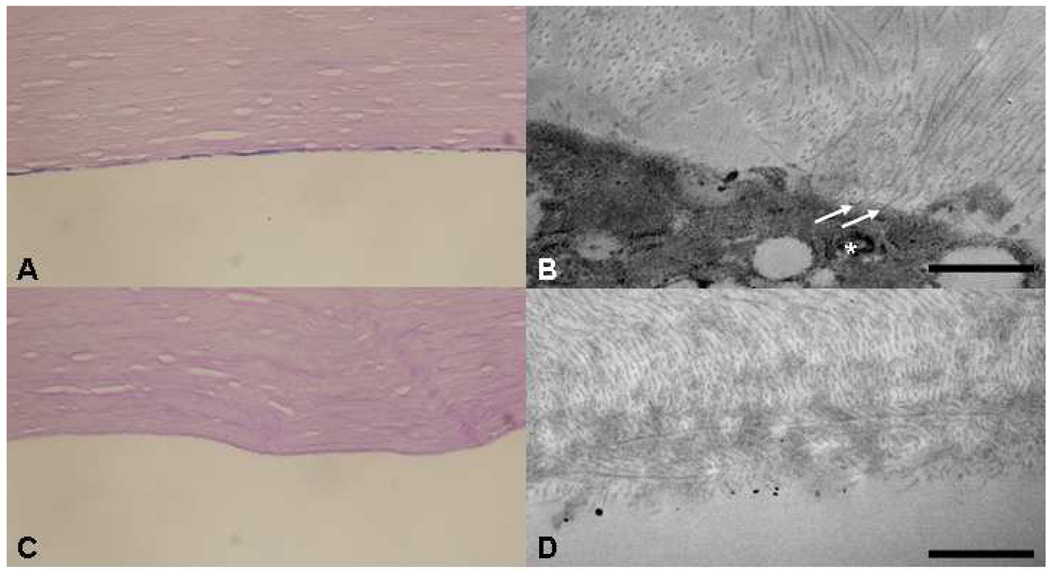
Fluorescence microscopy showed donor HCECs, labeled with CM-DiI, were present on the posterior corneal stroma of each experimental anterior segment with the magnetic field, whereas no or very few donor HCECs were detected on the posterior corneal stroma of the fellow control anterior segments (Figure 7). HCEC density after cell transplantation in the presence of a magnetic field ranged from 812–1525 cells/mm2 (Table 3); endothelial cell density was determined by counting the DAPI-labeled nuclei of CM-DiI-labeled cells in a defined area of fluorescence microscopy images, or by counting donor HCECs in a defined area of scanning electron microscopy images. HCECs formed a monolayer on the posterior corneal stroma of each experimental recipient cornea, but not control recipient corneas (Figure 8). Transmission electron microscopy showed that HCECs incorporated with SPMs were associated with collagen fibrils in the stromal extracellular matrix of experimental recipient corneas (Figure 8). Scanning electron microscopy confirmed donor HCECs flattening and establishing a confluent monolayer on bare corneal stroma (Figure 9); however, uniformity of donor HCEC attachment was not consistent over recipient corneas.
Figure 7. Fluorescence microscopy of posterior corneal stroma after human corneal endothelial cell (HCEC) transplantation.
A. Many DiI-labeled (red) donor HCECs were detected on corneas of anterior segments subjected to the magnetic field (magnification, 40x). B. At higher magnification (200x), donor HCEC density was 981 cells/mm2 in this recipient (nuclei are stained blue with DAPI and donor cell cytoplasm is stained red with Di-I). C and D. No donor HCECs were detected on control corneas not subjected to a magnetic field (magnification, 40x and 200x, respectively).
Figure 8. Human corneal endothelial cell (HCEC) attachment to human recipient stroma.
HCECs incorporated with 100 nm superparamagnetic microspheres (SPMs) were transferred to anterior segments of human eyes. A. Corneas of anterior segments that were subjected to an external magnetic field in the perfusion organ culture model showed HCECs with incorporated SPMs associating with posterior corneal stroma. Descemet’s membrane was removed prior to HCEC transplantation; Periodic Acid Schiff, 400x. B. Transmission electron microscopy shows that HCECs with incorporated SPM (*) were attached to corneal stromal collagen fibrils (arrows) in anterior segments subjected to an external magnetic field; Bar 1 µm. C and D. Light and transmission electron microscopy of paired (fellow) control corneas (anterior segments not subjected to a magnetic field) showed collagen fibrils at the posterior bare stromal surface and absence of any significant HCEC attachment; C, Periodic Acid Schiff, 400x; D, Bar 1 µm.
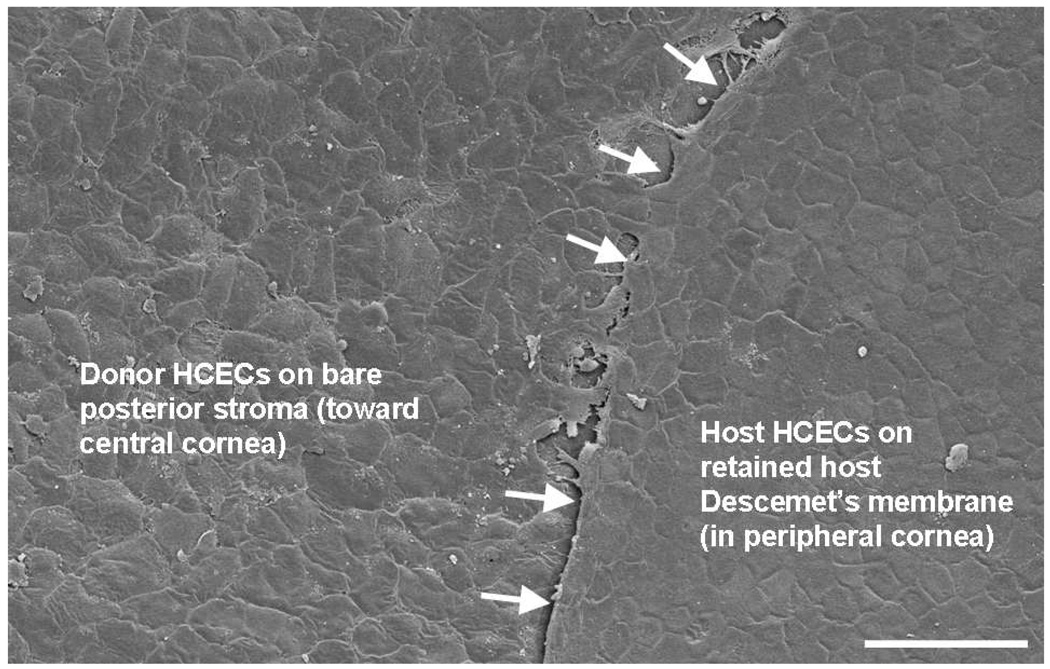
Figure 9. Formation of a confluent monolayer of human corneal endothelial cells (HCECs) on recipient human corneal stroma.
Scanning electron microscopy showed donor HCECs flattening and establishing a confluent monolayer on bare corneal stroma 3 days after transplantation; donor HCEC density was 1,525 cells/mm2. Donor HCECs were incorporated with 100 nm superparamagnetic microspheres and were transferred to the recipient human anterior segment in the presence of a magnetic field for 48 hours. Host HCECs on retained Descemet’s membrane were present peripherally; the stripped edge of Descemet’s membrane was evident (arrows). Bar, 100 µm.
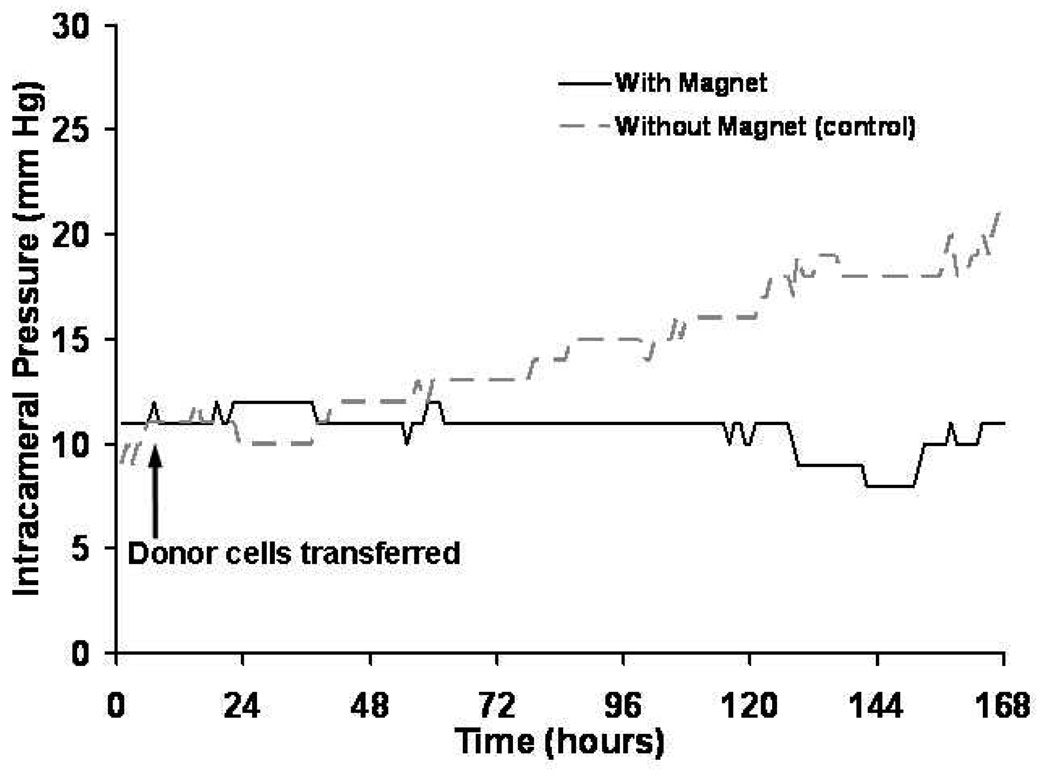
Intracameral (intraocular) pressure was continuously recorded during and after addition of HCECs to the perfusion organ culture model. Intraocular pressure remained stable in experimental anterior segments with the magnetic field, whereas intraocular pressure was noted to increase in fellow control anterior segments without the magnetic field (Figure 10).
Figure 10.
Intracameral (intraocular) pressure after human corneal endothelial cell (HCEC) transplantation.In the presence of a magnetic field, human anterior segments (without Descemet’s membrane) perfused with HCECs incorporated with SPMs did not result in an increase in intraocular pressure (solid line), suggesting that cells were localized toward the cornea and away from the aqueous drainage pathway. In contrast, addition of HCECs with incorporated SPMs to human anterior segments without a magnetic field resulted in increased intraocular pressure (dashed line), presumably because cells occluded the aqueous drainage pathway.
DISCUSSION
Corneal transplantation for endothelial dysfunction has evolved over the last decade from penetrating keratoplasty to posterior lamellar keratoplasty techniques,4 but these techniques are dependent on the availability of good quality cadaveric corneal tissue for transplantation. Development of cultured HCEC transplantation techniques would expand the donor pool and enable delivery of the cells in a minimally invasive procedure. The present study suggests that cultured HCECs can incorporate SPMs without affecting the short-term viability or light transmittance of the cells, and that HCECs can be successfully delivered and seeded to recipient human corneal stroma by using forces of attraction between intracellular SPMs and an external magnetic field. The use of the human anterior segment organ culture model as a method of studying the short-term results of endothelial cell transplantation was also verified.
Superparamagnetic microspheres are presently used in clinical practice as an intravenous contrast agent for magnetic resonance imaging studies. Experimental applications of SPMs have included incorporation into various cell types as a cell tracer,26–28 and into vascular endothelial cells to localize the cells to magnetized coronary and femoral artery stents.21, 22 For corneal disease, Mimura et al. previously described using magnetic forces of attraction to localize rabbit corneal endothelial cells to rabbit recipient corneas for transplantation,16, 29 but several differences exist between their study and ours. First, we incorporated cells with superparamagnetic (magnetite oxide) particles whereas Mimura et al. incorporated cells with ferromagnetic (iron) particles. Ferromagnetic particles retain magnetic properties after removal of an external magnetic field, whereas superparamagnetic particles do not, preventing self-aggregation of the particles and the cells incorporating them.20, 30 Second, we used the technique to promote cell attachment to bare stroma whereas Mimura et al. promoted cell attachment to Descemet’s membrane. Although Descemet’s membrane is the natural substrate for corneal endothelial cells, in conditions such as Fuchs’ endothelial dystrophy, Descemet’s membrane is abnormal because of collagenous excresences (guttae), which must be removed to improve vision.31 Developing strategies to promote corneal endothelial cell attachment to bare stroma will be beneficial for treating Fuchs’ dystrophy, a major indication for corneal transplantation.2 Third, we used a human model for our study, whereas Mimura et al. used a rabbit model despite the well-known regenerative capacity of rabbit corneal endothelial cells18 compared to human.
Toxicity to cells and other ocular tissues from magnetite oxide particles is a concern if they are to facilitate HCEC transplantation. We did not find any effect of low SPM concentrations on cellular viability or light transmittance up to 8 days in culture. Although no changes were identified in this short period, additional studies in an animal model will help determine the long-term effects of the SPMs. It is encouraging that at one year after rabbit corneal endothelial cell transplantation facilitated by iron particles, Mimura et al. demonstrated the absence of ocular toxicity.16 To minimize toxicity, using the lowest concentration of the smallest SPM would be most appropriate, and would possibly allow elimination of the particles from the anterior chamber should they be extruded from endothelial cells.16 Our results indicate that using the 100 nm SPM at 16–32 µL per culture well provided adequate cell migration without toxicity or loss of transmittance in vitro, and facilitated cell transplantation in our preliminary studies in organ culture. The 100 nm SPM (Feridex I.V.®) is an FDA-approved magnetic resonance imaging-contrast agent that can be injected intravenously in humans to localize hepatic and splenic tumors without systemic toxicity.20, 30, 32 Nevertheless, when delivered to the anterior segment of the eye, the toxicity profile of this agent is likely to be different than when used as a magnetic resonance imaging contrast agent and warrants further evaluation.
Light transmittance was not significantly affected by incorporating SPMs into HCECs, except for a trend toward decreased transmittance with the largest (900 nm) SPM at the higher concentrations. Transmittance of suspended HCECs without SPMs was approximately 70% and was consistent in all experiments. Although the transmittance of HCECs in vitro was lower than transmittance of the cornea in vivo,33 the apparently low transmittance of HCECs might be caused by conformational differences of the cells being in suspension and not being flat in monolayer in their normal environment in vivo. Corneal transmittance can be measured in vivo,33 and future animal studies will help determine whether corneal transmittance is affected by transplanting cultured endothelial cells incorporated with SPMs.
For cell transplantation to be effective, HCECs must attach to corneal stroma in sufficient density and retain adequate cell function. We demonstrated that HCECs can form flat, single cell layers along the corneal stroma with apparent association with collagen fibrils in a human organ culture model of anterior segments. Although promoting HCEC attachment directly to corneal stroma is possible, we have yet to determine the optimum number of transplanted cells, the strength and duration of the magnetic field, and other unknown factors, that will yield a higher, uniform endothelial cell density and a functional monolayer to maintain corneal transparency and function. Further studies are planned to investigate these variables and their effect on endothelial cell density, uniformity of cell attachment, corneal thickness, and corneal transmittance. We were unable to find other investigative studies that have attempted to attach cultured HCECs directly to corneal stroma, but anecdotal clinical observations indicate that HCECs can attach to corneal stroma with deposition of new Descemet’s membrane.34–36 Nevertheless, we recognize that formation of a functional HCEC monolayer after attachment to bare corneal stroma might be slow and our technique might require modification to make this approach more efficient.
The perfusion organ culture model of human anterior segments was developed in our laboratory as an ex vivo model to study the corneoscleral angle and aqueous drainage pathway,23, 24, 37–40 and in this study we demonstrated its applicability to endothelial cell transplantation. Organ culture (without perfusion) is the standard method of preserving human corneas for transplantation in Europe41, 42 with good clinical outcomes even after a month of preservation,43 and therefore clearly has a role in research. A similar perfusion model to that described in the present study has been used to examine isolated corneas in organ culture,44 but the absence of the aqueous drainage pathway in the latter model prevents intraocular pressure examination in response to placing cells in the anterior chamber of the model. Development of HCEC transplantation by injection of a bolus of cells into the anterior chamber of the eye will require continuous intraocular pressure monitoring to detect dangerous elevations in intraocular pressure, and to help determine the optimum number of cells delivered and the frequency of delivery. We previously showed that a bolus of 30,000 trabecular meshwork cells resulted in acute intraocular pressure elevation in our organ culture model45; a similar increase in intraocular pressure occurred in the present study after transfer of HCECs to anterior segments without a magnetic field, and HCECs were present in the trabecular meshwork (data not shown), presumably occluding the aqueous outflow pathway. However, in the presence of a magnetic field, as many as 1,000,000 HCECs with incorporated SPMs did not result in an elevation in intraocular pressure, which might be a favorable result of localizing the cells toward the cornea by using the magnetic field, but clearly warrants further examination to determine the repeatability of this result. The perfusion organ culture model will enable human-to-human endothelial cell transplantation studies and is less expensive than animal models. However, this technique can only examine short-term outcomes because anterior segments cannot be cultured for longer than 28 days.23 Therefore, the development of an animal model will be important to understand the long-term effects on cell viability, transmittance, and function after HCEC transplantation.
This study has rigorously examined the in vitro effects of incorporating SPMs into HCECs with the goal of using magnetic forces of attraction to facilitate HCEC transplantation. We demonstrated proof of concept of this technique in a human ex vivo model, by showing attachment of HCECs directly to corneal stroma. Further studies are planned to refine the technique and to examine in more detail the attachment and function of HCECs after direct cell seeding to Descemet’s membrane and bare stroma.
Acknowledgments
Supported by Research to Prevent Blindness, Inc., New York, NY (SVP as Olga Keith Wiess Special Scholar, and an unrestricted grant to the Department of Ophthalmology, Mayo Clinic); National Institutes of Health, Bethesda, MD (EY 07065 [MPF] and EY 15736 [MPF]); and Mayo Foundation, Rochester, MN.
Footnotes
Presented in part at the Association for Research in Vision and Ophthalmology Annual Meeting, Fort Lauderdale, FL, 2008
References
- 1.Eye Bank Association of America. Annual statistical report. 2003
- 2.Eye Bank Association of America. Annual statistical report. 2006
- 3.Price MO, Price FW. Descemet's stripping endothelial keratoplasty. Curr Opin Ophthalmol. 2007;18(4):290–294. doi: 10.1097/ICU.0b013e3281a4775b. [DOI] [PubMed] [Google Scholar]
- 4.Patel SV. Keratoplasty for endothelial dysfunction. Ophthalmology. 2007;114(4):627–628. doi: 10.1016/j.ophtha.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Jumblatt MM, Maurice DM, McCulley JP. Transplantation of tissue-cultured corneal endothelium. Invest Ophthalmol Vis Sci. 1978;17(12):1135–1141. [PubMed] [Google Scholar]
- 6.Gospodarowicz D, Greenburg G, Alvarado J. Transplantation of cultured bovine corneal endothelial cells to species with nonregenerative endothelium. The cat as an experimental model. Arch Ophthalmol. 1979;97(11):2163–2169. doi: 10.1001/archopht.1979.01020020481016. [DOI] [PubMed] [Google Scholar]
- 7.McCulley JP, Maurice DM, Schwartz BD. Corneal endothelial transplantation. Ophthalmology. 1980;87(3):194–201. doi: 10.1016/s0161-6420(80)35259-7. [DOI] [PubMed] [Google Scholar]
- 8.Chen KH, Azar D, Joyce NC. Transplantation of adult human corneal endothelium ex vivo: a morphologic study. Cornea. 2001;20(7):731–737. doi: 10.1097/00003226-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Zhu C, Joyce NC. Proliferative response of corneal endothelial cells from young and older donors. Invest Ophthalmol Vis Sci. 2004;45(6):1743–1751. doi: 10.1167/iovs.03-0814. [DOI] [PubMed] [Google Scholar]
- 10.Engelmann K, Drexler D, Bohnke M. Transplantation of adult human or porcine corneal endothelial cells onto human recipients in vitro. Part I: Cell culturing and transplantation procedure. Cornea. 1999;18(2):199–206. doi: 10.1097/00003226-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Gospodarowicz D, Greenburg G, Alvarado J. Transplantation of cultured bovine corneal endothelial cells to rabbit cornea: clinical implications for human studies. Proc Natl Acad Sci U S A. 1979;76(1):464–468. doi: 10.1073/pnas.76.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koizumi N, Sakamoto Y, Okumura N, et al. Cultivated corneal endothelial cell sheet transplantation in a primate model. Invest Ophthalmol Vis Sci. 2007;48(10):4519–4526. doi: 10.1167/iovs.07-0567. [DOI] [PubMed] [Google Scholar]
- 13.Mimura T, Yamagami S, Yokoo S, et al. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Invest Ophthalmol Vis Sci. 2004;45(9):2992–2997. doi: 10.1167/iovs.03-1174. [DOI] [PubMed] [Google Scholar]
- 14.Shimmura S, Miyashita H, Konomi K, et al. Transplantation of corneal endothelium with Descemet's membrane using a hyroxyethyl methacrylate polymer as a carrier. Br J Ophthalmol. 2005;89(2):134–137. doi: 10.1136/bjo.2004.050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hitani K, Yokoo S, Honda N, Usui T, Yamagami S, Amano S. Transplantation of a sheet of human corneal endothelial cell in a rabbit model. Mol Vis. 2008;14:1–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Mimura T, Yamagami S, Usui T, et al. Long-term outcome of iron-endocytosing cultured corneal endothelial cell transplantation with magnetic attraction. Exp Eye Res. 2005;80(2):149–157. doi: 10.1016/j.exer.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Mimura T, Yamagami S, Usui T, Seiichi, Honda N, Amano S. Necessary prone position time for human corneal endothelial precursor transplantation in a rabbit endothelial deficiency model. Curr Eye Res. 2007;32(7):617–623. doi: 10.1080/02713680701530589. [DOI] [PubMed] [Google Scholar]
- 18.Van Horn DL, Sendele DD, Seideman S, Buco PJ. Regenerative capacity of the corneal endothelium in rabbit and cat. Invest Ophthalmol Vis Sci. 1977;16(7):597–613. [PubMed] [Google Scholar]
- 19.Joyce NC, Zhu CC. Human corneal endothelial cell proliferation: potential for use in regenerative medicine. Cornea. 2004;23(8 Suppl):S8–S19. doi: 10.1097/01.ico.0000136666.63870.18. [DOI] [PubMed] [Google Scholar]
- 20.Wang YX, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol. 2001;11(11):2319–2331. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- 21.Pislaru SV, Harbuzariu A, Agarwal G, et al. Magnetic forces enable rapid endothelialization of synthetic vascular grafts. Circulation. 2006;114(1 Suppl):I314–I318. doi: 10.1161/CIRCULATIONAHA.105.001446. [DOI] [PubMed] [Google Scholar]
- 22.Pislaru SV, Harbuzariu A, Gulati R, et al. Magnetically targeted endothelial cell localization in stented vessels. J Am Coll Cardiol. 2006;48(9):1839–1845. doi: 10.1016/j.jacc.2006.06.069. [DOI] [PubMed] [Google Scholar]
- 23.Johnson DH, Tschumper RC. Human trabecular meshwork organ culture. A new method. Invest Ophthalmol Vis Sci. 1987;28(6):945–953. [PubMed] [Google Scholar]
- 24.Johnson DH, Tschumper RC. The effect of organ culture on human trabecular meshwork. Exp Eye Res. 1989;49(1):113–127. doi: 10.1016/0014-4835(89)90080-8. [DOI] [PubMed] [Google Scholar]
- 25.Brubaker RF. Flow of aqueous humor in humans [The Friedenwald Lecture] Invest Ophthalmol Vis Sci. 1991;32(13):3145–3166. [PubMed] [Google Scholar]
- 26.Hill JM, Dick AJ, Raman VK, et al. Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells. Circulation. 2003;108(8):1009–1014. doi: 10.1161/01.CIR.0000084537.66419.7A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinds KA, Hill JM, Shapiro EM, et al. Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood. 2003;102(3):867–872. doi: 10.1182/blood-2002-12-3669. [DOI] [PubMed] [Google Scholar]
- 28.Tai JH, Foster P, Rosales A, et al. Imaging islets labeled with magnetic nanoparticles at 1.5 Tesla. Diabetes. 2006;55(11):2931–2938. doi: 10.2337/db06-0393. [DOI] [PubMed] [Google Scholar]
- 29.Mimura T, Shimomura N, Usui T, et al. Magnetic attraction of iron-endocytosed corneal endothelial cells to Descemet's membrane. Exp Eye Res. 2003;76(6):745–751. doi: 10.1016/s0014-4835(03)00057-5. [DOI] [PubMed] [Google Scholar]
- 30.Clement O, Siauve N, Cuenod CA, Frija G. Liver imaging with ferumoxides (Feridex): fundamentals, controversies, and practical aspects. Top Magn Reson Imaging. 1998;9(3):167–182. [PubMed] [Google Scholar]
- 31.Patel SV, Baratz KH, Hodge DO, Maguire LJ, McLaren JW. The effect of corneal light scatter on vision after Descemet-stripping with endothelial keratoplasty. Arch Ophthalmol. 2008 doi: 10.1001/archophthalmol.2008.581. In Press. [DOI] [PubMed] [Google Scholar]
- 32.Schultz JF, Bell JD, Goldstein RM, Kuhn JA, McCarty TM. Hepatic tumor imaging using iron oxide MRI: comparison with computed tomography, clinical impact, and cost analysis. Ann Surg Oncol. 1999;6(7):691–698. doi: 10.1007/pl00021736. [DOI] [PubMed] [Google Scholar]
- 33.McLaren JW, Brubaker RF. Measurement of transmission of ultraviolet and visible light in the living rabbit cornea. Curr Eye Res. 1996;15(4):411–421. doi: 10.3109/02713689608995832. [DOI] [PubMed] [Google Scholar]
- 34.Stone DL, Kenyon KR, Stark WJ. Ultrastructure of keratoconus with healed hydrops. Am J Ophthalmol. 1976;82(3):450–458. doi: 10.1016/0002-9394(76)90494-3. [DOI] [PubMed] [Google Scholar]
- 35.Inomata H, Smelser GK, Polack FM. Fine structure of regenerating endothelium and Descemet's membrane in normal and rejecting corneal grafts. Am J Ophthalmol. 1970;70(1):49–64. [PubMed] [Google Scholar]
- 36.Watson SL, Abiad G, Coroneo MT. Spontaneous resolution of corneal oedema following Descemet's detachment. Clin Experiment Ophthalmol. 2006;34(8):797–799. doi: 10.1111/j.1442-9071.2006.01319.x. [DOI] [PubMed] [Google Scholar]
- 37.Erickson-Lamy K, Rohen JW, Grant WM. Outflow facility studies in the perfused human ocular anterior segment. Exp Eye Res. 1991;52(6):723–731. doi: 10.1016/0014-4835(91)90024-9. [DOI] [PubMed] [Google Scholar]
- 38.Pang IH, McCartney MD, Steely HT, Clark AF. Human ocular perfusion organ culture: a versatile ex vivo model for glaucoma research. J Glaucoma. 2000;9(6):468–479. doi: 10.1097/00061198-200012000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Fautsch MP, Bahler CK, Jewison DJ, Johnson DH. Recombinant TIGR/MYOC increases outflow resistance in the human anterior segment. Invest Ophthalmol Vis Sci. 2000;41(13):4163–4168. [PubMed] [Google Scholar]
- 40.Fautsch MP, Bahler CK, Vrabel AM, et al. Perfusion of his-tagged eukaryotic myocilin increases outflow resistance in human anterior segments in the presence of aqueous humor. Invest Ophthalmol Vis Sci. 2006;47(1):213–221. doi: 10.1167/iovs.05-0334. [DOI] [PubMed] [Google Scholar]
- 41.Bohnke M. Corneal preservation in organ culture. Curr Opin Ophthalmol. 1991;2:432–442. [Google Scholar]
- 42.Pels E, Schuchard Y. Organ-culture preservation of human corneas. Doc Ophthalmol. 1983;56(1–2):147–153. doi: 10.1007/BF00154722. [DOI] [PubMed] [Google Scholar]
- 43.Frueh BE, Bohnke M. Corneal grafting of donor tissue preserved for longer than 4 weeks in organ-culture medium. Cornea. 1995;14(5):463–466. [PubMed] [Google Scholar]
- 44.Brunette I, Nelson LR, Bourne WM. A system for long-term corneal perfusion. Invest Ophthalmol Vis Sci. 1989;30(8):1813–1822. [PubMed] [Google Scholar]
- 45.Bahler CK, Fautsch MP, Hann CR, Johnson DH. Factors influencing intraocular pressure in cultured human anterior segments. Invest Ophthalmol Vis Sci. 2004;45(9):3137–3143. doi: 10.1167/iovs.04-0154. [DOI] [PubMed] [Google Scholar]