Abstract
Context: Calcitonin (CT) is the main medullary thyroid carcinoma (MTC) tumor marker. However, it has several limitations, including a concentration-dependent biphasic half-life, sensitivity to rapid in vitro degradation, and the presence of different isoforms/fragments. Procalcitonin (PCT), the prohormone of calcitonin, is free of these limitations but is currently used only as a sepsis marker.
Objectives: The objective of the study was to determine whether PCT is suited as a MTC tumor marker by comparing the diagnostic performance of PCT with that of CT in MTC.
Design: PCT and CT were measured in a total of 835 subjects, including normal volunteers (n = 197) and patients with active-MTC (n = 91), cured-MTC (n = 42), neuroendocrine tumors (n = 225), mastocytosis (n = 48), follicular cell-derived thyroid carcinoma (cured = 120, persistent/recurrent = 55), and benign thyroid disease (n = 57).
Results: PCT levels were significantly higher in the active-MTC patients (mean 126.4 ng/ml) than the cured-MTC patients (mean <0.1 ng/ml). The overall concordance between the two markers was 95.7% (κ = 0.81). Receiver-operating characteristic curve analysis showed no significant difference in diagnostic performance between CT and PCT. PCT’s diagnostic sensitivity and specificity were 91 and 96%, respectively. The corresponding values for CT were 99 and 98%. Analyte stability studies showed that CT is very unstable in vitro with a decrease of 35–50% from the original value 24 h after the blood draw, whereas PCT levels did not significantly change during this time.
Conclusions: A strong correlation was observed between PCT and CT levels in patients with MCT. Given PCT’s greater analytical stability, we conclude that it represents a promising complementary MTC tumor marker.
Procalcitonin is a complementary tumor marker to calcitonin in patients with medullary thyroid carcinoma.
Medullary thyroid carcinoma (MTC) is a malignant tumor of the thyroid cells C cells, accounting for about 3% of thyroid cancers (1). Most MTCs overproduce the main C cell secretory product, calcitonin (CT). CT is considered a sensitive and specific marker for MTC and is therefore used for the diagnosis and the follow-up of MTC (1). Serial measurements of calcitonin are useful for quantification of tumor mass and disease progression, with doubling times of less than 2 yr being associated with a poorer prognosis (2). Carcinoembryonic antigen (CEA) is also used for monitoring MTC patients. CEA, although a nonspecific and insensitive marker for detection of early MTC, is a useful follow-up marker, with increases being a possible predictor of disease progression (3,4).
One of the shortcomings of CT is that modest increases in serum CT concentrations might be observed in C cell hyperplasia, other neuroendocrine tumors, certain leukemias, systemic mastocytosis, small cell carcinoma of the lung, breast or pancreatic cancer, renal failure, hyperparathyroidism, autoimmune thyroiditis, pregnancy, lactation, and during the neonatal period (4,5,6,7,8). CT also presents several analytical challenges: 1) a concentration-dependent and biphasic half-life (∼15 and ∼40 min at physiological concentrations; ∼3 and ∼30 h at elevated concentrations) (9), which complicates its use for monitoring success of treatment interventions; 2) sensitivity to relatively rapid degradation by serum proteases, which can lead to false-low or false-negative test results if samples are not processed expeditiously after a blood draw; and 3) the presence of various different immunoreactive isoforms and fragments (10,11,12), which can lead to inaccurate results (usually false low) as well as poor comparability of results obtained by different assays.
A possible alternative tumor marker that is largely free of these problems is the precursor of CT, procalcitonin (PCT). Expression of PCT in healthy individuals is limited to thyroid C cells and it is found at low concentrations in the circulation. Unlike CT, PCT has a concentration-independent and highly predictable in vivo half-life of 20–24 h (13), and it displays excellent in vitro stability in serum or plasma (14).
PCT elevations are known to occur in severe bacterial infections or sepsis due to PCT production by nonthyroidal tissues (15). PCT assays are currently approved by the Food and Drug Administration to aid in the risk assessment of critically ill patients for progression to severe sepsis and septic shock. Although increased levels of PCT have been reported in patients with MTC (16), PCT has not been systematically evaluated for its utility as a potential MTC tumor marker.
The aim of this study was to validate PCT as a MTC tumor marker. This included: 1) establishing a healthy reference range for PCT and reestablishing a CT reference range on the same population, 2) establishing an athyrotic reference range for CT and PCT, and 3) comparing the clinical performance of CT and PCT for the diagnosis of MTC.
Subjects and Methods
This study was approved by the Mayo Clinic Institutional Review Board. A total of 835 subjects were included:
1. Normal subjects (n = 197; 98 males, aged 22–76 yr; 99 females, aged 22–79 yr).
2. Athyrotic individuals (n = 120; 32 males, ages 15–84 yr; 88 females, aged 20–75 yr) who had undergone total thyroidectomy for follicular cell-derived thyroid carcinoma and who had an unstimulated serum thyroglobulin level of less than 0.1 ng/ml, no detectable thyroglobulin autoantibodies by sensitive immunometric assays, and no clinical or imaging evidence of recurrence.
3. Patients with confirmed active MTC (n = 91, 48 males, aged 31–93 yr; 43 females, aged 22–77 yr), based on clinical, imaging, or histological evidence of persistent or recurrent disease or continuous elevation of serum CT without evidence for a non-MTC cause of the elevation. Patients with active MTC included eight cases of newly diagnosed disease, 43 cases of recurrent or metastatic disease, and 40 cases with stable disease. Patients were classified with stable disease if they had persistent elevations of CT without a significant increase in the levels on multiple serial measurements. These patients had either been monitored for a small, unchanged lesion for at least 5 yr or a lesion had not been identified by imaging studies. Multiple samples from some patients with active MTC were available for a follow-up period of up to 1 yr (stable disease n = 6; metastatic disease n = 6). Six of the eight patients with newly diagnosed disease had specimens available prior and after surgery. Four of these patients were considered to be disease free after surgery, based on imaging and biochemical studies, whereas two patients had residual disease based on the same criteria.
4. Patients with confirmed MTC, deemed disease free by clinical assessment, imaging, and serum CT determinations (cured MTC; n = 42; 14 males, aged 12–75 yr; 28 females, aged 20–80 yr).
5. Patients with non-MTC neuroendocrine tumors (n = 225; 123 males, aged 18–83 yr; 102 females, aged 25–85 yr), mainly carcinoids and islet cell carcinomas.
6. Patients with mastocytosis (n = 48; 13 males, aged 20–71 yr; 35 females, aged 28–81 yr).
7. Patients with high serum thyroglobulin levels (40–6959 ng/ml) (n = 55; 22 males, aged 19–82 yr; 33 females, aged 22–82 yr) who were confirmed to have persistent or recurrent follicular cell-derived malignancy, including follicular thyroid carcinoma (n = 8), Huerthle cell carcinoma (n = 12), papillary thyroid carcinoma (n = 32), and anaplastic thyroid carcinoma (n = 3).
8. Patients with benign thyroid diseases, including benign thyroid nodules, hyperthyroidism, hypothyroidism, and goiter (n = 57; 14 males, aged 28–82 yr; 43 females, aged 17–79 yr).
The normal subjects were recruited through the Mayo Clinic Department of Laboratory Medicine and Pathology reference value program. The specimens from all other subjects were obtained from residual samples from Mayo Clinic patients who had undergone CT, thyroglobulin/thyroglobulin autoantibodies, or chromogranin A testing. All samples were stored at −20 C immediately after clinical testing, until testing for PCT (all samples) or CT (samples not previously tested for CT) was performed. The final diagnosis was obtained by reviewing the medical records from the time when the initial analyte measurement was performed to the last available follow-up visit. Patients with renal failure were excluded because this will result in serum CT and PCT elevations.
Analyte measurements
CT was measured by an immunochemiluminometric assay using an Immulite 2000 analyzer (Siemens Healthcare Diagnostics, Deerfield, IL). The assay has a functional sensitivity (lowest analyte concentration with an interassay coefficient of variation <20%) of 5.0 pg/ml. Values greater than 50.0 pg/ml were serially diluted to confirm the results because occasional values above 50.0 pg/ml have shown nonlinear dilution due to CT isoform or fragment interferences.
PCT was measured on a Kryptor system (BRAHMS USA, Annapolis, MD) by a homogenous time-resolved amplified cryptate emission immunometric fluorescent assay (17). The functional sensitivity of the assay was determined to be 0.1 ng/ml. Values greater than 2.0 ng/ml were automatically diluted by the instrument to obtain a value within the linear range of the assay.
CEA was measured by immunochemiluminometric assay on a UniCel DXI 800 (Beckman Coulter, Brea, CA). Values greater than 3.0 ng/ml were considered positive.
Analyte stability and CT accuracy at elevated analyte concentrations
Five patient samples were tested for CT and PCT immediately after being drawn and after being stored at room temperature, 4 C and −20 C for 24 h.
Two patients in the active-MTC cohort were found to have CT values inconsistent with previous results. These samples were repeated after dilution. In addition, over a 2-month period, all samples with undiluted serum CT concentrations of greater than 50 pg/ml were reviewed (n = 300), and original results were compared with their respective backcalculated diluted values. All samples were serially diluted until a backcalculated value was obtained that differed less than 20% from the previous dilution.
Data analysis
CT and PCT values below the functional sensitivity of the assays were replaced with 4.99 pg/ml and 0.09 ng/ml, respectively, for data analysis purposes. Data analysis, including the κ statistic calculation, was performed using JMP version 7 (SAS Institute, Inc., Cary, NC).
Analytical assay performance of CT and PCT were compared by linear regression of all CT and PCT measurements above the functional sensitivities of the assays after log transforming the data to obtain a normally distributed data set.
P values were calculated using a two-sided unpaired t test for the comparison of PCT levels in cured-MTC and active-MTC patients and a two-sided paired t test for the log-transformed PCT and CT correlation by linear regression.
Normal population reference ranges were calculated using nonparametric analysis following the guidelines of the Clinical Laboratory Standards Institute C28-A3 document (18). The 97.5th percentile of the population was set as the upper reference limit.
Receiver-operating characteristic (ROC) curves were constructed and analyzed using Analyze-it (Leeds, UK). In addition to comparing PCT and CT diagnostic performance in the entire subject cohort, ROC curve analysis was used to analyze test performance for several subgroups that correspond to possible clinical scenarios: 1) work-up of a thyroid nodule or suspected thyroid malignancy (patients with MTC compared with patients with follicular cell derived carcinomas or benign thyroid illness); 2) false-positive CT or PCT elevations (patients with MTC compared with patients with mastocytosis or neuroendocrine tumors); and 3) MTC clinical follow-up (patients with active MTC vs. patients with cured MTC).
Results
A summary of the diagnosis, demographic data and CT, PCT, and CEA measurements for the patient cohort is provided in Table 1.
Table 1.
PCT, CT, and CEA levels in the study groups
| Mean PCT ng/ml | Median PCT ng/ml | PCT positive levels | Mean CT pg/ml | Median CT pg/ml | CT positive levels | Mean CEA ng/ml | Median CEA ng/ml | CEA positive levels | |
|---|---|---|---|---|---|---|---|---|---|
| Active MTC | |||||||||
| Newly diagnosed | 13.8 | 5.2 | 8/8 | 1336.0 | 777.5 | 8/8 | 53.1 | 17.8 | 8/8 |
| Recurrent/metastatic | 241.7 | 13.1 | 42/43 | 20447.9 | 3403.0 | 42/43 | 435.5 | 32.7 | 26/30 |
| Stable | 3.3 | 0.6 | 33/40 | 334.7 | 78.0 | 40/40 | 19.2 | 3.7 | 18/32 |
| Cured MTC | <0.1 | <0.1 | 0/42 | 5.5 | <5.0 | 1/42 | 3.5 | 1.3 | 3/30 |
| Follicular cell-derived carcinomas | 0.1 | <0.1 | 3/55 | <5.0 | <5.0 | 0/55 | NT | NT | NT |
| Benign thyroid illnesses | 0.1 | <0.1 | 1/57 | <5.0 | <5.0 | 0/57 | NT | NT | NT |
| Athyroid | <0.1 | <0.1 | 0/120 | <5.0 | <5.0 | 0/120 | NT | NT | NT |
| Neuroendocrine tumors | 0.17 | <0.1 | 22/225 | 16.2 | <5.0 | 8/225 | NT | NT | NT |
| Mastocytosis | <0.1 | <0.1 | 2/48 | 6.1 | <5.0 | 2/48 | NT | NT | NT |
| Healthy donors | <0.1 | <0.1 | 1/197 | 5.2 | <5.0 | 0/197 | NT | NT | NT |
NT, Not tested.
Reference ranges for CT and PCT
The reference intervals for CT in healthy volunteers were determined to be less than 8.0 pg/ml for females and less than 16.0 pg/ml for males. For PCT, the upper limit for normal volunteers was 0.15 ng/ml or less.
For athyrotic individuals, the upper limit of the reference range for both genders was less than 5.0 pg/ml for CT and less than 0.1 ng/ml for PCT.
Serum CT and PCT concentrations in patients with neuroendocrine tumors, mastocytosis, and active follicular cell-derived thyroid carcinomas
In patients with neuroendocrine tumors, elevations in PCT and CT were observed in 10% (22 of 225) (range 0.16–9.60 ng/ml) and 4% (eight of 225) (range 8.9–2261 pg/ml) of patients, respectively (Table 1). In patients with a diagnosis of mastocytosis, only two (different) patients each had elevated serum CT and PCT levels (Table 1).
To further test the specificity of CT and PCT, patients diagnosed with follicular cell-derived carcinoma (n = 55) and benign thyroid illness (n = 57) were evaluated. CT was within reference range limits in all patients tested. PCT levels were slightly elevated (between 0.21 and 0.34 ng/ml) in four patients (one with hyperthyroidism and three with metastatic follicular cell derived thyroid carcinoma).
Serum CT and PCT concentrations in active-MTC and cured-MTC patients
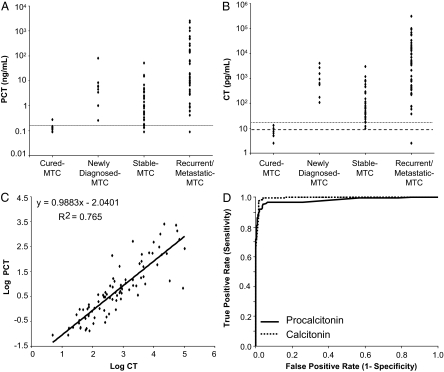
PCT levels were above the reference range cutoff in 83 of the 91 active-MTC patients (range 0.16–2585 ng/ml; Fig. 1A). The mean PCT levels were significantly higher (P = 0.006) in the active-MTC patients (126.4 ng/ml) compared with the cured-MTC patients (<0.1 ng/ml). Among active-MTC patients, those with stable disease had the lowest mean PCT levels (3.6 ng/ml) followed by patients with newly diagnosed MTC (13.8 ng/ml) and patients with recurrent/metastatic disease (241.7 ng/ml). Because CT levels are currently used as a key criterion to establish the diagnosis of MTC, the CT levels were above the reference range in 90 of the 91 patients with active MTC. The lowest mean CT levels were detected in patients with stable disease (334.7 pg/ml) followed by patients with newly diagnosed MTC (1336.0 pg/ml) and patients with recurrent and metastatic disease (20447.9 pg/ml) (Fig. 1B). Log-transformed paired CT and PCT measurements correlated significantly with each other (P < 0.0001; Fig. 1C).
Figure 1.
A, Distribution of PCT in cured-MTC and active-MTC patients. The horizontal line corresponds to the upper limit of the normal population reference range (0.15 ng/ml). B, Distribution of CT in cured-MTC and active-MTC patients. The horizontal lines correspond to the upper limit of the reference range for males (dotted, 16 pg/ml) and females (dashed, 8 pg/ml). C, Relationship between log-transformed CT (Log10 pg/ml) and corresponding PCT (Log10 ng/ml) concentrations for samples containing CT and PCT concentrations above the respective functional sensitivity limits of the two assays. The equation for the linear fit of the log-transformed data and the r2 are shown. D, ROC curve analysis for CT and PCT in the 835 specimens included in the study. Nearly identical ROC curves were obtained when the diagnostic performance of CT and PCT were compared in various subgroups (see Subjects and Methods and Results for details).
In all patient subgroups, ROC curve analysis of PCT and CT diagnostic performance yielded statistically indistinguishable areas under the curve (AUCs). For the entire cohort, these were 0.99 [95% confidence interval (CI) 0.99–1.0] for CT and 0.98 (95% CI 0.97–0.99) for PCT (Fig. 1D). In the subset of MTC patients compared with patients with other malignant or benign thyroid conditions, the respective AUCs were 0.99 (95% CI 0.98–1.00) for CT and 0.98 (95% CI 0.96–0.99) for PCT, whereas the comparison of MTC patients with patients with mastocytosis and neuroendocrine tumors showed AUCs of 0.99 (95% CI 0.98–1.00) for CT and 0.98 (95% CI 0.96–0.99) for PCT. In the follow-up scenario of patients with active MTC vs. cured MTC, CT showed an AUC of 0.98 (95% CI 0.97–1.0) and PCT an AUC of 0.97 (95% CI 0.95–1.0). The PCT concentration that provided the best diagnostic accuracy was 0.16 ng/ml, which corresponds closely to the laboratory established upper limit of the normal reference population of 0.15 ng/ml or less.
Using the in-house established reference ranges for CT and PCT for healthy volunteers, the agreement between the two markers was 96.5% for active-MTC cases and 95.8% for cured-MTC cases, which gave 95.7% overall agreement and a κ of 0.81 (95% CI 0.74–0.86). The seven discordant active-MTC cases had PCT values within the reference range and CT values of 10, 12, 16, 18, 43, 60, and 80 pg/ml. These cases had been followed up for a period of 4–22 yr without changes in CT and considered to have stable disease. In all these cases, the CEA levels were negative.
In the patients without MTC, the most common causes for discrepancies between CT and PCT measurements were mastocytosis (CT positive and PCT negative or vise versa; n = 4) and neuroendocrine tumors (CT negative and PCT positive; n = 17).
PCT had a sensitivity of 91% (83 of 91) for the detection of active MTC. The eight cases with active MTC that were missed by PCT included the seven cases with long-term stable disease described above and one case missed by both PCT and CT. PCT specificity was calculated using various subgroups. The assay specificity was 99.5% when compared with healthy individuals, 98% when compared against patients with other benign or malignant thyroid diseases, and 96% when compared against all the cases included in the study. CT had a sensitivity of 99% (85 of 86) and specificities of 100, 100, and 99%, respectively, in the same groups.
Because CEA is often used to monitor patients with MTC, we also determined the performance of CEA in these patients. CEA was positive in 100% of newly diagnosed MTC, 87% of cases with stable disease, 56% of cases with recurrent/metastatic disease, and 10% of cured-MTC cases (Table 1).
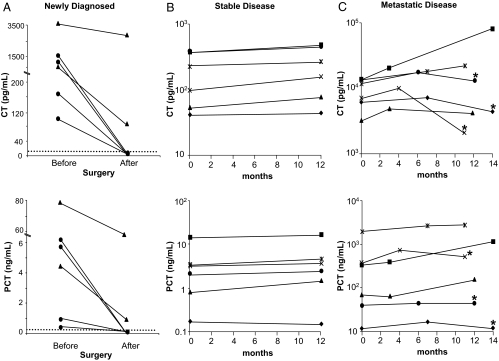
In the subgroup of MTC patients, for whom more than one residual CT sample was available, both CT and PCT levels mirrored the clinical course. In patients who were disease free after surgery, PCT and CT levels decreased in a similar fashion from values above the reference range to values within the reference range (Fig. 2A, circles). In the two patients with residual metastatic disease, the levels of PCT and CT remained elevated (Fig. 2A, triangles). In patients with stable disease, the levels of CT and PCT remained constant during a 1-yr follow-up period (Fig. 2B). In contrast, in patients with metastatic disease (n = 6), the levels of CT and PCT increased over the 1-yr follow-up period in those patients who did not receive any further treatment (Fig. 2C). By contrast, three patients in which ZD6474 (Zactima; vandetanib, AstraZeneca Pharmaceuticals LP, Wilmington, DE) treatment was initiated exhibited a decrease in the levels of CT after 6 months of treatment, although the levels remained above the upper limit of the healthy population reference range. PCT levels were significantly decreased in two of these patients, whereas one patient did not show a change in PCT (Fig. 2C).
Figure 2.
A, PCT and CT concentrations in six patients newly diagnosed with MTC. Measurements were taken prior and after surgery. Three patients were tested 1 month after surgery and three patients 6 months after surgery. The circles represent patients who were disease free after surgery, and the triangles represent patients with residual metastatic disease after surgery. B, PCT and CT concentrations in six patients with stable disease. Measurements were taken 12 months apart. C, PCT and CT concentrations in six patients with metastatic disease. At least three measurements were available for each patient during a 14-month period. Asterisks represent the level of CT and PCT after 6–7 months of the initiation of ZD6474 therapy.
Analytical challenges of CT measurements
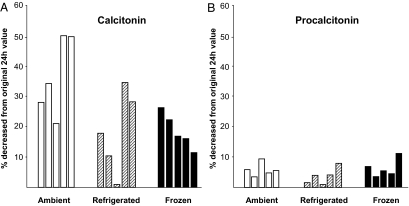
As previously described, CT proved to be a very unstable analyte showing up to 50% decrease in concentration when samples were stored at room temperature and up to 35% when stored refrigerated for 24 h (Fig. 3A). Freezing improved stability to a variable, but incomplete degree. PCT showed much better stability under all conditions, with levels decreasing no more that 10% (Fig. 3B).
Figure 3.
Stability of PCT and CT at various storage conditions. Five donors were tested for CT (A) and PCT (B) immediately after the blood draw. Samples were aliquoted and stored ambient, refrigerated, or frozen for 24 h. Thereafter samples were tested for CT and PCT. The graphs indicate the percent decrease in analyte levels compared with the initial measurement done immediately after the blood draw.
Another reported problem of CT is false-low results, mostly due to a pseudohook effect caused by CT fragments, leading to seemingly chaotic serial CT measurements (10,11,12). Two study patients with recurrent/metastatic disease showed CT levels inconsistent with previous results and the clinical picture (Table 2), whereas their PCT values were consistent between measurements. Retesting of these samples after dilution yielded CT levels consistent with the previous measurements, suggesting the presence of an analytical interference, likely CT fragments. In addition, during a 2-month period, we observed 27 from a total of 300 patients with CT levels greater than 50 pg/ml, in whom the initial value would have been underestimated by as much as 70% if the laboratory had not diluted the original sample (Table 3).
Table 2.
Inconsistent serial calcitonin levels in patients with progressive MTC
| Patient | Collection date | CT (pg/ml) | CT after dilution (pg/ml) | PCT (ng/ml) |
|---|---|---|---|---|
| 1 | October 11, 2006 | 3116 | NT | 69.8 |
| November 17, 2006 | 9.3 | 2289 | 75.6 | |
| January 3, 2007 | 4816 | NT | 61.6 | |
| 2 | November 20, 2006 | 48 | NT | 0.9 |
| July 23, 2007 | 220 | NT | 3.5 | |
| December 5, 2007 | 2.5 | 457 | 7.6 |
NT, Not tested
Table 3.
Nonlinear calcitonin levels due to pseudohook effect of the immunoassay
| Patient | CT (pg/ml), undiluted value | CT (pg/ml) × 5 dilution | CT (pg/ml) fold increase | CT (pg/ml) × 10 dilution | CT (pg/ml) fold increase |
|---|---|---|---|---|---|
| 1 | 1583 | 2774 | 1.8 | 3046 | 1.9 |
| 2 | 1345 | 2923 | 2.2 | 3454 | 2.6 |
| 3 | 1708 | 2167 | 1.3 | 2378 | 1.4 |
| 4 | 418 | 841 | 2.0 | 1084 | 2.6 |
| 5 | 1699 | 2776 | 1.6 | 3184 | 1.9 |
| 6 | 1223 | 1581 | 1.3 | 1685 | 1.4 |
| 7 | 1182 | 1631 | 1.4 | 1750 | 1.5 |
| 8 | 875 | 1166 | 1.3 | 1264 | 1.4 |
| 9 | 455 | 1213 | 2.7 | 1556 | 3.4 |
| 10 | 1169 | 1558 | 1.3 | 1724 | 1.5 |
| 11 | 1736 | 2314 | 1.3 | 2596 | 1.5 |
Eleven representative cases of the 27 patients that showed nonlinear CT levels. Samples were serially diluted until the values of the dilutions match within 20%. Notice the underestimation of CT levels in the undiluted samples.
Discussion
This retrospective study evaluated the use of PCT as a tumor marker in patients with MTC. Good analytical and clinical correlation was observed between PCT and CT. ROC curve analysis showed no statistically significant performance difference between the two analytes. However, AUCs under the ROC curves, as well as clinical sensitivity and specificity at defined cutoffs, tended to be slightly better for CT than PCT. This is likely due to the fact that patients were classified as active MTC vs. cured MTC based on clinical, imaging, histological, and biochemical (CT levels) criteria. Because serum CT is a very good MTC marker and its levels were used as part of this diagnostic gold standard, CT’s clinical performance was biased toward 100% sensitivity. Based on this study design, the best performance an alternative marker can show is equal to that of CT.
There were seven patients with stable disease with PCT levels within the reference interval. These patients were classified as having stable disease due to long-term slight elevations of CT (4–22 yr follow-up), but no lesions were detectable by imaging. Their serum CEA levels were within the reference interval. Patients with stable disease represent a challenge to physicians, especially when biochemical evidence of disease is present, but lesions cannot be identified by imaging. Because the number of patients with stable disease in whom a discrepancy between the two analytes was observed is small, any conclusions on whether PCT is an inferior marker in this population will be premature. Further prospective studies are necessary to establish whether PCT is an inferior or superior analyte in this patient population.
Our data, although limited, suggest that PCT could produce false-positive results in many of the same conditions that can cause CT elevations unrelated to MTC. However, it appears that the spectrum of PCT elevations is different from what is observed for CT. One might therefore speculate that in some instances testing a patient with both CT and PCT could help eliminate some false-positive results. For example, in mastocytosis two samples were positive by CT, whereas two different samples were positive by PCT. Ten percent of neuroendocrine tumors had elevated PCT levels, whereas 4% were CT positive with an overlap of 2% between the two groups. The patients with neuroendocrine tumors or follicular-cell derived thyroid carcinomas, who had elevated PCT levels, had liver, lung, and lymph node metastasis, respectively. Recently it has been suggested that PCT might serve as an early indicator of metastatic disease (19). Further studies might be warranted to establish the utility of PCT, if any, as a general marker of cancer metastasis.
Our study is in agreement with two smaller reports that determined serum PCT concentrations in patients with various diseases including MTC (16,17). Elevations of PCT were observed in patients with MTC and also in patients with neuroendocrine tumors and hepatocellular carcinomas.
The biggest potential advantages of PCT lie in its lack of susceptibility to interfering isoforms or fragments, which cause false-low results, and its in vitro preanalytical stability. Both of these issues are highlighted in our study. In two of the study patients, false-low CT levels were observed, which corrected after dilution. By contrast, PCT levels in these patients were consistently elevated. Similar observations were made over a 2-month period in 27 of 300 routine CT samples with levels greater than 50 pg/ml. CT measurements of the undiluted samples underestimated the likely true CT concentration by as much as 70%.
With regard to samples stability, our studies showed a decrease in CT of as much as 50% in samples that are not stored properly. It is suggested that specimens that are being tested for CT be refrigerated for a maximum of 8 h or otherwise stored frozen to prevent this degradation. However, many laboratories do not perform CT testing in-house and processing of send-out samples, shipping, sorting, and receiving at the reference laboratory are likely to result collectively in a significant risk of prolonged exposure to ambient temperatures or multiple freeze-thaws, potentially leading to false low CT results.
Finally, a common problem with CT assays, is the lack of comparability between different assays (20,21). This is due to the many different assay formats and assay antibodies used. In concert with the various and variable CT isoforms and fragments that might be found in some patients, this result in a significant disagreement between different CT assays, despite the facts that assays are now commonly calibrated to the second World Health Organization international standard preparation (89/620). By contrast, the intellectual property for commercial PCT assays is held and licensed out by a single company, and therefore, all available commercial assays are nearly identical in terms of antibodies used, making for much improved between-assay comparability. In a modern medical world, in which referral laboratories might be changed and in which patient mobility between institutions continues to grow, this has the potential to facilitate greatly the follow-up of MTC patients.
In summary, our study suggests that PCT shows comparable clinical performance with CT in MTC. The strengths of PCT are consistent and predictable half-life, lack of fragment/isoform interference, in vitro stability, and analytical consistency between assays. Additional prospective studies will be required to define more precisely in which clinical situations these analytical advantages will support PCT measurement in addition to, or in lieu of, that of CT. At the very least, it appears that PCT might be useful in those individuals with chaotic serial CT measurement patterns.
Footnotes
This work was supported by National Institutes of Health Grant CA80117 (to S.K.G.G., coprincipal investigator) and Mayo Clinic Departments of Medicine and Laboratory Medicine and Pathology funds.
Disclosure Statement: The authors have no disclosures.
First Published Online December 16, 2008
Abbreviations: AUC, Area under the curve; CEA, carcinoembryonic antigen; CI, confidence interval; CT, calcitonin; MTC, medullary thyroid carcinoma; PCT, procalcitonin; ROC, receiver-operating characteristic.
References
- Giuffrida D, Gharib H 1998 Current diagnosis and management of medullary thyroid carcinoma. Ann Oncol 9:695–701 [DOI] [PubMed] [Google Scholar]
- Laure Giraudet A, Al Ghulzan A, Auperin A, Leboulleux S, Chehboun A, Troalen F, Dromain C, Lumbroso J, Baudin E, Schlumberger M 2008 Progression of medullary thyroid carcinoma: assessment with calcitonin and carcinoembryonic antigen doubling times. Eur J Endocrinol 158: 239–246 [DOI] [PubMed] [Google Scholar]
- Saad MF, Fritsche HA, Jr., Samaan NA 1984 Diagnostic and prognostic values of carcinoembryonic antigen in medullary carcinoma of the thyroid. J Clin Endocrinol Metab 58:889–894 [DOI] [PubMed] [Google Scholar]
- Leboulleux S, Baudin E, Travagli JP, Schlumberger M 2004 Medullary thyroid carcinoma. Clin Endocrinol (Oxf) 61:299–310 [DOI] [PubMed] [Google Scholar]
- Niccoli P, Conte-Devolx B, Lejeune PJ, Carayon P, Henry JF, Roux F, Wion-Barbot N, Bigorgne JC 1996 [Hypercalcitoninemia in conditions other than medullary cancers of the thyroid]. Ann Endocrinol (Paris) 57:15–21 [PubMed] [Google Scholar]
- Iacobone M, Niccoli-Sire P, Sebag F, De Micco C, Henry JF 2002 Can sporadic medullary thyroid carcinoma be biochemically predicted? Prospective analysis of 66 operated patients with elevated serum calcitonin levels. World J Surg 26:886–890 [DOI] [PubMed] [Google Scholar]
- Silva OL, Broder LE, Doppman JL, Snider RH, Moore CF, Cohen MH, Becker KL 1979 Calcitonin as a marker for bronchogenic cancer: a prospective study. Cancer 44:680–684 [DOI] [PubMed] [Google Scholar]
- Zaidi M, Moonga BS, Bevis PJ, Bascal ZA, Breimer LH 1990 The calcitonin gene peptides: biology and clinical relevance. Crit Rev Clin Lab Sci 28:109–174 [DOI] [PubMed] [Google Scholar]
- Fugazzola L, Pinchera A, Luchetti F, Iacconi P, Miccoli P, Romei C, Puccini M, Pacini F 1994 Disappearance rate of serum calcitonin after total thyroidectomy for medullary thyroid carcinoma. Int J Biol Markers 9:21–24 [DOI] [PubMed] [Google Scholar]
- Becker KL, Snider RH, Silva OL, Moore CF 1978 Calcitonin heterogeneity in lung cancer and medullary thyroid cancer. Acta Endocrinol 89:89–99 [DOI] [PubMed] [Google Scholar]
- Goltzman D, Tischler AS 1978 Characterization of the immunochemical forms of calcitonin released by a medullary thyroid carcinoma in tissue culture. J Clin Invest 61:449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin LA, Heath 3rd H 1981 Calcitonin: physiology and pathophysiology. N Engl J Med 304:269–278 [DOI] [PubMed] [Google Scholar]
- Meisner M, Schmidt J, Huttner H, Tschaikowsky K 2000 The natural elimination rate of procalcitonin in patients with normal and impaired renal function. Intensive Care Med 26(Suppl 2):S212–S216 [DOI] [PubMed] [Google Scholar]
- Meisner M, Tschaikowsky K, Schnabel S, Schmidt J, Katalinic A, Schuttler J 1997 Procalcitonin—influence of temperature, storage, anticoagulation and arterial or venous asservation of blood samples on procalcitonin concentrations. Eur J Clin Chem Clin Biochem 35:597–601 [DOI] [PubMed] [Google Scholar]
- Schneider HG, Lam QT 2007 Procalcitonin for the clinical laboratory: a review. Pathology 39:383–390 [DOI] [PubMed] [Google Scholar]
- Bihan H, Becker KL, Snider RH, Nylen E, Vittaz L, Lauret C, Modigliani E, Moretti JL, Cohen R 2003 Calcitonin precursor levels in human medullary thyroid carcinoma. Thyroid 13:819–822 [DOI] [PubMed] [Google Scholar]
- Ghillani PP, Motte P, Troalen F, Jullienne A, Gardet P, Le Chevalier T, Rougier P, Schlumberger M, Bohuon C, Bellet D 1989 Identification and measurement of calcitonin precursors in serum of patients with malignant diseases. Cancer Res 49:6845–6851 [PubMed] [Google Scholar]
- 2008 Defining, establishing, and verifying reference intervals in the clinical laboratory, Approved Guideline, third edition. Clinical and Laboratory Science Institute (CLSI), Wayne, PA, Doccument C28-A3 [Google Scholar]
- Matzaraki V, Alexandraki KI, Venetsanou K, Piperi C, Myrianthefs P, Malamos N, Giannakakis T, Karatzas S, Diamanti-Kandarakis E, Baltopoulos G 2007 Evaluation of serum procalcitonin and interleukin-6 levels as markers of liver metastasis. Clin Biochem 40:336–342 [DOI] [PubMed] [Google Scholar]
- Martinetti A, Seregni E, Ferrari L, Pallotti F, Aliberti G, Coliva A, Fracassi S, Bombardieri E 2003 Evaluation of circulating calcitonin: analytical aspects. Tumori 89:566–568 [DOI] [PubMed] [Google Scholar]
- Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, LiVosli VA, Niccoli-Sire P, John R, Ruf J, Smyth PP, Spencer CA, Stockigt JR 2003 Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 13:3–126 [DOI] [PubMed] [Google Scholar]