Abstract
Objective
To examine peripheral leukocyte Dectin-1 regulation in clinically relevant models of fungal and polymicrobial sepsis.
Design
Prospective animal study
Setting
University medical school research laboratory
Subjects
Age, weight and sex matched ICR/HSD mice
Interventions
Mice were infected with Candida albicans (1 × 105, IV) or were subjected to cecal ligation and puncture to induce polymicrobial sepsis.
Measurements
Blood, spleen, and peritoneal exudate were harvested and leukocytes were isolated. Leukocytes were evaluated for membrane associated Dectin-1 expression and cell phenotype by flow cytometry.
Main Results
In C. albicans infection, Dectin-1 positive blood and splenic leukocytes were increased from 23.5 to 58.9% over the course of infection. The increased percentage of Dectin expressing cells was primarily attributable to neutrophilia. However, the amount of Dectin-1 expressed by blood and splenic neutrophils in C. albicans infected mice was decreased by a range of 49.0 to 53.3%. C. albicans infection also resulted in an infiltration of Dectin-1 positive macrophages and neutrophils into the kidney. In contrast, polymicrobial sepsis decreased blood leukocyte Dectin-1 expressing cells by up to 51.4%. This reduction was due to a decrease in Dectin-1 positive neutrophils in the periphery. However, the percentage of Dectin-1 expressing cells in the peritoneal cavity increased by 774% with CLP. Treatment of isolated neutrophils with three soluble glucans, mannan, LPS, or a variety of cytokines revealed that glucans, alone or in combination, were the only treatment that resulted in a decrease in Dectin-1 positive neutrophils.
Conclusions
We conclude that peripheral leukocyte Dectin-1 expression is differentially regulated in fungal versus polymicrobial sepsis. These data demonstrate that leukocyte Dectin-1 levels are modulated in response to infections of fungal and non-fungal origin.
Keywords: DECTIN-1, GLUCAN, CANDIDA ALBICANS, POLYMICROBIAL SEPSIS, LEUKOCYTES
1. Introduction
The critically ill patient frequently develops a complex disease spectrum that can include sepsis syndrome and/or septic shock (1). Critically ill patients also can exhibit profound immunosuppression which leads to increased susceptibility to opportunistic pathogens, such as Candida species (2;3). We do not fully understand the cellular mechanisms that predispose the critically ill host to infection; however, recent evidence has provided significant insights into the cellular mechanisms that are involved in pathogen recognition.
The innate immune system recognizes pathogens by means of evolutionarily conserved pattern recognition receptors (PRRs) (4). PRRs recognize and interact with pathogen associated molecular patterns (PAMPs) (4). Glucans are fungal PAMPs that are present in the cell wall of fungi, plants, and certain bacteria (5;6). Purified glucans are known to stimulate innate immunity and to promote survival in a variety of infection models (7-9). Dectin-1 is the primary PRR for glucans (10-14). Dectin-1 is expressed at high levels on monocytes, neutrophils and macrophages and at lower levels on dendritic cells and some T cells (11;12;14). Interaction of glucan with Dectin-1 results in internalization of the complex in vitro (15;16) and decreases Dectin-1 expression on blood neutrophils and monocytes for seven days in vivo (17).
Dectin-1 is thought to be an important sentinel receptor for fungal infections (13). The fungal cell wall contains large amounts of glucan (5;6). Additionally, fungi release glucan into the extracelluar milieu (18). Binding of C. albicans blastospores to Dectin-1 is glucan dependent and results in internalization of the yeast and production of tumor necrosis factor (TNF) α and reactive oxygen species (13;19). However, the importance of Dectin-1 in the response to C. albicans infection is controversial. Taylor, et al. have recently reported that mice which are genetically deficient in Dectin-1 show increased mortality following C. albicans infection (20). In striking contrast, Saijo, et al. reported that survival in C. albicans infection is similar inDectin-1 knock-out and wild type mice (21). However, Saijo et al. reported that Dectin-1 plays a role in the response to Pneumocystis carinii infection (21). Thus, both of these studies indicate a role for Dectin-1 in response to fungal infection. However, these data also indicate that additional studies are required to more precisely determine the role of Dectin-1 in response to fungal infection. These data also suggest that there may be differential responses of Dectin-1 to various infections.
Dectin-1 may also be involved in the response to bacterial and/or polymicrobial infection. Administration of the Dectin-1 ligand glucan phosphate (GP) improves survival rates in cecal ligation and puncture (CLP) induced polymicrobial sepsis (22). The molecular mechanisms responsible for protection are not fully known, but glucan ligands blunt the early increase in nuclear factor kappa B and nuclear factor IL-6 activation (7) and activate the phosphoinositide-3-kinase pathway (22), thereby limiting the host pro-inflammatory response to the septic injury. Since Dectin-1 is the primary receptor for glucans and the first step in the response to glucan is binding of the ligand by PRRs, it is currently thought that Dectin-1 is involved in the protective response of glucans in surgical sepsis (13). How Dectin-1 levels may be affected by ongoing sepsis has not been investigated.
The majority of studies addressing the interaction of microbes and Dectin-1 have been conducted in vitro (10;13;19). We have established that in vivo administration of highly purified glucan decreases leukocyte membrane associated Dectin-1 (17), however the effect of clinically relevant fungal or bacterial infections on in vivo Dectin-1 levels has not been examined. The goal of the present study was to determine the effect of clinically relevant polymicrobial sepsis on in vivo leukocyte Dectin-1 expression and to compare and contrast this effect with that of a clinically relevant fungal infection.
2. Materials and Methods
Mice
Age- and weight-matched adult male outbred mice (ICR/HSD) were obtained from Harlan Sprague Dawley (Indianapolis, IN). The animals were maintained on standard laboratory chow and water ad libitum with a 12-hour light/dark cycle. Serologic testing confirmed that the mice were virus free. All animal procedures were reviewed and approved by the Institutional Review Board at the James H. Quillen College of Medicine, East Tennessee State University.
Polymicrobial Sepsis
Polymicrobial sepsis was induced by CLP (3 animals per time point) as previously described (23;24). Survival analysis of this model of sepsis resulted in an onset of mortality at 22 h and a median survival time of ~30 h with 20% long term survival (data not shown). Sham surgery (laparotomy alone) and animals that underwent no surgery or anesthesia were employed as controls (3 animals per group per time point). Mice were euthanized by CO2 asphyxiation 0, 3, 6, 12, and 24 h post-operatively, and peritoneal cells, spleens and blood were harvested and screened for Dectin-1 expression by flow cytometry.
Fungal Infection
Fungal infection was induced (3 animals per time point) by intravenous (IV) administration of C. albicans (1 × 105 cfu). The C. albicans used in this study is a clinical isolate used as a reference control in the Microbiology Clinical Laboratory at the Quillen College of Medicine. Survival analysis of this strain and dose of C. albicans resulted in a median survival time of 13 days and time to 100% mortality of 21 days (data not shown). Mice were euthanized by CO2 asphyxiation on days 1, 3, 5, 7, and 14, and blood, kidneys, and spleens were harvested from control and infected mice and screened for Dectin-1 expression by flow cytometry (blood and spleens) or immunohistochemistry (kidneys). Control mice (3 animals per time point) were administered 5% dextrose in water.
Dectin-1 expression on cultured primary murine neutrophils
Peritoneal neutrophils were harvested from ICR/HSD mice 18 h after IP injection of thioglycollate. After removal of adherent cells, the purity of neutrophils was 50-70%. The neutrophils were cultured in the presence of 1 μg/ml Saccharomyces cerevisiae derived glucan phosphate (GP) (25), C. albicans blastospore or hyphal derived GP (26), mannan (Sigma, St. Louis, MO) or LPS (Sigma) alone or in combination for 3 h. In a separate study, neutrophils were incubated with GP (1 μg/ml), TNFα (0.1, 1, 10 ng/ml; BD Pharmingen, San Diego, CA), interleukin (IL)-6 (0.1, 1, 10 ng/ml; BD Pharmingen), IL-17 (0.1, 1, 10 ng/ml; R and D Systems, Minneapolis, MN), or L cell conditioned media (30% in RPMI) for 3 h at 37°C. The cells were stained with anti-Dectin-1 (R and D Systems) and anti-neutrophil (Serotec, Oxford, UK) antibodies and analyzed by flow cytometry. After gating to the neutrophil population, the percentage of cells positive for Dectin-1 was determined.
Apoptosis of cultured primary murine neutrophils
Peritoneal neutrophils were harvested and treated as for the Dectin-1 expression study. The cells were washed and pelleted. The pellets were suspended in 70% ice cold ethanol and stored at -20°C for at least 24 h. The cells were washed three times and then incubated with 50 μg/ml propidium iodide (Molecular Probes, Eugene, OR) and 10 μg/ml RNaseA (Thermo Scientific, Surrey, UK) for 30 m at 37°C. Apoptosis was measured by determining the sub-G0 population by flow cytometry.
Flow Cytometry
Whole blood was collected into EDTA Microtainers (BD Vacutainer Systems, Franklin Lakes, NJ). Splenocytes were isolated by teasing and erythrocytes were lysed with PharmLyse buffer (BD Pharmingen). Resident peritoneal cells were obtained by lavage. Leukocytes and neutrophils were blocked with 5% rabbit serum, 0.5% bovine serum albumin (BSA), and 5mM EDTA with anti-murine CD16/32 (BD Pharmingen) prior to staining. Leukocytes were stained with biotinylated rat anti-Dectin-1 (14) or biotinylated goat anti-Dectin-1 (R and D Systems), anti-neutrophil (clone 7/4) (Serotec Oxford, UK), anti-F4/80 (Caltag, Burlingame, CA), and anti-CD3 (BD Pharmingen) or their isotype control antibodies. Staining was performed according to the protocol described by BD Pharmingen. Biotinylated antibodies were detected by streptavidin-phycoerythrin (BD Pharmingen). Cells were suspended in Pharmingen Stain Buffer and analyzed using a FACScalibur flow cytometer with CellQuest software (BD Biosciences, Mountain View, CA).
Immunohistochemistry
Kidneys were embedded in “Tissue-Tek” OCT compound (Miles Inc., Elkhart, IL), snap frozen, sectioned (8 μ) onto Vectabond slides (Vector Laboratories, Burlingame, CA), and fixed in 95% ethanol. The sections were blocked with 10% donkey serum, followed by incubation with rat anti-Dectin-1 (14), anti-neutrophil (clone 7/4) (Serotec), anti-F4/80 (Caltag) or their isotype control antibodies in 1% bovine serum albumin in phosphate buffered saline. After washing in phosphate buffered saline the sections were incubated with anti-rat antibody (Molecular Probes) and 300nM DAPI (Molecular Probes). Cover slips were mounted with Prolong Anti-Fade (Molecular Probes), and the slides were examined on a fluorescent microscope.
Statistics
Data were summarized by the mean and standard error of the mean (sem). Group mean responses were compared by analysis of variance and pair-wise multiple comparison testing (the least significant difference procedure or Tukey's procedure for cases where analysis of variance was not significant). Flow data were normalized to the mean percent positive cells for the untreated control for each time interval. Control values were set to 1.0. Probability levels of 0.05 or smaller were considered significant.
3. Results
Dectin-1 expression on blood, spleen, and peritoneal leukocytes in mice with polymicrobial sepsis
The effect of polymicrobial sepsis on Dectin-1 levels was determined by measuring Dectin-1 expression on the blood leukocytes of mice that had undergone CLP. Initial examination of Dectin-1 expression in control mouse leukocytes revealed three populations of peripheral blood Dectin-1 expressing cells (Fig. 1A). Approximately 30% - 60% of blood leukocytes were negative for Dectin-1 (Fig. 1A). Most Dectin-1 positive blood leukocytes have high levels of Dectin-1 expression (Dectin-1 high, Fig. 1A). Following CLP there was a shift to a lower level of Dectin-1 expression (Dectin-1low, Fig. 1A). This shift reflects a reduction in the number of Dectin-1 receptors found on the surface of the cells. The data described below focus on the leukocyte populations that show a shift from normal levels of Dectin-1 expression (Dectin-1high) to decreased Dectin-1 expression (Dectin-1low).
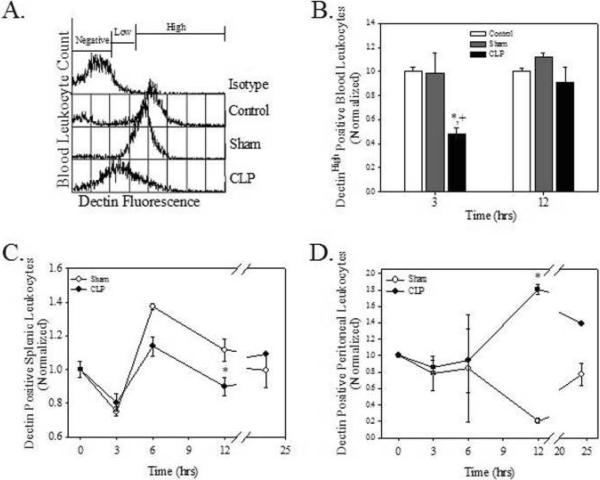
Figure 1.
Sepsis results in decreased Dectin-1 expression on peripheral leukocytes. Mice underwent no surgery (control), laparotomy (sham) control or CLP at time 0. Blood leukocytes (A and B), splenocytes (C), and peritoneal cells (D) were isolated and assayed for Dectin-1 surface expression. A. Representative histogram of 3 h blood data. B, C, and D. Data are normalized to the no surgery control, which was set a 1.0. Values are means +/- SEM. N=3/group/time interval. *p<0.05 compared to control and + p<0.05 compared to sham surgery.
CLP significantly decreased Dectin-1high expressing blood leukocytes by 51.4 and 50.9% at 3 h when compared to control and sham surgery, respectively (Fig. 1B, p<0.05). In contrast, Dectin-1low expressing cells increased in response to CLP at 3 h post-operatively when compared to control (146%) and to sham (54.5%) (data not shown, p<0.05). The percentages of Dectin-1high and Dectin-1low positive cells returned to baseline by 12 h (Fig. 1B and data not shown). Splenic leukocytes from CLP animals showed a significant decrease in the percentage of Dectin-1 expressing cells (19.6%) when compared to Sham at 12 h post-operatively (Fig. 1C, p<0.05). The percentage of Dectin-1 positive peritoneal leukocytes from mice with CLP sepsis showed a significant 774% increase 12 h post-operatively when compared to sham surgery mice (Fig. 1D, p<0.05). These data indicate that CLP alters leukocyte Dectin-1 expression in the blood, spleen, and peritoneal cavity.
Dectin-1 expression on blood neutrophils in mice with polymicrobial sepsis
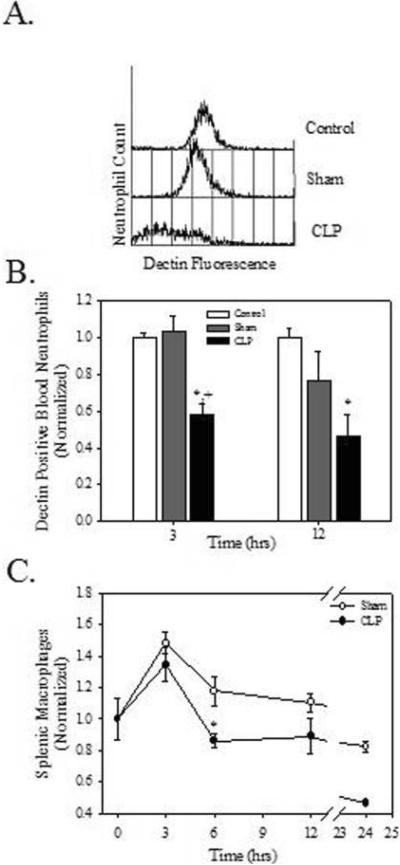
Leukocyte phenotype was determined to more critically examine changes in Dectin-1 expression in CLP sepsis. Though there was no change in blood neutrophil numbers, CLP decreased Dectin-1 expressing blood neutrophils when compared to control and sham animals (Figure 2A). Specifically, CLP decreased the percentage of Dectin-1 positive blood neutrophils by 41.8% (vs control) and 43.8% (vs sham surgery) at 3 h (Fig. 2B, p<0.05). By 12 h the percentage of Dectin-1 positive blood neutrophils was decreased by 53.2% versus control (Fig. 2B, p<0.05). There were no significant changes in the percentage of blood monocytes or lymphocytes or in the percentage of these cells that expressed Dectin-1 at either 3 or 12 h post-operatively (data not shown). In the spleen CLP altered only the percentage of macrophages (11.2% decrease, p<0.05) at 6 h post-operatively (Fig. 2C). There were no changes in the percentage of neutrophils or lymphocytes, nor were there changes in the percentage of neutrophils, macrophages, or lymphocytes that expressed Dectin-1 at any of the time intervals (data not shown).
Figure 2.
Sepsis results in a decrease in Dectin-1 expressing peripheral blood neutrophils. Mice underwent no surgery (control), laparotomy (sham) control or CLP at time 0. Blood and splenocytes were harvested, stained with anti-Dectin-1, anti-neutrophil, and anti-macrophage antibodies, and analyzed by flow cytometry. A. Representative histogram of 3 h blood data. B. Histograms were gated to the neutrophil population and the percentage of Dectin-1 positive cells was determined. C. The percentage of macrophages was determined. B and C. Data are normalized to the no surgery control, which was set at 1.0. Values are means +/- SEM. N=3/group/time interval. *p<0.05 compared to control and + p<0.05 compared to sham surgery.
Blood and splenic leukocyte Dectin-1 expression in mice with systemic C. albicans infection
To compare the in vivo effects of another clinically relevant infection model to polymicrobial sepsis, fungal sepsis was induced in mice by injection of C. albicans, and Dectin-1 expression was analyzed. Overall, the percentage of Dectin-1high positive cells was increased in both the blood and spleens from C. albicans infected mice when compared to controls (Fig. 3A and B). Increases of 71.8%, 65.7%, 82.9%, 118% (p<0.05) were observed in Dectin-1 high positive blood leukocytes on days 3, 5, 7, and 14 of infection, respectively (Fig. 3C). The percentage of Dectin-1 low positive cells was decreased by 20.9% at 5 d and increased by 26.3% at 14 d (p<0.05; data not shown). The percentage of Dectin-1 expressing splenic leukocytes showed an 86.5% (p<0.05) increase at day 7 post-infection (Fig. 3D). Since Dectin-1 expression has been reported to vary among the different leukocytes (11;12;14), cells were analyzed for phenotype. In the blood the percentage of neutrophils was significantly increased by 107, 98.3, and 76.2% on days 3, 5, and 14, respectively with C. albicans infection (p<0.05, data not shown). C. albicans infection increased the percentage of splenic neutrophils by 353 and 216% at 7 and 14 days, respectively (p<0.05, data not shown). There was a corresponding decrease in the percentage of blood and splenic T lymphocytes with C. albicans infection, though there was no change in the percentage of blood or splenic monocytes (data not shown). Therefore, one means by which C. albicans infection increased Dectin-1 in the blood and spleen was by increasing the percentage of neutrophils.
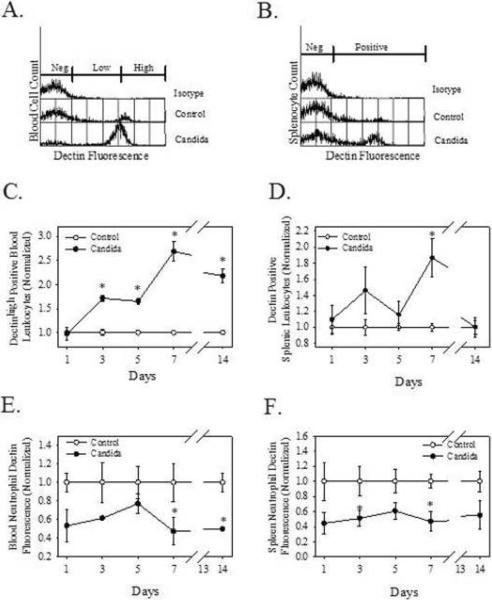
Figure 3.
Infection with C. albicans increases Dectin-1 positive cells in the blood and spleen, but decreases Dectin-1 expression on neutrophils. Mice were injected at time 0 with 1 × 105 cfu C. albicans, IV. Blood cells (A) were harvested on day 3 and splenocytes (B) were harvested on day 7 of infection and stained with anti-Dectin-1 antibody (2a11) prior to flow cytometric analysis (representative histograms). In panels C (blood) and D (spleen) cells were harvested, stained with 2a11, and analyzed for percent positive cells by flow cytometry. In panels E (blood) and F (spleen) cells were harvested, stained with 2a11 and anti-neutrophil, and analyzed for mean fluorescence of Dectin-1 on the neutrophil population by flow cytometry. The data were normalized to the unchallenged control group, which was set at 1. N=3/group/time interval, values are means ± SEM, * indicates p<0.05 compared to control at each time point.
Alterations in neutrophil population dynamics and Dectin-1 expression in mice with systemic infection with C. albicans
As noted above, C. albicans infection results in an influx of neutrophils which normally express high levels of Dectin-1 (12). However, the amount of membrane associated Dectin-1 expressed by neutrophils, as measured by mean fluorescence of Dectin-1, was decreased in response to C. albicans infection (Fig. 3E and 3F). On days 7 and 14 post-infection, Dectin-1 fluorescence on blood neutrophils was decreased by 52.6 and 50.2%, respectively (p<0.05) (Fig. 3E). Dectin-1 fluorescence was decreased on splenic neutrophils on days 3 and 7 post-infection (49.0 and 53.3%, respectively, p<0.05) (Fig. 3F). Though C. albicans infection decreased the percentage of T cells, it increased the percentage of splenic T lymphocytes that express Dectin-1. The percentage of Dectin-1 positive splenic T cells was increased on days 3 (36.6%) and 7 (23.3%) when compared to controls (p<0.05, data not shown). From these data we conclude that leukocyte Dectin-1 response to C. albicans infection is complex and involves both alterations in leukocyte population dynamics and membrane Dectin-1 expression. However, the overall effect is an increase in blood and splenic Dectin-1 levels.
Dectin-1 positivity in C. albicans induced renal lesions
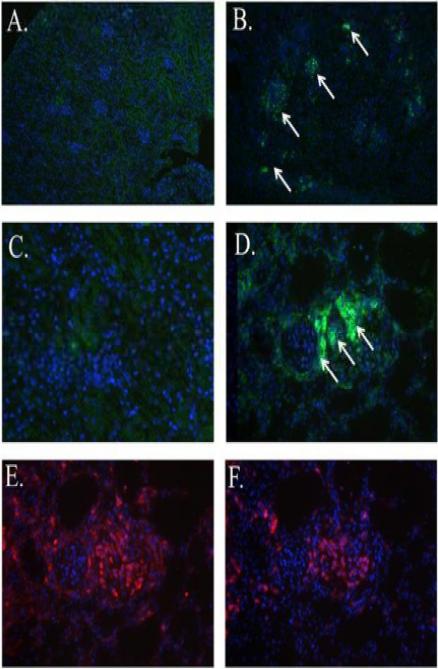
The kidneys are a primary site of infection and colonization in murine C. albicans infection (9;27). Therefore, renal Dectin-1 expression was examined in C. albicans infection by immunohistochemistry. Dectin-1 expression (green) was increased in the kidneys of C. albicans infected mice (Fig. 4B, white arrows). Specifically, Dectin-1 expression was increased in the area of renal lesions (Fig. 4B, white arrows) when compared to kidneys from normal control mice (Fig. 4A). Higher magnification shows Dectin-1 positive cells (Fig. 4D, arrow) in the infected kidney, while the control kidney shows very little Dectin-1 positivity (Fig. 4C). Immunophenotyping of sequential sections revealed that Dectin-1 positivity co-localized to areas containing primarily macrophages (in red) (Fig. 4E) and small numbers of neutrophils (in red) (Fig. 4F). Thus, the increased Dectin-1 expression observed in the kidneys of mice with C. albicans infection is due to an influx of Dectin-1 positive macrophages, and, to a lesser extent, neutrophils.
Figure 4.
Dectin-1 positive macrophages and neutrophils are found in association with C. albicans induced renal lesions. Mice were injected on day 0 with 1 × 105 cfu C. albicans or diluent. Kidneys were harvested on day 3 of infection. Immunohistochemistry using anti-Dectin-1 antibody (green) and the DAPI nuclear counterstain (blue) showed Dectin-1 positivity (white arrows) in kidneys from infected mice (B). Control kidneys (A) did not show Dectin-1 positivity (100X). Higher magnification (400X) images reveal the lack of Dectin-1 positive cells in the control kidney (C) and a focus of Dectin-1 positive cells in the infected kidney (D). Staining of consecutive sections with anti-macrophage F4/80 or anti-neutrophil antibodies revealed that the Dectin-1 positive areas were predominantly co-localized with macrophages (red) (E) and to a lesser extent with neutrophils (red) (F). 400X. Representative images.
The effect of glucan and non-glucan PAMPs on Dectin-1 expression on primary neutrophils in vitro
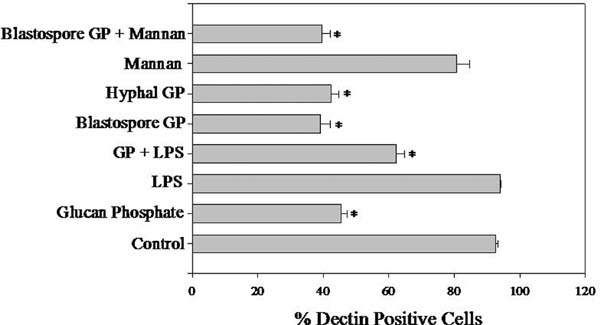
Our data indicate that clinically relevant infections alter Dectin-1 levels via two mechanisms, i.e. changes in the number of Dectin-1 positive leukocytes and modulation of Dectin-1 expression on leukocytes. In an effort to elucidate the mechanisms responsible for in vivo changes in Dectin-1 membrane expression, neutrophils were treated with S. cerevisiae glucan, C. albicans blastospore glucan, C. albicans hyphal glucan, LPS, mannan, or combinations of the above and evaluated for Dectin-1 surface expression. Glucan from all sources resulted in a loss of Dectin-1 from the neutrophil cell surface (Fig. 5). S. cerevisiae glucan reduced Dectin-1 levels by 51.0% (p<0.05) (Fig. 5). C. albicans glucan had similar effects on Dectin-1 expression with the blastospore glucan decreasing Dectin-1 levels by 57.6%, and the hyphal glucan decreased Dectin-1 by 54.2% (Fig. 5, p<0.05). Neither the LPS alone, nor the mannan alone, significantly altered the Dectin-1 expression (Fig. 5). However, when neutrophils were treated with either LPS or mannan in combination with glucan changes in Dectin-1 levels that were comparable to treatment with glucan alone were observed, i.e. mannan with the C. albicans blastospore glucan reduced Dectin-1 levels by 57.2% (p<0.05), and LPS and S. cerevisiae glucan reduced Dectin-1 levels by 32.8% (Fig. 5, p<0.05). Thus, glucan is the only PAMP tested that altered Dectin-1 on neutrophils. To ensure that the observed decreases in Dectin-1 expression were not due to increased apoptosis induced by the PAMPs, the percentage of apoptotic neutrophils was measured 3 hours after PAMP treatment. LPS treatment decreased the percentage of apoptotic cells by 61.0% and the LPS and glucan treatment decreased the percentage of apoptotic cells by 62.3% when compared to control (data not shown). None of the glucans alone or in combination with mannan significantly altered the percentage of apoptotic cells when compared to media treated cells (data not shown). Thus the effects of the glucans on neutrophils Dectin-1 is not due to induction of apoptosis.
Figure 5.
S. cerevisiae and C. albicans derived glucans reduce the percentage of Dectin-1 expressing elicited neutrophils alone and in combination with other PAMPs. Thioglycollate elicited neutrophils were incubated with 1 μg/ml S. cerevisiae GP, C. albicans blastospore or hyphal GP, LPS, or S. cerevisiae mannan alone or in combination for 3 h. The cells were stained with anti-Dectin-1 and anti-neutrophil antibodies and analyzed by flow cytometry. The percentage of Dectin-1 positive cells within the neutrophil positive population was determined. Average of three independent experiments with N=6. Values are means +/- SEM. * indicates p<0.05 compared to control.
The effect of inflammatory cytokines on Dectin-1 expression on primary neutrophils in vitro
Willment et al. have reported that Dectin-1 expression can be modulated by cytokines in vitro (28). CLP sepsis and C. albicans infection are known to up regulate a variety of cytokines including TNFα{10869}, IL-6 (22) and IL-17 (29). To determine if circulating cytokines were responsible for the changes in Dectin-1 expression, neutrophils were treated with varying concentrations of TNFα, IL-6, IL-17, or L cell conditioned media and analyzed for Dectin-1 expression. None of the cytokines or the L cell conditioned media altered neutrophil Dectin-1 levels (data not shown). Therefore, the effect of sepsis on leukocyte Dectin-1 expression may not be attributable to circulating cytokines.
4. Discussion
This is the first report describing regulation of leukocyte Dectin-1 expression in response to clinically relevant models of in vivo fungal or polymicrobial infection. We observed that C. albicans infection resulted in an increase in blood and splenic Dectin-1. In contrast, Dectin-1 was decreased with polymicrobial sepsis. Thus, the regulation of leukocyte Dectin-1 appears to be a dynamic process that is influenced by the type of infection the host encounters. Dectin-1 has been proposed to be a pivotal PRR for fungal infections (30). The fact that leukocyte Dectin-1 is modulated in response to polymicrobial sepsis may indicate a role for this PRR in non-fungal infections as well.
Following C. albicans infection there was an overall increase in Dectin-1 levels in the blood, spleen, and kidneys of infected mice. Recent work from our laboratory revealed that systemic administration of pure glucan resulted in loss of Dectin-1 expression on blood leukocytes due to internalization of the Dectin-1/glucan complex (17). It has been shown that systemic fungal infections increase levels of circulating glucan (31). Therefore, the increase in Dectin-1 levels in fungal sepsis may seem counter-intuitive. However, the increase in Dectin-1 levels in C. albicans infection was due primarily to an increase in the proportion of blood and splenic neutrophils and a decrease in the proportion of T lymphocytes. As neutrophils express large levels of Dectin-1 while T cells express none except in a small subset (12), this alteration in cell population dynamics would, in part, explain the increase in Dectin-1. We have reported that administration of fungal mannans, but not glucans, induces neutrophilia in vivo (17). Additionally, in the spleen there is an increase in the percentage of T lymphocytes that express Dectin-1 with C. albicans infection. This could be due to an expansion of the subset of T cells that express Dectin-1 or due to a loss of splenic T cells that are Dectin-1 negative. The cause of this increase in Dectin-1 positive splenic T lymphocytes is not known, however this increase does contribute to the overall increase in Dectin-1 found in the spleen of C. albicans infected mice. In contrast, we found that levels of cell surface Dectin-1 on blood and splenic neutrophils were decreased. We speculate that this was due to the internalization of Dectin-1 upon binding C. albicans glucan, a supposition supported by the in vitro neutrophil experiments. Thus, the overall increase in Dectin-1 levels observed in the blood and spleens of C. albicans infected mice may be the result of the opposing influences of two fungal PAMPs, glucan and mannan, on Dectin-1, i.e. mannan induces neutrophilia, while glucan results in decreased membrane Dectin-1 positivity. The net effect of fungal infection was increased Dectin-1 levels systemically. We also observed an increase in Dectin-1 positive cells surrounding fungal foci in C. albicans infected mice. The increased Dectin-1 expression was due to an influx of Dectin-1 positive macrophages and, to a lesser extent, neutrophils. We speculate that this is an attempt to contain the fungal pathogen in the renal parenchyma.
Neutrophils and macrophages are integral to the defense against fungal infections (32). These cells express high levels of Dectin-1, which is a proposed sentinel receptor for fungal infections (33;34). Therefore, an increase in Dectin-1 expressing cells in the blood, spleen, and kidney may be interpreted as an innate immune response to candidal infection. However, the increase in Dectin-1 positive cells was not sufficient to clear the fungal pathogen, nor was it sufficient to alter disease outcome. There are several possible explanations for this observation. First, the C. albicans challenge dose may be of sufficient magnitude to overwhelm innate host defenses. Second, it is possible that the reduced expression of Dectin-1 by neutrophils leaves these cells less able to respond to the fungal organisms. The latter supposition is supported by work by Taylor, et al. who have demonstrated that Dectin-1 knock-out mice are more susceptible to C. albicans infection (20).
In contrast to fungal infection, we found that polymicrobial sepsis decreased blood Dectin-1 by decreasing the percentage of Dectin-1 expressing peripheral neutrophils. The mechanisms responsible for the decrease in Dectin-1 expression are unclear; however, it is possible that CLP may result in elevated levels of circulating glucans. Digby, et al. demonstrated that septic patients have increased circulating glucan levels, regardless of infectious etiology (31). Polymicrobial sepsis was induced by perforation of the ileocecal pouch, resulting in the introduction of cecal contents into the abdominal cavity inducing peritonitis. Glucans from the gut microflora may have been absorbed into the systemic circulation and bound to Dectin-1 with subsequent internalization of the receptor-ligand complex (17). Alternatively, other factors have been shown to affect Dectin-1 expression (28). Willment et al have reported that certain cytokines and LPS negatively regulate Dectin-1 RNA expression on macrophages in vitro (28). Studies have shown that septic patients have elevated levels of cytokines as well as circulating LPS (35). Any or all of these factors might contribute to the decreased cell surface expression of Dectin-1 that was observed; however, the in vitro studies revealed that glucan administration alone resulted in loss of Dectin-1 from the neutrophil cell surface. Non-glucan PAMPs such as LPS and mannan did not alter neutrophil Dectin-1 unless the cells were also treated with glucan. Additionally, treatment with TNFα, IL-6, IL-17, or L cell conditioned media had no effect on neutrophil cell surface Dectin-1. Thus, to date only glucans seem to decrease Dectin-1 surface expression on neutrophils. Therefore, it is likely that the loss of Dectin-1 positive neutrophils in polymicrobial sepsis is due to elevated levels of circulating glucans.
Of further interest is the change in Dectin-1 expression by peritoneal cells with CLP sepsis. In contrast to the blood data, the percentage of Dectin-1 positive peritoneal macrophages was increased when compared to sham operated animals. One factor in the difference between the sham and CLP values is a decrease in the sham value as compared to the other time intervals. The cause of this decrease is not known, but is likely due to factors released into the peritoneal cavity by the trauma of surgery. Though this study has demonstrated that only glucans seem to affect Dectin-1 expression in neutrophils, the same is not the case with macrophages. Previous work has shown that IL-10, dexamethasone, and LPS will all negatively affect Dectin-1 expression by macrophages (28). The second factor in the difference would be the increased percentage of Dectin-1 positive cells with CLP. Previous studies have shown that resident peritoneal macrophages express Dectin-1 at low levels, but that macrophages elicited by an inflammatory stimulus, i.e. sterile peritonitis, express Dectin-1 at high levels (12). Additionally, GM-CSF treated macrophages express higher levels of Dectin-1 (28). It is possible that the levels of GM-CSF in the local milieu are increased with an inflammatory insult originating in the peritoneal cavity, and this leads to an increase in the expression of Dectin-1 by the resident peritoneal macrophages.
The consequences of the decrease in Dectin-1 positive neutrophils in polymicrobial sepsis are unclear. Critically ill patients have been shown to be more susceptible to opportunistic fungal infection (2;3). It is possible that reduced Dectin-1 expression is responsible for this increase in susceptibility. Further studies examining human Dectin-1 expression following critical illness will be necessary to determine if this is the case. Additional studies are also warranted to better determine what role Dectin-1 might be playing in response to polymicrobial sepsis.
One possible limitation of this study as a comparison of different models of infection is the difference in kinetics between the two models of infection. CLP induced polymicrobial sepsis is an initially localized infection which results in acute shock with rapid mortality occurring as soon as 22 hours post-operatively. Thus, only the immediate response of Dectin-1 to acute shock could be examined without skewing the results toward survivors. With IV administration of C. albicans, however, the infection is rapidly disseminated via the blood. There is no demonstrable acute shock phase, and candidemia results in a more chronic disease in which the mice are unlikely to succumb prior to day six of infection. This limitation does not alter the significance of the findings, but future studies are warranted to compare infectious diseases with similar clinical courses.
5. Conclusion
In conclusion, this is the first report of the effects of clinically relevant infections on in vivo regulation of leukocyte Dectin-1 levels. We found that Dectin-1 expression is differentially and dynamically regulated in fungal versus polymicrobial sepsis. These data support the concept that Dectin-1 plays a role in the innate immune response to fungal infection and may also be involved in the pathophysiology of polymicrobial sepsis.
Acknowledgments
This work was supported, in part, by Public Health Service grants GM53522 from the National Institute of General Medical Sciences to DLW. This work was also supported, in part, by Public Health Service grant HL071837 from the National Heart, Lung and Blood Institute and American Heart Association grants 0051489B and 0255038B to C.L.
References
- (1).Oberholzer A, Oberholzer C, Moldawer LL. Sepsis Syndromes: Understanding the Role of Innate and Acquired Immunity. Shock. 2001;16(2):83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- (2).Eggimann P, Pittet D. Postoperative Fungal Infections. Surgical Infections. 2006;7(Supplement 2):S-53–S-56. doi: 10.1089/sur.2006.7.s2-53. [DOI] [PubMed] [Google Scholar]
- (3).De Pauw BE. Increasing Fungal Infections in the Intensive Care Unit. Surgical Infections. 2006;7(Supplement 2):S-93–S-96. doi: 10.1089/sur.2006.7.s2-93. [DOI] [PubMed] [Google Scholar]
- (4).Gordon S. Pattern Recognition Receptors: Doubling Up for the Innate Immune Response. Cell. 2002 December 27;111:927–30. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- (5).Williams DL, Rice PJ, Herre J, Willment JA, Taylor PR, Gordon S, Brown GD. Recognition of fungal glucans by pattern recognition receptors. Recent Developments in Carbohydrate Research. 2003;1:49–66. [Google Scholar]
- (6).Chauhan N, Li D, Singh P, Calderone R, Kruppa M. The Cell Wall of Candida spp. In: Calderone RA, editor. Candida and Candidiasis. 1st ed. ASM Press; Washington, DC: 2002. pp. 159–75. [Google Scholar]
- (7).Williams DL, Ha T, Li C, Kalbfleisch JH, Laffan JJ, Ferguson DA. Inhibiting early activation of tissue nuclear factor-κB and nuclear factor interleukin 6 with (1-->3)-β-D-glucan increases long-term survival in polymicrobial sepsis. Surgery. 1999;126:54–65. doi: 10.1067/msy.1999.99058. [DOI] [PubMed] [Google Scholar]
- (8).Williams DL, Browder W, McNamee R, Di Luzio NR. Glucan immunomodulation in experimental E. coli sepsis. In: Normann SJ, Sorkin E, editors. Macrophages and Natural Killer Cells. Plenum Publishing Corporation; New York: 1982. pp. 701–6. [DOI] [PubMed] [Google Scholar]
- (9).Williams DL, Cook JA, Hoffmann EO, DiLuzio NR. Protective effect of glucan in experimentally induced candidiasis. J Reticuloendothel Soc. 1978;23:479–90. [PubMed] [Google Scholar]
- (10).Brown GD, Gordon S. Immune Recognition: A new receptor for beta-glucans. Nature. 2001 September 6;413(6851):36–7. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- (11).Willment JA, Gordon S, Brown GD. Characterization of the human β-glucan receptor and its alternatively spliced isoforms. Journal of Biological Chemistry. 2001;276(47):43818–23. doi: 10.1074/jbc.M107715200. [DOI] [PubMed] [Google Scholar]
- (12).Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, Wong SWC. The β-glucan receptor, Dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol. 2002;169(7):3876–82. doi: 10.4049/jimmunol.169.7.3876. [DOI] [PubMed] [Google Scholar]
- (13).Brown GD, Herre J, Williams DL, Willment JA, Marshall ASJ, Gordon S. Dectin-1 mediates the biological effects of β-glucans. J Exp Med. 2003;197:1119–24. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SYC, Gordon S. Dectin-1 is a major β-glucan receptor on macrophages. J Exp Med. 2002;296(407):412. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Mueller A, Rice PJ, Ensley HE, Coogan PS, Kalbfleisch JH, Kelley JL, Love EJ, Portera CA, Ha T, Browder IW, Williams DL. Receptor binding and internalization of watersoluble (1-->3)-beta-D-glucan biologic response modifier in two monocyte/macrophage cell lines. J Immunol. 1996;156:3418–25. [PubMed] [Google Scholar]
- (16).Herre J, Marshall ASJ, Caron E, Edwards AD, Williams DL, Schweighoffer E, Tybulewicz V, Reis e Sousa C, Gordon SE, Brown GD. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood. 2004 December 15;104(13):4038–45. doi: 10.1182/blood-2004-03-1140. [DOI] [PubMed] [Google Scholar]
- (17).Ozment-Skelton TR, Goldman MP, Gordon S, Brown GD, Williams DL. Prolonged reduction of leukocyte membrane-associated Dectin-1 levels following β-glucan administration. Journal of Pharmacolology and Experimental Therapeutics. 2006;318(2):540–6. doi: 10.1124/jpet.106.102293. [DOI] [PubMed] [Google Scholar]
- (18).Hiyoshi M, Tagawa S, Hashimoto S, Sakamoto C, Tatsumi N. Evaluation of a new laboratory test measuring plasma (1→3)-β-D-glucan in the diagnosis of Candida deep mycosis: comparison with a serologic test. Kansenshogaku Zasshi. 1999;73(1):1–6. doi: 10.11150/kansenshogakuzasshi1970.73.1. [DOI] [PubMed] [Google Scholar]
- (19).Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. The EMBO Journal. 2005 March 23;24(6):1277–86. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nature Immunology. 2006;8(1):31–8. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Saijo S, Fujikado N, Furuta T, Chung S, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, Kinjo T, Nakamura K, Kawakami K, Iwakura Y. Dectin-1 is required for host defense against Pneumocystic carinii but not against Candida albicans. Nature Immunology. 2006;8(1):39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- (22).Williams DL, Li C, Ha T, Ozment-Skelton T, Kalbfleisch JH, Preiszner J, Brooks L, Breuel K, Schweitzer JB. Modulation of the phosphoinositide 3-Kinase pathway alters innate resistance to polymicrobial sepsis. J Immunol. 2004;172:449–56. doi: 10.4049/jimmunol.172.1.449. [DOI] [PubMed] [Google Scholar]
- (23).Williams DL, Ha T, Li C, Kalbfleisch JH, Ferguson DA., Jr Early activation of hepatic NFkB and NF-IL6 in polymicrobial sepsis correlates with bacteremia, cytokine expression and mortality. Ann Surg. 1999;230(1):95–104. doi: 10.1097/00000658-199907000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Yang S, Chung CS, Ayala A, Chaudry IH, Wang P. Differential alterations in cardiovascular responses during the progression of polymicrobial sepsis in the mouse. Shock. 2002;17(1):55–60. doi: 10.1097/00024382-200201000-00010. [DOI] [PubMed] [Google Scholar]
- (25).Williams DL, McNamee RB, Jones EL, Pretus HA, Ensley HE, Browder IW, Di Luzio NR. A method for the solubilization of a (1-3)-β-D-glucan isolated from Saccharomyces cerevisiae. Carbohyd Res. 1991;219:203–13. doi: 10.1016/0008-6215(91)89052-h. [DOI] [PubMed] [Google Scholar]
- (26).Lowman DW, Ferguson DA, Williams DL. Structural characterization of (1-->3)-beta-D-glucans isolated from blastospore and hyphal forms of Candida albicans. Carbohydr Res. 2003 July 4;388(14):1491–6. doi: 10.1016/s0008-6215(03)00169-1. [DOI] [PubMed] [Google Scholar]
- (27).Rogers TJ, Balish E. Immunity to experimental renal candidiasis in rats. Infec Immun. 1978;19:737–40. doi: 10.1128/iai.19.2.737-740.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Willment JA, Lin H-H, Reid DM, Taylor PR, Williams DL, Wong SYC, Gordon S, Brown GD. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J Immunol. 2003;171:4569–73. doi: 10.4049/jimmunol.171.9.4569. [DOI] [PubMed] [Google Scholar]
- (29).Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 190(3):624–31. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- (30).Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nature Reviews Immunology. 2006;6(1):33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- (31).Digby J, Kalbfleisch J, Glenn A, Larsen A, Browder W, Williams D. Serum Glucan Levels Are Not Specific for Presence of Fungal Infections in Intensive Care Unit Patients. Clinical and Diagnostic Laboratory Immunology. 2003;10(5):882–5. doi: 10.1128/CDLI.10.5.882-885.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Romani L. Immunity to fungal infections. Nat Rev Immunol. 2004;4(1):1–23. doi: 10.1038/nri1255. [DOI] [PubMed] [Google Scholar]
- (33).Herre J, Gordon S, Brown GD. Dectin-1 and its role in the recognition of β-glucans by macrophages. Molecular Immunology. 2004;40:869–76. doi: 10.1016/j.molimm.2003.10.007. [DOI] [PubMed] [Google Scholar]
- (34).Herre J, Willment JA, Gordon S, Brown GD. The role of Dectin-1 in antifungal immunity. Critical Reviews in Immunolology. 2004;24(3):193–203. doi: 10.1615/critrevimmunol.v24.i3.30. [DOI] [PubMed] [Google Scholar]
- (35).Marchant A, Deviere J, Byl B, De-Groote D, Vincent JL, Goldman M. Interleukin-10 production during septicaemia. Lancet. 1994;343(8899):707–8. doi: 10.1016/s0140-6736(94)91584-9. [DOI] [PubMed] [Google Scholar]