Abstract
Context: Protein is an essential component of muscle and bone. However, the acidic byproducts of protein metabolism may have a negative impact on the musculoskeletal system, particularly in older individuals with declining renal function.
Objective: We sought to determine whether adding an alkaline salt, potassium bicarbonate (KHCO3), allows protein to have a more favorable net impact on intermediary indices of muscle and bone conservation than it does in the usual acidic environment.
Design: We conducted a 41-d randomized, placebo-controlled, double-blind study of KHCO3 or placebo with a 16-d phase-in and two successive 10-d metabolic diets containing low (0.5 g/kg) or high (1.5 g/kg) protein in random order with a 5-d washout between diets.
Setting: The study was conducted in a metabolic research unit.
Participants: Nineteen healthy subjects ages 54–82 yr participated.
Intervention: KHCO3 (up to 90 mmol/d) or placebo was administered for 41 d.
Main Outcome Measures: We measured 24-h urinary nitrogen excretion, IGF-I, 24-h urinary calcium excretion, and fractional calcium absorption.
Results: KHCO3 reduced the rise in urinary nitrogen excretion that accompanied an increase in protein intake (P = 0.015) and was associated with higher IGF-I levels on the low-protein diet (P = 0.027) with a similar trend on the high-protein diet (P = 0.050). KHCO3 was also associated with higher fractional calcium absorption on the low-protein diet (P = 0.041) with a similar trend on the high-protein diet (P = 0.064).
Conclusions: In older adults, KHCO3 attenuates the protein-induced rise in urinary nitrogen excretion, and this may be mediated by IGF-I. KHCO3 may also promote calcium absorption independent of the dietary protein content.
Supplementation of older adults with potassium bicarbonate attenuates the urinary nitrogen excretion that accompanies an increase in dietary protein, suggesting that the net effect of dietary protein on muscle may be enhanced by reducing its accompanying acid load.
Protein is an essential component of skeletal muscle, and severe protein deficiency causes muscle wasting (1). Studies have demonstrated a positive association between dietary protein intake and lean body mass (2,3), perhaps mediated by the anabolic hormone, IGF-I (4). Other studies have suggested that high-protein diets cause increased urinary nitrogen (UNi) excretion due in part to muscle breakdown from the acidogenic component of dietary protein (5,6). Diets rich in protein and low in fruits and vegetables result in a low-grade, chronic metabolic acidosis because the metabolism of protein releases noncarbonic acids (e.g. sulfuric acid) into the bloodstream in amounts that override the alkalinizing effect of potassium in vegetable foods (7). Conditions of chronic metabolic acidosis, such as chronic kidney disease and ketogenic weight loss diets, stimulate muscle breakdown (5,8). Reversal of metabolic acidosis by administration of alkaline salts has been shown to decrease UNi excretion, suggesting an attenuation of muscle breakdown (9,10), but only one prior study has demonstrated such an effect in healthy adults on a high-protein diet (11).
Dietary protein promotes peripubertal bone growth (4,12) and has been positively associated with higher bone mass (13,14) and lower hip fracture rates in adults (15,16). However, dietary protein also appears to have some potentially adverse effects on calcium and bone metabolism. For example, protein has consistently been shown to increase urinary calcium (UCa) excretion (17,18), whereas it has had varying effects on calcium absorption (19,20,21,22,23,24). In addition, experimental increases in amino acid intakes have been shown to negatively influence bone remodeling (25). These adverse calcium and bone effects may result from the metabolic acid load that accompanies a high dietary protein intake. An acidic environment reduces osteoblastic activity (26), increases osteoclastic activity (27), and appears to have a direct physicochemical effect on bone. Studies have found that the addition of alkaline salts lowered UCa excretion (28,29) and biochemical markers of bone turnover during short-term administration (29,30), suggesting beneficial effects on bone preservation. A recent study showed that administration of an alkaline salt of potassium in rats in combination with a high-protein diet improved calcium retention but failed to demonstrate beneficial skeletal effects (31). To our knowledge, there are no similar studies in humans.
The purpose of this study was to investigate whether the addition of an alkaline salt of potassium, potassium bicarbonate (KHCO3), allows dietary protein to have a more favorable impact on indices of muscle and bone conservation than is observed in its usual acidic environment. We studied healthy older men and women because typical age-related declines in renal function may decrease their ability to compensate for protein-induced metabolic acidosis, and alkali therapy may prevent this from occurring.
Subjects and Methods
Study design and subjects
This was a double-blind, randomized, placebo-controlled study that was conducted at the Metabolic Research Unit at the Jean Mayer United States Department of Agriculture Human Nutrition Research Center on Aging at Tufts University. The Tufts Medical Center-Tufts University Health Sciences Campus Institutional Review Board approved the study, and written informed consent was obtained from each subject.
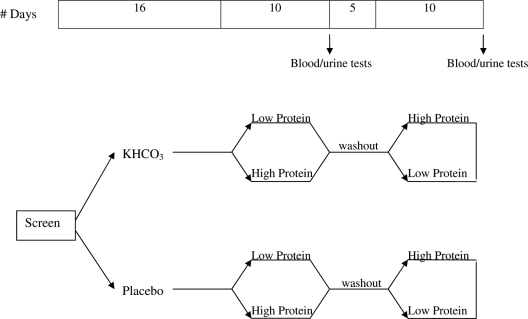
Subjects were given up to 90 mmol/d KHCO3 or placebo for 41 d. A computer-generated randomization scheme was used for block randomization of subjects within sex and age (50 to 64 and 65 and older) strata. Subjects underwent a 16-d phase-in to reach a maximal KHCO3 (or placebo) dose of 90 mmol/d, and then two successive 10-d metabolic diet periods containing either low (0.5 g/kg · d) or high (1.5 g/kg · d) protein in random order, with a 5-d washout period in between on their usual diets (Fig. 1). Blood, urine, and fractional calcium absorption analyses were performed after each diet period.
Figure 1.
Study design. Randomization to placebo or KHCO3 on d 1. Sixteen-day phase-in period followed by two successive 10-d metabolic diets (low protein, 0.5 g/kg · d; or high protein, 1.5 g/kg · d) in random order with a 5-d washout period in between.
Healthy men and postmenopausal women age 50 and older were recruited through direct mailings and local newspaper advertisements, and they were prescreened by telephone. Before study entry, subjects were screened with a medical history, physical examination, and fasting blood and urine tests within 6 months of the study start date. Exclusion criteria included body mass index of 38 kg/m2 of more; vegetarianism; use of medications including oral glucocorticoids, estrogen, osteoporosis medications, thiazide diuretics, and nonsteroidal antiinflammatory drugs; medical conditions including kidney stones, cirrhosis, gastroesophageal reflux, active hyperparathyroidism, untreated thyroid disease, significant immune disorders, unstable heart disease, adrenal insufficiency, primary aldosteronism, Bartter’s syndrome, and diabetes mellitus; total hip bone mineral density (BMD) T-score below −3.0; creatinine clearance below 50 ml/min/1.73 × m2 of body surface area; 24-h UCa excretion greater than 300 mg/d; abnormal serum calcium; elevated alkaline phosphatase; and serum 25-hydroxyvitamin D [25(OH)D] level below 16 ng/ml.
Twenty-six subjects were screened, and 23 were enrolled. Subjects were asked to maintain their usual diet, exercise level, and body weight; to discontinue their own calcium and vitamin D supplements; and to avoid bicarbonate- or potassium-rich products during the study. Three individuals randomized to the KHCO3 group discontinued the study for reasons unrelated to treatment. Twenty subjects (11 in the placebo group and nine in the KHCO3 group) completed the study. One subject on placebo was excluded from this analysis because of a suspected acid-base disorder as indicated by a 10-fold higher net acid excretion (NAE) compared with the mean in other subjects, a low urine pH, and a 3-fold higher N-telopeptide level. Characteristics of the 19 subjects included in this analysis are shown in Table 1.
Table 1.
Subject characteristics before study entry
| Placebo (mean ± sd) | KHCO3 (mean ± sd) | |
|---|---|---|
| n | 10 | 9 |
| Females (n) | 8 | 5 |
| Age (yr) | 62 ± 7 | 62 ± 9 |
| Height (cm) | 164.15 ± 6.27 | 164.78 ± 8.74 |
| Weight (kg) | 64.08 ± 3.73 | 72.38 ± 12.50 |
| BMI (kg/m2) | 23.9 ± 2.3 | 26.6 ± 3.7 |
| Lean body mass (kg) | 40.45 ± 5.63 | 43.39 ± 11.51 |
| Total hip T-score | −0.43 ± 0.72a | −0.08 ± 0.74 |
| 25(OH)D (ng/ml) | 26.20 ± 6.75 | 23.11 ± 6.25 |
| PTH (pg/ml) | 46.5 ± 7.7b | 47.4 ± 10.4b |
| Serum calcium (mg/dl) | 9.11 ± 0.37 | 9.12 ± 0.19 |
| 24-h UCa (mg) | 102.9 ± 55.5 | 115.5 ± 61.1 |
| 24-h UCa/Cr (mg/g) | 87.4 ± 45.3 | 99.9 ± 40.0 |
| Total energy intake (kcal/d) | 1491.5 ± 412.1 | 1558.0 ± 551.8 |
| Dietary protein intake (g/d) | 69.1 ± 22.1 | 73.0 ± 29.9 |
| Dietary potassium intake (mg/d) | 2909.1 ± 650.0 | 2605.7 ± 1023.6 |
| Dietary calcium intake (mg/d) | 697.9 ± 258.4 | 898.6 ± 635.2 |
| Dietary sodium intake (mg/d) | 2830.0 ± 997.1 | 2471.3 ± 1091.9 |
| PASE score | 175.3 ± 49.3c | 167.9 ± 74.1c |
To convert values for 25(OH)D to nmol/liter, multiply by 2.5; serum PTH to pmol/liter, multiply by 0.11; serum calcium to mmol/liter, multiply by 0.25; UCa to mmol, multiply by 0.025; UCa/Cr ratio to mmol/mol, multiply by 2.82.
Total hip T-score measurements available on eight subjects in the placebo group.
PTH levels were drawn on eight KHCO3 and eight placebo subjects approximately 5 months before the start of the study.
PASE was available for eight subjects in the KHCO3 group and nine subjects in the placebo group.
Diet, supplements, and physical activity
Usual nutrient intakes were assessed by food frequency questionnaire (32) before subjects started the study pills. During the two 10-d metabolic diet cycles, all food and caloric beverages were provided by the Metabolic Research Unit as a 3-d cycle menu. Each subject was studied on a low- and a high-protein diet. The low-protein diet contained 0.5 g/kg · d of protein from natural foods, mainly meat. The high-protein diet contained an additional 1.0 g/kg · d of dietary protein, as lean meat. The contents of the daily diet, calculated with version 4.05 of the University of Minnesota Food and Nutrient Database 34, are shown in Table 2. Phosphorus intake was higher on the high-protein diet because meat contains significant amounts of phosphorus. We chose not to balance the phosphorus in the two diets to simulate the real-life setting. During the study, caffeine-containing beverages were limited to 12 ounces daily, and alcohol was not permitted. Subjects came in at least three times per week to eat a meal, pick up their food, and be weighed. The research dietitian assessed adherence to the diet by reviewing self-report food intake checklists and returned uneaten food and food containers at each visit. Adjustments in the foods provided were made to optimize adherence and maintain body weight. Each day during the study, subjects took a supplement tablet containing 600 mg of calcium, 266 mg of phosphorus, 125 IU of vitamin D3, 50 mg of magnesium (Posture D; US Rhodia, Cranbury, NJ) and a multivitamin (CVS brand) containing 400 IU of vitamin D3 with the evening meal.
Table 2.
Daily nutrient contents of the 10-d low-protein and high-protein metabolic diets in the two groups
| Placebo (mean ± sd) | KHCO3 (mean ± sd) | |
|---|---|---|
| Energy (kcal/d) | ||
| Low | 1990.7 ± 142.3 | 2168.1 ± 240.9 |
| High | 2160.9 ± 72.9 | 2401.1 ± 271.0a |
| Total fat (g/d) | ||
| Low | 105.4 ± 8.7 | 117.7 ± 16.0a |
| High | 84.0 ± 3.8 | 100.6 ± 16.0a |
| Total carbohydrate (g/d) | ||
| Low | 238.7 ± 16.0 | 251.4 ± 20.7 |
| High | 255.6 ± 6.6 | 265.8 ± 16.1 |
| Total protein (g/d) | ||
| Low | 32.1 ± 2.02 | 36.1 ± 5.2a |
| High | 96.7 ± 5.7 | 109.2 ± 17.7a |
| Protein (g/kg · d) | ||
| Low | 0.50 ± 0.01 | 0.50 ± 0.02 |
| High | 1.51 ± 0.02 | 1.51 ± 0.04 |
| Total dietary fiber (g/d) | ||
| Low | 15.6 ± 1.4 | 16.2 ± 2.1 |
| High | 14.8 ± 0.4 | 15.2 ± 1.1 |
| Calcium (mg/d)b | ||
| Low | 586.1 ± 6.3 | 583.8 ± 13.7 |
| High | 602.0 ± 4.9 | 590.1 ± 10.3a |
| Phosphorus (mg/d)b | ||
| Low | 688.3 ± 50.6 | 739.8 ± 63.6 |
| High | 1125.0 ± 55.4 | 1210.4 ± 133.4 |
| Magnesium (mg/d)b | ||
| Low | 197.5 ± 12.6 | 209.3 ± 21.4 |
| High | 244.1 ± 9.0 | 254.0 ± 21.7 |
| Sodium (mg/d) | ||
| Low | 2496.7 ± 140.0 | 2624.3 ± 143.4 |
| High | 2565.0 ± 106.9 | 2704.7 ± 139.5a |
| Potassium (mg/d) | ||
| Low | 2298.5 ± 128.2 | 2396.4 ± 235.0 |
| High | 2348.9 ± 43.0 | 2504.4 ± 225.3a |
Differs from the placebo group within diet P < 0.05.
Each subject also received a supplement containing 600 mg calcium, 266 mg phosphorus, and 50 mg magnesium.
Leisure, household, and occupational activity were assessed on d 1 and 41 with the Physical Activity Scale for the Elderly (PASE) questionnaire (33).
Study capsules and dosing schedule
Capsules containing 7.5 mmol of KHCO3 and matching placebo capsules were made by a local compounding pharmacy. Subjects started on three capsules daily (one after each meal), and gradually increased the dose by three capsules every 3 d to a maximum daily dose of 12 capsules (90 mmol/d; four capsules after each meal with 8 ounces of water), which they took thereafter throughout the study. If a subject developed gastrointestinal distress on the pills, the dose was cut back by three capsules per day, and escalated again 3 d later, as tolerated. A safety serum potassium level was drawn after the 16-d phase-in, but no hyperkalemia was observed.
Biochemical measurements
Blood was drawn after a 12-h overnight fast and between 0700 and 1000 h. All samples from individual subjects were batched for analyses, with the exception of the serum potassium measurement on d 17 (a safety measurement). Serum 25(OH)D was measured with RIA kits from Diasorin (Stillwater, MN) with coefficients of variation (CV) of 5.6–7.7%. Serum intact PTH, IGF-I, IGF binding protein-3 (IGFBP-3), and osteocalcin were measured by chemiluminescent immunoradiometric assays on an automated immunoassay system, (IMMULITE 1000, Diagnostic Product Corporation, Los Angeles, CA). The CV for this assay ranged from 3.0–9.0%. Serum calcium, potassium, phosphorus, and 24-h urinary sodium (UNa), potassium (UK) and creatinine (UCr) were measured on an automated clinical chemistry analyzer (Olympus AU400; Olympus America Inc., Melville, NY). The CVs for these assays ranged from 3.0–6.0%. The 24-h UCa was measured by direct-current plasma emission spectroscopy (Beckman SpectraSpan VI Direct Current Plasma Emission Spectrophotometer; Beckman Instruments, Fullerton, CA) with a CV of 3–5%. Twenty-four-hour urinary N-telopeptide (UNTX) was measured by ELISA (Wampole, Princeton, NJ), with a CV of 5.6–7.7%. Twenty-four-hour UNi was measured with a model FP-2000 nitrogen/protein determinator (LECO, St. Joseph, MI), which employs a Dumas combustion method and detection using a thermal conductivity cell. It measures nitrogen with a precision of 15 ppm. Twenty-four-hour NAE was measured by a modification of the Jorgensen titration method (34), as described by Chan (35): NAE = titratable acid + NH4+ − HCO3−. Briefly, titratable acid − HCO3− was assessed after addition of HCl, boiling the sample, and then titrating the sample to neutral pH. To measure the NH4+, formol was added to the sample to release the H+ from NH4+, and the sample was again titrated to neutral pH. All titrations were carried out with a TIM 900 Titration Manager (Radiometer Analytical, Loveland, CO). The precision of NAE measurements in our laboratory was determined by analyzing aliquots of a single 24-h urine collection on 15 different days. The aliquots were stored frozen at −20 C and thawed only once. The CV of these measurements was 10.1%.
Calcium absorption
Calcium absorption was measured in each subject on the last day of each metabolic diet period using dual tracer stable isotope technique (36). A 2-wk interval was needed between these measurements to clear much of the stable isotopes after the first administration (thus the 5-d gap between the two diet cycles). On the last day of each diet cycle, subjects arrived at the center after an overnight fast, had a peripheral iv catheter placed, and were given breakfast. Toward the end of breakfast, subjects were given 44Ca (15 mg for subjects weighing <80 kg and 23 mg for those ≥80 kg) that had been mixed in 240 ml of calcium-fortified Minute Maid orange juice (340 mg of calcium). The breakfast and tracer drink combined contained a total of 400 mg of calcium. Two hours after breakfast, 42Ca (1–1.5 mg for subjects weighing <80 kg and 2.3 mg for those weighing ≥80 kg) was infused iv over 2 min. A 24-h urine collection began immediately after the oral tracer was administered with breakfast. When the collection was completed, an aliquot was prepared and analyzed by the method of Chen et al. (37). The 42Ca/44Ca ratio was measured by a magnetic sector inductively coupled plasma mass spectrometer (ICP-MS, Bremen, Germany). Fractional calcium absorption was determined as the ratio of the cumulative oral tracer recovery to the cumulative iv tracer recovery in the 24-h urine collections obtained after dosing. The precision of this method is less than 1%. The stable isotopes were purchased from Trace Sciences International Corp. (Richmond Hill, Ontario, Canada). The isotopic enrichments for these tracers were greater than 95%. Tracers were prepared by the Tufts Medical Center Research Pharmacy and were tested for sterility and pyrogenicity before use.
Dual-energy x-ray absorptiometry
BMD of the total hip and whole skeleton and whole-body soft-tissue composition were measured at the beginning of the study with a model Prodigy dual-energy x-ray absorptiometry scanner (GE-Lunar, Madison, WI). CVs were less than 1% for the hip BMD and lean body mass, as described previously (38).
Statistical analysis
For the analyses of the dietary protein effect, urine measurements were not corrected for creatinine because UCr excretion is dependent upon protein intake; in analyses of the KHCO3 effect, urine measurements were expressed as a ratio to UCr. Subject characteristics in the KHCO3 and placebo groups and unadjusted differences between groups were compared using t-tests for two independent samples. Paired t-tests were used to assess the effects of dietary protein on outcome variables in the placebo group. Pearson correlation coefficients were used to describe linear associations. Analysis of covariance was used to compute and compare means adjusted for various variables across groups. Two-sided P values less than 0.05 were considered to indicate statistical significance. Statistical analyses were conducted with SPSS version 15.0 (SPSS Inc., Chicago, IL).
Results
The placebo group included a few more women; as a result, a trend toward lower body weight was observed in this group (P = 0.061; Table 1), and intakes of total energy and some nutrients were lower (Table 2). Otherwise, characteristics of the two groups did not differ significantly.
Neither body weight nor physical activity changed significantly during the study in either treatment group (P > 0.270), and changes in these measures did not differ between groups (P > 0.545). The 25(OH)D levels at the end of the study were similar in the two groups (27.9 ± 3.3 ng/ml in the placebo group, 26.3 ± 3.9 ng/ml in the KHCO3 group, P = 0.353). Adherence (mean ± sd) to study pills for the placebo and KHCO3 groups was 95 ± 8% and 94 ± 8%, respectively, during the 41-d study period. Adherence to the dietary supplements during the same period was 99 ± 1% in the placebo group and 97 ± 8% in the KHCO3 group.
Effects of dietary protein
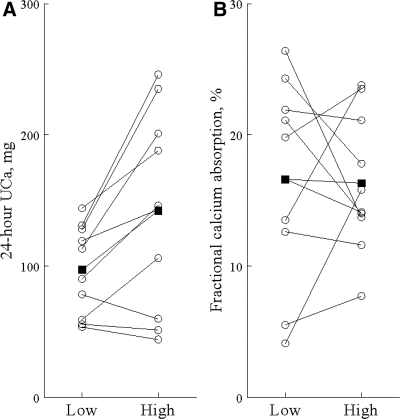
The effects of dietary protein on indices of muscle and bone metabolism were examined in the 10 placebo subjects. In these subjects, an increase in dietary protein intake was associated with significant increases in NAE (by 12.6 ± 8.1 mEq/liter; P = 0.001), UNi (by 9.1 ± 2.2 g; P < 0.001), IGF-I (by 24.3 ± 18.1 ng/ml; P = 0.002), and UCa (by 44.8 ± 47.9 mg; P = 0.016) (Fig. 2A) and a decrease in PTH (by −6.9 ± 8.8 pg/ml; P = 0.037). In contrast, changes in fractional calcium absorption (Fig. 2B), IGFBP-3, serum calcium, phosphorus, osteocalcin, and UNTX were not statistically significant.
Figure 2.
UCa (A) and fractional calcium absorption (B) on the low- and high-protein diets in the placebo group (n = 10). Each line with open circles represents an individual subject. The line with the black-filled square is the mean. The P value for the difference between levels of protein: P = 0.016 (A); P = 0.913 (B).
Effects of KHCO3
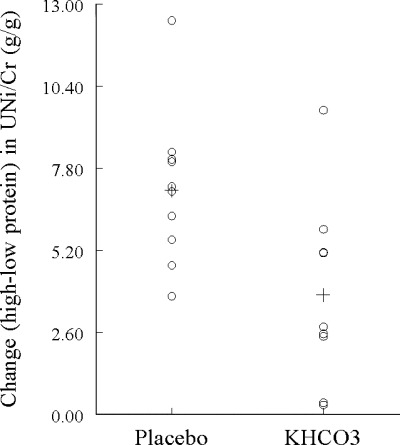
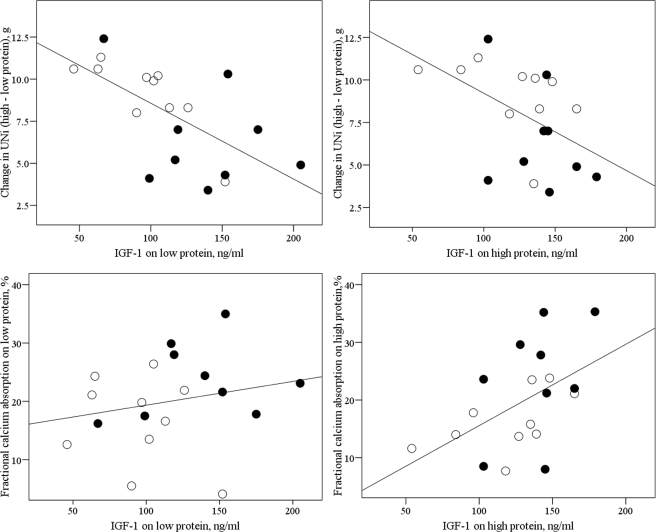
Supplementation with KHCO3 had the expected effects of lowering NAE and increasing UK on both diets (Table 3). The muscle, calcium, and bone indices during each diet cycle and the changes in these indices with an increase in dietary protein were compared across the two treatment groups (Table 3). The rise in UNi to creatinine ratio (UNi/Cr) with an increase in protein intake was less in the KHCO3 group than the placebo group (P = 0.015; Fig. 3). KHCO3 supplementation was also associated with higher IGF-I levels on the low-protein diet (P = 0.027), with a similar trend on the high-protein diet (P = 0.050). There was no statistically significant difference in IGFBP-3 levels between the KHCO3 group and the placebo group (Table 3). Notably, adjustment for IGF-I level on either diet eliminated the statistically significant effect of KHCO3 on the change in UNi with an increase in protein intake (P > 0.125 for treatment effect after adjustment). In addition, in all 19 subjects, the change in UNi with an increase in protein intake was inversely correlated with IGF-I level both on the low-protein diet (r = −0.650; P = 0.003; Fig. 4, top left) and the high-protein diet (r = −0.480; P = 0.036; Fig. 4, top right).
Table 3.
Serum and urine biochemistry at the end of the low- and high-protein diets and changes in these measurements with an increase in dietary protein in the two groups
| Low protein (mean ± sd) | High protein (mean ± sd) | Change (mean ± sd) | |
|---|---|---|---|
| Serum | |||
| IGF-I (ng/ml) | |||
| Placebo | 95.9 ± 31.7 | 120.2 ± 33.2 | 24.3 ± 18.1 |
| KHCO3 | 136.4 ± 41.3a | 139.4 ± 25.3b | 3.0 ± 25.6c |
| IGFBP-3 (μg/ml) | |||
| Placebo | 4.12 ± 0.69 | 4.24 ± 0.72 | 0.12 ± 0.44 |
| KHCO3 | 4.57 ± 1.09 | 4.57 ± 0.70 | 0.00 ± 0.54 |
| Osteocalcin (ng/ml) | |||
| Placebo | 6.2 ± 2.6 | 6.9 ± 4.3 | 0.6 ± 3.6 |
| KHCO3 | 6.7 ± 3.8 | 6.6 ± 2.9 | −0.1 ± 2.6 |
| PTH (pg/ml) | |||
| Placebo | 46.2 ± 10.6 | 39.3 ± 12.8 | −6.9 ± 8.8 |
| KHCO3 | 35.2 ± 13.8 | 38.8 ± 13.3 | 3.6 ± 8.9c |
| Calcium (mg/dl) | |||
| Placebo | 9.15 ± 0.40 | 9.00 ± 0.27 | −0.14 ± 0.39 |
| KHCO3 | 9.02 ± 0.21 | 8.96 ± 0.54 | −0.07 ± 0.60 |
| Phosphorus (mg/dl) | |||
| Placebo | 3.71 ± 0.40 | 3.49 ± 0.42 | −0.22 ± 0.38 |
| KHCO3 | 3.57 ± 0.52 | 3.43 ± 0.55 | −0.13 ± 0.37 |
| 24-h urine | |||
| UCr (g) | |||
| Placebo | 1.03 ± 0.25 | 1.20 ± 0.29 | 0.17 ± 0.42 |
| KHCO3 | 1.04 ± 0.39 | 1.30 ± 0.39 | 0.26 ± 0.21 |
| 24-h urine corrected for creatinine | |||
| UNi/Cr (g/g) | |||
| Placebo | 4.2 ± 0.8 | 11.3 ± 2.2 | 7.1 ± 2.4 |
| KHCO3 | 5.9 ± 3.0 | 9.7 ± 2.4 | 3.8 ± 3.0c |
| UCa/Cr (mg/g) | |||
| Placebo | 101.0 ± 48.4 | 121.3 ± 70.9 | 20.3 ± 52.4 |
| KHCO3 | 108.7 ± 60.4 | 110.0 ± 62.1 | 1.3 ± 26.8 |
| UNTX/Cr (nmol/mmol) | |||
| Placebo | 41.0 ± 15.2 | 40.4 ± 19.1 | −0.6 ± 14.0 |
| KHCO3 | 37.1 ± 10.5 | 35.1 ± 7.0 | −2.0 ± 5.8 |
| UNa/Cr (mEq/g) | |||
| Placebo | 99.1 ± 28.5 | 93.8 ± 24.1 | −5.2 ± 37.4 |
| KHCO3 | 136.4 ± 42.8a | 107.3 ± 25.2 | −29.2 ± 34.9 |
| UK/Cr (mEq/g) | |||
| Placebo | 53.9 ± 15.1 | 49.5 ± 17.7 | −4.3 ± 18.7 |
| KHCO3 | 140.2 ± 55.0a | 110.9 ± 28.2a | −29.3 ± 38.1 |
| NAE/Cr (mEq/g) | |||
| Placebo | 10.2 ± 9.1 | 33.9 ± 8.2 | 31.9 ± 24.3 |
| KHCO3 | −55.0 ± 34.6a | −23.1 ± 22.0a | 23.7 ± 12.4 |
| Calcium absorption | |||
| Fractional calcium absorption (%) | |||
| Placebo | 16.6 ± 7.6 | 16.3 ± 5.2 | −0.27 ± 7.6 |
| KHCO3 | 23.7 ± 6.3a | 23.5 ± 10.0d | −0.3 ± 7.0 |
To convert values for IGF-1to μg/liter, multiply by 1.0; osteocalcin to μg/liter, multiply by 0.17; serum PTH to pmol/liter, multiply by 0.11; serum calcium to mmol/liter, multiply by 0.25; serum phosphorus to mmol/liter, multiply by 0.32; UCr to mmol, multiply by 8.84; UNi/Cr to mmol/mmol, multiply by 4.04; UCa/Cr ratio to mmol/mol, multiply by 2.82.
Differs from placebo group within diet at P < 0.05.
Differs from placebo group within diet at P = 0.05.
Change differs from placebo group at P < 0.05.
Differs from placebo group within diet at P = 0.064.
Figure 3.
Dot plot of the change (high–low protein diet) in UNi/Cr by treatment group. Each open circle represents the change for an individual subject. The cross (+) represents the mean change in each group. P for difference between groups = 0.015.
Figure 4.
Top left, IGF-I on low protein and change (high–low protein) in UNi (r = −0.650; P = 0.003; n = 19). ○, Placebo; •, KHCO3. Top right, IGF-I on high protein and change (high–low protein) in UNi (r = −0.480; P = 0.036). Bottom left, IGF-I on low-protein diet and fractional calcium absorption on low protein (r = 0.216; P = 0.374). Bottom right, IGF-I on high protein and fractional calcium absorption on high protein (r = 0.507; P = 0.027).
Fractional calcium absorption was higher in the KHCO3 group than the placebo group on the low-protein diet (P = 0.041), and there was a similar trend on the high-protein diet (P = 0.064, Table 3). In all 19 subjects, fractional calcium absorption and IGF-I were positively correlated on the high-protein diet (r = 0.507; P = 0.027; Fig. 4, bottom right) but not on the low-protein diet (r = 0.216; P = 0.374; Fig. 4, bottom left). The change in fractional calcium absorption with an increase in protein had no association with the change in IGF-I with an increase in protein (r = 0.079; P = 0.747; n = 19).
The UCa to creatinine ratio (UCa/Cr) did not differ significantly in the two groups on either diet (Table 3), and the change in UCa/Cr with an increase in protein intake also did not differ significantly in the two groups (P = 0.252; Table 3). Adjustment for UNa to creatinine (UNa/Cr) excretion did not substantially alter these results.
The groups did not differ in mean serum PTH on either diet, and the groups had mixed changes in PTH with an increase in protein (Table 3). We did not observe significant effects of either KHCO3 treatment or protein intake on serum calcium, phosphorus, or markers of bone turnover (Table 3). UNa/Cr was higher in the KHCO3 group than the placebo group on the low-protein diet (P = 0.037; Table 3), but not on the high-protein diet.
Adverse effects
Two subjects in the KHCO3 group reported transient gastroesophageal complaints (one had epigastric discomfort and one had two episodes of emesis).
Discussion
Supplementation with 90 mmol/d KHCO3, which resulted in a net alkali-producing intake, reduced by almost 50% the rise in UNi excretion that accompanied increased protein intake in healthy older men and women. In our subjects, who were on fixed protein intakes and had stable weight and physical activity, this reduction in UNi excretion can be considered an indicator of reduced muscle wasting. These findings add to those from a study by Frassetto et al. (11) in which 60–120 mmol of KHCO3 daily for 18 d in 14 healthy postmenopausal women on constant high-protein diets (about 1.6 g/kg · d) resulted in a significant reduction in total UNi excretion from 14.0 ± 0.6 to 13.2 ± 0.5 g/d (P < 0.001).
The fact that IGF-I was higher in the KHCO3 group than the placebo group after each metabolic diet period suggests that it was increased by KHCO3 supplementation. IGFBP-3 levels in the KHCO3 group did not differ significantly from the placebo group. These results are consistent with a study in which healthy adults given ammonium chloride to induce metabolic acidosis had significant decreases in serum IGF-I and no change in IGFBP-3 (39). That decrease in IGF-I was attributed to an impaired hepatic IGF-I response to circulating GH, similar to that seen in prolonged fasting and in protein deprivation. Our study provides the first evidence that ingestion of alkali may increase serum IGF-I levels in healthy older men and women. Because adjustment for IGF-I eliminated the significant effect of KHCO3 treatment on protein-induced increases in nitrogen excretion, our study also provides evidence that IGF-I may be the mediator of a beneficial KHCO3 effect on muscle.
Calcium absorption on low protein was greater in the KHCO3 group than the placebo group with a similar trend on high protein, suggesting that it may be increased by KHCO3. However, Sebastian et al. (29) studied 18 women on and then off KHCO3 and observed no change in calcium absorption 12 d after the KHCO3 treatment was stopped. The positive correlation that we observed between calcium absorption and IGF-I suggests that IGF-I may mediate a positive effect of KHCO3 on calcium absorption. IGF-I has been shown to promote calcium absorption in aged female rats (40), but there are no comparable data in humans. Contrary to our expectations, given our diet-induced rise in IGF-I and previous evidence that protein promotes an increase in calcium absorption (22), we did not observe an effect of increased protein intake on calcium absorption. Differences in our results are not likely to be methodological because we used a dual-tracer stable isotope method as used in the prior study (22). Possible explanations are that Kerstetter et al. (22) kept phosphorus content constant, whereas we allowed it to increase on a high-protein diet, and that both studies were quite small. Our null finding is in agreement with prior balance studies (23,24), two isotopic studies (19,20), and a radiotracer study (21).
In healthy adults, KHCO3 and other alkali therapy have been found to reduce UCa excretion in the setting of acidogenic diets (28,29). The hypocalciuric effect of KHCO3 is presumably due to its neutralization of the acidic environment known to release calcium from bone as a buffer (30). There was a small decrease in UCa excretion in the KHCO3 group compared with the placebo group, but this reduction was not large enough to be statistically significant in this study. We did, however, confirm previous observations that increased dietary protein intake leads to increased calcium excretion (18,22). The source of the calciuria does not appear to be from increased intestinal absorption; other possibilities include altered endogenous fecal calcium excretion and bone. Notably, the studies that have documented calciuria have generally been short in duration.
We did not confirm prior reports of a beneficial KHCO3 effect on markers of bone turnover (29,30) and detected no effect of increased dietary protein intake on osteocalcin and UNTX, in agreement with some (21,22,31), but not other prior studies (29).
This pilot study had some important strengths, including the fact that the dose of KHCO3 effectively neutralized the protein-related acid load, our subjects’ adherence to and persistence in the study were high, and we used the gold-standard dual-tracer stable isotope method for measuring calcium absorption. We chose a parallel arm design to study the KHCO3 effect. A crossover design, as used to study the dietary protein effect, would have added power to observe a KHCO3 effect; however, we wanted to avoid potential carryover effects of KHCO3, which has no well-established washout period. The primary limitation of this study was that we did not have baseline samples for the KHCO3 and placebo groups for study endpoints. In addition, the small sample size may have prevented us from detecting some clinically meaningful effects. Lastly, our findings pertain to high-dose KHCO3 supplementation that caused a net alkali-producing intake; thus, we cannot comment on the degree to which these findings may vary at other doses.
In conclusion, supplementation with KHCO3 attenuates the UNi excretion that accompanies an increase in dietary protein, suggesting that the net effect of dietary protein on muscle may be enhanced by reducing its accompanying acid load. KHCO3 supplementation was also associated with higher fractional calcium absorption, independent of protein intake. Moreover, the KHCO3-induced nitrogen sparing and enhanced calcium absorption appear to be mediated by IGF-I, which was higher on the KHCO3 supplementation. A higher protein intake increased UCa excretion, but not calcium absorption. Larger long-term studies are needed to establish whether KHCO3 supplementation is a worthwhile strategy for reducing age-related muscle wasting and bone loss and to test the hypothesis that IGF-I is a mediator of such effects.
Acknowledgments
We thank the Metabolic Research Unit at the Jean Mayer United States Department of Agriculture (USDA) Human Nutrition Research Center on Aging at Tufts University and the staff at the USDA/Agricultural Research Service Children’s Nutrition Research Center at Baylor College of Medicine for their work on the study.
Footnotes
L.C. was supported by Grant DK007651. This research was supported by the Unilever Corporate Research, Bedfordshire, United Kingdom. This work was also supported by the USDA/ARS under agreement no. 58-1950-7-707. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the USDA.
Disclosure Statement: The authors have nothing to disclose.
First Published Online December 2, 2008
Abbreviations: BMD, Bone mineral density; CV, coefficients of variation; IGFBP-3, IGF binding protein-3; NAE, net acid excretion; 25(OH)D, 25-hydroxyvitamin D; UCa, urinary calcium; UCr, urinary creatinine; UK, urinary potassium; UNa, urinary sodium; UNi, urinary nitrogen; UNTX, urinary N-telopeptide.
References
- Balint JP 1998 Physical findings in nutritional deficiencies. Pediatr Clin North Am 45:245–260 [DOI] [PubMed] [Google Scholar]
- Piatti PM, Monti F, Fermo I, Baruffaldi L, Nasser R, Santambrogio G, Librenti MC, Galli-Kienle M, Pontiroli AE, Pozza G 1994 Hypocaloric high-protein diet improves glucose oxidation and spares lean body mass: comparison to hypocaloric high-carbohydrate diet. Metabolism 43:1481–1487 [DOI] [PubMed] [Google Scholar]
- Leidy HJ, Carnell NS, Mattes RD, Campbell WW 2007 Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring) 15:421–429 [DOI] [PubMed] [Google Scholar]
- Rizzoli R, Bonjour JP 2004 Dietary protein and bone health. J Bone Miner Res 19:527–531 [DOI] [PubMed] [Google Scholar]
- Bell JD, Margen S, Calloway DH 1969 Ketosis, weight loss, uric acid, and nitrogen balance in obese women fed single nutrients at low caloric levels. Metabolism 18:193–208 [DOI] [PubMed] [Google Scholar]
- Vazquez JA, Adibi SA 1992 Protein sparing during treatment of obesity: ketogenic versus nonketogenic very low calorie diet. Metabolism 41:406–414 [DOI] [PubMed] [Google Scholar]
- Lennon EJ, Lemann Jr J, Litzow JR 1966 The effects of diet and stool composition on the net external acid balance of normal subjects. J Clin Invest 45:1601–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibotto G, Russo R, Sofia A, Sala MR, Sabatino C, Moscatelli P, Deferrari G, Tizianello A 1996 Muscle protein turnover in chronic renal failure patients with metabolic acidosis or normal acid-base balance. Miner Electrolyte Metab 22:58–61 [PubMed] [Google Scholar]
- Gougeon-Reyburn R, Lariviere F, Marliss EB 1991 Effects of bicarbonate supplementation on urinary mineral excretion during very low energy diets. Am J Med Sci 302:67–74 [DOI] [PubMed] [Google Scholar]
- Papadoyannakis NJ, Stefanidis CJ, McGeown M 1984 The effect of the correction of metabolic acidosis on nitrogen and potassium balance of patients with chronic renal failure. Am J Clin Nutr 40:623–627 [DOI] [PubMed] [Google Scholar]
- Frassetto L, Morris Jr RC, Sebastian A 1997 Potassium bicarbonate reduces urinary nitrogen excretion in postmenopausal women. J Clin Endocrinol Metab 82:254–259 [DOI] [PubMed] [Google Scholar]
- Bonjour JP, Ammann P, Chevalley T, Rizzoli R 2001 Protein intake and bone growth. Can J Appl Physiol 26 Suppl:S153–S166 [DOI] [PubMed] [Google Scholar]
- Promislow JH, Goodman-Gruen D, Slymen DJ, Barrett-Connor E 2002 Protein consumption and bone mineral density in the elderly: the Rancho Bernardo Study. Am J Epidemiol 155:636–644 [DOI] [PubMed] [Google Scholar]
- Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PW, Kiel DP 2000 Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 15:710–720 [DOI] [PubMed] [Google Scholar]
- Wengreen HJ, Munger RG, West NA, Cutler DR, Corcoran CD, Zhang J, Sassano NE 2004 Dietary protein intake and risk of osteoporotic hip fracture in elderly residents of Utah. J Bone Miner Res 19:537–545 [DOI] [PubMed] [Google Scholar]
- Munger RG, Cerhan JR, Chiu BC 1999 Prospective study of dietary protein intake and risk of hip fracture in postmenopausal women. Am J Clin Nutr 69:147–152 [DOI] [PubMed] [Google Scholar]
- Allen LH, Oddoye EA, Margen S 1979 Protein-induced hypercalciuria: a longer term study. Am J Clin Nutr 32:741–749 [DOI] [PubMed] [Google Scholar]
- Kerstetter JE, Allen LH 1990 Dietary protein increases urinary calcium. J Nutr 120:134–136 [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Harris SS 2002 Calcium intake influences the association of protein intake with rates of bone loss in elderly men and women. Am J Clin Nutr 75:773–779 [DOI] [PubMed] [Google Scholar]
- Heaney RP 2000 Dietary protein and phosphorus do not affect calcium absorption. Am J Clin Nutr 72:758–761 [DOI] [PubMed] [Google Scholar]
- Roughead ZK, Johnson LK, Lykken GI, Hunt JR 2003 Controlled high meat diets do not affect calcium retention or indices of bone status in healthy postmenopausal women. J Nutr 133:1020–1026 [DOI] [PubMed] [Google Scholar]
- Kerstetter JE, O'Brien KO, Caseria DM, Wall DE, Insogna KL 2005 The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab 90:26–31 [DOI] [PubMed] [Google Scholar]
- Hegsted M, Linkswiler HM 1981 Long-term effects of level of protein intake on calcium metabolism in young adult women. J Nutr 111:244–251 [DOI] [PubMed] [Google Scholar]
- Schuette SA, Linkswiler HM 1982 Effects on Ca and P metabolism in humans by adding meat, meat plus milk, or purified proteins plus Ca and P to a low protein diet. J Nutr 112:338–349 [DOI] [PubMed] [Google Scholar]
- Zwart SR, Davis-Street JE, Paddon-Jones D, Ferrando AA, Wolfe RR, Smith SM 2005 Amino acid supplementation alters bone metabolism during simulated weightlessness. J Appl Physiol 99:134–140 [DOI] [PubMed] [Google Scholar]
- Sprague SM, Krieger NS, Bushinsky DA 1994 Greater inhibition of in vitro bone mineralization with metabolic than respiratory acidosis. Kidney Int 46:1199–1206 [DOI] [PubMed] [Google Scholar]
- Arnett TR, Spowage M 1996 Modulation of the resorptive activity of rat osteoclasts by small changes in extracellular pH near the physiological range. Bone 18:277–279 [DOI] [PubMed] [Google Scholar]
- Lemann Jr J, Gray RW, Pleuss JA 1989 Potassium bicarbonate, but not sodium bicarbonate, reduces urinary calcium excretion and improves calcium balance in healthy men. Kidney Int 35:688–695 [DOI] [PubMed] [Google Scholar]
- Sebastian A, Harris ST, Ottaway JH, Todd KM, Morris Jr RC 1994 Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. N Engl J Med 330:1776–1781 [DOI] [PubMed] [Google Scholar]
- Maurer M, Riesen W, Muser J, Hulter HN, Krapf R 2003 Neutralization of Western diet inhibits bone resorption independently of K intake and reduces cortisol secretion in humans. Am J Physiol Renal Physiol 284:F32–F40 [DOI] [PubMed] [Google Scholar]
- Mardon J, Habauzit V, Trzeciakiewicz A, Davicco MJ, Lebecque P, Mercier S, Tressol JC, Horcajada MN, Demigne C, Coxam V 2008 Long-term intake of a high-protein diet with or without potassium citrate modulates acid-base metabolism, but not bone status, in male rats. J Nutr 138:718–724 [DOI] [PubMed] [Google Scholar]
- Block G, Woods M, Potosky A, Clifford C 1990 Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 43:1327–1335 [DOI] [PubMed] [Google Scholar]
- Washburn RA, Smith KW, Jette AM, Janney CA 1993 The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46:153–162 [DOI] [PubMed] [Google Scholar]
- Jorgensen K 1957 Titrimetric determination of the net excretion of acid/base in urine. Scand J Clin Lab Invest 9:287–291 [DOI] [PubMed] [Google Scholar]
- Chan JC 1972 The rapid determination of urinary titratable acid and ammonium and evaluation of freezing as a method of preservation. Clin Biochem 5:94–98 [DOI] [PubMed] [Google Scholar]
- Yergey AL, Abrams SA, Vieira NE, Aldroubi A, Marini J, Sidbury JB 1994 Determination of fractional absorption of dietary calcium in humans. J Nutr 124:674–682 [DOI] [PubMed] [Google Scholar]
- Chen Z, Griffin IJ, Kriseman YL, Liang LK, Abrams SA 2003 Inductively coupled plasma mass spectrometric analysis of calcium isotopes in human serum: a low-sample-volume acid-equilibration method. Clin Chem 49:2050–2055 [DOI] [PubMed] [Google Scholar]
- White J, Harris SS, Dallal GE, Dawson-Hughes B 2003 Precision of single vs bilateral hip bone mineral density scans. J Clin Densitom 6:159–162 [DOI] [PubMed] [Google Scholar]
- Brungger M, Hulter HN, Krapf R 1997 Effect of chronic metabolic acidosis on the growth hormone/IGF-1 endocrine axis: new cause of growth hormone insensitivity in humans. Kidney Int 51:216–221 [DOI] [PubMed] [Google Scholar]
- Fleet JC, Bruns ME, Hock JM, Wood RJ 1994 Growth hormone and parathyroid hormone stimulate intestinal calcium absorption in aged female rats. Endocrinology 134:1755–1760 [DOI] [PubMed] [Google Scholar]