Abstract
This Perspective chronicles the conceptual development, proof of principle, exploration of scope, mechanistic investigations and applications in natural product total synthesis of palladium-catalyzed cross-coupling reactions of silicon derivatives. The explication of how this new class of cross-coupling reactions evolved from problem formulation to use in complex molecule synthesis serves as one goal of the essay. The other goal is the presentation of the various stages of this methodological enterprise such that the reader gleans a first hand look at one approach to the creation of new synthetic reactions. These two goals are woven together such that the underlying thought processes that guide a program of reaction development emerge in clear view and imbue the chemical tapestry with a cohesive logic.
Of course, men make much use of excuses for activities which lead to discovery, and the lure of unknown structures has in the past yielded a huge dividend of unsought fact, which has been of major importance in building organic chemistry as a science. Should a surrogate now be needed, we do not hesitate to advocate the case for synthesis.
R. B. Woodward (1963)1
Introduction
Woodward’s visionary insights in the oft-quoted passage above are as relevant nowadays as they were nearly half a century ago. The revolution Woodward advocated, namely that the new challenge for organic synthesis begins at the advanced vantage point of an established structure,1b inspired generations of organic chemists and thrives still today. Even though the organic chemistry of the 21st century is as rich and varied as anything Woodward could have imagined, it is nonetheless appropriate once again to ask, “what excuses for activities that lead to discovery currently capture the imagination of organic chemists?”. Although the answers to this question will naturally reflect each individual’s perspective, some consensus can be found in what defines the frontier for the major themes of structure, function, synthesis, reactivity, and mechanism. As Woodward (and many others) predicted, the creative function of organic chemistry continues to provide solutions to the challenges associated with all of these categories.
However, in the sub-domain of synthesis, the frontier has appropriately shifted from the synthesis of structures, both naturally inspired and theoretically challenging, to the synthesis of properties, i.e. structures with programmed function.2 The desired function can take on the myriad manifestations of non-natural substances that enhance life on earth. And although the approaches to the creation of molecules expressing those functions are many and varied (e.g. computer aided design, diversity oriented synthesis), the fundamental fact remains that the ability to rapidly, efficiently, selectively, and inexpensively synthesize compounds defines the horizon for the success of all of these enterprises. We therefore submit, that the introduction of new concepts and the creation of useful methods (i.e. issues of reactivity and mechanism) for the synthesis of (organic) compounds constitute a chemical evergreen.
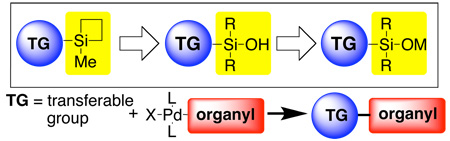
If one accepts this premise, then the question naturally arises, “how does one go about introducing concepts and creating new methods?”. For the purposes of this personal perspective, we shall address the latter question in the form of a narrative explication of an ongoing research program in our laboratories, namely the invention of cross-coupling reactions of organosilanols.3 The evolution of this program nicely illustrates one of many successful paradigms that the creation of new synthetic reactions can follow. We have chosen this one because, as implied in the title, it represents a real-life case study of the interplay of invention, discovery, development and application.4
Invention Stage I: An Interesting Concept Finds a Practical Application
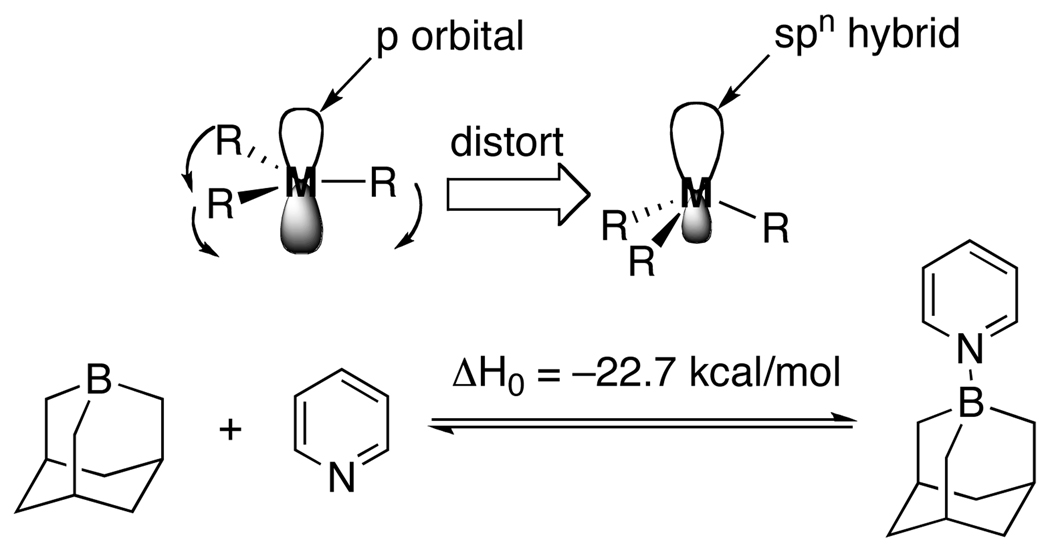
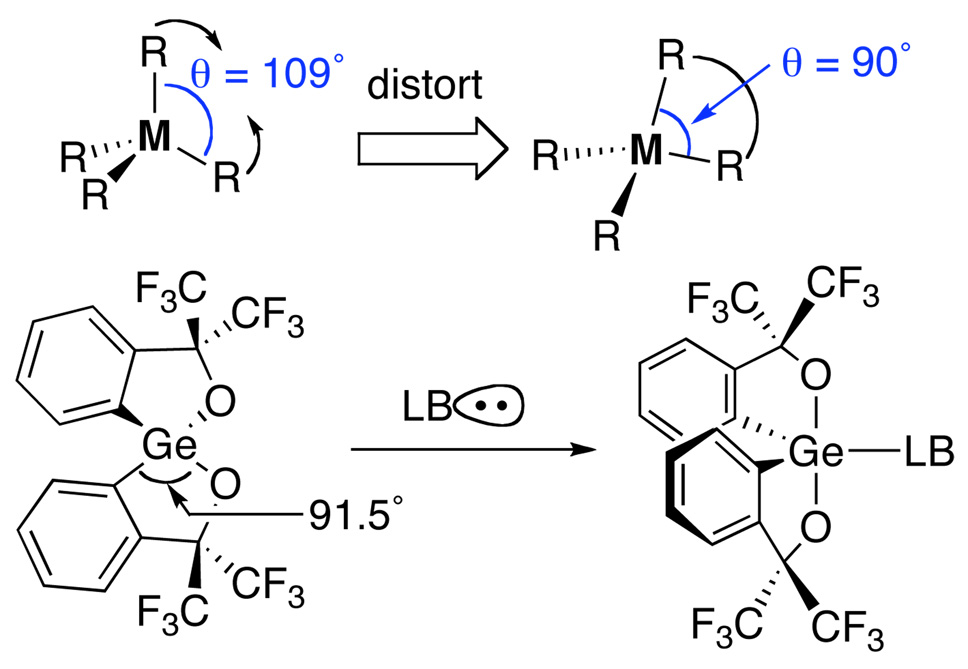
A recurring theme in this research group is the introduction of new strategies to modulate chemical reactivity. One such strategy is known as “strain-release Lewis acidity”. Whereas the concept of Lewis acidity (i.e. the tendency of a substance to employ a lone pair from another molecule in completing the stable group of one of its own atoms5) is most commonly associated with electronic perturbation of the acceptor molecule, “strain-release Lewis acidity” is founded on the structural perturbations that enhance the affinity of the acidic atom for expanding its coordination number.6 Invariably, expansion of coordination number also changes ligand field geometry. Therefore, distortion of the ground state geometry of the nascent Lewis acid toward the geometry of the Lewis acid-base adduct should result in an energy release upon binding and attendant increase in the equilibrium favoring association. The concept is general for elements in the Main Group. For example, in Group 13 the hyper Lewis acidity of 1-boraadamantane7 results from geometrical inhibition of planarity of the boron atom that causes a mixing of s-character into the unoccupied orbital and attendant exposure of the nuclear charge, Scheme 1. In Group 14 the enhanced Lewis acidity of spirogermane (Scheme 2) results from the distortion of the C-Ge-O angles from their normal value of 109° to 91.5° closely approximating the idealized value in a trigonal bipyramidal adduct.8
Scheme 1.
Scheme 2.
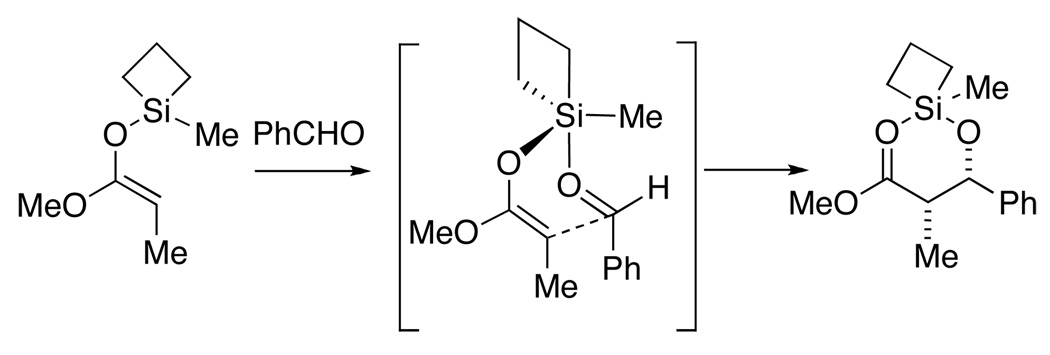
Early attempts to harness this potential of “strain release Lewis acidity” for chemical reactions focused on the use of enoxy derivatives of silacyclobutanes for uncatalyzed aldol additions (Scheme 3).9 The angle strain in a four coordinate siletane (79° vs. 109°) is partially relieved upon binding a fifth ligand to produce a trigonal bipyramidal species (79° vs. 90°) in which the siletane bridges an apical and a basal position. The affinity of the silicon atom for the aldehyde oxygen lone pairs served to activate and organize the assembly for a spontaneous aldol addition reaction.10
Scheme 3.
To expand the utility of the “strain release Lewis acidity” concept, we considered other chemical reactions that required access to a hypercoordinate silicon moiety for reaction to take place. It was during this period (late 1990’s) that transition metal catalyzed cross-coupling was undergoing spectacular development11 and our attention was drawn to the possibility of using siletanes as donor groups in this process. The pioneering work of Hiyama and Hatanaka had demonstrated that organosilanes, when suitably functionalized (with heteroatoms) and in the presence of a nucleophilic activator, can undergo cross-coupling reactions with palladium catalysis.12 The crucial feature for the success of the “Hiyama Coupling” was believed to be the ability to generate the reactive, pentacoordinate siliconate intermediate that was needed to effect the rate limiting transmetalation.13 However, the fluoro- and chlorosilanes used for these couplings had not been widely adopted most likely because of their sensitivity and the need for elevated temperatures in the presence of fluoride source to effect coupling. Thus, under the reasonable assumption that transmetalation is the turnover limiting step, our efforts focused on the design of a new silicon subunit with enhanced reactivity and better chemical stability. In view of the Hiyama-Hatanaka Paradigm, our initial proposal was to investigate the ability of siletanes to serve as the coupling partners. Siletanes offered two critical advantages: (1) they are chemically more robust than halo silanes and (2) their propensity to become pentacoordinate in the presence of Lewis bases (e.g. fluoride) should facilitate transmetalation.
Discovery Stage I: Always Question Your Assumptions
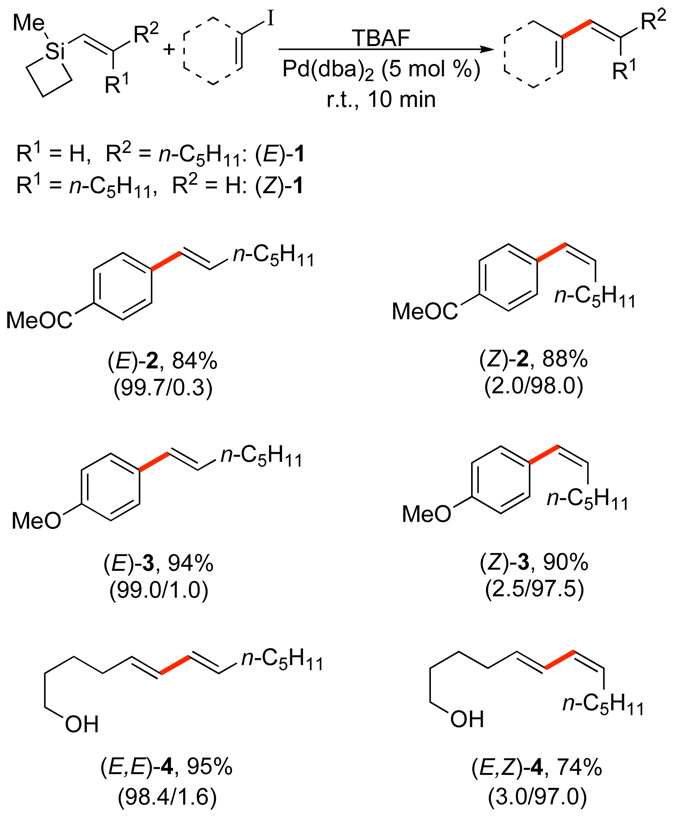
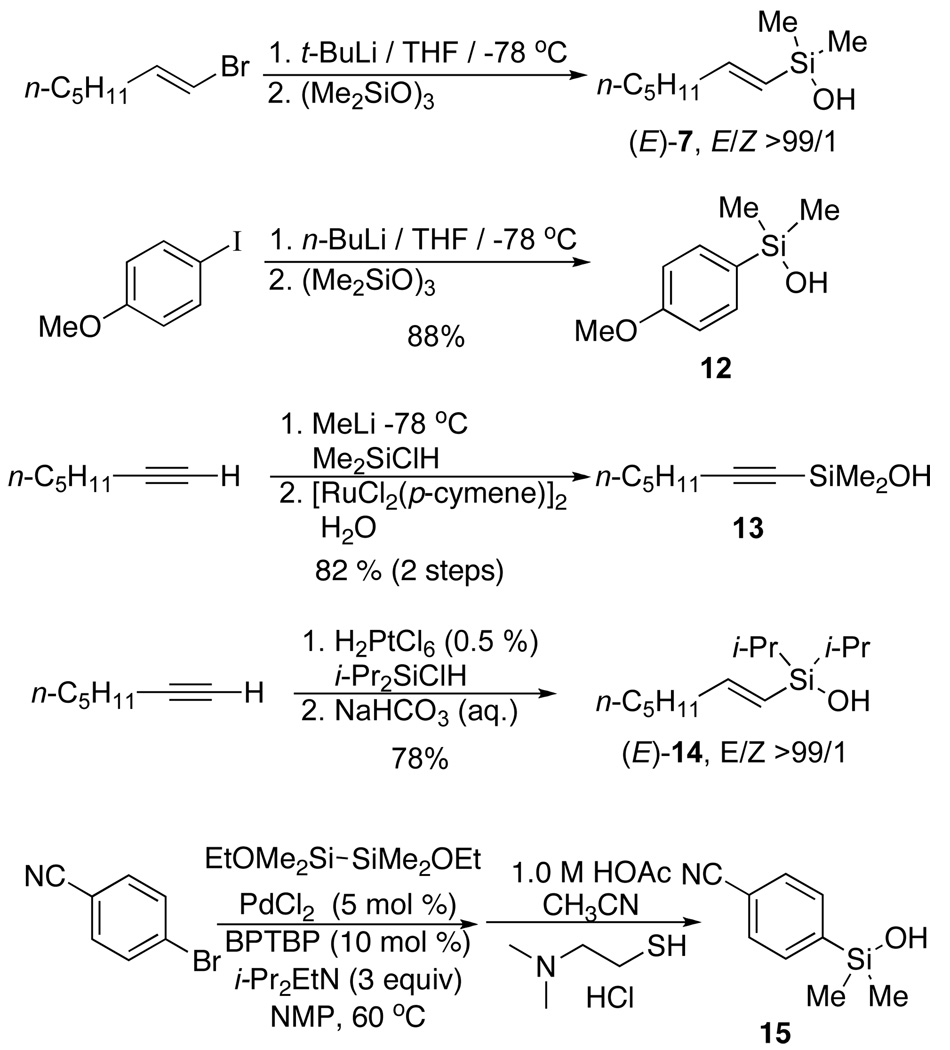
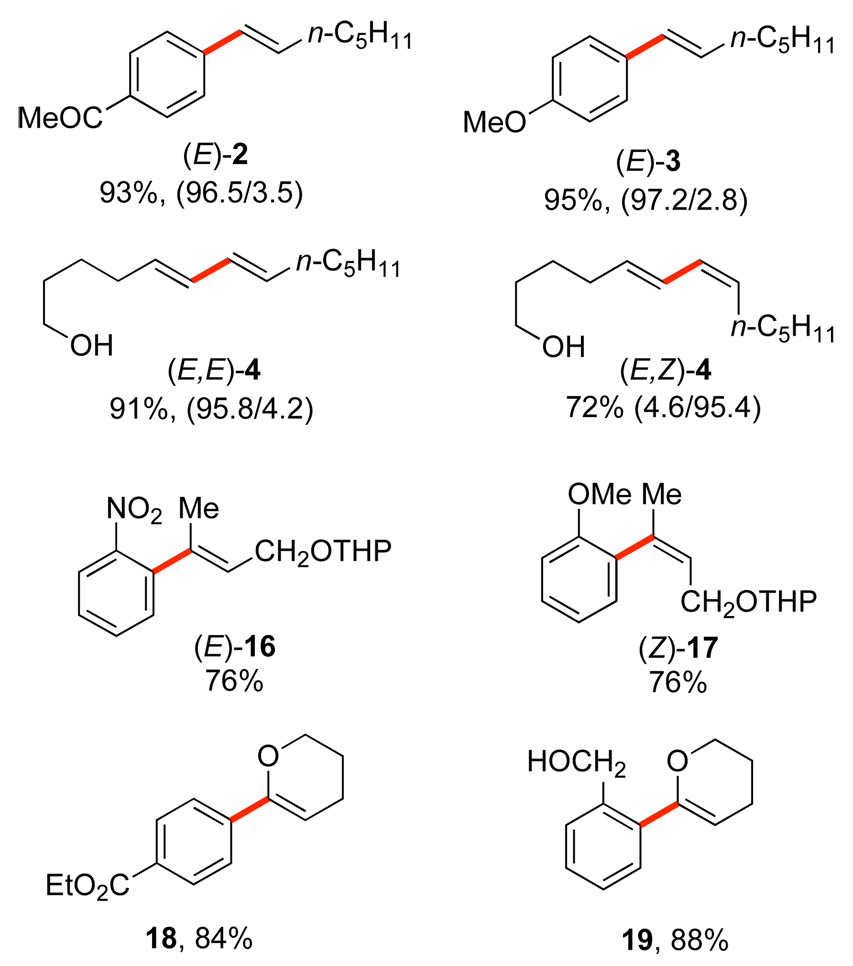
Thus, with the conceptual framework established, we began the testing of our hypothesis in earnest. To our delight, silacyclobutanes (E)-1 and (Z)-1 (readily prepared from the commercially available silacyclobutyl chloride) underwent cross-coupling reactions with aryl halides in combination with tetrabutylammonium fluoride (TBAF) and in the presence of a palladium catalyst (Scheme 4).14 The use of TBAF as a nucleophilic activator was most effective, while other fluoride activators (TASF, TBAT and KF) were incapable of promoting the reaction. A survey of catalysts revealed the “ligandless” palladium(0) as Pd(dba)2 or Pd2(dba)3 to be superior to other palladium sources. The reactions were remarkable for the mild conditions and high stereospecificity (greater than 98% in most cases) of the TBAF promoted coupling to a variety of alkenyl and aryl iodides (ca. 10 min at ambient temperature). Even in the cross-coupling of alkenyl iodides to form (E,E)-4 and (E,Z)-4, the olefin geometry of both coupling partners is highly conserved. The successful coupling of alkenylsiletanes was extended to vinyl-, 2-propenyl-15 and even arylsiletanes.16
Scheme 4.
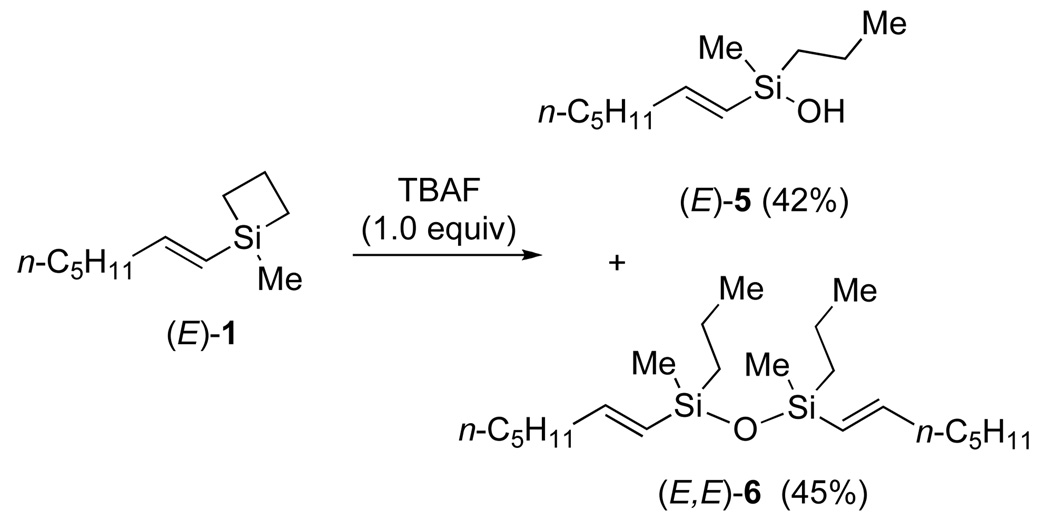
These initial successes, although highly satisfying, were also mystifying. Were the unexpectedly high rate, selectivity, and generality of these cross-coupling reactions truly due to the “strain release Lewis acidity” of the siletane moiety?17 An early experimental observation of a significant exotherm when combining the siletane with the TBAF solution was an important clue. The products isolated from combination of (E)-1 with TBAF are the silanol (E)-5 and disiloxane (E,E)-6. These products are clearly derived from ring opening of (E)-1 by the combined action of TBAF and water (from the crystal hydrates in TBAF•3H2O) (Scheme 5).18
Scheme 5.
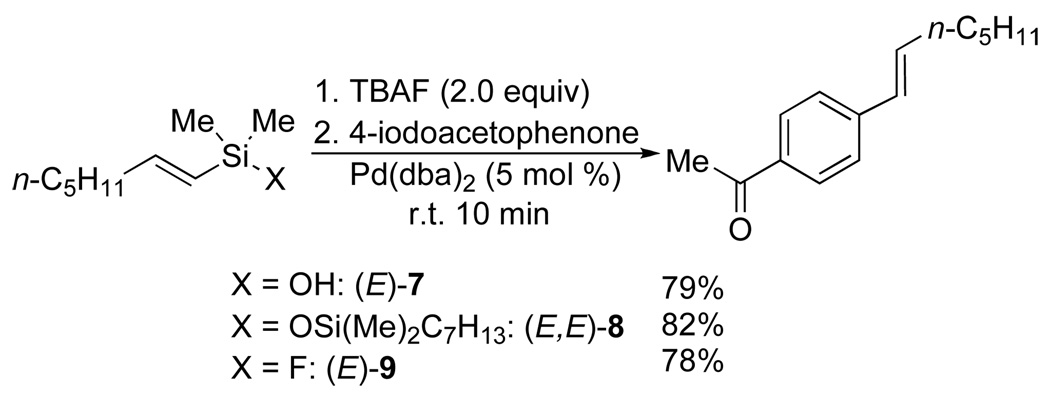
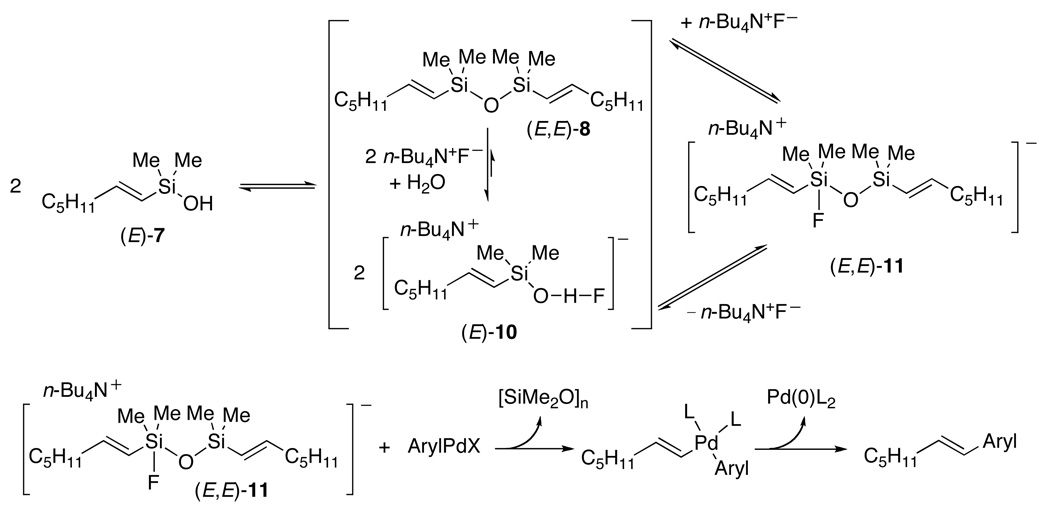
The destruction of the siletane ring upon exposure to TBAF clearly invalidated our original formulation of the process and compelled a reevaluation of our mechanistic hypotheses. The primary question now became whether the silanol 5, disiloxane 6 or even a fluorosilane were responsible for the cross-coupling. This question was addressed by independent synthesis of 7, 8 and 9, the dimethyl analogs of the three most likely candidates for that putative reactive intermediate. All three substrates are competent coupling partners and provided nearly identical results in a typical coupling with 4-iodoacetophenone (Scheme 6).
Scheme 6.
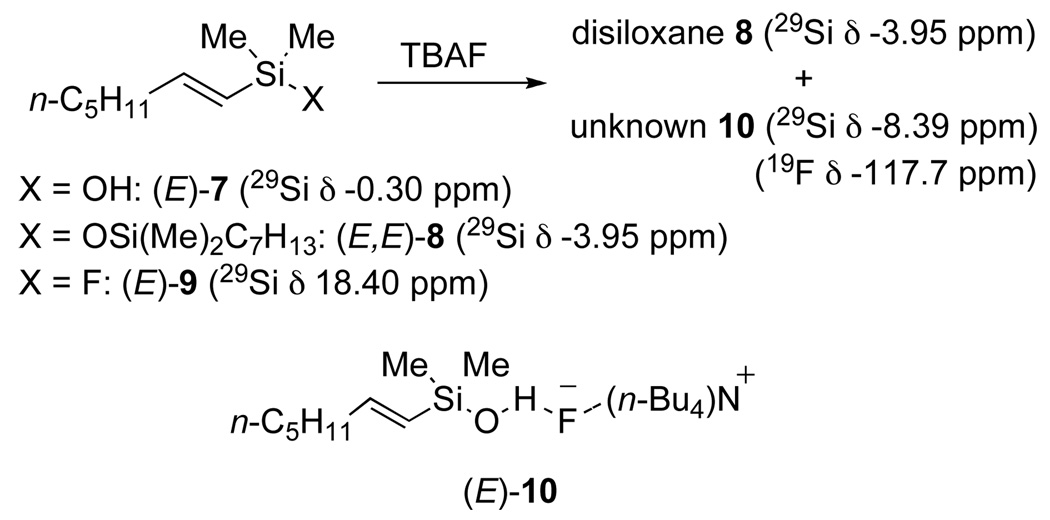
The similarity of reaction rates and yields could be explained by either interconversion of these species to one another, or conversion of each of them to a more advanced, common reactive intermediate. Interestingly, 1H NMR analysis of a mixture of TBAF with siletane 1, silanol 7, disiloxane 8, or fluorosilane 9, showed only two species which are formed almost instantaneously. One species is easily identified as the disiloxane 8 and the other species 10 as a compound containing both silicon and fluorine as confirmed by 29Si and 19F NMR analysis (Scheme 7). Moreover, the ratio of 10 to disiloxane increased with increasing amounts of TBAF; under typical conditions for cross-coupling the ratio heavily favors 10.
Scheme 7.
The identity of 10 proved quite difficult to establish. All attempts to isolate this material provided only the silanol 7. The sign and magnitude of the 29Si NMR chemical shift was indicative of tetracoordinate silicon species, yet it did not match any of the previously synthesized tetracoordinate silanes. After elimination of many alternatives, we concluded that the unknown species is a hydrogen bonded complex between an organosilanol and TBAF, (E)-10 (Scheme 7). Because (E)-10 contains a hydrogen-bonded fluorine atom, 19F NMR analysis of a sample generated from (E)-7 and TBAF should indicate the presence of a fluorine at a resonance different from TBAF. At room temperature, the spectrum displayed only a single resonance at −117.7 ppm. Cooling the solution to −95 °C, however, allowed the observation of two signals, one at −113.8 ppm for TBAF and one at −150.8 ppm which is very close to the chemical shift for bifluoride (FHF−).19
Although indirect, all of the available data are consistent with structure (E)-10 as the best fit for the unknown reaction component. However, the ability to observe a species spectroscopically does not guarantee that it lies on the reaction pathway. Thus, to demonstrate if (E)-10 is truly a reactive intermediate and also secure a deeper understanding of the reaction mechanism a full kinetic analysis of the process was undertaken.20 For these studies, silanol (E)-7 was used because it represents the simplest way to generate (E)-10 and test its kinetic competence. From initial rate kinetic studies the following conclusions were secured: (1) the reactions showed first order dependence on [Pd] and zeroth order dependence on aryl halide, (2) a second order dependence on (E)-7 was found and (3) two difference regimes for the dependence on [TBAF] were found; first order at low [TBAF] and inverse first order at high [TBAF].
A self-consistent picture of the reaction pathway could now be formulated that explained this unusual kinetic dependence and the intermediacy of (E)-10 (Scheme 8). The first order dependence on [Pd] and zero order dependence on aryl halide suggest a rapid, irreversible oxidative addition and a rate limiting transmetalation of the ArylPdX species. Moreover, the second order dependence on (E)-7 reveals that two silanol moieties must be present in the turnover limiting transition structure. The intermediacy of (E)-10 nicely explains this as well. Given that (E)-10 is formed together with (E,E)-8 from (E)-7 in a TBAF dependent equilibrium, we now see that the species most likely responsible for the turnover limiting transmetalation is a fluoride activated disiloxane (E,E)-11. At typical TBAF concentration the resting state of the silanol is almost completely in the form of (E)-10. Thus, to access the key intermediate (E,E)-11 it must dimerize and lose one molecule of TBAF, thus explaining the second order behavior in (E)-7 and inverse order behavior in TBAF. This picture is consistent with the Hiyama-Hatanaka paradigm, though the details of the actual species involved are now more refined.
Scheme 8.
Development Stage I: From Siletanes to Silanols and their Derivatives
Although strain-release Lewis acidity was not the underlying basis for the facile cross-couplings of siletanes, careful detective work uncovered an important structural clue that might explain the facility of these reactions, namely the presence of an oxygen atom which allowed access to a fluoride activated disiloxane such as (E,E)-11. Thus, on the basis these findings, a new vista opened before us to explore the uncharted chemistry of dimethylsilyloxy compounds as cross-coupling partners. If indeed, all such silyloxy compounds converged to a common intermediate in the presence of TBAF•3H2O, then not only silanols, but di- and polysiloxanes, and silyl ethers should participate in cross-coupling reactions.
To implement this program, the first requirement was a general, high yielding synthesis of organosilanols. Fortunately, the enormous importance of organosilicon compounds in both polymer and synthetic organic chemistry21 provided satisfactory methods for the installation of the dimethylsilanol unit through: (1) addition of organometallic compounds to silicon electrophiles followed by simple or oxidative hydrolysis,22 (2) transition metal catalyzed hydrosilylation,23 and (3) silyl insertion,24 Scheme 9. Silanols are not well known as reagents in organic synthesis but they are air and water stable reagents that can be chromatographed on silica gel and distilled.25 They can undergo dimerization to disiloxanes with widely varying rates, but in the absence of acidic or basic catalysts are stable indefinitely. Moreover, the disiloxanes are equally competent in cross-couplings.
Scheme 9.
Fluoride-promoted cross-couplings of alkenylsilanols with aryl halides under catalysis by Pd(dba)2 are general, high yielding and stereospecific processes (Scheme 10).26 Even heteroatom-substituted alkenylsilanols give coupling products 18 and 19 readily at room temperature.26b
Scheme 10.
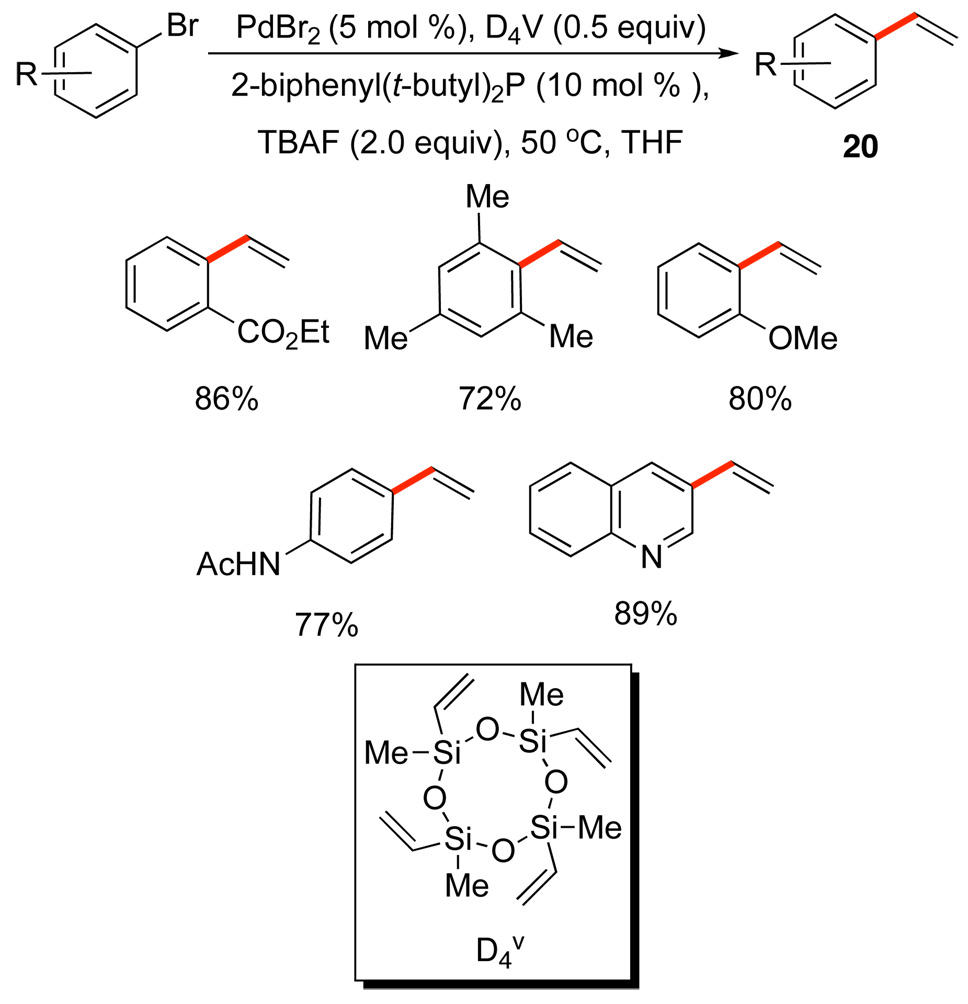
The success of these simple couplings stimulated the search for a silanol equivalent of the siletane vinylating agent reported previously. The direct synthesis of styrenes by cross-coupling provides direct access to these important building blocks and atom-efficient, economical methods are needed.27 Fortunately, one of the most inexpensive sources of vinylsilyl groups is D4 v 28 which is also is a highly effective vinylating agent of both iodides29a and bromides29b, Scheme 11. For the less reactive bromides, an electron rich ligand is needed to facilitate oxidative addition and an excess of D4 v (0.5 equiv) is needed to suppress a secondary Heck reaction of the products 20 to form stilbenes.
Scheme 11.
Tandem Reactions
One of the unique advantages of silicon-based activating groups for cross-coupling reactions is the ability to introduce fresh carbon-silicon bonds into organic substrates by a variety of selective processes. Some of these sequences can be executed as “one-pot” processes and some are better carried out after isolation of silicon-containing intermediates. For brevity, only a single example (of many reported) from each process will be illustrated, but together, the diversity of structural changes serves to emphasize the power of the tandem reactions.
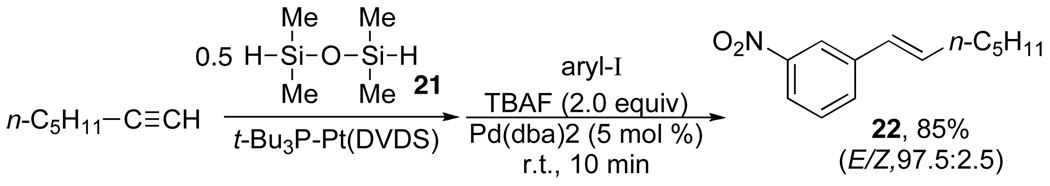
The simplest tandem reaction involves the combination of hydrosilylation of terminal alkynes and the cross-coupling of the resulting alkenylsilanols or equivalents, Scheme 12.30 The site and stereoselectivity of the process is controlled by the hydrosilylation step, which for our purposes was optimized using t-Bu3P•Pt(DVDS)31 and tetramethyldisiloxane, 21.
Scheme 12.
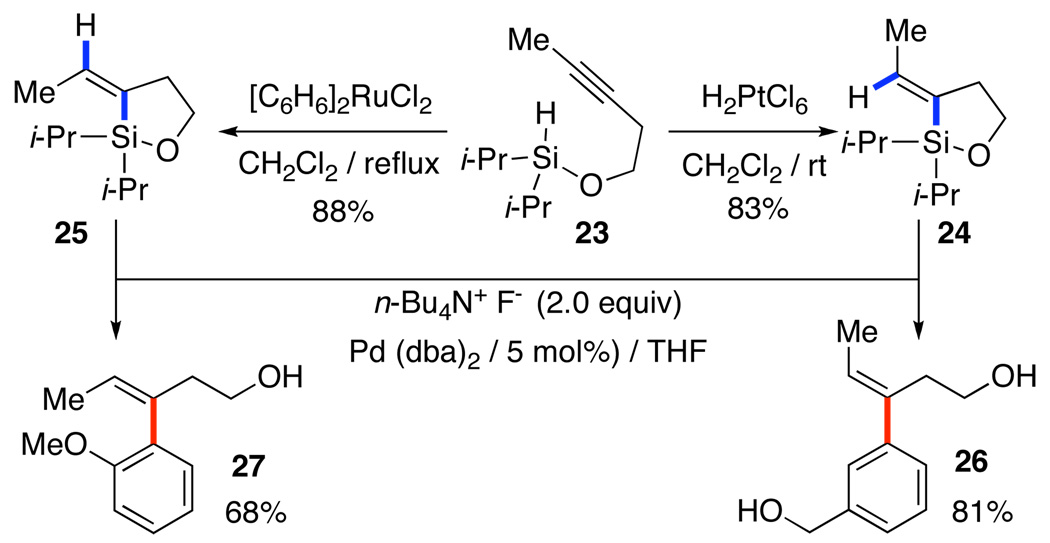
The tandem hydrosilylation/cross-coupling becomes more interesting when executed intramolecularly. A number of different variations on this theme have been developed that allow the stereocontrolled construction of homoallylic alcohols from silylated 3-pentynols, 23.32 Here again, the configuration of the double bond is controlled by the hydrosilylation step which can be done in either syn (Pt to 24) or anti (Ru to 25) addition pathways, Scheme 13.
Scheme 13.
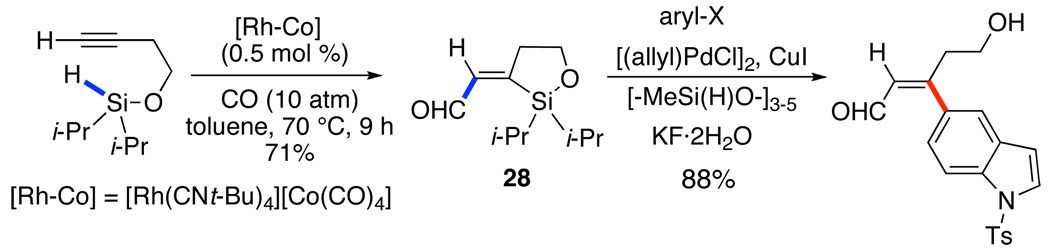
A slight modification of the reaction conditions elevates the intramolecular hydrosilylation to a silylformylation process that increases the molecular complexity of the product. The use of a more active catalyst under a mild pressure of CO allows the efficient incorporation of a formyl group in 28.33 Because siloxane 28 is highly deactivated by the enal, new conditions for the coupling were developed that suppressed protiodesilylation, Scheme 14.
Scheme 14.
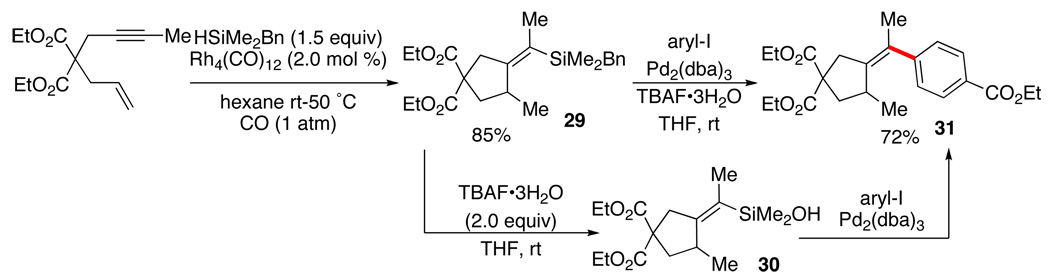
The versatility of the hydrosilylation reaction can be extended still further by the addition of the Si-H bond across multiple unsaturated units. Thus, the silylcarbocyclization reaction can create rings by joining the internal carbons of tethered dienes, enynes or diynes while functionalizing the terminal carbons.34 Once again, a stereodefined alkylidene unit is created that can serve as a locus for carbon-carbon bond formation. For this application, we employed benzyldimethylsilane (Scheme 15) secure in the knowledge that the benzyl group in 29 would suffer rapid protiodesilylation by TBAF•3H2O to 30 under the conditions for cross-coupling.35 In this way, 5-membered carbocycles and heterocycles could be prepared with a highly substituted exoarylidene group appended.36,37
Scheme 15.
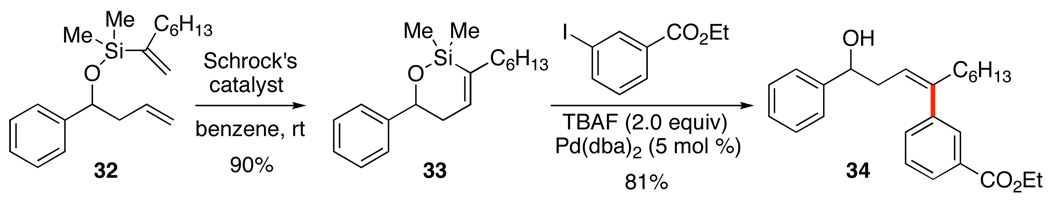
In the foregoing examples the alkene geometry is set as an exo alkylidene unit on the newly formed siloxane ring. An alternative approach that creates a defined cis geometry endocyclic to a siloxane ring takes advantage of the power of ring closing metathesis. Schrock’s catalyst efficiently closed unsaturated silyl ethers such as 32 to form 5-, 6- and 7-membered rings (Scheme 16).38a These stable siloxanes 33 underwent smooth cross-coupling with a variety of aryl and alkenyl halides to produce functionalized and geometrically defined products, 34.
Scheme 16.
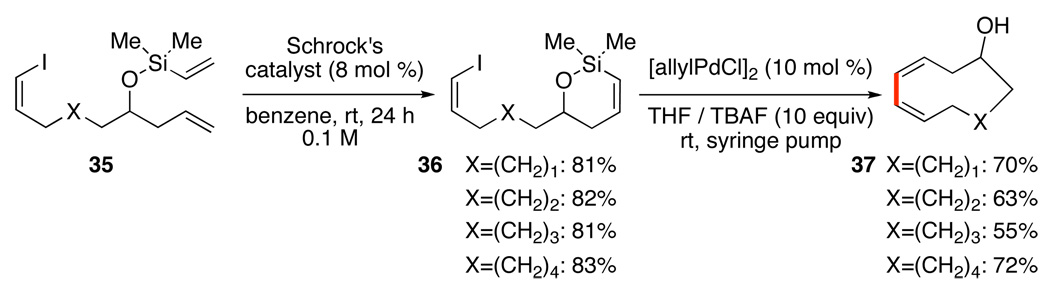
Extension of the tandem RCM/cross-coupling sequence to a double intramolecular mode using polyunsaturated precursors 35, allows construction of medium-sized rings containing a difficult-to-access cis-cis diene unit (Scheme 17).38b,c By this approach 9-, 10-, 11- and 12-membered rings containing the Z,Z-diene, 37, could be efficiently prepared from simple precursors. The relative position of the hydroxyl group is controlled by the size of the siloxane ring.
Scheme 17.
Although incomplete, this brief summary of the successful development of silanol-based cross-coupling should convince the reader that the scope, generality and efficiency of the process warrant consideration for application in the challenging arena of natural product total synthesis. Indeed, the siren call of this seductive activity is often impossible to resist when a newly developed method can enable the simplification of a complex molecular target.39
Application Stage I: The Method Comes of Age
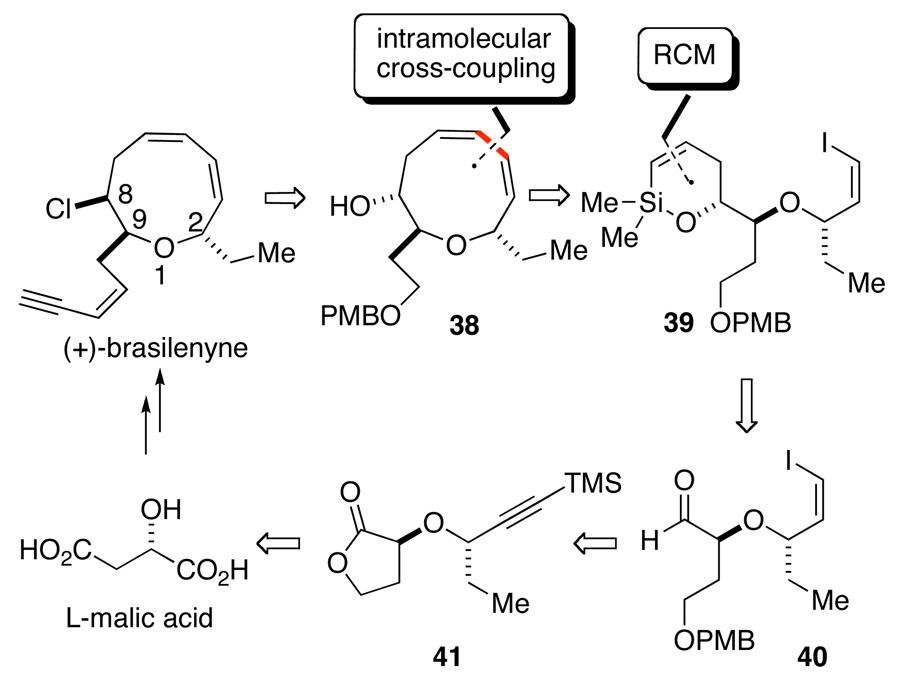
Among the various implementations of the silanol-based cross-coupling, the tandem RCM/cross-coupling process held a special fascination for its ability to construct compounds that are otherwise very difficult to prepare. Thus, a target molecule was identified whose synthesis would feature this construction as the key strategic maneuver. (+)-Brasilenyne, isolated from the digestive gland of a sea hare (Aplysia brasiliana) by Fenical et al. in 1979,40 has a novel nine-membered cyclic ether skeleton containing a 1,3–cis–cis diene unit. We recognized that (+)-brasilenyne would be an ideal target to illustrate this synthetic method because the coupling process is well suited to generate the oxonin core of brasilenyne. The synthetic plan introduces several challenges that require additional manipulations, most notably the side chain at C(9), the ethyl group at C(2) and the presence of the chlorine-bearing center at C(8). The retrosynthetic analysis in Scheme 18 illustrates how these components were to be assembled.41
Scheme 18.
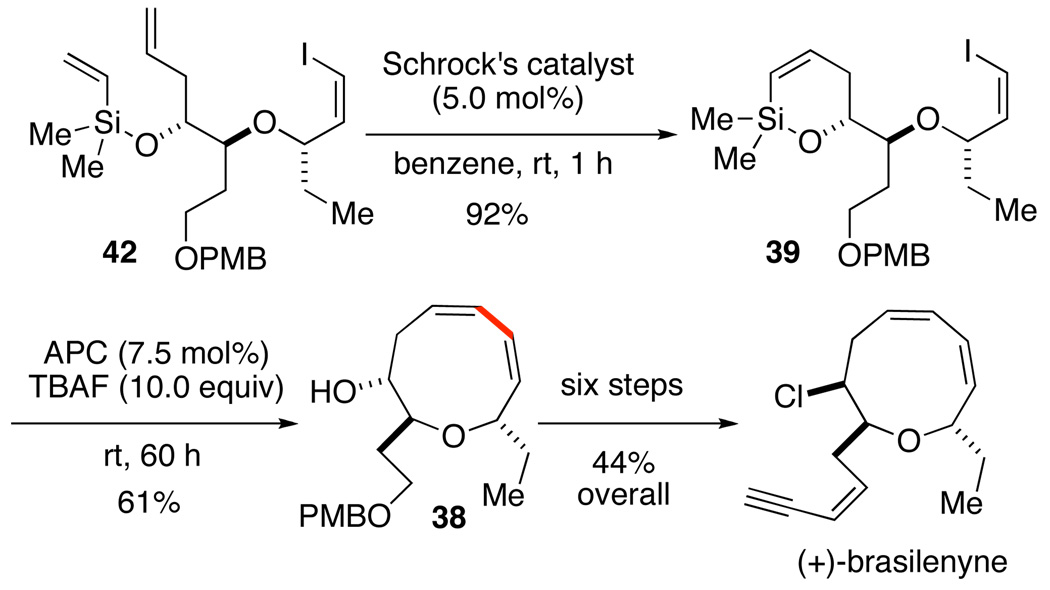
The details of the assembly of the various intermediates with high levels of stereocontrol have been described in detail elsewhere.41b The crucial application of the tandem RCM/cross-coupling process with advanced intermediates 42 and 39 proceeded smoothly to afford key intermediate 38 (Scheme 19). Routine manipulations (protection, enyne elaboration and hydroxyl inversion) afforded (+)-brasilenyne.
Scheme 19.
The total synthesis of (+)-brasilenyne successfully illustrated the suitability of the tandem RCM/cross-coupling sequence, but it also highlighted a serious limitation to the method, namely the continued use of TBAF to activate the cross-coupling event. Thus, the design of any complex molecule total synthesis must avoid the use of silicon protecting groups. Moreover, for anticipated larger scale applications, the need for the expensive and aggressive reagent would certainly discourage prospective users. These concerns stimulated a concerted effort to find a more practical and milder method of activating silanol cross-coupling reactions. Although the motivation for this next stage in the development process was purely of preparative origin, we were unaware of the fundamental mechanistic insights that lay waiting to be discovered.
Invention Stage II: Embracing the Hiyama-Hatanaka Paradigm
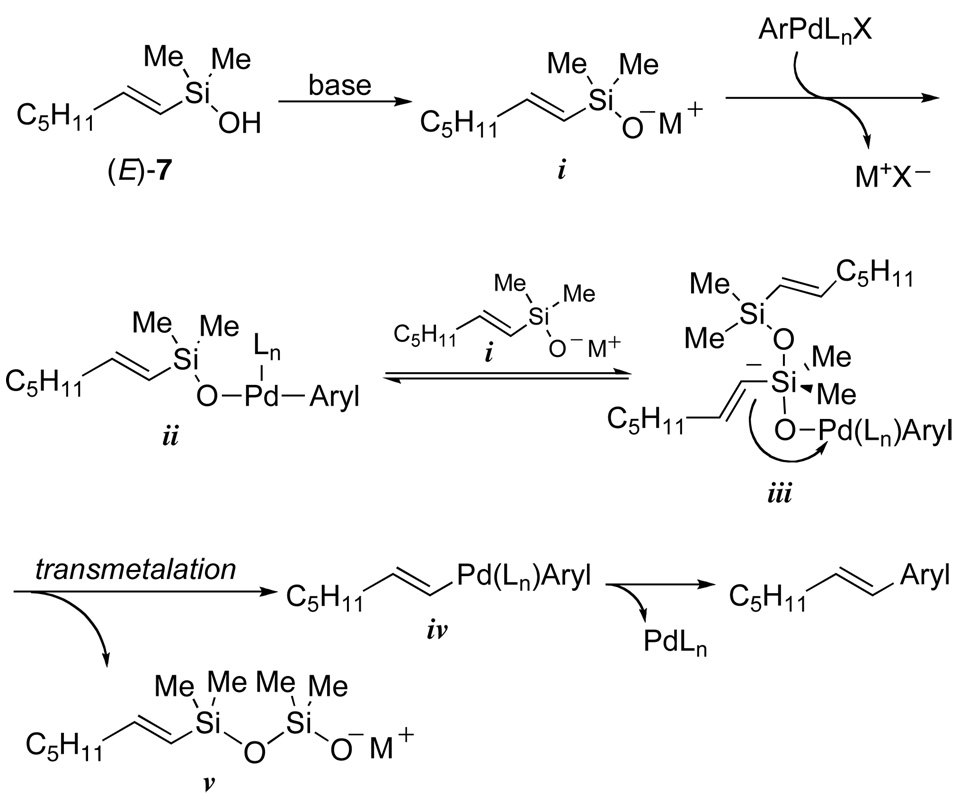
The development of non-fluoride-based cross-coupling reactions of organosilanes was a challenging prospect given the reigning dogma that a pentacoordinate siliconate was required to effect transmetalation to an organopalladium halide. How else can the Si-C bond be activated for transmetalation in the absence of fluoride? Initial hypotheses proposed that the conjugate base of silanol (E)-7 could serve this function in two ways (Figure 1): (1) the in-situ-generated silanolate (i) could form an organopalladium(II) silanolate complex (ii) by the displacement of the halide from the organopalladium(II) complex (ArylPdLnX), and (2) a second equiv of i could serve as the nucleophilic activator to form the desired pentacoordinate siliconate (iii). This species would undergo intramolecular transmetalation with simultaneous formation of polysiloxanes. In this proposal, the role of the second silanolate is analogous to the established role of fluoride in the preceding transformation.
Figure 1.
Early proposal for the mechanism of the fluoride-free cross-coupling of silanols.
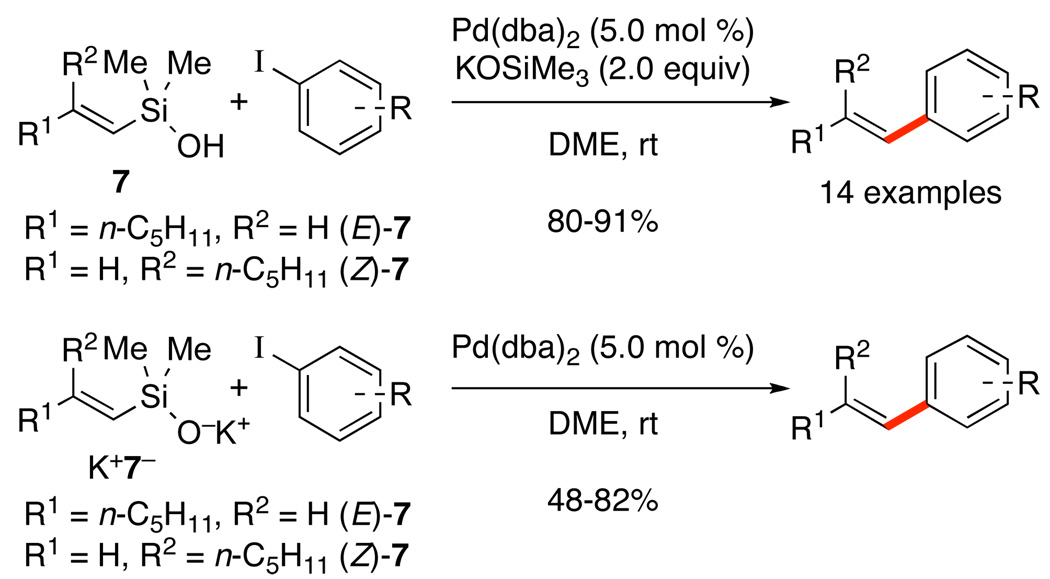
In the event, the use of potassium trimethylsilanolate (KOSiMe3) with (E)-7 and (Z)-7 or their independently prepared conjugate bases underwent clean cross-coupling at room temperature with a range of aryl iodides (Scheme 20).42 These early observations launched campaigns on both preparative and mechanistic fronts to map out the uncharted landscape. Although we thought that a simple solution to the “fluoride problem” had been found, we did not anticipate that this discovery would represent a new paradigm for silicon-based cross-coupling reactions.
Scheme 20.
Discovery Stage II: Continue to Question Your Assumptions
Once again we were faced with the self-affirming, successful demonstration of a reasonable hypothesis, in this case that non-fluoride bases could activate the cross-coupling of silanols. If our experience in general and this project in particular has taught us anything, it is never to become too enamored with your own hypotheses; always experiment fervently, and then listen carefully. The first cautionary message arrived in the observation that the Brønsted base-promoted reaction exhibited significantly greater rate dependence on the reaction conditions and on the steric and electronic properties of the silicon center.43 We felt it was not prudent to assume that the fluoride-free process operates by the same mechanism as the TBAF-promoted cross-coupling. 44 Accordingly, a full kinetic analysis was undertaken with the following objectives in mind: (1) determine the intermediacy of a tetracoordinate species (ii, Figure 1) (2) determine if ii undergoes anionic activation by a second equivalent of silanolate and, (3) isolate or spectroscopically characterize the catalyst resting state.
To test the validity of the proposed mechanism, the reaction order with respect to each component in the cross-coupling reaction of K+(E)-7− with 2-iodothiophene was determined. The experimentally derived rate equation is: 44
| (eq. 1) |
| (eq. 2) |
| (eq. 3) |
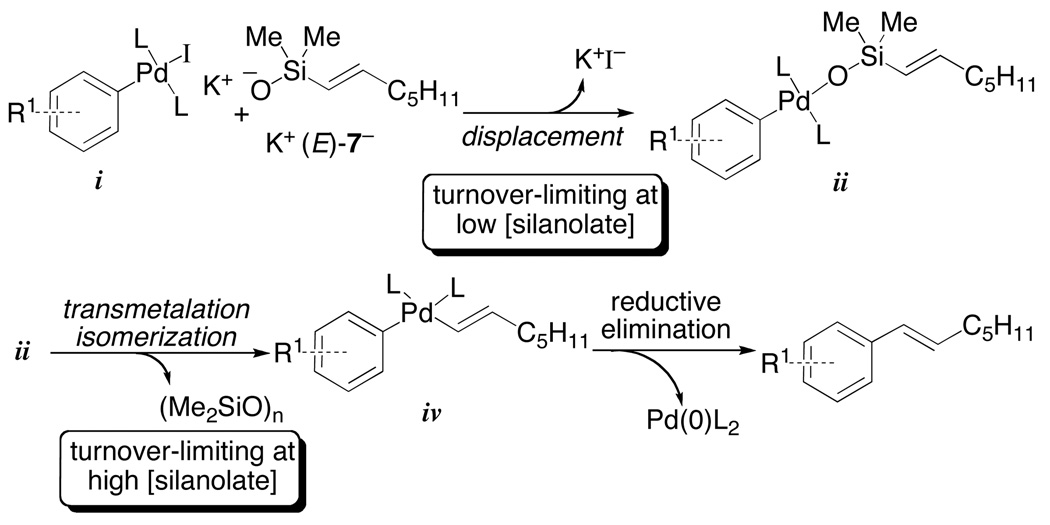
This equation is consistent with the saturation of a reactive intermediate prior to transmetalation, but it was not clear whether that species is ii or iii. These possibilities were distinguished by kinetic analysis using a superstoichiometric amount of palladium relative to K+(E)-4−. Under these conditions, a first-order dependence on silanolate was observed. These critical experiments revealed an inconsistency with our original proposal (Figure 1).44 If iii were a reactive intermediate prior to transmetalation a second order dependence on K+(E)-4− should be observed with a superstoichiometric loading of palladium and substoichiometric amount of K+(E)-4−. This result suggests that the transmetalation proceeds directly from an arylpalladium(II)silanolate complex ii. These data also imply that the first-order dependence on K+(E)-4− (under catalytic conditions) is consistent with a turnover-limiting bimolecular displacement of halide by K+(E)-4− (i to ii). Therefore, at high concentrations of K+(E)-4− intramolecular transmetalation from an arylpalladium(II) silanolate (ii to iv) becomes turnover limiting.
These results mandated a revision of the original mechanistic proposal to incorporate an intramolecular transmetalation from a neutral species (Figure 2, ii to iv), thus contradicting the dogma that silicon-based cross-coupling reactions require the generation of a pentacoordinate siliconate prior to transmetalation (Hiyama-Hatanaka Paradigm). Furthermore, these observations illustrate the critical importance of the Si-O-Pd linkage for this new transmetalation pathway. To confirm this new hypothesis, additional evidence was needed to support the intermediacy of ii by isolation and demonstration of its kinetic competence in the absence of additional silanolate. Unfortunately, the high reactivity of K+(E)-7− precluded the isolation of any intermediates.
Figure 2.
Revised mechanistic proposal for the fluoride-free cross-coupling.
The problem was addressed by making recourse to the palladium-catalyzed cross-coupling reactions of aryldimethylsilanolates, which are considerably slower compared to their alkenyl congeners. Kinetic analysis of the reaction of cesium silanolate (Cs+12−) with 2-bromotoluene in the presence of allylpalladium chloride dimer ([allylPdCl]2), at 100 °C established that the cross-coupling is in the same mechanistic regime as K+(E)-7− (equation 4).
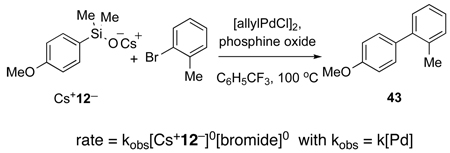 |
(4) |
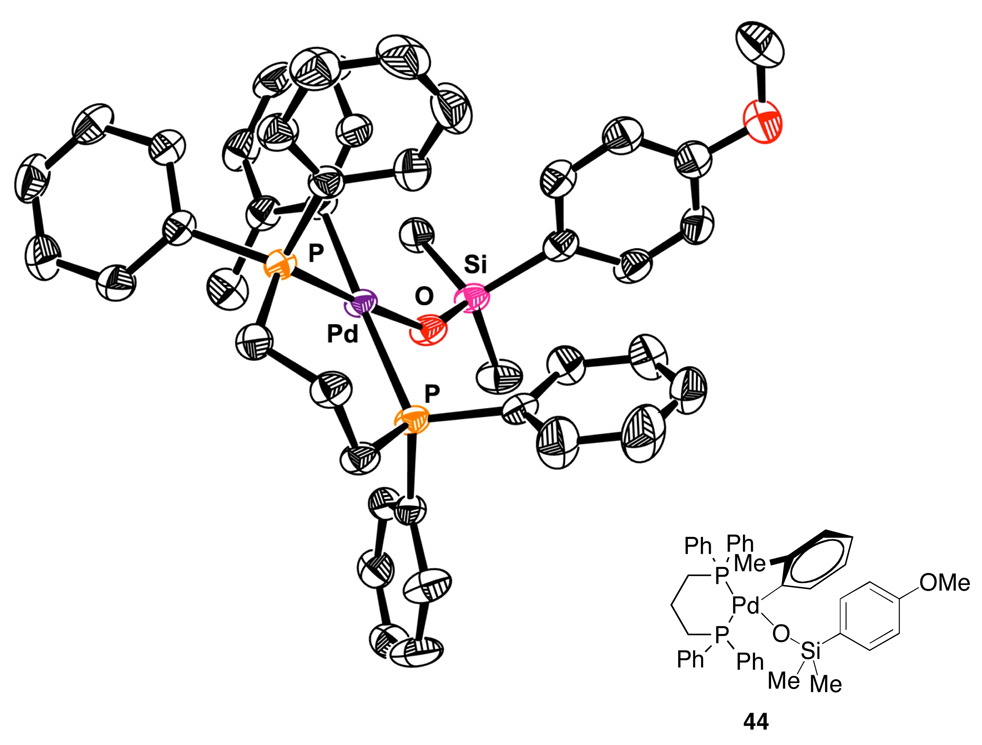
This rate equation is consistent with a turnover-limiting transmetalation of an arylpalladium(II) silanolate and is similar to the observed rate equation for the cross-coupling of K+(E)-7− (eq. 3). These data once again rule out activation by a second equivalent of silanolate to generate a pentacoordinate siliconate prior to transmetalation. Moreover, the key arylpalladium(II) silanolate intermediate 44 could be detected by 31P NMR spectroscopy in reaction mixtures. Finally, complex 44 was independently synthesized and fully characterized including single crystal X-ray analysis (Figure 3).45
Figure 3.
X-ray crystal structure of complex 44 (ORTEP drawing with thermal ellipsoids drawn at 50% probability level).
The isolation of 44 allowed for the demonstration that arylpalladium(II) silanolate complexes can undergo transmetalation in the absence of an activator. Heating 44 to 100 °C provides the biaryl product in quantitative yield. This result unambiguously demonstrates that a neutral arylpalladium(II) silanolate complex can undergo direct transmetalation to give a cross-coupled product.
The mechanistic studies forced a radical rethinking of how the fluoride-free cross-coupling reactions occur. Through a combination of kinetics, spectroscopy and synthesis, a new pathway was elucidated in which transmetalation occurs from a tetracoordinate species containing an Si-O-Pd linkage. This discovery led to the development of new silicon-based cross-coupling reactions and application in total synthesis.
Development Stage II: From Silanols to Silanolates
With the discovery that silanolate salts are the active agents in the fluoride free cross-coupling process, a number of different methods were developed to prepare the alkali metal salts. A wide variety of Brønsted bases have been implemented for the formation of silanolates including: KOSiMe3, Cs2CO3, NaOt-Bu, KOt-Bu, NaH, KH and NaHMDS. To date, four different modes of activation have been developed, three for in situ generation of the silanolate: (1) reversible deprotonation with the weaker bases, (2) irreversible deprotonation with the stronger bases, and (3) silanolate exchange isolation; and one for independent generation and isolation of the silanolate. The presentation of the methodological development will follow the structural variation in the silanol donor. The first two classes are those for which fluoride-promoted couplings are known, but are vastly improved in the form of silanolate couplings because of substrate scope and/or mildness of conditions. The remaining classes are those that have been enabled by the introduction of the silanolate coupling protocol.
Alkenylsilanolates
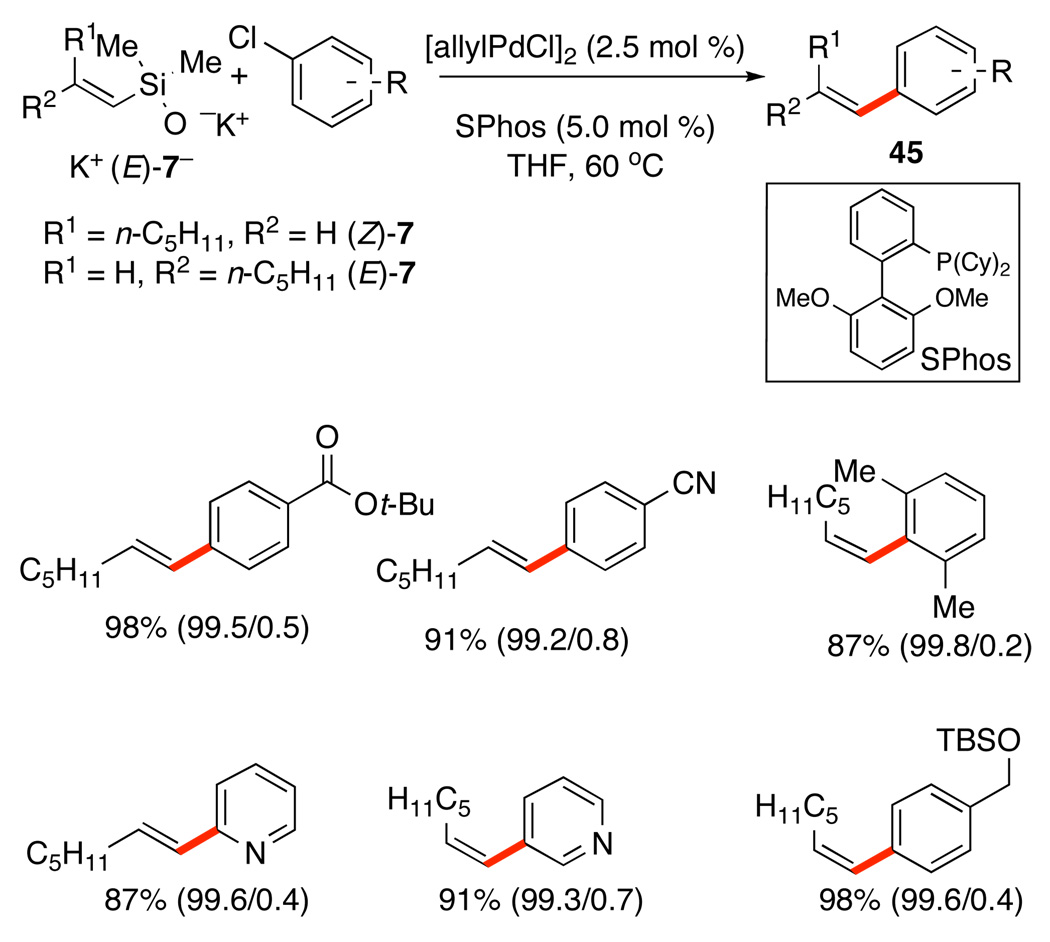
Three different methods of silanolate generation have been conscripted into service for this class of donors. The coupling of simple alkenylsilanols in the presence of KOTMS (reversible deprotonation) was the initial development and has been presented in Scheme 20.42 A second method (irreversible deprotonation with KH) is employed for the coupling of silanolates with aromatic chlorides. The use of in situ generated K+(E)-7−, [allylPdCl]2, and (SPhos) in THF at 60 °C provides excellent yield of 45 with a variety of aryl chlorides bearing nitrile, ester, nitro and ketone substituents.46 Furthermore, pyridines react smoothly, as do mono- and diortho-substituted substrates (Scheme 21). In all cases the cross-coupling reaction is highly stereospecific, even for K+(Z)-7− (ratios of the major/minor geometrical isomer in parentheses). The scope in silanolate also includes (E)- and (Z)-styrylsilanolates, and tri- and tetrasubstituted alkenylsilanolates with similar results.
Scheme 21.
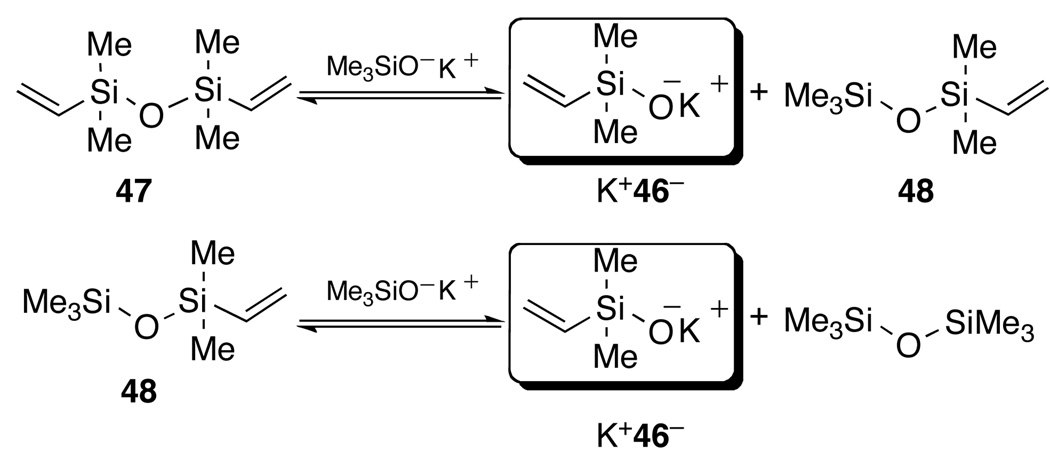
The special case of vinylation represents a significant challenge because of the propensity of dimethylvinylsilanol (46) to undergo spontaneous dehydrative dimerization to form divinyltetramethyldisiloxane (DVDS, 47). Although it should be possible to prepare the alkali metal salt of this delicate silanol, occasionally, one is well advised to embrace this kind of adversity and use nature as an ally. In this case, we chose to take advantage of the high thermodynamic stability of both DVDS and hexamethyldisiloxane to establish an equilibrium that would generate K+46− in situ as shown in Scheme 22. Since we know that KOTMS is not deleterious to the coupling reaction, only K+46− should participate.
Scheme 22.
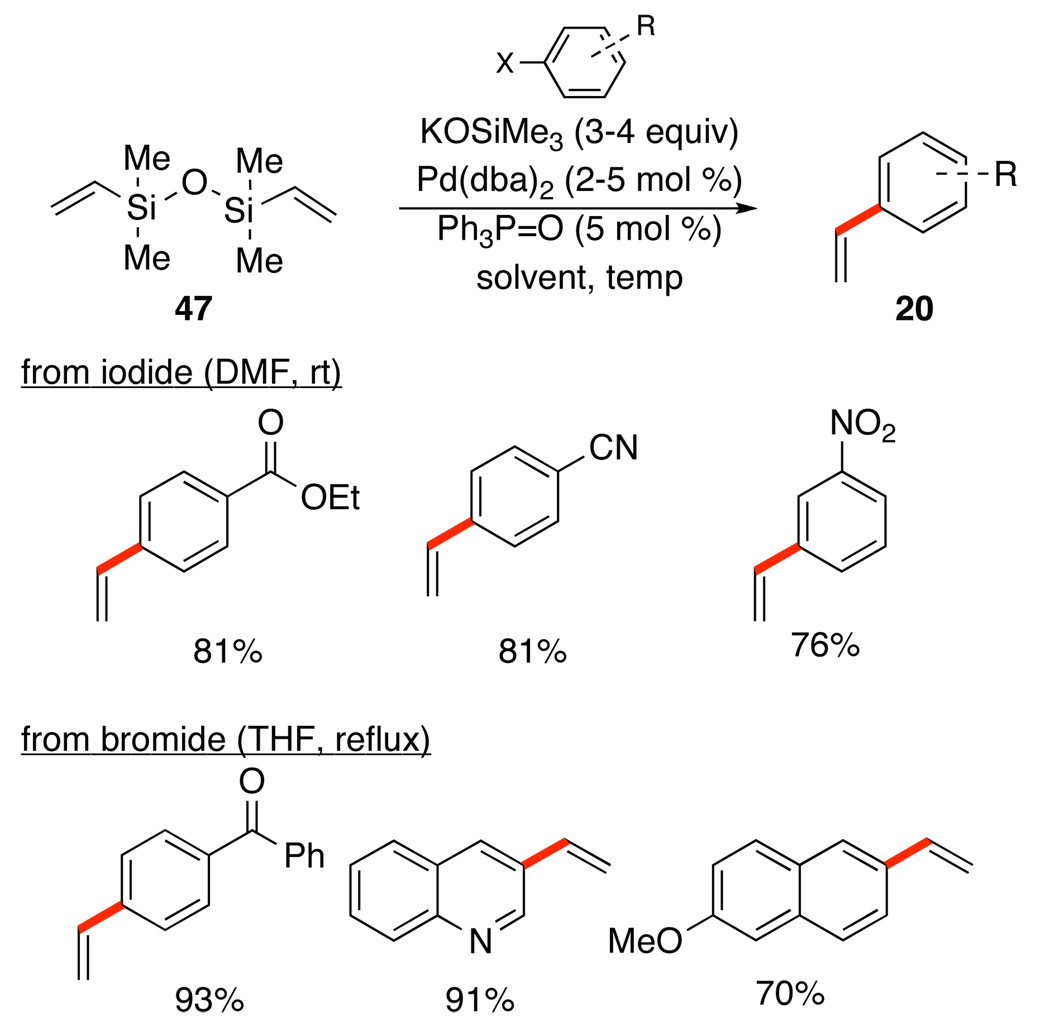
Gratifyingly, this strategy performed admirably for the vinylation of both aromatic iodides and bromides, Scheme 23.47 A broader range of bromides could be engaged by the use of 2-(biphenyl)di(t-Bu)2P and a slight modification of the reaction conditions.
Scheme 23.
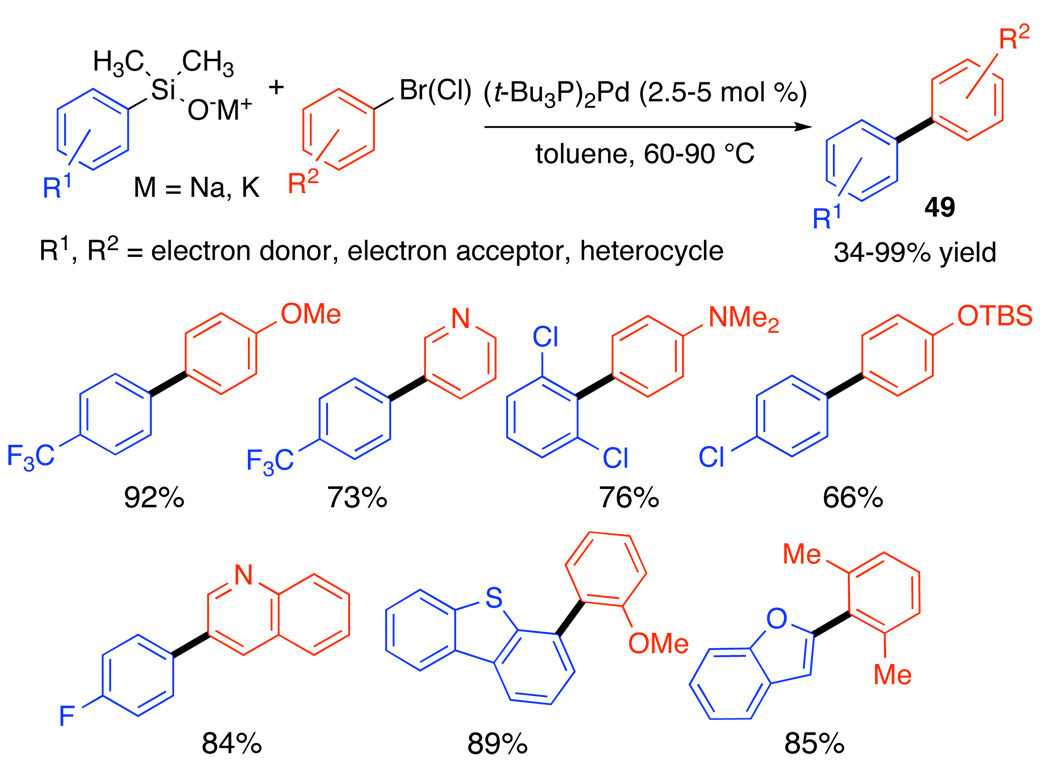
Arylsilanolates
This extremely important class of cross-coupling partners is conspicuously absent from the ranks of the successful silanol couplings previously described. Indeed, under fluoride activation, the arylsilanols give poor yields and much protiodesilylation. Even the silanolates generated by reversible deprotonation (Cs2CO3) do not have a significant substrate scope.48 Fortunately, two critical discoveries have dramatically expanded the scope of the aryl-aryl cross-coupling, namely, the use of isolated silanolate salts and the use of (t-Bu3P)2Pd (“Fu’s catalyst).49 The advantages of using the preformed, isolated salts include: (1) the reagents are isolable, free-flowing solids that are indefinitely stable in a moisture free environment (similar to sodium methoxide), (2) their physical properties facilitate handling, transportation, and storage, (3) they require no additional activation and (4) the anhydrous conditions avoid the problems of disiloxane formation and protiodesilylation. The new cross-coupling process displays significant scope in the electronic and steric demands of both donor and acceptor (including both bromides and iodides), Scheme 24.
Scheme 24.
Heterocyclic Silanolates
Five-membered (π-excessive) heterocycles are ubiquitous in pharmaceutical, materials and natural product chemistry and cross-coupling has taken on a heightened significance in the elaboration of their structures.50 Accordingly a significant effort has been invested in the synthesis and manipulation of heterocyclic silanolates. All three methods for generation of silanolates that involve Brønsted base deprotonation have been employed for this class of coupling partners.
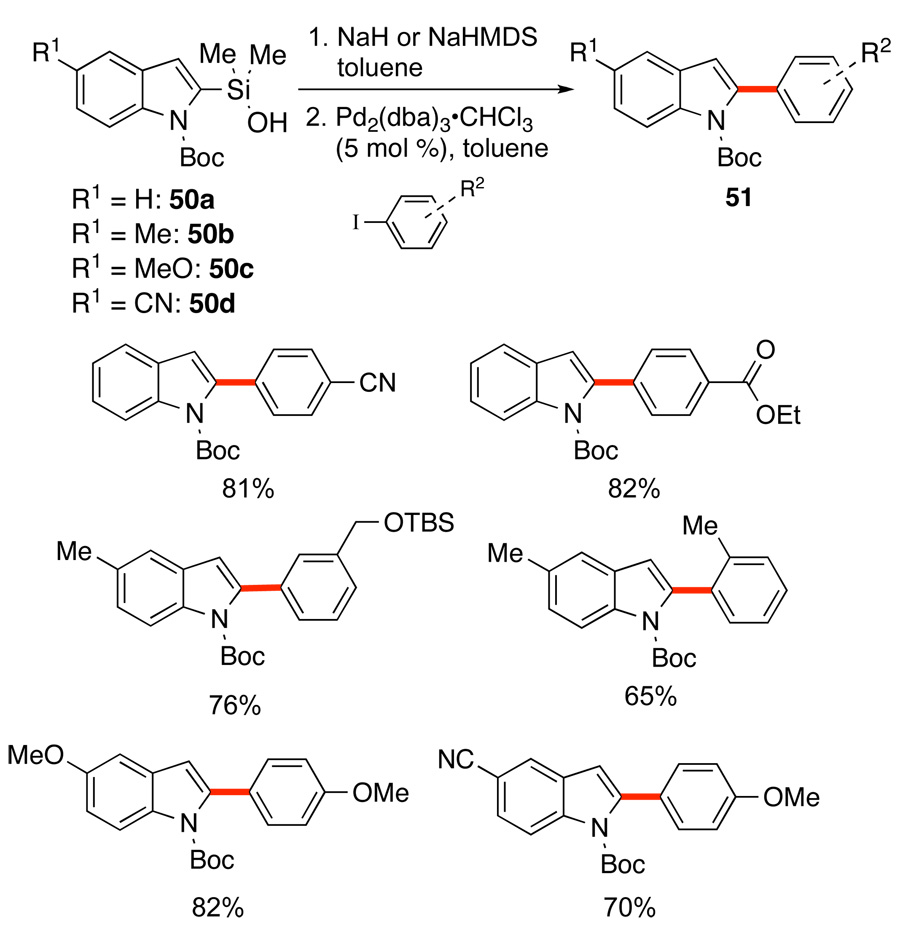
The greatest effort has been directed to the coupling of indoles and early studies demonstrated the ease of synthesis and stability of protected 2-indolyldimethylsilanols and their sodium salts. Reversible deprotonation of the silanols with NaOt-Bu gave satisfactory results with both aromatic bromides and iodides, but a stoichiometric amount of CuI was needed to suppress protiodesilylation.51 This limitation was removed by making recourse to the sodium silanolates generated in situ by irreversible deprotonation with NaH or NaHMDS, Scheme 25. The in-situ preparation of Na+50a− provides an active reagent for the cross-coupling of aryl iodides and extends the scope of the cross-coupling partners to aryl iodides containing esters and nitriles without the need for CuI. The preformation protocol could be extended to other silanolate precursors that are sensitive to protiodesilylation such as 50c and 50d.
Scheme 25.
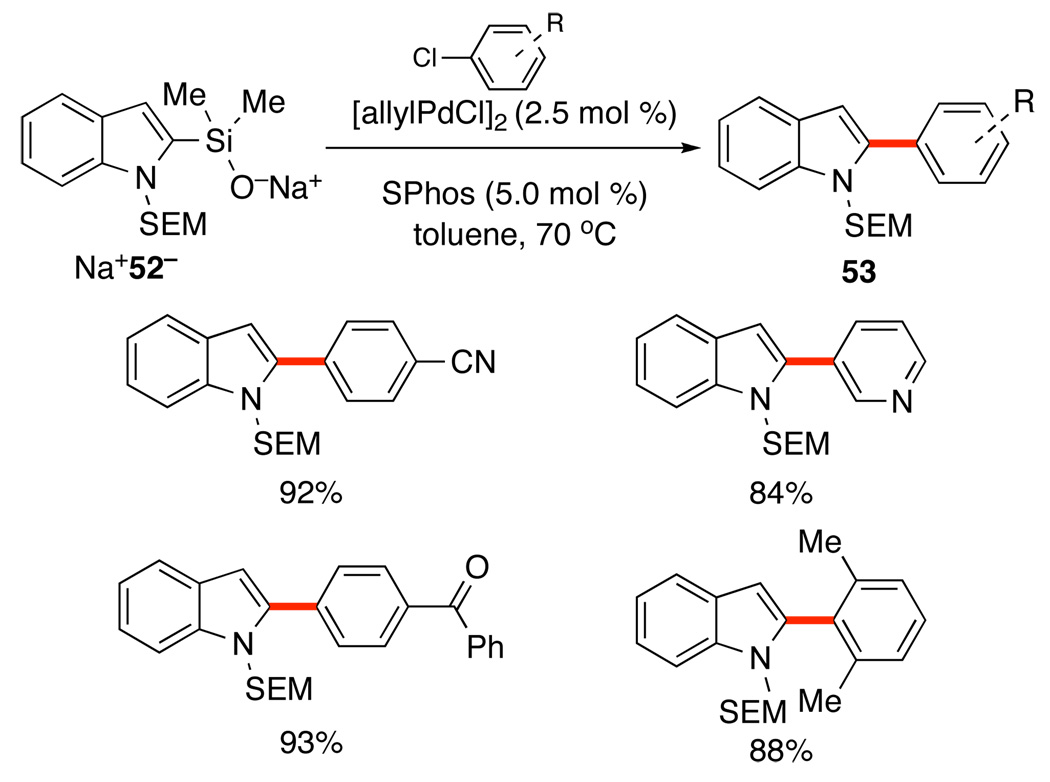
To facilitate the manipulation of (heteroaryl)dimethylsilanolates, their salts can be isolated from the irreversible deprotonation protocol. The salts are stable, storable, solids that can be charged directly into a reaction mixture and are competent nucleophiles for a broad range of organic halides. The sodium salt of N-SEM-dimethyl(2-indolyl)silanolate (52) is easily prepared following the irreversible deprotonation protocol using NaH. Removal of the solvent affords Na+52− as a colorless, free-flowing solid. This silanolate undergoes smooth cross-coupling with aryl chlorides (under conditions developed for K+7−) to provide excellent yields of 2-substituted indoles 53 with a broad substrate scope (Scheme 26). 51
Scheme 26.
Allylic Silanolates
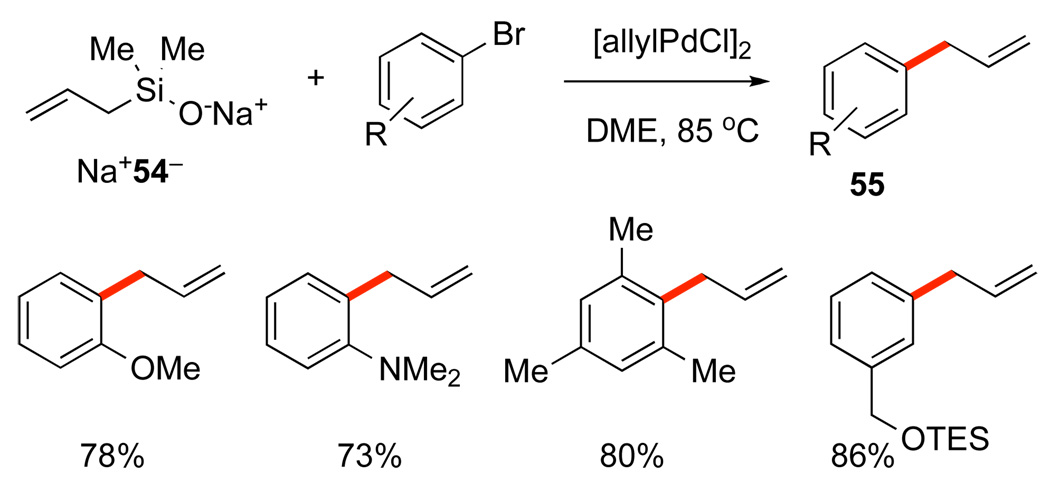
This new class of cross-coupling partners introduces another level of site- and stereoselectivity to the coupling process. Moreover, early attempts to induce fluoride-promoted coupling of allylsilanols lead exclusively to protiodesilylation. The sodium salts of both allyl- (54) and 2-butenylsilanols (56) are stable, free-flowing powders that effect cross-coupling with a wide range of aromatic bromides (Scheme 27 and Scheme 28).52 An important limitation in the allylation reaction is the isomerization of products derived from electron-deficient arenes to propenylated arenes. Notably, a TES protecting group survives the reaction conditions.
Scheme 27.
Scheme 28.
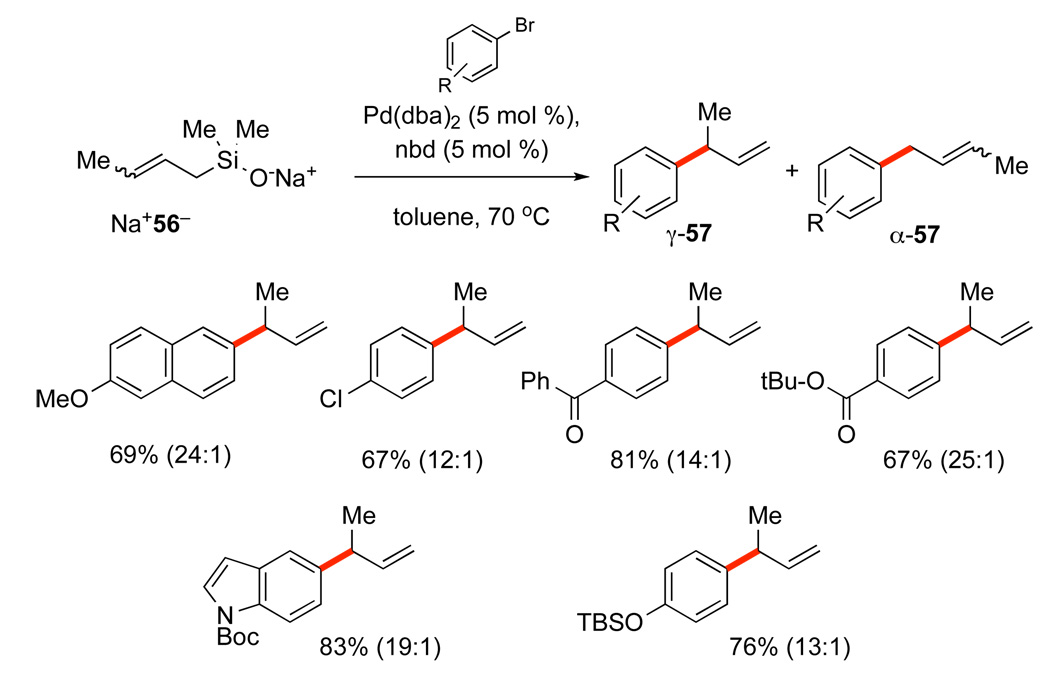
The “crotylation” of aromatic bromides with Na+56− required extensive optimization to maximize the formation of the desired γ-substitution product, γ-57 (Scheme 29). A remarkable effect of π-acidic ligands (dba and nbd) allows for preparatively useful selectivities and yields to be realized for a wide range of aromatic bromides bearing both electron-donating and electron-withdrawing substituents. The double bond configuration of Na+56− has only a modest effect on the yield and site selectivity of the process.52
Scheme 29.
Tandem Reactions
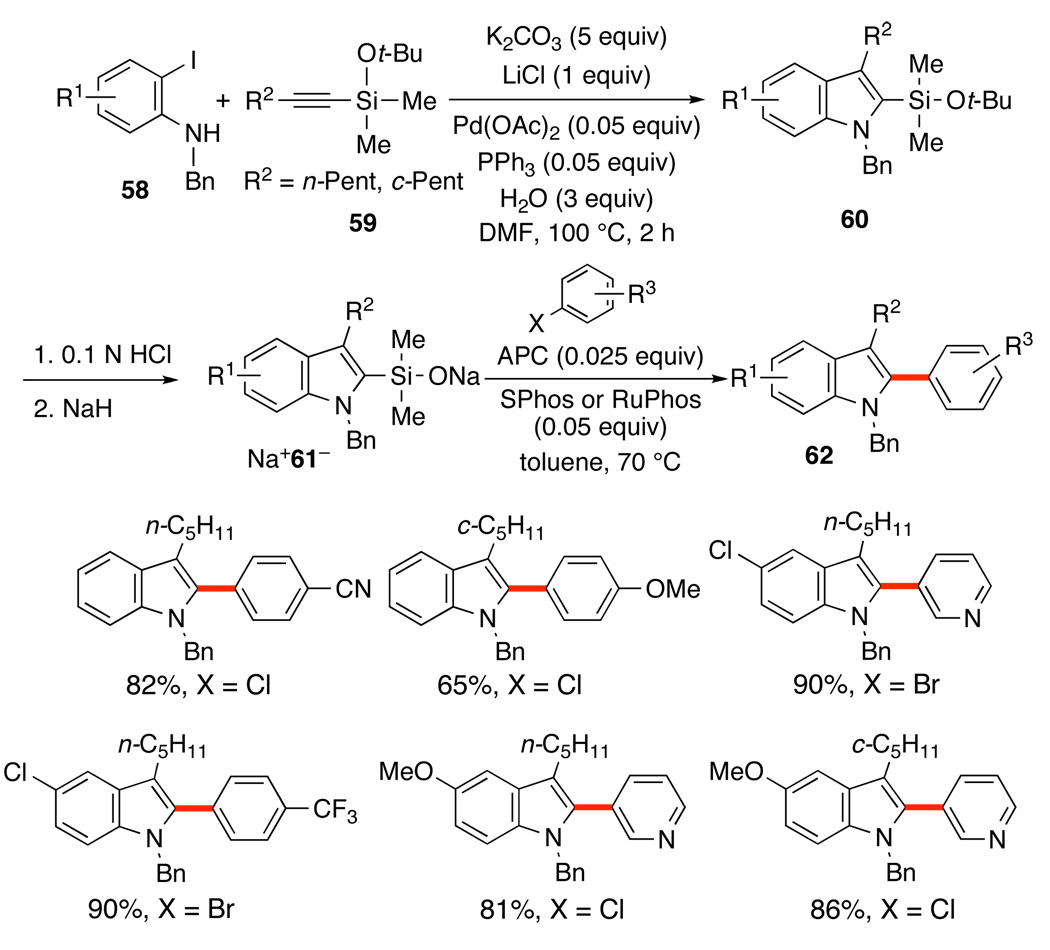
Because of the need for a free silanol, fewer tandem processes have been developed compared to the fluoride-activated variant. Nevertheless, one such tandem sequence has recently been reported that involves the combination of the Larock indole synthesis with a cross-coupling reaction, Scheme 29.53 The regioselectivity of the Larock heteroannulation is controlled by the size of the alkyne substituents. Thus, a bulky silyl ether in 59 always resides at the C(2) position of the newly created indole ring. After mild hydrolysis and sodium salt formation, the coupling reactions of Na+61− proceeds extremely well, even with aromatic chlorides. Overall the combination of these two reactions allows the controlled construction of 2,3-disubstituted indoles 62 from 2-iodoanilines 58 and silylated alkynes, 59.
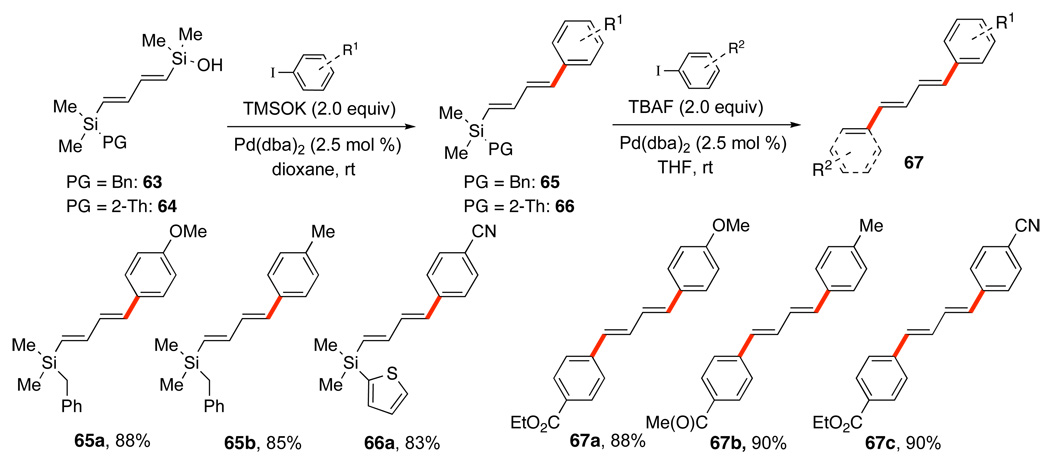
A second tandem process that involves two silicon-based cross-coupling reactions serves to create unsymmetrical polyenes rapidly and selectively. This process combines both fluoride-free and fluoride-promoted reactions for the sequential cross-coupling reaction of differently functionalized 1,4-bissilylbutadienes.54 One end of the 1,4-bissilylbutadiene bears a silanol that can easily be converted to the potassium silanolate with KOSiMe3. The other end bears a silanol surrogate (benzyl or 2-thienyl) that is revealed to the corresponding silanol upon treatment with TBAF.
Under KOSiMe3 activation, silanols 63 and 64 undergo rapid cross-coupling at ambient temperature with a wide range of aryl iodides provide the dienylsilanes 65 and 66 in good yields (Scheme 30). Under fluoride activation, the silanol is revealed from the benzyl- or 2-thienylsilane55 and the cross-coupling proceeds smoothly with a variety of aryl iodides in good yields. The 2-thienyl group in 66a is needed to suppress benzyl group migration in couplings of 65 in which R1 is an electron withdrawing group (e.g. nitrile, ester).35b The 2-thienyl group survives the initial Brønsted base-promoted cross-coupling and provides the desired coupling product, e.g. 67c, upon treatment with TBAF without the undesired migration.
Scheme 30.
Application Stage II: More Ambitious Targets
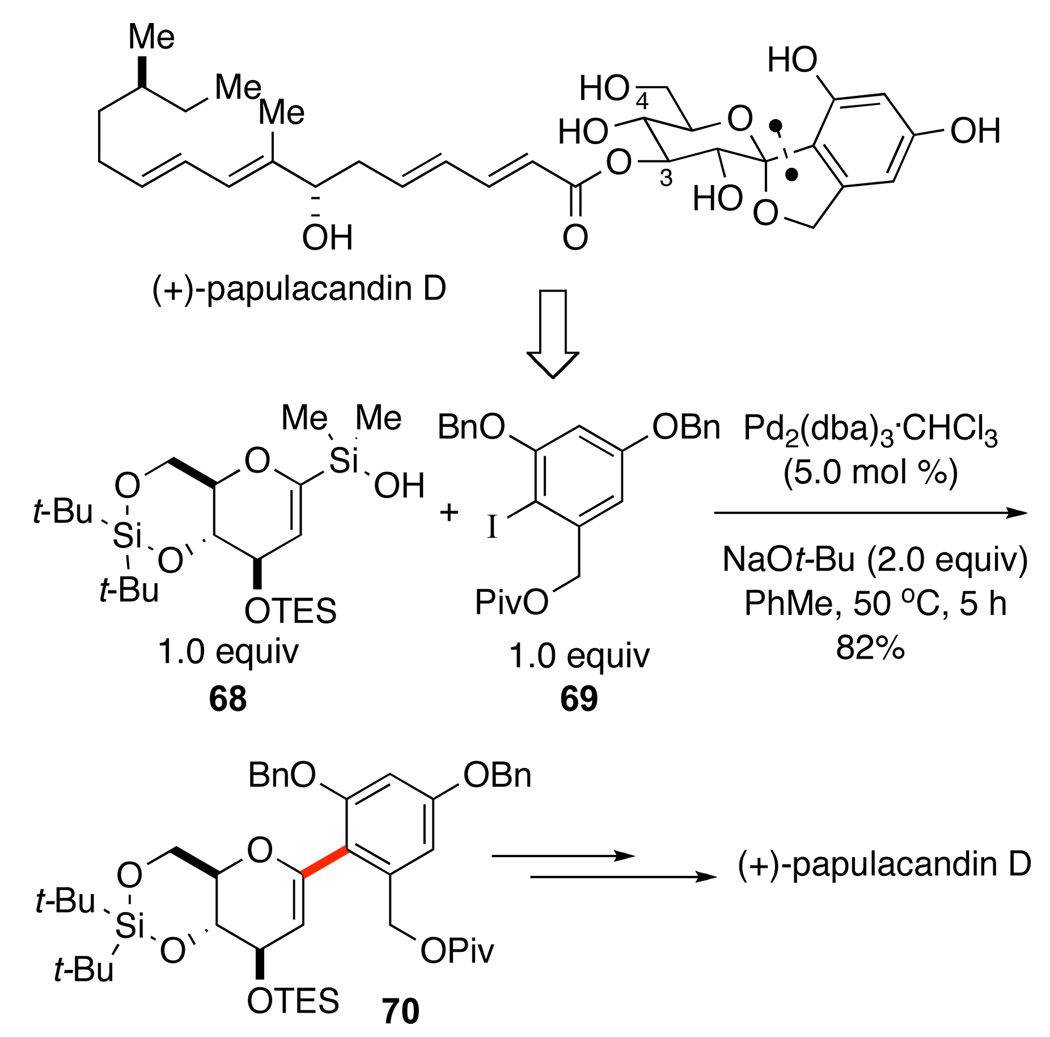
The use of silanolate cross-coupling reactions in target-oriented synthesis is intended to highlight the usefulness of the process where fluoride activation (and, indeed other cross-coupling modes) would most likely fail. All of these features were illustrated in the total synthesis of the antifungal agent, (+)-papulacandin D (Scheme 31).56 Retrosynthetic analysis identified the formation of the C-arylglycoside bond as a critical strategic disconnection in the synthesis. Previous studies had demonstrated the feasibility of cross-coupling of 2-pyranylsilanols (Scheme 10),26b but a fluoride promoted coupling was clearly incompatible with the silylated glucal intermediate 68. Moreover the desired cross-coupling reaction is very challenging because the aromatic iodide is both electron rich and 2,6-disubstituted. Fortunately, the use of NaOt-Bu (2.0 equiv) and Pd2(dba)3•CHCl3 (5 mol %) with an equal molar ratio of 68 and 69 in toluene at 50 °C provides the desired (1-aryl)hexenopyranose 70 in 82% yield. In this single transformation the entire carbon-skeleton of the spiroketal portion of (+)-papulacandin D was assembled. The mildness of this method is highlighted by the compatibility of the sodium silanolate of 68 with different silicon protecting groups.
Scheme 31.
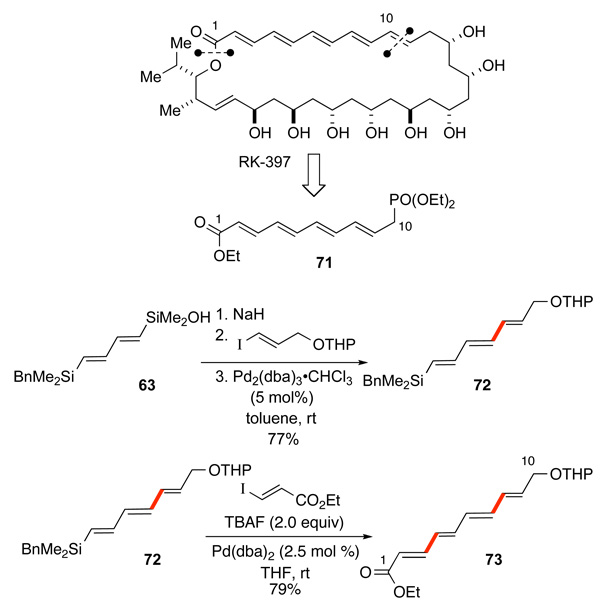
The synthetic utility of the tandem silicon-based cross-coupling process is illustrated in the total synthesis of the polyene macrolide RK-397.57 Retrosynthetic analysis revealed that the polyene chain building block, 71, could be rapidly constructed through the sequential cross-coupling of 63 (Scheme 32). The first cross-coupling reaction employs the in situ generated salt Na+63− which reacts smoothly with the protected 3-iodopropenol to afford 72 in 77% yield. The second cross-coupling reaction requires in situ unmasking of the benzylsilane in 72 with TBAF•3H2O which also promotes the coupling of the generated silanol with ethyl (E)-iodopropenoate to provide the tetraene 71 in 79% yield. Standard functional group manipulations provided the phosphonate building block 71.
Scheme 32.
Epilog
This Perspective was written to serve two purposes, one chemical and one philosophical. The chemical purpose is fulfilled if the reader has gained an appreciation of the scope, utility and practical advantages of silicon-based cross-coupling reactions, particularly those that employ silanolate salts. The philosophical purpose is to illustrate, by way of a real life example, how a program of organic synthetic methodology can be conceived and executed. Obviously, many different approaches to this important activity are viable and hopefully this forum will allow those alternative views to be described in detail. Nevertheless, it is hoped that this latter, overarching purpose has also been fulfilled, and that new investigators embarking on their own journey will find that the case for creative invention of new chemical reactions provides the “excuses”1b they need that will lead them to new and exciting discoveries.
Acknowledgements
We are pleased to acknowledge the experimental and intellectual contributions of many outstanding co-workers over the years in the cross-coupling subgroup: Aaron Bailey, John Baird, Christopher Butler, Timothy Chang, Jun Young Choi, Matthew Christy, Christophe Eggertswyler, Jack Liu, Jeff Kallemeyn, Tetsuya Kobayashi, Joseck Muhuhi, Luc Neuville, Michael Ober, Weitao Pan, Christopher Regens, Russell Smith, Ramzi Sweis, Steve Tymonko, Nathan Werner, Zhigang Wang, Daniel Wehrli, Zhicai Wu, and Shih-Ming Yang, without whose dedicated efforts this story could never have been told. Funding for this research was provided by the National Institutes of Health (GM63167), Boehringer-Ingelheim, Johnson & Johnson, Merck, Aldrich and Johnson-Matthey.
References
- 1.Woodward RB, Cava MP, Ollis WD, Hunger A, Daeniker HU, Schenker K. Tetrahedron. 1963;19:247–288. [Google Scholar]; (b) Readers are directed to footnote § on page 248 of ref. 1a.
- 2.In my opinion, there is a problem that is central to organic chemistry alone and in `which biologists cannot help us. We all agree….that the emphasis in synthetic research is shifting toward the synthesis of properties, and not just compounds. Eschenmoser A. In: Chemical Synthesis, Gnosis to Prognosis. Chatgilialoglu C, Snieckus V, editors. Dodrecht: Kluwer; 1996. p. 231.
- 3.Those readers interested in the introduction of new concepts are directed to a recent, comprehensive account. Denmark SE, Beutner GL. Angew. Chem., Int. Ed. 2008;47:1560–1638. doi: 10.1002/anie.200604943.
- 4.This Perspective borrows heavily from previous reviews and accounts. We have taken some literary license to bring them together with a more pedagogical flavor to allow the overall methodological process to suffuse through the chemical details. Denmark SE, Sweis RF. Chem. Pharm. Bull. 2002;50:1531–1541. doi: 10.1248/cpb.50.1531. Denmark SE, Sweis RF. Acc. Chem. Res. 2002;35:835–846. doi: 10.1021/ar020001r. Denmark SE, Ober MH. Aldrichimica Acta. 2003;36:75–85. Denmark SE, Baird JD. Chem. Eur. J. 2006;12:4954–4963. doi: 10.1002/chem.200600034. Denmark SE, Regens CR. Acc. Chem. Res. 2008;41:1486–1499. doi: 10.1021/ar800037p.
- 5.Lewis GN. Valence and The Structure of Atoms and Molecules. New York: Chemical Catalog Co., Inc; 1923. [Google Scholar]
- 6.(a) Perozzi EF, Michalak RS, Figuly GD, Stevenson WH, Dess DB, Ross MR, Martin JC. J. Org. Chem. 1980;45:1049. [Google Scholar]; (b) Stevenson WH, Wilson S, Martin JC, Farnham WB. J. Am. Chem. Soc. 1985;107:6430. [Google Scholar]; (c) Stevenson WH, Martin JC. J. Am. Chem. Soc. 1985;107:6352. [Google Scholar]
- 7.Mikhailov BM, Bubnov YuN. Organoboron Compounds in Organic Synthesis. London: Harwood; 1984. pp. 645–668. [Google Scholar]
- 8.Denmark SE, Jacobs RT, Dai-Ho G, Wilson S. Organometallics. 1990;9:3015–3019. [Google Scholar]
- 9.(a) Denmark SE, Griedel BD, Coe DM, Schnute ME. J. Am. Chem. Soc. 1994;116:7026–7043. [Google Scholar]; (b) Denmark SE, Griedel BD. J. Org. Chem. 1994;59:5136–5138. [Google Scholar]
- 10.See also Myers AG, Widdowson KL. J. Am. Chem. Soc. 1990;112:9672. Myers AG, Widdowson KL, Kukkola PJ. J. Am. Chem. Soc. 1992;114:2765.
- 11.Diederich F, Stang PJ, editors. Metal-Catalyzed Cross-Coupling Reactions. Weinheim: Wiley-VCH; 1998. [Google Scholar]
- 12.Hiyama T. Chapter 10. In: Diederich F, Stang PJ, editors. Metal Catalyzed Cross-Coupling Reactions. Weinhein: Wiley-VCH; 1998. [Google Scholar]
- 13.To facilitate discussion and to acknowledge their pioneering contributions, we have dubbed this insight, the “Hiyama-Hatanaka Paradigm”.
- 14.Denmark SE, Choi JY. J. Am. Chem. Soc. 1999;121:5821–5822. [Google Scholar]
- 15.Denmark SE, Wang Z. Synthesis. 2000:999–1003. [Google Scholar]
- 16.Denmark SE, Wu Z. Org. Lett. 1999;1:1495–1498. [Google Scholar]
- 17.An important cautionary note to all young investigators was provided to the author on the occasion of his first group meeting on Oct 1975: “the more unexpected and surprising the result, the more certain you must be of its validity,” A. Eschenmoser. Quoted with the permission of A. E.
- 18.Denmark SE, Wehrli D, Choi JY. Org. Lett. 2000;2:2491–2494. doi: 10.1021/ol006170y. [DOI] [PubMed] [Google Scholar]
- 19.Martin JS, Fujiwara FY. Can. J. Chem. 1971;49:3071–3073. [Google Scholar]
- 20.Denmark SE, Sweis RF, Wehrli D. J. Am. Chem. Soc. 2004;126:4865–4875. doi: 10.1021/ja037234d. [DOI] [PubMed] [Google Scholar]
- 21.Brook MA. Silicon in Organic, Organometallic, and Polymer Chemistry. New York: John Wiley; 2000. and referenced cited therein. [Google Scholar]
- 22.(a) Denmark SE, Tymonko SA. J. Org. Chem. 2003;70:9151–9154. doi: 10.1021/jo0351771. [DOI] [PubMed] [Google Scholar]; (b) Lee M, Ko S, Chang S. J. Am. Chem. Soc. 2000;122:12011–12012. [Google Scholar]
- 23.Ojima I, Li Z, Zhu J. In: The chemistry of organic silicon compounds. Rappoport Z, Apeloig Y, editors. Vol. 2. Great Britain: John Wiley & Sons; 1998. pp. 1687–1792. [Google Scholar]
- 24.Denmark SE, Kallemeyn JM. Org. Lett. 2003;5:3483–3486. doi: 10.1021/ol035288m. [DOI] [PubMed] [Google Scholar]
- 25.Lickiss PD. Adv. Inorg. Chem. 1995;42:147–262. [Google Scholar]
- 26.(a) Denmark SE, Wehrli D. Org. Lett. 2000;2:565–568. doi: 10.1021/ol005565e. [DOI] [PubMed] [Google Scholar]; (b) Denmark SE, Neuville L. Org. Lett. 2000;2:3221–3224. doi: 10.1021/ol0064112. [DOI] [PubMed] [Google Scholar]
- 27.Denmark SE, Butler CR. Chem. Commun. 2009:20–33. doi: 10.1039/b809676g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.2,4,6,8-Tetraethenyl-2,4,6,8-tetramethylcyclotetrasiloxane. Denmark SE, Butler CR. e-EROS Encyclopedia of Reagents for Organic Synthesis. 2007
- 29.(a) Denmark SE, Wang Z. J. Organomet. Chem. 2001;624:372–375. [Google Scholar]; (b) Denmark SE, Butler CR. Org. Lett. 2006;8:63–66. doi: 10.1021/ol052517r. [DOI] [PubMed] [Google Scholar]
- 30.(a) Denmark SE, Wang Z. Org. Lett. 2001;3:1073–1076. doi: 10.1021/ol0156751. [DOI] [PubMed] [Google Scholar]; (b) Denmark SE, Wang Z. Org. Synth. 2004;81:54–62. [Google Scholar]
- 31.DVDS = divinyldimethyldisiloxane.
- 32.(a) Denmark SE, Pan W. Org. Lett. 2001;3:61–64. doi: 10.1021/ol006769y. [DOI] [PubMed] [Google Scholar]; (b) Denmark SE, Pan W. Org. Lett. 2002;4:4163–4166. doi: 10.1021/ol026933c. [DOI] [PubMed] [Google Scholar]; (c) Denmark SE, Pan W. Org. Lett. 2003;5:1119–1122. doi: 10.1021/ol0342002. [DOI] [PubMed] [Google Scholar]
- 33.Denmark SE, Kobayashi T. J. Org. Chem. 2003;68:5153–5159. doi: 10.1021/jo034064e. [DOI] [PubMed] [Google Scholar]
- 34.(a) Varchi G, Ojima I. Cur. Org. Chem. 2006;10:1341–1362. [Google Scholar]; (b) Fujiwara M, Ojima I. Chapter 7. In: Evans PA, editor. Modern Rhodium-Catalyzed Organic Reactions. Weinheim: Wiley-VCH; 2005. [Google Scholar]
- 35.(a) Trost BM, Machacek MR, Ball Z. Org. Lett. 2003;5:1895–1898. doi: 10.1021/ol034463w. [DOI] [PubMed] [Google Scholar]; (b) Trost BM, Ball ZT. J. Am. Chem. Soc. 2004;126:13942–13944. doi: 10.1021/ja045971j. [DOI] [PubMed] [Google Scholar]
- 36.Denmark SE, Liu JH-C. J. Am. Chem. Soc. 2007;129:3737–3744. doi: 10.1021/ja067854p. [DOI] [PubMed] [Google Scholar]
- 37.This process also can be executed to terminate with a formylation step if the pressure of CO is raised and the conditions suitably adjusted.
- 38.(a) Denmark SE, Yang S-M. Org. Lett. 2001;3:1749–1752. doi: 10.1021/ol015950j. [DOI] [PubMed] [Google Scholar]; (b) Denmark SE, Yang S-M. J. Am. Chem. Soc. 2002;124:2102–2103. doi: 10.1021/ja0178158. [DOI] [PubMed] [Google Scholar]; (c) Denmark SE, Yang S-M. Tetrahedron. 2004;60:9695–9708. [Google Scholar]
- 39.It is worth a moment’s pause to consider the historical significance of this passage. The triumphs of the Woodwardian era (well past his death through the second half of the 20th century), i.e. the conquest of natural products of greater and greater molecular complexity created the credo that the ultimate purpose of any new synthetic method is to serve the greater objective of improving the ability of the master practitioner to take on these Herculean tasks. This attitude gave rise to the oft-leveled criticism that a synthetic method has not been thoroughly vetted until it is applied in the more rigorous arena of total synthesis. While we acknowledge that executing a new transformation on benzaldehyde is less challenging than on an advanced synthetic intermediate adorned with e.g. sensitive functional groups and labile stereocenters, we argue that the constellation of pitfalls provided by a natural product structure is arbitrary. The seemingly limitless bounty of organic chemical structures produced in nature has provided different generations of chemists ample motivation to expand their knowledge of structure, (bio)synthesis, and of course function. Thus, the edifice of knowledge in organic chemistry is inextricably linked with natural products. However, if we accept Berthelot’s premise that "La Chimie crée son objet. Cette faculté créatrice, semblable à celle de l’art lui-même, la distingue essentiellement des sciences naturelles et historiques" – "Chemistry creates its object. This creative capability, resembling that of art itself, distinguishes it essentially from the natural and historical sciences – " then we submit that the challenge to all chemists and methodologist in particular transcends the molecules created in nature. We further submit that newly introduced methods should adhere to a set of guidelines that enable a potential use of the method to gauge the likelihood that the method will be applicable for their particular need. The criteria include, but are not limited to: (1) demonstration of broad scope in substrate structure, (2) illustration of response to steric and electronic perturbations, (3) demonstration of compatibility with common functional groups, (4) demonstration of robustness of conditions, i.e. if the method can operate under a wide range of solvents and temperatures, (5) demonstration of scalability to at least pass the mmol threshold, (6) an understanding of the origin of stereocontrol if applicable and (7) a working mechanistic hypothesis based minimally on sound physical organic principles and ideally on experimental data including kinetics, reactive intermediates and computational analysis. Berthelot, M. "La synthèse chimique", Librairie Germer Baillière et Cie, 1876.
- 40.(a) Kinnel RB, Dieter RK, Meinwald J, Engen DV, Clardy J, Eisner T, Stallard MO, Fenical W. Proc. Natl. Acad. Sci. USA. 1979;76:3576. doi: 10.1073/pnas.76.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fenical W, Sleeper HL, Paul VJ, Stallard MO, Sun HH. Pure and Appl. Chem. 1979;51:1865. [Google Scholar]
- 41.(a) Denmark SE, Yang S-M. J. Am. Chem. Soc. 2002;124:15196. doi: 10.1021/ja028936q. [DOI] [PubMed] [Google Scholar]; (b) Denmark SE, Yang S-M. J. Am. Chem. Soc. 2004;126:12432. doi: 10.1021/ja0466863. [DOI] [PubMed] [Google Scholar]; (c) Denmark SE, Yang S-M. Chapt. 4. In: Harmata MA, editor. Strategies and Tactics in Organic Synthesis. Amsterdam: Elsevier; 2005. [Google Scholar]
- 42.Denmark SE, Sweis RF. J. Am. Chem. Soc. 2001;123:6439–6440. doi: 10.1021/ja016021q. [DOI] [PubMed] [Google Scholar]
- 43.Denmark SE, Neuville L, Christy MEL, Tymonko SA. J. Org. Chem. 2006;71:8500–8509. doi: 10.1021/jo061481t. [DOI] [PubMed] [Google Scholar]
- 44.Denmark SE, Sweis RF. J. Am. Chem. Soc. 2004;126:4876–4882. doi: 10.1021/ja0372356. [DOI] [PubMed] [Google Scholar]
- 45.Denmark SE, Tymonko SA, Smith RC, Ober MH. Manuscript submitted. [Google Scholar]; (b) The crystallographic coordinates of 44 have been deposited with the Cambridge Crystallographic Data Centre; deposition no. 645665. These data can be obtained free of charge via from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; via www.ccdc.cam.ac.uk/conts/retrieving.html or deposit@ccdc.cam.ac.uk).
- 46.Denmark SE, Kallemeyn JM. J. Am. Chem. Soc. 2006;128:15958–15959. doi: 10.1021/ja065988x. [DOI] [PubMed] [Google Scholar]
- 47.Denmark SE, Butler CR. J. Am. Chem. Soc. 2008;130:3690–3704. doi: 10.1021/ja7100888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.(a) Denmark SE, Ober MH. Org. Lett. 2003;5:1357–1360. doi: 10.1021/ol034328j. [DOI] [PubMed] [Google Scholar]; (b) Denmark SE, Ober MH. Adv. Synth. Catal. 2004;346:1703–1714. [Google Scholar]
- 49.Denmark SE, Smith RC, Chang W-tT, Muhuhi JM. J. Am. Chem. Soc. 2009 doi: 10.1021/ja8091449. manuscript in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li JJ, Gribble GW. Palladium in Heterocyclic Chemistry. Oxford: Pergamon; 2000. [Google Scholar]
- 51.Denmark SE, Baird JD, Regens CS. J. Org. Chem. 2008;73:1440–1455. doi: 10.1021/jo7023784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Denmark SE, Werner NS. J. Am. Chem. Soc. 2008;130:16382–16393. doi: 10.1021/ja805951j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Denmark SE, Baird JD. Tetrahedron. 2009 doi: 10.1016/j.tet.2008.10.043. manuscript in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Denmark SE, Tymonko SA. J. Am. Chem. Soc. 2005;127:8004–8005. doi: 10.1021/ja0518373. [DOI] [PubMed] [Google Scholar]
- 55.Hosoi K, Nozaki K, Hiyama T. Chem. Lett. 2002;31:138–139. [Google Scholar]
- 56.Denmark SE, Regens CS, Kobayashi T. J. Am. Chem. Soc. 2007;129:2774–2776. doi: 10.1021/ja070071z. [DOI] [PubMed] [Google Scholar]
- 57.Denmark SE, Fujimori S. J. Am. Chem. Soc. 2005;127:8971–8973. doi: 10.1021/ja052226d. [DOI] [PubMed] [Google Scholar]