Abstract
Context: Studies have shown that labor occurs primarily in the night/morning hours. Recently, we identified the human myometrium as a target for melatonin (MEL), the neuroendocrine output signal coding for circadian night.
Objective: The purpose of this study was to determine the effects of MEL on contractility and the contractile machinery in telomerase-immortalized human myometrial cells.
Design: To ascertain the effect of MEL on myometrial contractility in vitro, we performed gel retraction assays with cells exposed to iodomelatonin ± oxytocin (OT). The effects of iodomelatonin on gap junctions were also investigated. Additionally, expression levels of the type 2 MEL receptor (MT2R) were assessed in myometrial biopsies from term pregnant women with or without labor.
Results: MEL was found to synergistically enhance OT-induced contractility via the MT2R, which is coupled to a protein kinase C-dependent increase in phosphorylation of the myosin light chain protein. MT2R expression was markedly elevated in samples from pregnant women who had entered labor, as compared to matched nonlaboring pregnant women. MEL increased expression of the gap junction protein, connexin 43. In vitro dye spread assays showed that MEL-treated cells displayed substantially increased intercellular coupling. Increases in connexin 43 mRNA and cell to cell coupling were also found to be mediated via the MT2R in a protein kinase C-dependent manner.
Conclusions: MEL synergizes with OT to promote myometrial cell contractions and to facilitate gap junction activity in vitro. Such a synergy in vivo would promote coordinated and forceful contractions of the late term pregnant uterus necessary for parturition.
Melatonin synergizes with oxytocin to enhance myometrial cell contractions via increased activation of myosin light chain and elevated gap junction activity.
Continuous monitoring of normal uterine contractile activity during late term pregnancy in humans has shown increased frequency between 2030 h and 0200 h (1). Studies on the timing of human labor onset and delivery show that the initiation of labor peaks between 2400 h and 0500 h, regardless of gestational age (2). Current models describe parturition as a multistep process beginning with myometrial activation in late pregnancy, followed by stimulation leading to uterine contraction and subsequent delivery of the infant. Myometrial activation encompasses cellular remodeling with appropriate changes in gene expression. The increased expression of these “contraction-associated proteins” marks the transition of the myometrium from a quiescent to activated state. These proteins facilitate the powerful uterine contractions necessary to deliver the infant by increasing the excitability of the myometrial cells, enhancing smooth muscle myosin-actin interactions, and increasing intercellular connectivity, thereby facilitating synchronous myometrial contractions (3).
After its activation, the myometrium can be stimulated by multiple factors including oxytocin (OT), prostaglandins, and noradrenaline (4,5). OT, a nonapeptide hormone secreted by the pituitary gland, is one of the most potent uterine contractants. OT, upon binding to its Gq protein-coupled receptor [OT receptor (OTR)], activates the membrane-bound phospholipase C (PLC) and subsequently protein kinase C (PKC). Inositol trisphosphate, cleaved from membrane phospholipids, binds receptors on the sarcoplasmic reticulum, triggering the release of Ca2+ from intracellular stores as well as the influx of extracellular Ca2+ via membrane calcium channels. Increases in intracellular calcium concentrations result in activation of the Ca2+/calmodulin-dependent enzyme, myosin light chain kinase, thereby leading to the phosphorylation of the myosin light chain and myometrial contraction (6,7).
Melatonin (MEL), a monoamine hormone secreted by the epithalamic pineal gland, is a major molecular messenger of the nocturnal phase of the light-dark cycle. MEL signals via two G protein-coupled receptors, MEL receptor 1 (MT1R) and MEL receptor 2 (MT2R) (8). We have previously shown that human myometrium is a target for MEL and expresses both MEL receptors (9,10). These studies pointed to a potential point of interaction between OT and MEL signaling pathways. An earlier report demonstrated that MEL potentiates norepinephrine-induced contractility in a dose-dependent manner in human myometrial strips (11).
Our previous work showed striking similarities between MEL regulation of OTR mRNA expression and the regulation of OTR mRNA expression by OT (10,12), leading us to explore further the similarities between the MEL and OT signaling pathways in the myometrium. Herein, we investigated the effects of MEL on myometrial contractility in vitro by conducting experiments with the well-characterized telomerase-immortalized human myometrial (hTERT) smooth muscle cell line that has been shown to express OTRs (13). The results of the present studies show that MEL acts synergistically via the MT2R/PLC/PKC signaling pathway to significantly increase sensitivity of myocytes to OT and increase OT-induced contractility in a dose-dependent manner. In addition, we also investigated the potential effects of MEL on expression of the gap junction protein, connexin 43 (Cx43). Expression of Cx43 is known to increase late in human pregnancy, thereby facilitating myometrial cell coupling and synchronization of uterine contraction (3). Our data reveal that MEL treatment of cultured myometrial cells increased both mRNA and protein levels of Cx43 via the MT2R signaling cascade. Taken together, these studies point to a novel regulatory function of the circadian hormone MEL in “gating” human myometrial activity. More specifically, our data provide a model system to investigate the mechanism through which MEL interacts with the OT pathway to promote uterine contractility and parturition.
Materials and Methods
Cell culture
hTERT cells were maintained in DMEM/F12 (Mediatech, Manassas, VA) medium with 10% fetal bovine serum (Hyclone, Logan, UT) with penicillin/streptomycin and gentamycin at 37 C and 5% CO2. Cells were trypsinized at 90% confluency and plated in T175 cell culture flasks at a 1:5 dilution or six-well plates at 20,000 cells per well. For pharmacological experiments, cells were treated with iodomelatonin (I-MEL) (Tocris Bioscience, Ellisville, MO) or OT (Sigma-Aldrich, St. Louis, MO) or cotreated as described in Results. Pharmacological inhibitors, 4-phenyl-2-propionamidotetralin (4P-PDOT), a MT2R-specific antagonist, the general PKC inhibitor C1, and the PLC inhibitor U73122 (all from Tocris) were applied as a pretreatment 1 h before application of I-MEL or OT. After treatment, the cells were trypsinized, pelleted, washed in PBS, and frozen at −20 C until further analysis.
Immunoblotting
Frozen myometrial samples from pregnant women before and after the onset of labor and from nonpregnant women were obtained from a National Institutes of Health-funded tissue repository as described previously (14). Term pregnancy without labor was defined as gestational wk 38–40 with no sign of uterine contractions or cervical changes, whereas term pregnancy with true labor was defined as undergoing at least three spontaneous (not induced), regular uterine contractions in a 10-min interval in association with progressive increases in cervical effacement and dilation, or dilation of more than 4 cm. Uterine contractions in the absence of cervical change were considered “false labor.” Myometrial tissues from women with clinical or histological chorioamnionitis, rupture of membranes more than 12 h, placenta previa, antiphospholipid antibody syndrome, abnormal vaginal discharge, or positive myometrial cultures for β-strep, gonorrhea, trichomonas, or syphilis were also excluded.
For in vitro investigations, cultured hTERT cells were collected by trypsinization and gentle scraping. Cells were suspended in PBS and pelleted by centrifugation. Protein extraction was performed according to the method of Shearman et al. (15). After electrophoretic separation on a 10% sodium dodecyl sulfate-polyacrylamide gel, proteins were semi-dry blotted in buffer onto a Whatman PROTRAN Nitrocellulose membrane (Whatman GmbH, Dassel, Germany). Molecular size markers (Amersham, Arlington Heights, IL) were included. The membrane was incubated for 60 min at 20 C in blocking buffer containing 5% milk powder before overnight incubation at 4 C with anti-OTR, anti-MT2R (Santa Cruz Biotechnology, Santa Cruz, CA), anti-Cx43 antiserum (Millipore, Bedford, MA), or anti-actin (Sigma) at a dilution of 1:1000 in blocking buffer. After washing in buffer [20 mm Tris (pH 7.6) + 137 m NaCl + 0.05% Tween-20], the membrane was incubated at 20 C for 1 h with a peroxidase-conjugated affinity purified goat antirabbit Ig (Sigma) in a 1:2000 dilution. Chemiluminescent signals were then detected with the Pierce ECL Western Blotting Substrate (Pierce, Rockford, IL) using CL-X Posure film (Pierce). Western blotting for phosphomyosin light chain kinase (Ser19) (Millipore) was performed with the following exceptions. Membranes were blocked with a 5% BSA/TBS solution for 1 h. The primary antibody was diluted in TBS at a 1:500 dilution at 4 C overnight with shaking. The goat antirabbit Ig was diluted 1:2000 in a 5% milk/TBST solution. Densitometric analysis was performed using AIS image analysis software (AIS, Toronto, Ontario, Canada) of images acquired with a digital camera. Criterion for assessment of samples as immunonegative was the absence of a band after 1 h of exposure. Western blots were repeated a minimum of three times to ensure reproducibility.
Myometrial cell contractility assay
Myometrial cell contractility was assayed using a collagen disk retraction assay as described by Devost and Zingg (16), plating 10,000 hTERT cells per well. Samples were treated as described in the Results, and each treatment was performed in triplicate. Myometrial cell contractility was quantified by capturing images of the fixed collagen disks with a digital camera and analyzing for total area using AIS image analysis software. The results were normalized to the cell-free control sample areas and expressed as a percentage of untreated control area.
Quantitative PCR
Cellular total RNA was extracted with the RNEasy kit (QIAGEN, Valencia, CA) according to the manufacturer’s protocol. The RNA concentration was measured with the Nanodrop photometer and then reverse transcribed to cDNA by means of the iScript RT system (Bio-Rad Laboratories, Hercules, CA). Quantitative real-time PCR was performed on a Bio-Rad iCycler using iQ SYBR Green Supermix (Bio-Rad), together with 1 μl of sense and antisense primers (10 pmol/μl) of the transcript of interest and 2 μl of template cDNA. The following thermal cycling parameters were used: initial heat activation of the DNA-polymerase was performed at 95 C for 5 min. Thereafter, 40 cycles at 95 C (15 sec), 58 C (30 sec), and 72 C (30 sec) were run. After thermocycling, the iCycler performs an automatic melting curve, which entails cooling to 55 C for 10 sec and then increasing temperatures in 0.2 C increments up to 90 C. This controls for primer-dimer formation and other nonspecific effects. Quantification of the data was achieved by the Bio-Rad iCycler software using a standard curve from a primer-specific dilution series for the PCR product. Data were normalized against expression of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primer sequences used for Cx43 and GAPDH quantification are as follows: Cx43 forward, 5′-ATG AGC AGT CTG CCT TTC GT-3′; Cx43 reverse, 5′-TCT GCT TCA AGT GCA TGT CC-3′; GAPDH forward, 5′-GTC TTC ACC ACC ATG GAG-3′; and GAPDH reverse, 5′-GTC ATG GAT AAC CTT GGC-3′.
Lucifer yellow dye migration assay
Lucifer yellow scrape loading assays were performed in accordance with the procedures of El-Fouly et al. (17). Cells were grown to confluency in 30 mm cell culture dishes, washed with warm PBS, and treated with 2 ml of prewarmed 0.05% lucifer yellow in PBS solution. The cells were then scraped by drawing a scalpel across the plate. After 2 min at room temperature, the plates were washed three times with prewarmed PBS and returned to normal medium. The plates were then photographed at 10 min after scraping using a digital camera through a Zeiss Axiovert 40CFL microscope (Zeiss, Thornwood, NY) at a 100× and 200× magnification. Each treatment condition was repeated three times to ensure reproducibility.
Statistical analyses
Experiments were repeated a minimum of three times. Replicate values for each data point were averaged, and differences were statistically analyzed using a one-way ANOVA followed by the Bonferroni post hoc test (Prism; GraphPad, San Diego, CA) with P < 0.05 as the criterion level for significance. For testing the observed vs. expected percentages of MT2R-immunopositive myometrial samples, a χ2 test was employed with P < 0.01 as criterion level.
Results
Human myometrium is a target for MEL
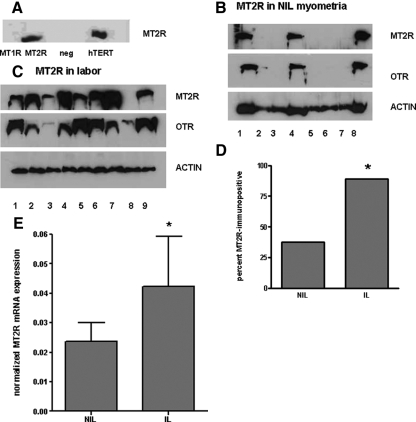
Myometrial tissues express both isoforms of MEL receptors at the transcript and ligand binding levels (9,10). Using frozen myometrial samples, we conducted immunoblotting experiments and in some cases radioreceptor assays. Western blot analysis was inconclusive using commercial antibodies for MT1R, but clearly confirmed the presence of MT2R both in hTERT cells (Fig. 1A) and in term pregnant myometrium. Remarkably, MT2R immunoreactive signals were detected in 89% of myometrial samples from women in labor, compared with only 38% of tissues from pregnant women before the onset of labor (Fig. 1, B–D). Myometrial tissues from nonpregnant women were completely devoid of MT2R mRNA and immunoreactivity (data not shown). Additional immunoblotting experiments were also conducted to ascertain a potential correlation between OTR and MT2R expression. As shown in Fig. 1B, the MT2R-immunopositive myometria were all likewise OTR-immunopositive, whereas MT2R-negative tissues were typically OTR-negative or in a single case only very weakly OTR-positive. We also found that MT2R mRNA levels were elevated in tissues from laboring patients (Fig. 1E). These results point to a certain degree of regulated coexpression of MT2R and OTR in human myometrial tissue at the time of labor. Additionally, 125I-MEL binding was increased significantly in MT2R-immunopositive tissues (from laboring women), whereas MEL binding was barely detectable in samples (from nonlaboring women) in which the MT2R was not detected by Western blot (data not shown). This large difference in specific 125I-MEL binding implicates the MT2R as the primary site of MEL binding in myometrial smooth muscle (data not shown).
Figure 1.
Functional MEL receptors in the human myometrium. A, Western blot for the MT2R in hTERT cells, uteroleioma cells stably transfected with MT1R, MT2R, or neither (neg). B and C, MT2R immunoreactivity in myometrial punches from pregnant nonlaboring patients (B) and patients in active labor (C). Numbers in B and C represent individual donor samples. D, Results from panels B and C in histogram form. *, P < 0.05 by χ2 statistic. E, MT2R mRNA expression in tissues from the same laboring (IL) and nonlaboring (NIL) patients as in B and C. *, P < 0.05 relative to NIL.
MEL increases OT sensitivity and myometrial contractility in vitro via the MT2R/PLC/PKC signaling pathway
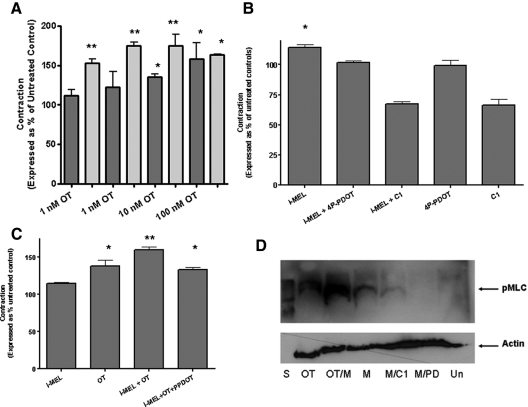
Because MEL has been reported to signal via the MT2R to activate Gq mechanisms involving PLC and PKC (8) and these same signaling pathways are used by the OTR, we hypothesized that cross-talk between the MT2R and OTR might modulate myometrial contractions induced by OT. To investigate this possibility, we performed collagen gel retraction assays in accordance with the published protocols of Devost and Zingg (18). Cotreatment of cultured hTERT myometrial smooth muscle cells with 1 nm I-MEL and 1 nm OT resulted in a 2-fold statistically significant increase in contractility compared with treatment with OT alone. I-MEL acted to increase OT-induced contractility in a dose-dependent manner as well as increasing the sensitivity to OT (Fig. 2A). Pharmacological experiments were then conducted with the MT2R-specific antagonist 4P-PDOT (10 nm) and the general PKC inhibitor, C1 (10 μm). Treatment with 4P-PDOT reduced the synergistic effect of I-MEL to levels corresponding to treatment with OT alone (Fig. 2C). Treatment with C1 reduced myometrial cell contractility when cells were treated alone or in combination with OT, I-MEL, or both (Fig. 2B). Treatment with the PLC inhibitor, U73122, completely abolished contractility in response to all treatments (data not shown). Western blot analysis for phosphorylated myosin light chain at Ser19 indicated that although I-MEL treatment alone resulted in modest increases in myosin light chain phosphorylation, OT-induced increases in myosin light chain phosphorylation were increased dramatically by I-MEL (Fig. 2D). I-MEL-induced increases in myosin light chain phosphorylation were abolished by 4P-PDOT or C1 pretreatment (Fig. 2D), suggesting that MT2R and PKC are essential for I-MEL-induced increases in myosin light chain phosphorylation.
Figure 2.
Effects of I-MEL on OT-induced contractility. A, Contractility of hTERT myometrial cells treated with OT (dark bars) or cotreated with 1 nm I-MEL (light bars). *, P < 0.05 relative to control values; **, P < 0.05 relative to all columns marked with single asterisk. B, Effects of treatment with 10 μm of the PKC inhibitor C1, or 10 nm of the MT2R-specific antagonist 4P-PDOT, on I-MEL induced contractility. *, P < 0.05 relative to control. C, Effect of 4P-PDOT pretreatment on the contractility of samples cotreated with I-MEL and OT. **, P < 0.05 relative to OT-treated and I-MEL/OT/4P-PDOT-treated samples. *, P < 0.05 significantly elevated over controls. D, Effects of treatment with 1 nm of I-MEL (M) and/or OT on the phosphorylation of the myosin light chain regulatory subunit and the effect of cotreatment with 10 nm 4P-PDOT (PD) or 10 μm C1. Un, Untreated control cells; S, size markers.
MEL increases expression of the gap junction protein Cx43 in vitro through a PKC-dependent pathway
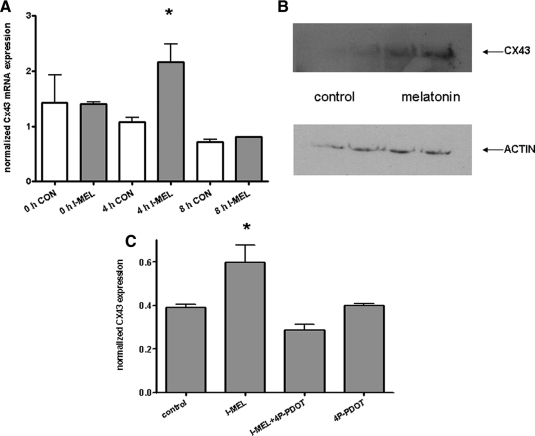
Cx43 expression has been shown to be up-regulated in human myometrium at term and by PKC activation in cultured cells (19). Initially, we investigated the acute effects of I-MEL on Cx43 mRNA levels in cultured hTERT myocytes by quantitative PCR. Treatment with I-MEL (1 nm) resulted in significant but transient increases in Cx43 mRNA after 4 h (Fig. 3A). Western blot analysis confirmed up-regulation of Cx43 protein levels in I-MEL-treated cells (Fig. 3B). Cotreatment with 4P-PDOT abolished the effect of I-MEL on Cx43 expression, as did treatment with U73122 or C1 (Fig. 3C). These results indicate that induction of myometrial cell Cx43 by I-MEL involves MT2R signaling via PLC and PKC.
Figure 3.
Effects of I-MEL on the expression of the gap junction protein, Cx43. A, Effects of treatment with 1 nm I-MEL (filled bars) or control vehicle (open bars) on Cx43 mRNA levels in hTERT cells collected at 0, 4, and 8 h (mean ± sem; n = 9). B, Effects of treatment with I-MEL on Cx43 protein levels. C, Effect of cotreatment with 4P-PDOT on Cx43 mRNA levels.
To ascertain the effects of I-MEL in a physiological context, we investigated the effects of I-MEL on Cx43 mRNA expression in an 8-h time course experiment. Cells were treated with I-MEL (1 nm) for 8 h to mimic a natural nocturnal phase of the light-dark cycle. Cx43 mRNA levels were assayed at times 0, 4, and 8 h. Cx43 mRNA expression was elevated at 4 h after MEL treatment but had returned to untreated levels 8 h after treatment. These data indicate that Cx43 mRNA expression may be nocturnally stimulated by physiological exposures to MEL (Fig. 3A). The return of Cx43 mRNA expression to control levels after 8 h indicates the likely presence of additional methods of regulation of Cx43 mRNA expression.
MEL increases intercellular connectivity in vitro
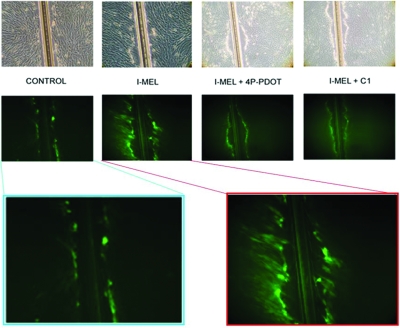
I-MEL-induced increases in Cx43 mRNA and protein led us to predict that intercellular communication would be increased due to the formation of additional gap junctions. To test this hypothesis, we performed lucifer yellow dye migration assays on I-MEL-treated hTERT cells. Results of these experiments show that the cells from MEL-pretreated plates have increased levels of cell coupling, allowing for a greater spread of the lucifer yellow dye from the site of uptake, an effect that can be blocked by pretreatment with 4P-PDOT or C1 (Fig. 4). Treatment with 4P-PDOT or C1 alone resulted in dye migration comparable to control levels (data not shown).
Figure 4.
Effects of MEL on gap junction communication in hTERT cells. The top row shows the cells under bright field, whereas the remaining figures were photographed under fluorescent light to demonstrate lucifer yellow dye spread within 10 min after scrape loading. Treatments include 1 nm I-MEL, or I-MEL after pretreatment of the cells with 10 nm 4P-PDOT or 10 μm C1. The bottom two images at higher magnification reveal lucifer yellow dye spread in greater detail.
Discussion
The molecular mechanisms leading to forceful uterine contractions of labor involve many interacting factors and regulatory pathways. Our data present a novel mechanism of interaction of the nocturnal brain hormone MEL with the OT-signaling pathway in human myometrial cells. These interactions likely occur at multiple points in the signaling cascade. Herein, we demonstrate that cotreatment with physiological concentrations of MEL increases both basal- and OT-induced contractility of myometrial cells and that this stimulatory effect can be blocked by the application of the MT2R-specific antagonist 4P-PDOT, the general PKC inhibitor C1, and the PLC inhibitor U73122. From these data it can be concluded that MEL acts synergistically via MT2R to activate PLC, which likely triggers an increase in intracellular calcium. This increase in intracellular Ca2+, while presumably only modestly sufficient to augment basal contractility alone (Fig. 2), appears to greatly facilitate OT-induced contractility by sensitizing myometrial cells to OT. Kitazawa et al. (20) previously described a calcium sensitization phenomenon in which PKC resulted in increased force generation in permeabilized uterine strips clamped at a constant Ca2+ environment. Because MEL has also been shown to activate PKC in multiple tissues (21) and we have previously reported that MEL regulates OTR expression in myometrial cells through PKC (10), we suggest that MEL increases sensitivity to OT not only through Ca2+-sensitization but also through modulated OTR expression. This agrees with our model that MEL acts synergistically to promote contractility in uterine smooth muscle cells.
OT-induced contractility is initiated by increased phosphorylation of myosin light chain at Ser19, which facilitates conformational changes in myosin and interactions with actin. Investigation of I-MEL treatment on myosin light chain phosphorylation (Fig. 2D) showed changes in myosin light chain that agree with our observations from the contractility studies. These data support the conclusion that the responsible receptor is a Gq coupled MT2R rather than a Gi coupled MT2R or MT1R (8), which could theoretically augment contractility by suppressing cAMP levels. Although high levels of cAMP have been shown to relax myometrium (3), the myometrium is somewhat refractory to cAMP-induced relaxation relative to other smooth muscles. Our previous work has shown that in this system, MEL signaling is pertussis toxin-insensitive and PKC-dependent (10) and that MEL treatment inhibits forskolin-induced cAMP accumulation only in myometrial samples from nonpregnant women (9). Taken together, these findings further strengthen the notion that the synergistic effect of MEL on OT-induced contractility is via MT2R.
MTR and OTR expression levels were similarly different in myometrial samples from nonlaboring and laboring women. MT2R immunoreactive signals were detected in 89% of myometrial samples from women in labor but in only 38% of samples from pregnant women not in labor (P < 0.05). All samples that were immunopositive for MT2R were also OTR positive. This suggests that low MT2R levels during preterm pregnancy may preclude the synergistic input of MEL on contractility and thus serve to maintain myometrial quiescence. Activation of gene expression at labor also is consistent with the MT2R, like the OTR, being necessary to provide maximum uterine contractility during parturition, and that the nocturnal surges in MEL temporally gate these contractions to occur preferentially at night. To date, the regulatory mechanisms underlying gene expression for both the OTR and MTR are poorly understood; indeed our results appear to be the first report of positive regulation of MT2R expression in humans. The similar regulation of both OTR and MT2R and the fact that both serum OT (22) and MEL levels (23,24) increase over the course of pregnancy support our hypothesis that MEL acts synergistically to promote OT-induced contractility in pregnant women at term.
Activation of myometrium late in gestation is associated with marked increases in gap junction proteins. The increase in gap junctions is thought to facilitate greater communication between myometrial cells to allow for synchronized contractions. Cx43 protein is the primary component of myometrial gap junctions in term myometrium (3,19). We show here that unlike OT, which to date has been shown to have little effect on Cx43 protein levels and gap junction formation in humans (25), I-MEL treatment results in significant increases in Cx43 mRNA and protein levels, and that it leads to increased intercellular coupling between uterine myocytes. These data indicate that MEL not only promotes contractility through direct action on the contractile machinery but also acts to facilitate synchronized contractions via increased gap junction-mediated intercellular communication. Both actions would enhance nocturnal uterine contractility at term.
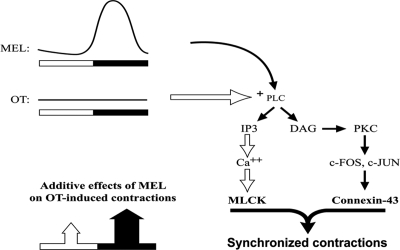
In conclusion, we propose the model shown in Fig. 5 to explain the action of MEL in promoting nocturnal contractility during labor. Release of MEL from the pineal gland into the circulation at night leads to binding of MEL to myometrial MT2Rs. Bound MEL activates PLC, which generates inositol trisphosphate and increases intracellular calcium, thus activating myosin light chain kinase. The phosphorylation of myosin light chain results in increased contractility and enhanced sensitivity to OTR-mediated signals. Additionally, the diacylglycerol released by MEL binding to the MT2R activates PKC, which has been shown to act via c-fos and c-jun to increase Cx43 expression (26). It is important to note that modest increases in hTERT cell contractility in vitro by I-MEL alone were always detected. However, the greatest increases in contractility were achieved when both I-MEL and OT were provided concomitantly. We propose that this neuroendocrine synergy plays a key role in the increase in births observed in the late evening and early morning in humans.
Figure 5.
Proposed model for the synergy between MEL and OT on nocturnal myometrial contractility in the laboring pregnant uterus. MEL acts synergistically with OT to increase PLC activity and associated signaling mechanisms, thereby enhancing myometrial contractility and gap junction-associated intercellular communication. DAG, Diacylglycerol.
The results of this study point to MEL playing a procontractile role in human myometrial physiology during pregnancy. Combined with our previous observations regarding the action of I-MEL on OTR mRNA expression, it appears that MEL and OT signaling is very similar in the human myometrium. These data provide new insights into the mechanisms underlying the timing of birth and regulation of the contractile machinery in the myometrium. Additionally, they reveal a novel physiological interaction whose further characterization may serve in the development of new pharmacological strategies for the management of preterm and/or delayed parturition.
Acknowledgments
The authors thank Dr. Dominic Devost, Dr. Hans Zingg, and Dr. Katrina Slaughter for their helpful advice and technical assistance.
Footnotes
This work represents a portion of the doctoral studies of J.T.S.
Disclosure Statement: J.T.S. and R.P. have nothing to disclose. R.A.W. received grant support (National Institutes of Health Grant PPG 11149) for procurement of the myometrial tissues. J.O. has received the provisional patent U.S. 60991866.
First Published Online November 11, 2008
Abbreviations: Cx43, Connexin 43; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; hTERT, telomerase immortalized human myometrial; I-MEL, iodomelatonin; MEL, melatonin; MT1R, type 1 MEL receptor; MT2R, type 2 MEL receptor; OT, oxytocin; OTR, OT receptor; PKC, protein kinase C; PLC, phospholipase C; 4P-PDOT, 4-phenyl-2-propionamidotetralin.
References
- Zahn V, Hattensperger W 1993 [Circadian rhythm of pregnancy contractions]. Z Geburtshilfe Perinatol 197:1–10 [PubMed] [Google Scholar]
- Seron-Ferre M, Ducsay CA, Valenzuela GJ 1993 Circadian rhythms during pregnancy. Endocr Rev 14:594–609 [DOI] [PubMed] [Google Scholar]
- Smith R 2007 Parturition. N Engl J Med 356:271–283 [DOI] [PubMed] [Google Scholar]
- Blanks AM, Shmygol A, Thornton S 2007 Preterm labour. Myometrial function in prematurity. Best Pract Res Clin Obstet Gynaecol 21:807–819 [DOI] [PubMed] [Google Scholar]
- Berg G, Andersson RG, Ryden G 1986 α-Adrenergic receptors in human myometrium during pregnancy. Am J Obstet Gynecol 154:601–606 [DOI] [PubMed] [Google Scholar]
- Arthur P, Taggart MJ, Mitchell BF 2007 Oxytocin and parturition: a role for increased myometrial calcium and calcium sensitization? Front Biosci 12:619–633 [DOI] [PubMed] [Google Scholar]
- Sanborn BM, Ku CY, Shlykov S, Babich L 2005 Molecular signaling through G-protein-coupled receptors and the control of intracellular calcium in myometrium. J Soc Gynecol Investig 12:479–487 [DOI] [PubMed] [Google Scholar]
- Masana MI, Dubocovich ML2001 Melatonin receptor signaling: finding the path through the dark. Sci STKE 2001, PE39 [DOI] [PubMed] [Google Scholar]
- Schlabritz-Loutsevitch N, Hellner N, Middendorf R, Muller D, Olcese J 2003 The human myometrium as a target for melatonin. J Clin Endocrinol Metab 88:908–913 [DOI] [PubMed] [Google Scholar]
- Sharkey J, Olcese J 2007 Transcriptional inhibition of oxytocin receptor expression in human myometrial cells by melatonin involves protein kinase C signaling. J Clin Endocrinol Metab 92:4015–4019 [DOI] [PubMed] [Google Scholar]
- Martensson LG, Andersson RG, Berg G 1996 Melatonin together with noradrenaline augments contractions of human myometrium. Eur J Pharmacol 316:273–275 [DOI] [PubMed] [Google Scholar]
- Phaneuf S, Asboth G, Carrasco MP, Linares BR, Kimura T, Harris A, Bernal AL 1998 Desensitization of oxytocin receptors in human myometrium. Hum Reprod Update 4:625–633 [DOI] [PubMed] [Google Scholar]
- Condon J, Yin S, Mayhew B, Word RA, Wright WE, Shay JW, Rainey WE 2002 Telomerase immortalization of human myometrial cells. Biol Reprod 67:506–514 [DOI] [PubMed] [Google Scholar]
- Word RA, Tang DC, Kamm KE 1994 Activation properties of myosin light chain kinase during contraction/relaxation cycles of tonic and phasic smooth muscles. J Biol Chem 269:21596–21602 [PubMed] [Google Scholar]
- Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, Reppert SM 2000 Interacting molecular loops in the mammalian circadian clock. Science 288:1013–1019 [DOI] [PubMed] [Google Scholar]
- Devost D, Zingg HH 2007 Novel in vitro system for functional assessment of oxytocin action. Am J Physiol Endocrinol Metab 292:E1–E6 [DOI] [PubMed] [Google Scholar]
- El-Fouly MH, Trosko JE, Chang C-C 1987 Scrape loading and dye transfer: a rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res 168:422–430 [DOI] [PubMed] [Google Scholar]
- Devost D, Zingg HH 2007 Novel in vitro system for functional assessment of oxytocin action. Am J Physiol Endocrinol Metab 292:E1–E6 [DOI] [PubMed] [Google Scholar]
- Geimonen E, Boylston E, Royek A, Andersen J 1998 Elevated connexin-43 expression in term human myometrium correlates with elevated c-Jun expression and is independent of myometrial estrogen receptors. J Clin Endocrinol Metab 83:1177–1185 [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Kobayashi S, Horiuti K, Somlyo AV, Somlyo AP 1989 Receptor-coupled, permeabilized smooth muscle. Role of the phosphatidylinositol cascade, G-proteins, and modulation of the contractile response to Ca2+. J Biol Chem 264:5339–5342 [PubMed] [Google Scholar]
- Sampson SR, Lupowitz Z, Braiman L, Zisapel N 2006 Role of protein kinase Cα in melatonin signal transduction. Mol Cell Endocrinol 252:82–87 [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F 2001 The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81:629–683 [DOI] [PubMed] [Google Scholar]
- Kivela A 1991 Serum melatonin during pregnancy. Acta Endocrinol (Copenh) 124:233–237 [PubMed] [Google Scholar]
- Kivela A, Kauppila A, Leppaluoto J, Vakkuri O 1989 Serum and amniotic fluid melatonin during human labor. J Clin Endocrinol Metab 69:1065–1068 [DOI] [PubMed] [Google Scholar]
- Ciray HN, Backstrom T, Ulmsten U 1998 Ineffectiveness of oxytocin on intercellular communication between term pregnant human myometrial cells before labor. Am J Obstet Gynecol 178:855–861 [DOI] [PubMed] [Google Scholar]
- Mitchell JA, Lye SJ 2001 Regulation of connexin 43 expression by c-fos and c-jun in myometrial cells. Cell Commun Adhes 8:299–302 [DOI] [PubMed] [Google Scholar]