Abstract
Exposure of the early gestation ovine fetus to exogenous glucocorticoids induces organ-specific alterations in postnatal cardiovascular physiology. To determine whether early gestation corticosteroid exposure alters coronary reactivity before the development of systemic hypertension, dexamethasone (0.28 mg·kg−1 · day−1) was administered to pregnant ewes by intravenous infusion over 48 h beginning at 27 days gestation (term, 145 days). Vascular responsiveness was assessed in endothelium-intact coronary arteries isolated from 1-wk-old steroid-exposed and age-matched control lambs (N = 6). Calcium imaging was performed in fura 2-loaded primary cultures of vascular smooth muscle cells (VSMC) from the harvested coronary arteries. Early gestation steroid exposure did not significantly alter mean arterial blood pressure or coronary reactivity to KCl, thromboxane A2 mimetic U-46619, or ANG II. Steroid exposure significantly increased coronary artery vasoconstriction to acetylcholine and endothelin-1. Vasodilatation to adenosine, but not nitroprusside or forskolin, was significantly attenuated following early gestation steroid exposure. Endothelin-1 or U-46619 stimulation resulted in a comparable increase in intracellular calcium concentration ([Ca2+]i) in coronary VSMC isolated from either dexamethasone-treated or control animals. However, the ANG II- or KCl-mediated increase in [Ca2+]i in control VSMC was significantly attenuated in VSMC harvested from dexamethasone-treated lambs. Coronary expression of muscle voltage-gated l-type calcium channel α-1 subunit protein was not significantly altered by steroid exposure, whereas endothelial nitric oxide synthase expression was attenuated. These findings demonstrate that early gestation glucocorticoid exposure elicits primary alterations in coronary responsiveness before the development of systemic hypertension. Glucocorticoid-induced alterations in coronary physiology may provide a mechanistic link between an adverse intrauterine environment and later cardiovascular disease.
Keywords: calcium imaging, endothelium, endothelial nitric oxide synthase, fetal programming
Exposure of the Early Gestation ovine fetus to exogenous glucocorticoids induces organ-specific changes in postnatal cardiovascular physiology. The hypertensive aspect of this model of fetal programming was first described by Dodic et al. in 1998 (3). In their studies, the offspring of ewes given dexamethasone for 48 h beginning at 27 days gestation (term, 145–150 days) were hypertensive at 4 mo of age and remained hypertensive at 10 and 18 mo of age, despite normal intrauterine and postnatal growth. Using an identical model, we recently reproduced the hypertensive phenotype and demonstrated unique alterations in coronary artery reactivity (17). More specifically, coronary arteries from the 4-mo-old lambs (8 mo following in utero exposure to dexamethasone) displayed increased vasoconstriction to the second messenger-dependent vasoconstrictors acetylcholine, ANG II, and the thromboxane A2 mimetic U-46619. Similar changes were not seen in the mesenteric arteries, demonstrating programming of vessel-specific alterations in vascular function. Furthermore, changes in coronary artery reactivity were not identified in dexamethasone-exposed fetal sheep studied at 123–126 days gestation (17), suggesting the development of changes in coronary artery reactivity begins in the postnatal period.
Given the concordance in the development of elevated arterial blood pressure and altered coronary artery reactivity following early gestation steroid exposure, we were previously unable to define whether the coronary alterations were a primary phenotype or a secondary phenomenon induced by the progressive systemic hypertension. The present study was designed to determine whether alterations in coronary function seen in our model of fetal programming are present in newborn sheep before the development of hypertension. We postulated that early gestation exposure to glucocorticoids leads to primary alterations in early postnatal coronary artery physiology even in the absence of systemic hypertension. To define the cell types potentially involved in programmed coronary dysfunction, we further evaluated both endothelial-dependent coronary artery responses and agonist-induced smooth muscle cell cytosolic calcium transients.
MATERIALS AND METHODS
Animal Model
All procedures were performed within the regulations of the Animal Welfare Act and the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Iowa Animal Care and Use Committee. Time-dated pregnant ewes were obtained from Iowa State University (ISU) and housed at the ISU Agricultural Station throughout the course of study. At 27–28 days gestation (term, ~145 days), dexamethasone (0.28 mg·kg−1 · day−1; Gensia Sicor Pharmaceuticals, Irvine, CA) was administered to the ewes by continuous intravenous infusion over 48 h. The ewes delivered naturally. Within the first week of life, the lambs and ewes were transferred from the ISU Agricultural Station to the University of Iowa Animal Care Unit. A single lamb from each litter was used for these studies, and steroid-exposed lambs were studied concurrently with nondexamethasone-exposed age-matched control lambs (N = 6 for each group).
Following transfer, the lambs were anesthetized with 12 mg/kg of thiopental sodium (Abbott Laboratories, Abbott Park, IL), intubated, and ventilated with a mixture of halothane (1%), oxygen (33%), and nitrous oxide (66%). A polyethylene catheter (PE-90) was then inserted into the lamb’s left femoral artery and sutured in place. The catheter was tunneled subcutaneously and secured to the back of the lamb using porous elastic bandages. Ampicillin (Sigma, St. Louis, MO) was administered at the completion of surgery (2 g im) and every 12 h for the subsequent 3 days (1 g im). After surgery, the lamb was returned to an individual pen with its mother and fed at liberty. After a 3-day recovery period, arterial blood pressure and heart rate were recorded using MacLab software (ADInstruments, Colorado Springs, CO) for 1 h while the lambs stood comfortably supported by a sling, allowing the transducer to be maintained at the level of the heart.
Coronary Artery Contractile Responses
Following measurement of blood pressure and heart rate, the lambs were euthanized with intravenous pentobarbital sodium (50 mg/kg; Abbott Laboratories, Abbott Park, IL), and coronary arteries were collected. On coronary artery harvest, circumflex coronary artery segments were cleansed of adherent connective tissue and immediately sectioned into eight 3-mm-long rings. The endothelium was left intact and the rings were mounted in individual 18-ml isolated organ chambers and connected to an isometric force transducer by 32-gauge stainless steel wire. Contractile responses were recorded with a MacLab 8E and stored on a Power Macintosh 8600 computer. The length-tension relationship was defined experimentally to 30 and 90 mM KCl at varying passive stretch. Passive stretch was set at 90% of the tension required to obtain peak responses to KCl (0.7 g), and the rings were allowed to equilibrate in bicarbonate-buffered physiological salt solution (PSS) at 37°C for 60 min before the start of experimentation. The composition of the PSS was as follows (in mM): 130 NaCl, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4·7 H2O, 14.9 NaHCO3, 1.6 CaCl2·H20, 5.5 dextrose and 0.03 CaNa2-EDTA (pH 7.30). PSS was aerated with a mixture of 95% O2-5% CO2.
Initially, to allow normalization of subsequent vasoconstriction data, the contractile response of each vessel to 120 mM KCl was recorded. The arteries were then reequilibrated to their baseline with multiple washes of PSS during 1 h before preconstriction of each vessel with the thromboxane A2 mimetic U-46619 (10−6 M). To investigate potential alterations in cyclic nucleotide-mediated vasodilatation, separate baths were used to assess cumulative concentration responses to sodium nitroprusside (10−10 to 10−5 M) or forskolin (10−11 to 10−6 M). Responses to adenosine (10−10 to 10−5 M) were similarly evaluated with the addition of increasing concentrations of the agent at 4-min intervals.
Arteries were once more reequilibrated to their baseline with multiple washes of PSS before measurement of vasoconstrictor responsiveness. Cumulative concentration-responses to acetylcholine (10−9 to 10−5 M), ANG II (10−11 to 10−7 M), or endothelin-1 (ET-1; 10−11 to 10−7 M) were conducted with the addition of increasing concentrations of the agent under study at 4-min intervals, before decline of the preceding vascular response. Concentration-responses of the coronary arteries to ET-1 were also determined following pretreatment with the Rho-dependent protein kinase inhibitor Y-27632 (10−5 M).
After completion of the study, vascular rings were dried and weighed to normalize the responses to 120 mM KCl and 10−6 M U-46619. Microsoft Excel 2000 was used to generate concentration-response curves for each vasoactive agent. Following data analysis, Excel formatting options were used to aesthetically smooth the contour of the curves. All PSS reagents and vasoactive compounds were acquired from Sigma with the exception of ET-1 and U-46619, both supplied by Alexis (San Diego, CA).
Coronary Artery Vascular Smooth Muscle Cell Culture
Left anterior descending coronary artery segments from the final three lambs in each group (N = 3) were cleansed of adherent connective tissue and placed in ice-cold PSS with penicillin G (100 U/ml) and streptomycin sulfate (100 µg/ml). The vessel was then subjected to enzymatic dispersion in MEM containing elastase (125 µg/ml), collagenase (1 mg/ml), BSA (2 mg/ml), and soybean trypsin inhibitor (375 µg/ml). The suspension was incubated at 37°C for 90 min with intermittent trituration followed by passage of the dispersed cells through a 100-µm nylon mesh. The filtered suspension was then centrifuged at 200 g for 10 min to yield a pellet of cells. The harvested vascular smooth muscle cells (VSMC) were then plated onto 25-mm coverslips bathed in DMEM containing 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin at a density of 5,000 cells/cm2. The culture dishes were subsequently placed in a humidified 95% air-5% CO2 incubator set at 37°C. The incubation media was changed at frequent intervals until the first passage cells reached 80% confluency (over 7–9 days).
Calcium Imaging of Cultured Coronary VSMC
Transient alterations in intracellular calcium concentration ([Ca2+]i) were measured by fura-2 fluorescence ratio imaging using a videomicroscopic imaging system (Photon Technology International, South Brunswick, NJ) as described previously (20). Calcium binding to fura-2 produces a shift in fluorescence intensity peak from 380 to 340 nm, and thus an increase in intracellular Ca2+ produces an increase in the 340-to-380 ratio used to compute [Ca]i. Briefly, semiconfluent VSMC grown on the 25-mm coverslips were placed in low serum medium (0.2% FBS) for 24 h and then loaded with fura-2 by incubating with 1 µm fura-2 AM (Molecular Probes, Eugene, OR) for 30 min at 37°C. [Ca2+]i was measured at 37°C in bicarbonate-free PSS containing 0.1% BSA and 20 mM NaHEPES (pH 7.35) by acquiring excitation image pairs (340 and 380 nm) every 5 s using an intensified charge-coupled device camera (model IC-200; Photon Technology International). Intracellular calcium levels were computed from the 340-nm and 380-nm excitation images acquired before and following stimulation with ET-1 (1 µM), ANG II (1 µM), U-46619 [10 µM, with and without nifedipine (10 µM) pretreatment], or KCl (90 mM). Agonists were added as a 2× concentrated solution to yield the final concentrations indicated above. After background correction, ratio images were obtained by dividing the 340-nm images by the 380-nm images on a pixel-by-pixel basis. It should be noted that cells failing to show at least a 50% increase in [Ca2+]i over basal values following agonist stimulation were excluded from analysis (~20% of the cells from both the dexamethasone-exposed and control groups met this criteria). Interestingly, from the dexamethasone-exposed lambs, all VSMC failed to elicit a response to KCl while maintaining robust responsiveness to other agonists. Therefore, these cells were considered responsive and were included to compare KCl responses between dexamethasone and control group. Ultimately, there were 9–34 VSMC (n) evaluated from 2–3 coverslips (N) for each agonist.
Immunoblotting
Western blot analysis for endothelial nitric oxide (NO) synthase (eNOS) or smooth muscle voltage-gated l-type calcium channel α-1 subunit (Cav1.2) was performed as previously described (16). The Cav1.2 antibody (Alomone Labs, Jerusalem, Israel) was raised in rabbits, whereas the eNOS-specific monoclonal antibody (BD Biosciences Pharmingen, San Diego, CA) was raised in mice. Nitrocellulose blots (20 µg protein/lane) were incubated with the primary antibody at a 1:200 (Cav1.2) or 1:1,000 (eNOS) dilution for 2 h at room temperature. Blots were rinsed, washed, and then incubated with either a 1:1,000 dilution of goat anti-rabbit (Cav1.2) or a 1:5,000 dilution of goat anti-mouse (eNOS) infrared-labeled secondary antibody (Molecular Probes) at room temperature for 1 h. Binding of the secondary antibody was quantified using the Odyssey infrared scanner (Li-Cor, Lincoln, NE). Results were normalized by arbitrarily setting the densitometry of control coronary arteries to 100.
Coronary Artery Morphometric Analysis
To further define the mechanisms responsible for the observed physiologic alterations, a second set of dexamethasone-exposed and age-matched control newborn lambs were studied (N = 6 per group). Following identical environmental and experimental exposures from conception through femoral artery catheterization at 1 wk of age, baroreflex evaluation was performed (for a related study), and the lambs were euthanized 2 days later with intravenous pentobarbital sodium (50 mg/kg). On coronary artery harvest, a 4-mm segment of the left anterior descending coronary artery was immediately incubated for 10 min in PSS containing 10−4 M sodium nitroprusside to allow maximal vasodilatation. The rings were then fixed in Pen-Fix (Richard Allen Scientific, Kalamazoo, MI) before paraffin embedment. The rings were microtomed in true cross section at a thickness of 6 µm and then mounted on glass slides and stained with hematoxylin and eosin. Sections were viewed under a brightfield microscope (Nikon Optiphot-2) and imaged using a digital camera and Spot software (Diagnostic Instruments, Sterling Heights, MI). External vessel diameter, luminal diameter, and media thickness were averaged from at least three sections per vessel.
Immunohistochemistry
The same sodium nitroprusside-dilated and paraffin-embedded coronary arteries described above were once again placed on glass slides in 6-µm sections. Control and dexamethasone-exposed arterial segments were studied concurrently. The sections were deparaffinized in xylenes and hydrated in an ethanol/PSS series. After a 5-min PSS rinse, the sections were incubated in H2O2 (3% in methanol) for 30 min before blocking with BSA (1% in PSS). Sections were incubated with monoclonal mouse anti-eNOS antibody, 1:400 dilution, (BD Biosciences Pharmingen) at room temperature for 60 min. The sections were then incubated for 30 min in horseradish peroxidase conjugated goat anti-mouse secondary antibody at 1:200 dilution (Santa Cruz Biotechnology, Santa Cruz, CA). After being washed in PSS, the sections were incubated in the peroxidase substrate diaminobenzidine (Sigma) for 5 min and then dehydrated in an ethanol series and mounted with glass coverslips. Sections were sequentially viewed under a brightfield microscope (Nikon Optiphot-2), and a representative portion was imaged using a digital camera and Spot software (Diagnostic Instruments) with uniform microscope and software settings. The images were then converted to 16-bit gray-scale images of unaltered contrast and brightness with Adobe Photoshop 8.0. The relative density of eNOS protein immunolabeling was measured with the National Institutes of Health public domain Image J image analysis software. Regions of lightly and darkly stained tissue were first identified among the entire set of analyzed images, and the density of pixels within those regions was measured. A linear calibration function was then established by assigning a gray value of zero to the pixel value of the lightly stained region and a gray value of 100 to the pixel value of the darkly stained region. For each of the analyzed sections, the visualized portions of the lumen, media, and endothelium were traced, and the mean gray values were measured by the Image J software. Results were then averaged for two separate sections from each vessel. Consistent with the rigid image acquisition protocol, the staining intensity of the coronary lumen was comparable between the dexamethasone-exposed and control groups (9 ± 2 and 8 ± 2 arbitrary densitometric units, respectively). To account for possible differences in background staining intensity, the mean lumen intensity for each slide was subtracted from the corresponding media and endothelium staining intensities before statistical analysis. Results were normalized by arbitrarily setting the mean immunostaining density of the control coronary endothelium at 100.
Data Analysis
Physiologic parameters, maximal vascular responses, calcium concentrations, and vascular dimensions were compared using Student’s unpaired, two-tailed t-test (with significance at P < 0.05). Concentration responses to the vasoactive agents were compared using ANOVA, factoring for treatment group, and the presence or absence of inhibitors, thereby comparing each set of data in its entirety (dexamethasone exposed vs. control and pretreated vs. uninhibited). ANOVA was also used to evaluate eNOS expression, factoring for treatment group and vessel layer (e.g., endothelium vs. media). If ANOVA identified significant differences (P < 0.05), pairwise comparisons were made using the Tukey test, with P < 0.05 considered significant. Given the present sample size limitations, subgroup analysis based on birth plurality or gender was not performed. All analyses were performed using SAS System 9 for Microsoft Windows (SAS Institute, Cary, NC). All values are presented as means ± SE, and N refers to the number of animals studied, whereas n refers to the number of VSMC evaluated.
RESULTS
Physiologic Parameters
There were no significant differences in age, carcass weight, heart weight, heart rate, or blood pressure between the steroid exposed and control lambs (Table 1). Within both treatment groups, twin deliveries were common and a majority of the sheep were male (Table 1).
Table 1.
Birth, growth, and hemodynamic parameters for age-matched control and early gestation dexamethasone-exposed lambs
| Control | Dexamethasone Exposed | |
|---|---|---|
| Plurality at birth | 3 singleton, 3 twins | 2 singleton, 4 twins |
| Gender | 2 female, 4 males | 1 female, 5 males |
| Postnatal age, days | 9±1 | 8±1 |
| Weight, kg | 7.7±1.0 | 7.6±0.9 |
| Heart weight, g/kg | 7.4±0.2 | 7.5±0.2 |
| LVFW weight, g/kg | 2.4±0.2 | 2.6±0.2 |
| RVFW weight, g/kg | 1.4±0.1 | 1.6±0.1 |
| MAP, mmHg | 74±3 | 77±1 |
| HR, beats/min | 266±21 | 249±9 |
Values are means ± SE; N = 6. There were no significant differences between the control and steroid-exposed groups. LVFW, left ventricle free wall; RVFW, right ventricle free wall; MAP, mean arterial blood pressure; HR, heart rate. Tissue weights are in proportion to body wt.
Vascular Dimensions
Morphometric analysis showed no alteration in lumen diameter, media thickness, or media-to-lumen ratio following dexamethasone exposure (Table 2).
Table 2.
Morphometric analysis of coronary arteries from newborn lambs with and without early gestation dexamethasone exposure
| Control | Dexamethasone Exposed | |
|---|---|---|
| External vessel diameter, µm | 1,154±87 | 1,254±84 |
| Lumen diameter, µm | 814±76 | 900±68 |
| Media thickness, µm | 170±9 | 177±10 |
| Media-to-lumen ratio, % | 22±2 | 20±1 |
Values are displayed as means ± SE; N = 6. There were no significant differences between the control and steroid-exposed groups.
Vascular Reactivity
Maximal coronary artery vasoconstriction
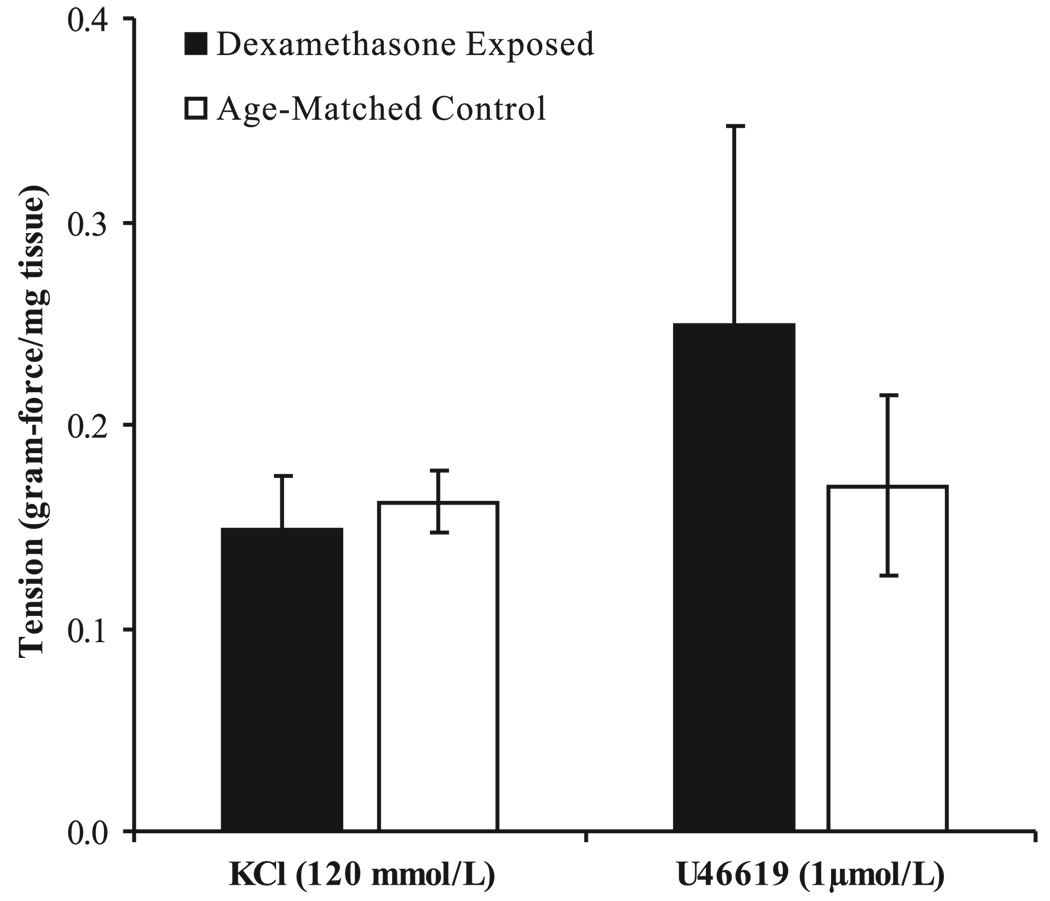
The vascular rings obtained from the control and treatment groups were of similar size (dry wt of 15 ± 2 and 13 ± 2 mg, respectively). Responses of the coronary artery segments to KCl (120 mM) and U-46619 (10−6 M) were not significantly altered by antenatal dexamethasone exposure (Fig. 1).
Fig. 1.
Coronary artery contractile responses to 120 mM KCl or 1 µM U-46619 normalized to vessel weight (N = 6). Values are displayed as means with vertical lines indicating SE. There were no significant differences between dexamethasone-exposed and control lambs.
Concentration responses to vasoconstrictors
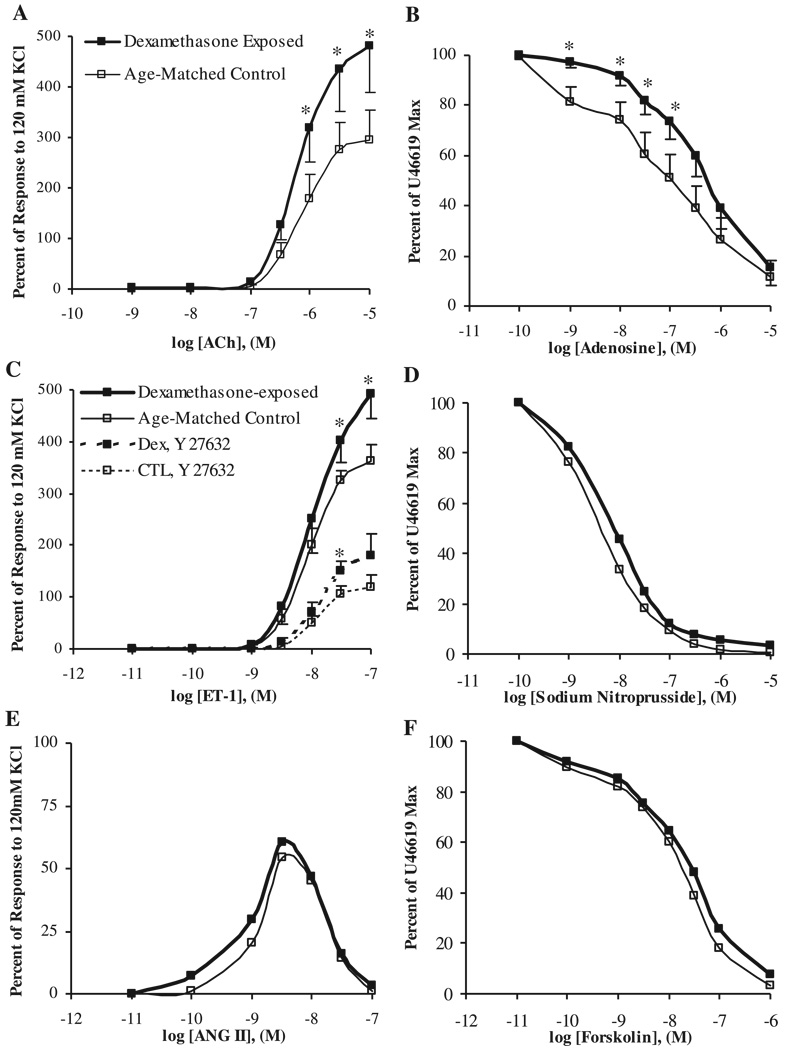
Coronary artery segments from lambs exposed to dexamethasone early in gestation exhibited significantly enhanced vasoconstrictive responses to acetylcholine and ET-1, relative to control vessels (Fig. 2, A and C). Pretreatment of the coronary artery segments with the Rho-dependent protein kinase inhibitor Y-27632 (10−5 M) significantly decreased the response of both steroid-exposed and control arteries to ET-1 but failed to mitigate the dexamethasone-induced alteration in vascular reactivity (Fig. 2C). There was no significant difference in coronary artery responses to ANG II following steroid exposure (Fig. 2E).
Fig. 2.
Coronary artery responsiveness to cumulative concentrations of acetylcholine (A), adenosine (B), endothelin-1 (ET-1; C; dashed lines noting responses following pretreatment with the Rho-kinase inhibitor Y-27632), sodium nitroprusside (D), ANG II (E), or forskolin (F). Responses were assessed in dexamethasone-treated or control lambs (N = 6). Values are displayed as means with vertical lines indicating SE. *Significant differences between dexamethasone-exposed and control lambs (P < 0.05).
Concentration-responses to vasodilators
Whereas dexamethasone exposure was associated with attenuated coronary artery responses to low concentrations of adenosine (Fig. 2B), there were no significant treatment group differences in coronary responses to sodium nitroprusside or forskolin (Fig. 2, D and F).
Agonist-Induced Changes in VSMC [Ca2+]i
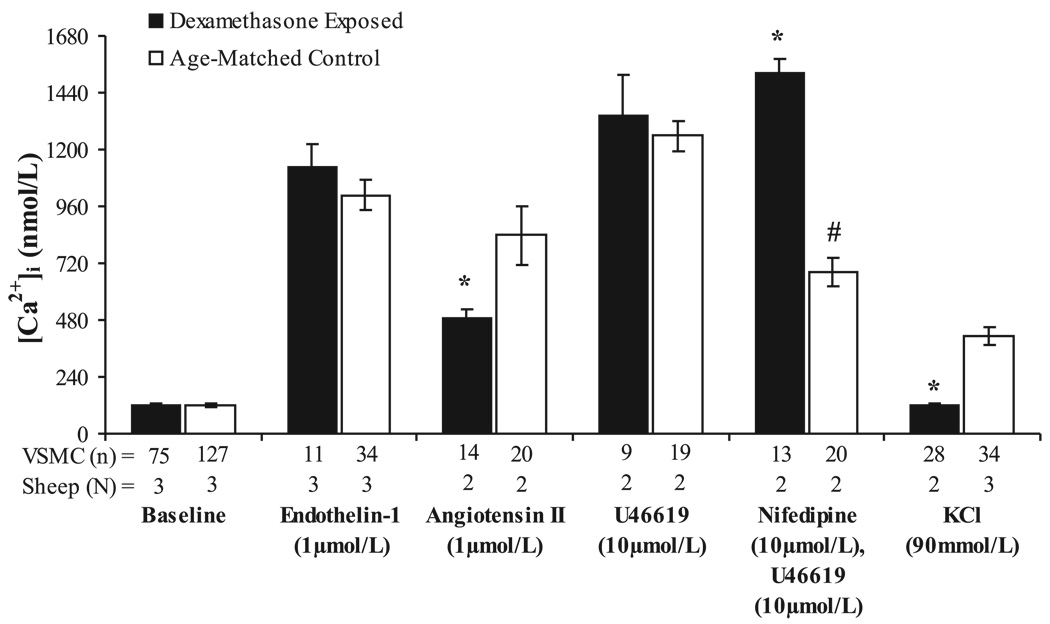
Baseline [Ca2+]i was similar in control and dexamethasone-exposed coronary VSMC (Fig. 3; 119 ± 5 and 123 ± 7 nM, respectively). There were no significant differences between the two groups in coronary VSMC peak [Ca2+]i responses to ET-1 or U-46619 (Fig. 3), whereas peak [Ca2+]i responses to ANG II were significantly lower in steroid-exposed coronary VSMC compared with cells from control vessels (Fig. 3, P < 0.05). Interestingly, VSMC obtained from two dexamethasone-exposed sheep appeared viable and responded to other agents but failed to respond to 90 mM KCl (peak [Ca2+]i 123 ± 6 nM), whereas VSMC harvested from all three control sheep responded to KCl (peak [Ca2+]i 413 ± 36 nM, P < 0.01). Similarly, the voltage-gated calcium channel antagonist nifedipine failed to inhibit U-46619-stimulated increases in peak [Ca2+]i in VSMC isolated from dexamethasone-treated coronaries, whereas a significant inhibition in peak [Ca2+]i response to U-46619 was seen in VSMC from control vessels following nifedipine pretreatment (Fig. 3, P < 0.01). Together, these findings suggest dexamethasone-mediated changes in the properties of voltage-gated calcium channels within coronary artery smooth muscle cells.
Fig. 3.
Vascular smooth muscle cell cytosolic calcium concentration measured at baseline and following stimulation with endothelin-1, ANG II, U-46619 with and without nifedipine pretreatment, or KCl in dexamethasone-treated and control coronary artery cells. Values are displayed as means with vertical lines indicating SE; N = number of lambs (and coverslips); n = number of VSMC analyzed. *Significant differences between dexamethasone-exposed and control lambs (P < 0.05). #Significantly different than response of control VSMC to U-46619 alone (P < 0.01).
Immunoblotting
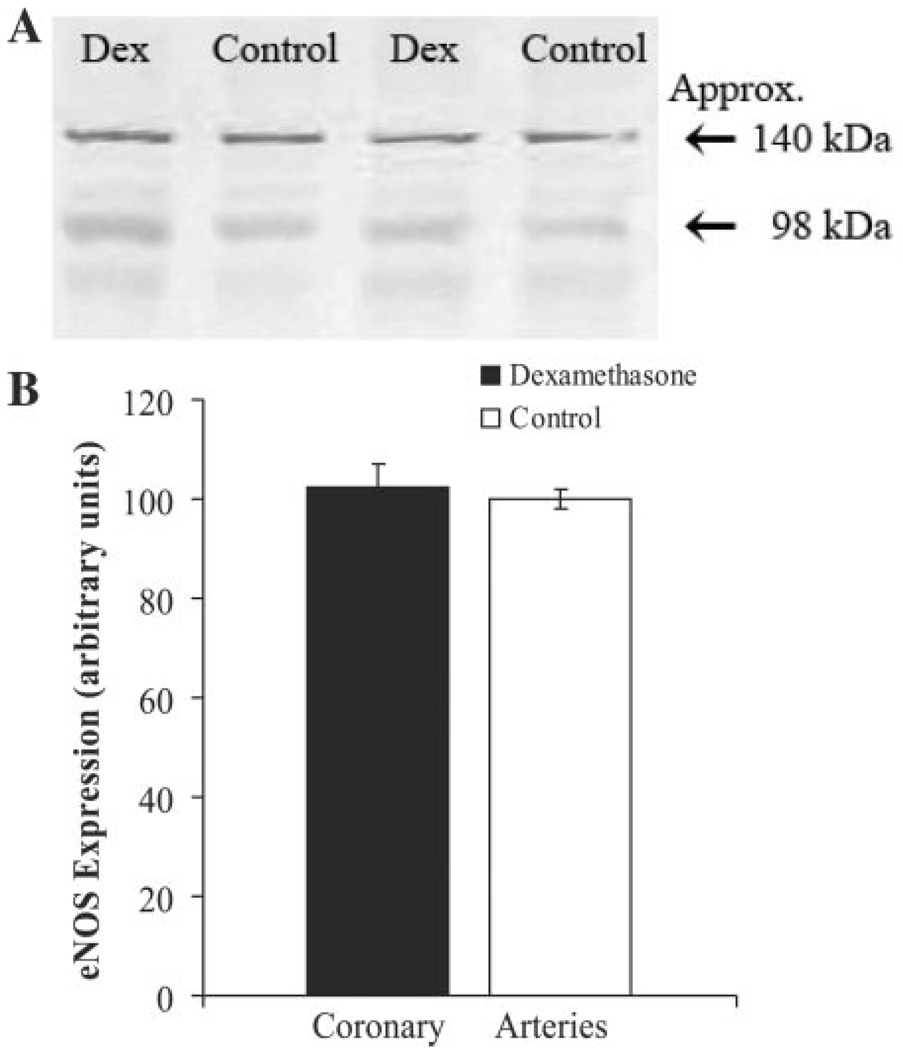
Western blot analysis consistently demonstrated the presence of Cav1.2 protein in both intact coronary arteries and isolated VSMC (data not shown). Although eNOS was abundant in the segments of whole coronary artery (Fig. 4A), there was no eNOS protein detected within the isolated VSMC. Dexamethasone exposure was not associated with any significant difference in Cav1.2 (data not shown) or eNOS (Fig. 4B) protein expression.
Fig. 4.
Representative gray scaled immunoblots for endothelial nitric oxide synthase (eNOS) protein in dexamethasone-exposed and control newborn lamb coronary arteries (A). eNOS protein was consistently detected at ~140 kDa within protein isolated from whole coronary arteries. There was no significant treatment effect on overall eNOS protein expression (B). Values are displayed as means with vertical lines indicating SE.
Immunostaining
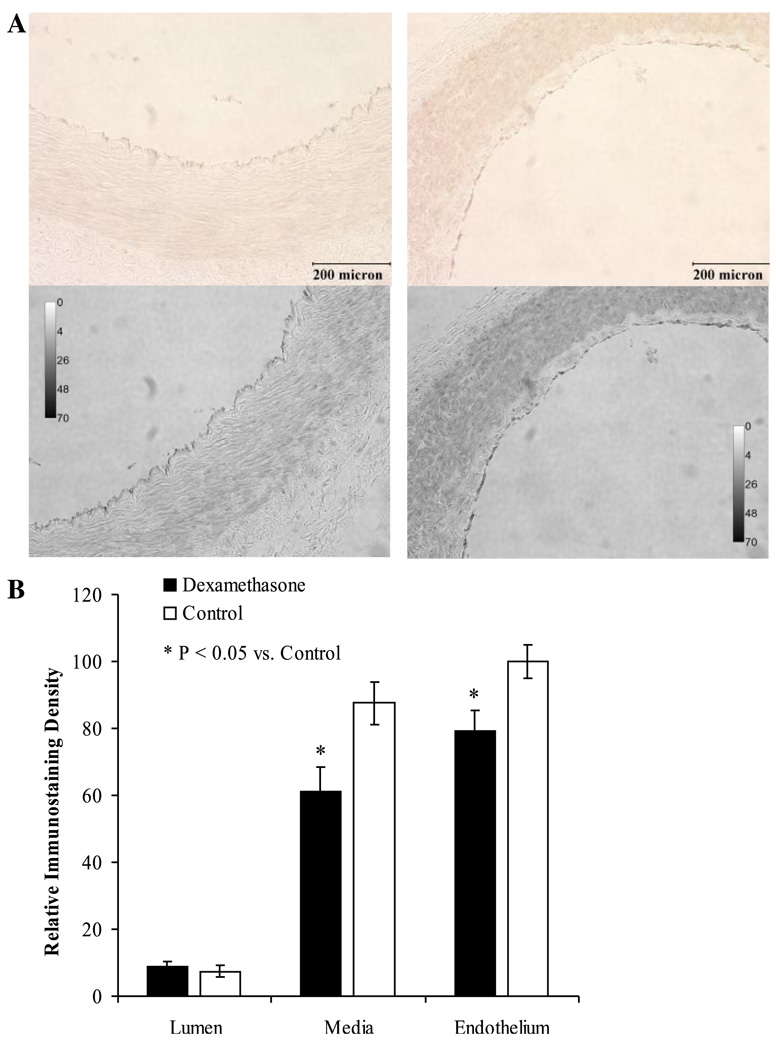
Coronary artery eNOS expression was localized within the endothelium for both treatment groups (Fig. 5; P < 0.01 vs. staining within the media). Dexamethasone exposure was associated with a significant decrease in eNOS staining within both the endothelium and media of the newborn lamb coronary arteries (Fig. 5B; P < 0.01).
Fig. 5.
Immunostaining for eNOS protein within coronary arteries harvested from dexamethasone-exposed (left) and control (right) newborn lambs (A). The first row demonstrates the brown immunostaining seen using the peroxidase substrate diaminobenzidine. The images were then converted to gray scale (A, second row) before quantification by densitometric analysis (B). Relative immunostaining density (as a reflection of eNOS expression) was significantly localized to the vascular endothelium (P < 0.01 vs. the media for each treatment group) and was significantly attenuated following early gestation dexamethasone-exposure. *Significantly different from control (P < 0.01). Values are displayed as means with vertical lines indicating SE and magnification ×200 was used for each image.
DISCUSSION
During the past 15 yr, there has been mounting evidence obtained through a variety of animal models verifying a causal relationship between early gestation adverse events and latent physiologic alterations (11, 19, 23). These studies have clearly documented adult onset blood pressure elevation following fetal nutrient deprivation or exposure to exogenous glucocorticoids (1, 3, 5, 17). In the present study, we describe antenatally programmed primary alterations in coronary artery physiology in the absence of systemic hypertension.
The major finding of this study is the observation that coronary arteries from 1-wk-old lambs briefly exposed to exogenous glucocorticoids early in gestation display altered agonist-specific vascular reactivity. Responsiveness was altered to a variety of agents, including acetylcholine, ET-1, and adenosine, acting through combined endothelium-dependent and endothelium-independent pathways. These changes in vascular responsiveness did not correspond with similar increases in VSMC calcium mobilization, suggesting early gestation dexamethasone exposure results in changes in endothelial function and/or coronary VSMC calcium sensitivity.
The dexamethasone-induced alterations in acetylcholine reactivity seen in the present study (using newborn sheep) closely mirror the changes we previously described in identically exposed 4-mo-old sheep (17). Given the persistence of this finding, both before and after the development of systemic hypertension, it is apparent that this vessel-specific effect is a primary and predictable adaptive response of the coronary vasculature to the early gestation steroid exposure. As with acetylcholine, ET-1-mediated net coronary artery reactivity occurs through a balance between VSMC-induced vasoconstriction and receptor-mediated endothelial NO production (15, 18). The dexamethasone-programmed increase in coronary reactivity to ET-1 that we observed is reminiscent of the dexamethasone-induced increase in femoral artery reactivity to ET-1 described both immediately and 6 mo following late gestation dexamethasone exposure (2, 12). Mechanistically, this enhanced vasoconstriction to ET-1 was not associated with alteration in ET-1-stimulated VSMC calcium transients. It is therefore unlikely that [Ca2+]i availability played a primary etiologic role in our observations.
In addition to intracellular calcium-mediated myosin light-chain kinase activation, myosin light-chain phosphorylation is regulated by Rho/Rho-kinase-mediated inhibition of myosin phosphatase. Abnormal activation of the Rho/Rho-kinase pathway has been shown to play a role in a variety of diseases, including hypertension and coronary vasospasm (7, 22). In both groups of lambs presently evaluated, the Rho-kinase inhibitor Y-27632 significantly decreased the responses to ET-1 (by 65–67%) but did not eliminate the dexamethasone-induced hypercontractile state. Despite the inability of Y-27632 to equalize coronary responsiveness, it is still possible that dexamethasone-exposure leads to a generalized increase in calcium sensitivity whereby a similar rise in VSMC intracellular calcium produces a greater degree of coronary vasoconstriction (as with ET-1) and equivalent vasoconstrictive forces can be generated in the presence of decreased cytosolic calcium transients (as with KCl and ANG II).
The steroid-induced abolishment of VSMC responsiveness to KCl was intriguing. Although difficult to interpret in concert with the lack of alteration in KCl vasoreactivity and the fact the VSMC were harvested from only two to three lambs, the apparent change in voltage-dependent calcium channel activity raised the possibility that the early gestation steroid exposure may have altered calcium influx through plasma membrane-based calcium channels. That hypothesis was further supported by the inability of nifedipine to inhibit the peak [Ca2+]i response to U-46619 within the dexamethasone-exposed coronary arteries. Based on these findings, immunoblots were used to assess for differential expression of the α-1 subunit of the smooth muscle voltage-sensitive calcium channel Cav1.2. This did not demonstrate any differences in Cav1.2 expression between control and dexamethasone-exposed coronary arteries. Although it is possible the study’s power may not have been adequate to detect differential protein expression, it is also possible that qualitative differences in calcium channel coupling to membrane potential may account for the observed changes. Regardless of the explanation for the steroid-induced attenuation in VSMC calcium mobilization, the direction of the effect (decrease in calcium mobilization following steroid exposure) supports our contention that early gestation glucocorticoid exposure may program primary alterations in coronary artery physiology through an increase in calcium sensitivity rather than an increase in calcium availability.
Alternatively, the absence of significant steroid-induced changes in responsiveness to the relatively endothelium-independent vasoconstrictors U-46619 and ANG II suggests alterations in vascular function may be agonist specific. The endothelial-dependent NO production that classically counterbalances the direct VSMC effects of acetylcholine and ET-1 is the most obvious difference between the general agonist pathways presently interrogated. Although we did not assess reactivity within endothelial-denuded vessels, adenosine was chosen to assess the relative contribution of endothelial-dependent and endothelial-independent vasodilatation in our studies. Coronary artery responsiveness to adenosine was significantly attenuated within the steroid-exposed group at concentrations up to 10−7 M, whereas maximal vasodilatation was unaltered at higher concentrations. Given recent studies within the coronary circulation showing adenosine consistently elicits endothelial-dependent vasodilatation via eNOS-mediated production of NO, but at concentrations greater than 10−7 M, VSMC-mediated vasodilatation plays a more dominant role (6), these results once more suggest the presence of a primary programming effect within steroid-exposed endothelial cells. Absence of significant glucocorticoid-related differences in vascular responses to the endothelium-independent vasodilators sodium nitroprusside or forskolin further reinforces the possibility that the observed alterations in vascular reactivity are a consequence of primary changes in endothelial cell-mediated pathways.
There is a growing body of literature suggesting that attenuated endothelial-dependent vasodilatation contributes to antenatally programmed alterations in vascular physiology. Among animal models of fetal programming, impaired vasorelaxation to acetylcholine has been well described in the offspring of undernourished rats (1, 8), and among children and adults with a history of low birth weight and subsequent increased risks of developing hypertension and coronary artery disease there is consistent demonstration of attenuated endothelial-dependent vasodilation (4, 9, 10, 13).
To further evaluate potential endothelial-dependent mechanisms underlying the observed differences, immunoblots for eNOS protein expression were completed. Absence of significant steroid-induced alteration in eNOS expression in this study may be related to the limited sensitivity of immunoblots on protein from whole coronary artery looking for relatively endothelial-specific gene expression. In the absence of endothelial cell culture availability, we proceeded to immunohistochemistry to better quantify cell type-specific eNOS expression. As expected, immunostains localized eNOS expression to the endothelial cell layer of sectioned coronary arteries. Further quantification of gray-scaled images revealed a significant dexamethasone-induced attenuation in signal intensity within both media and endothelium of coronary arteries. Although we were unable to quantify the relative contribution of eNOS-specific and nonspecific protein staining within complex vascular media, dexamethasone-induced reduction in eNOS staining within relatively simply structured endothelial cell monolayer is intriguing. This differential staining may have been due to a direct programming effect of dexamethasone on coronary artery and microvascular eNOS expression (as supported by our vascular reactivity data) or altered coronary architecture leading to differential nonspecific staining (despite a lack of gross morphometric differences). In light of these intriguing yet inconclusive findings, further evaluation of endothelial cell eNOS expression and activity will be needed to clarify the potential etiologic role of eNOS downregulation in the fetal programming of vascular dysfunction. Beyond eNOS, the physiologically complex coronary artery endothelial cells use a number of interrelated vasodilatory pathways. Most notably, prostaglandin E2 and hydrogen peroxide have recently been shown to play major roles in the regulation of porcine coronary arteriole tone (14, 21).
Perspectives
The present study provides novel information regarding primary glucocorticoid-induced alterations in coronary artery reactivity and suggests increased VSMC calcium sensitivity and/or endothelial-dependent processes are involved in the programming of coronary dysfunction. If these alterations are permanently programmed, their effects may be magnified by the progressive endothelial dysfunction that would likely result from the ensuing systemic hypertension, possibly linking an adverse intrauterine environment with future cardiovascular morbidity. Continued investigation into the effects of early gestational exposures on postnatal cardiovascular health is essential to develop methods of mitigating and ultimately preventing an important subset of cardiovascular morbidities.
ACKNOWLEDGMENTS
We thank Dr. Ramesh C. Bhalla, Department of Anatomy and Cell Biology, University of Iowa, for allowing the use of facilities for calcium determinations in our studies.
GRANTS
This study was supported by National Institutes of Health grants RO1-HL-62483 (to F. S. Lamb), R21-ES-012268 (to J. L. Segar), and T32-HD-041922 (Physician Training Program in Neonatal Biology to R. D. Roghair), as well as an Established Investigator Award from the American Heart Association (to F. S. Lamb). The facilities for calcium determinations were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant HL-14388 to Dr. Ramesh Bhalla.
REFERENCES
- 1.Brawley L, Itoh S, Torrens C, Barker A, Bertram C, Poston L, Hanson M. Dietary protein restriction in pregnancy induces hypertension and vascular defects in rat male offspring. Pediatr Res. 2003;54:83–90. doi: 10.1203/01.PDR.0000065731.00639.02. [DOI] [PubMed] [Google Scholar]
- 2.Docherty CC, Kalmar-Nagy J, Engelen M, Koenen SV, Nijland M, Kuc RE, Davenport AP, Nathanielsz PW. Effect of in vivo fetal infusion of dexamethasone at 0.75 GA on fetal ovine resistance artery responses to ET-1. Am J Physiol Regul Integr Comp Physiol. 2001;281:R261–R268. doi: 10.1152/ajpregu.2001.281.1.R261. [DOI] [PubMed] [Google Scholar]
- 3.Dodic M, May CN, Wintour EM, Coghlan JP. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci (Lond) 1998;94:149–155. doi: 10.1042/cs0940149. [DOI] [PubMed] [Google Scholar]
- 4.Goodfellow J, Bellamy MF, Gorman ST, Brownlee M, Ramsey MW, Lewis MJ, Davies DP, Henderson AH. Endothelial function is impaired in fit young adults of low birth weight. Cardiovasc Res. 1998;40:600–606. doi: 10.1016/s0008-6363(98)00197-7. [DOI] [PubMed] [Google Scholar]
- 5.Gopalakrishnan GS, Gardner DS, Rhind SM, Rae MT, Kyle CE, Brooks AN, Walker RM, Ramsay MM, Keisler DH, Stephenson T, Symonds ME. Programming of adult cardiovascular function after early maternal undernutrition in sheep. Am J Physiol Regul Integr Comp Physiol. 2004;287:R12–R20. doi: 10.1152/ajpregu.00687.2003. [DOI] [PubMed] [Google Scholar]
- 6.Hein TW, Kuo L. cAMP-independent dilation of coronary arterioles to adenosine: role of nitric oxide, G proteins, and KATP channels. Circ Res. 1999;85:634–642. doi: 10.1161/01.res.85.7.634. [DOI] [PubMed] [Google Scholar]
- 7.Kandabashi T, Shimokawa H, Miyata K, Kunihiro I, Eto Y, Morishige K, Matsumoto Y, Obara K, Nakayama K, Takahashi S, Takeshita A. Evidence for protein kinase C-mediated activation of rho-kinase in a porcine model of coronary artery spasm. Arterioscler Thromb Vasc Biol. 2003;23:2209–2214. doi: 10.1161/01.ATV.0000104010.87348.26. [DOI] [PubMed] [Google Scholar]
- 8.Lamireau D, Nuyt AM, Hou X, Bernier S, Beauchamp M, Gobeil F, Lahaie I, Varma DR, Chemtob S. Altered vascular function in fetal programming of hypertension. Stroke. 2002;33:2992–2998. doi: 10.1161/01.str.0000039340.62995.f2. [DOI] [PubMed] [Google Scholar]
- 9.Leeson CP, Kattenhorn M, Morley R, Lucas A, Deanfield JE. Impact of low birth weight and cardiovascular risk factors on endothelial function in early adult life. Circulation. 2001;103:1264–1268. doi: 10.1161/01.cir.103.9.1264. [DOI] [PubMed] [Google Scholar]
- 10.Martin H, Hu J, Gennser G, Norman M. Impaired endothelial function and increased carotid stiffness in 9-year-old children with low birth weight. Circulation. 2000;102:2739–2744. doi: 10.1161/01.cir.102.22.2739. [DOI] [PubMed] [Google Scholar]
- 11.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 12.Molnar J, Howe DC, Nijland MJ, Nathanielsz PW. Prenatal dexamethasone leads to both endothelial dysfunction and vasodilatory compensation in sheep. J Physiol. 2003;547:61–66. doi: 10.1113/jphysiol.2002.032565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norman M, Martin H. Preterm birth attenuates association between low birth weight and endothelial dysfunction. Circulation. 2003;108:996–1001. doi: 10.1161/01.CIR.0000085069.09770.3D. [DOI] [PubMed] [Google Scholar]
- 14.Oltman CL, Kane NL, Miller FJ, Jr, Spector AA, Weintraub NL, Dellsperger KC. Reactive oxygen species mediate arachidonic acid-induced dilation in porcine coronary microvessels. Am J Physiol Heart Circ Physiol. 2003;285:H2309–H2315. doi: 10.1152/ajpheart.00456.2003. [DOI] [PubMed] [Google Scholar]
- 15.Regoli D, Dion S, Rhaleb NE, Drapeau G, D’Orleans-Juste P. Vasoactive peptides and their receptors. Blood Vessels. 1990;27:137–145. doi: 10.1159/000158804. [DOI] [PubMed] [Google Scholar]
- 16.Roghair RD, Lamb FS, Bedell KA, Smith OM, Scholz TD, Segar JL. Late gestation betamethasone enhances coronary artery responsiveness to ANG II in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2004;286:R80–R88. doi: 10.1152/ajpregu.00421.2003. [DOI] [PubMed] [Google Scholar]
- 17.Roghair RD, Lamb FS, Miller FJ, Jr, Scholz TD, Segar JL. Early gestation dexamethasone programs enhanced postnatal ovine coronary artery vascular reactivity. Am J Physiol Regul Integr Comp Physiol. 2005;288:R46–R53. doi: 10.1152/ajpregu.00165.2004. [DOI] [PubMed] [Google Scholar]
- 18.Rubanyi GM, Polokoff MA. Endothelins: molecular biology biochemistry, pharmacology, physiology and pathophysiology. Pharmacol Rev. 1994;46:325–415. [PubMed] [Google Scholar]
- 19.Schwartz J, Morrison JL. Impact and mechanisms of fetal physiological programming. Am J Physiol Regul Integr Comp Physiol. 2005;288:R11–R15. doi: 10.1152/ajpregu.00698.2004. [DOI] [PubMed] [Google Scholar]
- 20.Sharma RV, Chapleau MW, Haiduczok G, Wachtel RE, Waite LJ, Bhalla RC, Abboud FM. Mechanical stimulation increases intracellular calcium concentration in nodose sensory neurons. Neuroscience. 1995;66:433–441. doi: 10.1016/0306-4522(94)00560-r. [DOI] [PubMed] [Google Scholar]
- 21.Thengchaisri N, Kuo L. Hydrogen peroxide induces endothelium-dependent and -independent coronary arteriolar dilation: role of cyclooxygenase and potassium channels. Am J Physiol Heart Circ Physiol. 2003;285:H2255–H2263. doi: 10.1152/ajpheart.00487.2003. [DOI] [PubMed] [Google Scholar]
- 22.Wettschureck N, Offermanns S. Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J Mol Med. 2002;80:629–638. doi: 10.1007/s00109-002-0370-2. [DOI] [PubMed] [Google Scholar]
- 23.Wintour EM, Johnson K, Koukoulas I, Moritz K, Tersteeg M, Dodic M. Programming the cardiovascular system, kidney and the brain—a review. Placenta. 2003;24 Suppl A:S65–S71. doi: 10.1053/plac.2002.0927. [DOI] [PubMed] [Google Scholar]