Abstract
Two diabetic patients with primary liver abscess, who initially responded unsatisfactorily to intravenous ceftriaxone or cefoxitin treatment and had abscess drainage, were found to be infected with a single clone of Klebsiella pneumoniae with two different colonial morphotypes and resistotypes. Primary liver abscess caused by second-generation cephalosporin-resistant K. pneumoniae strains may be an emerging problem in Taiwan.
Key words: Klebsiella pneumoniae, liver abscess, diabetes
Primary liver abscess caused by a single pathogen, Klebsiella pneumoniae, has long been an important infectious complication in diabetic patients in Taiwan (1,2). All K. pneumoniae strains causing primary liver abscess have a unique antimicrobial susceptibility pattern (resistant to ampicillin, ticarcillin, and carbenicillin but susceptible to other antibiotics including all cephalosporins and aminoglycosides) (1–4). Although multidrug-resistant strains of K. pneumoniae, whether community acquired or nosocomial, are not uncommon in Taiwan and other countries, these isolates had never been reported as the cause of primary liver abscess (5,6).
Case Summaries
A 42-year-old man (patient A) was admitted on July 31, 2000, for a fever of 2 weeks’ duration. He had had diabetes mellitus for 20 years and alcoholism for >10 years. Physical examination and abdominal echography showed hepatomegaly and a huge abscess (12 cm x 10 cm x 8 cm) over the right lobe of the liver. Laboratory tests showed leukocytosis (14,600/μL) with a left shift. After blood cultures, ceftriaxone (2 g every 12 hours) was given. A “pigtail” catheter was inserted for continuous drainage on the 5th hospital day. Abscess aspirate culture yielded K. pneumoniae with two colonial morphotypes (isolates on trypticase soy agar plates supplemented with 5% sheep blood [BBL Microbiology Systems, Cockeysville, MD] after 24 hours of incubation in ambient air) (Figure 1) and two resistotypes (by the routine disk diffusion method). One (isolate A1) had mucoid, opaque colonies and was resistant to ampicillin but susceptible to cefazolin and cefoxitin, and the other (isolate A2) had nonmucoid, white colonies and was resistant to ampicillin, cefazolin, and cefoxitin. Both isolates were susceptible to amoxicillin-clavulanate and cefotaxime. Blood cultures were negative. Because of persistent fever, the antibiotic was changed to imipenem (500 mg every 6 hours) on the 11th hospital day. Fever subsided 3 days after imipenem administration was begun. Imipenem was continued for a total of 24 days, followed by ciprofloxacin (750 mg every 12 hours) for 3 weeks. A follow-up echography 4 months after antibiotic treatment ended showed that the abscess had disappeared.
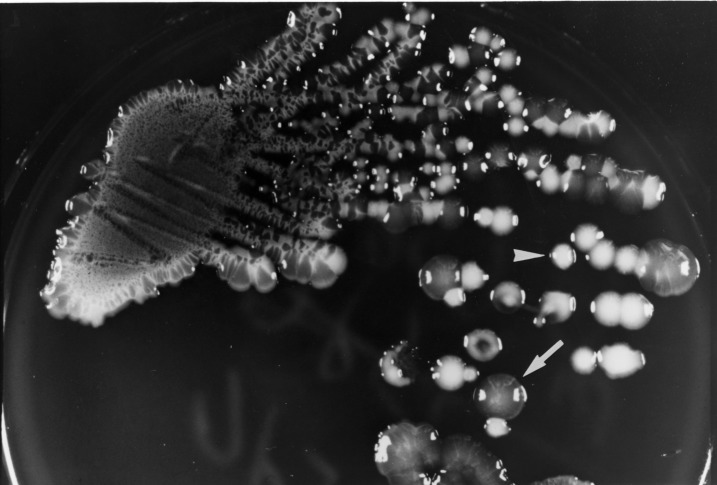
Figure 1.
Colonial morphology of Klebsiella pneumoniae grown on a primary isolation plate (trypticase soy agar supplemented with 5% sheep blood) from the abscess aspirate of patient A after 24 hours of incubation. The arrow shows a mucoid opaque colony (isolate A1). The arrowhead shows a nonmucoid white colony (isolate A2).
A 66-year-old man (patient B) with diabetes mellitus was admitted on August 4, 2000, with fever and hiccups of 4 days’ duration. Physical examination was unremarkable. Laboratory tests showed elevated levels of alkaline phosphatase (585 U/L; reference range 66-220 U/L) and gamma-glutamyltranspeptidase (301 U/L; reference range <52 U/L) but normal levels of aspartate and alanine aminotransferases. Abdominal echography and computed tomography revealed an abscess (6 cm x 4 cm x 4 cm) over the right lobe of the liver and a gallbladder stone. Cefoxitin (2 g every 8 hours) was given intravenously. The abscess was aspirated on the 4th day after hospitalization. Two isolates (isolates B1 and B2) of K. pneumoniae with different colonial morphotypes and resistotypes (as in isolates A1 and A2, respectively, from patient A) were found in one aspirate culture, and two isolates (isolates B3 and B4) of the same species with different phenotypes (as in isolates B1 and B2, respectively) were recovered from one set of blood cultures, performed at admission. Cefoxitin was discontinued, and cefotaxime (2 g every 6 hours) was administered. His fever resolved 2 days after cefotaxime administration was begun. The patient received cefotaxime for 15 days, followed by oral cefixime (200 mg every 12 hours) for 7 weeks. Follow-up echography showed the abscess had disappeared.
In vitro susceptibilities of 14 antimicrobial agents for these isolates were determined by using the standard agar dilution method (7). Biotyping of thee isolates was performed with the API ID32 GN system (bioMerieux, Marcy I'Etoile, France). Random amplified polymorphic DNA (RAPD) patterns of the six isolates were determined by means of arbitrarily primed polymerase chain reaction, as described in our previous report (8). A total of four primers were used: M13 (5'-TTATGTAAAACGACGGCCAGT-3'), OPH2, OPA3, and OPA9 (Operon Technologies, Inc., Alameda, CA). Pulsotypes of these isolates were determined by pulsed-field gel electrophoresis; plasmid analysis and conjugative experiment were performed as described (3). For comparisons, molecular typing of an additional two K. pneumoniae isolates recovered from two patients seen at our hospital in 1999 were performed simultaneously.
The cefoxitin- and cefuroxime-resistant isolates (isolates A2, B2, and B4) showed two- to four-fold higher MICs of cefotaxime and ceftriaxone (MICs 0.5-1 μg/mL), ceftazidime (MICs 1 μg/mL), flomoxef (MICs 0.5-2 μg/mL), ticarcillin-clavulanate (MICs 8 μg/mL), piperacillin-tazobactam (MICs 4-8 μg/mL), cefepime (MICs 0.5-1 μg/mL), cefpirome (MICs 0.12-1 μg/mL), imipenem (MICs 2 μg/mL), and meropenem (MICs 0.12 μg/mL) than those of the cefoxitin-susceptible isolates (isolates A1, B1, and B3). All isolates were susceptible to the above antibiotics and aminoglycosides (gentamicin and amikacin).
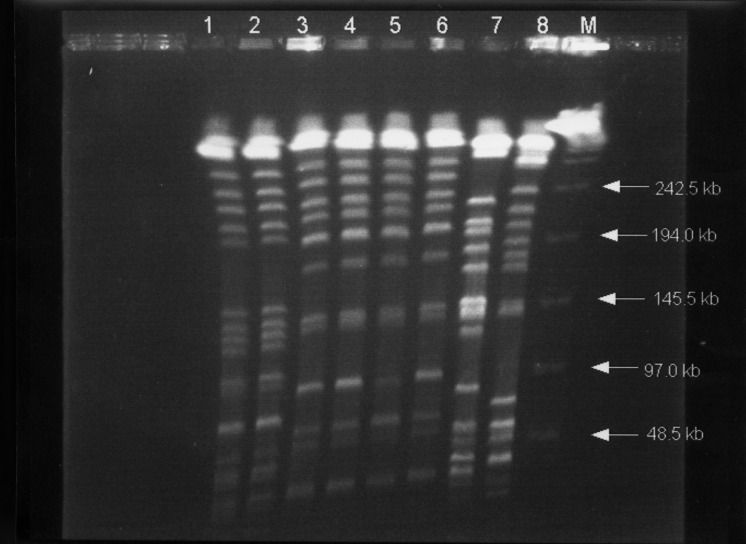
As shown in the Table, the identity of biotypes, RAPD patterns with four primers, and pulsotypes (Figure 2) was clearly demonstrated for the isolates with two different phenotypes from each patient, suggesting that they belonged to a single clone in each patient (clone 1, isolates A1 and A2; clone 2, isolates B1 to B4). Different clones isolated from two patients seen within 1 week indicate that no outbreak occurred. Isolates A1 and A2 both had two plasmids (50 kb and 5 kb). Only one plasmid (30 kb) was found in each of the four isolates (B1 to B4) of patient 2. All these plasmids cannot transfer to Escherichia coli C600.
Table. Clinical characteristics of two diabetic patients with liver abscess and microbiologic characteristics of Klebsiella pneumoniae isolates recovered from them.
| Patient designation (age, yr/gender) | Antibiotic treatment (days) | Sources of isolate/ designation | Characteristics of isolates | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Colonial morphotype | Res Resistotype (MIC, mg/mL)a | Bio Biotype | RAPD pattern | ||||||
| AMP | CZ | FOX (CXM) | CTX (CRO) | ||||||
| A (42/M) | Ceftriaxone (10) | Abscess fluid | |||||||
| Imipenem (24) | A1 | Mucoid | 64 | 4 | 16 | 0.12 | I | a/1 | |
| Ciprofloxacin (21) | A2 | Nonmucoid | 128 | 32 | 128 | 1 | I | a/1 | |
| B (66/M) | Cefoxitin (10) | Abscess fluid | |||||||
| Cefotaxime (15 | B1 | Mucoid | 32 | 2 | 4 | 0.06 | I | b/2 | |
| Cefixime (35) | B2 | Nonmucoid | 128 | 32 | 128 | 0.5 | I | b/2 | |
| Blood samples | |||||||||
| B3 | Mucoid | 32 | 2 | 4 | 0.03 | I | b/2 | ||
| B4 | Nonmucoid | 128 | 64 | 256 | 0.5 | I | b/2 | ||
aAMP, ampicillin; CZ, cefazolin; FOX, cefoxitin, CXM, cefuroxime, CTX, cefotaxime, CRO, ceftriaxone.
RAPD = random amplified polymorphic DNA.
Figure 2.
Pulsed-field gel electrophoresis profiles of XbaI-digested genomic DNAs from eight Klebsiella pneumoniae isolates. Lanes 1 and 2, profiles for isolates A1 and A2 (from patient A); lines 3 to 6, profiles for isolates B1 to B4 (from patient B), respectively; and lanes 7 and 8, isolates of K. pneumoniae from two other patients used as control strains. Lane M, bacteriophage lamdba DNA concatemers (GibcoBRL, Gaithersburg, MD)
Conclusions
Although infection caused by a single clone of one bacterial species that simultaneously possessed two obviously different colonial morphotypes has been noted previously (8), infection caused by a single clone of K pneumoniae exhibiting two discrete colonial morphotypes and resistotypes has never been reported. Primary liver abscess due to K. pneumoniae having resistance to second-generation cephalosporins (MIC up to 128 μg/mL for isolate B2 and 256 μg/mL for isolate B4 in patient B) and higher MICs of third-generation cephalosporins (MICs up to 1 μg/mL for isolate A2 in patient A) has also not been previously reported. We believe that this decreased in vitro susceptibility contributed to the unsatisfactory response to treatment with these agents.
The genetic basis for the resistance to cephalosporins and for the mucoid material synthesis by some gram-negative bacteria may be chromosomally determined or dependent on the acquisition of a specific plasmid or phage (5,9,10). The resistant and mucoid material synthesis may also be regulated by the environment in which the bacteria grow (5,9,10). The association of a specific plasmid with the presence of mucoid phenotype was not found in our isolates because both mucoid and nonmucoid strains of the two clones of K. pneumoniae had identical plasmid profiles. The mechanisms of the coexistence of two phenotypically distinct isolates within a single clone K. pneumoniae in abscess fluid, blood, or both should be investigated. The mechanism of cefoxitin resistance in our isolates was unclear because of the failure of transferability of the plasmids existing in our isolates to the susceptible recipient. Whether this resistance was chromosome mediated or caused by other mechanisms needs further investigations.
These two cases suggest that primary liver abscess caused by multiresistant strains of K. pneumoniae in diabetic patients may be an emerging problem in Taiwan. The belief that only strains of K. pneumoniae that are susceptible to cephalosporins cause primary liver abscess has now been disproved.
Biography
Dr. Hsueh is an assistant professor in the departments of Laboratory Medicine and Internal Medicine at National Taiwan University College of Medicine in Taipei, Taiwan. His research interests include the epidemiology of emerging and nosocomial infections and molecular mechanisms of antimicrobial drug resistance. He is actively involved in developing a national research program for antimicrobial drug resistance in Taiwan (Surveillance for Multicenter Antimicrobial Resistance in Taiwan-SMART).
Footnotes
Suggested citation: Hsueh PR, Wu JJ, Teng LJ, Chen YC, Yang PC, Ho SW, et al. Primary Liver Abscess Caused by One Clone of Klebsiella pneumoniae with Two Colonial Morphotypes and Resistotypes. Emerg Infect Dis. [serial on the Internet]. 2002 Jan [date cited]. Available from http://www.cdc.gov/ncidod/eid/vol8no1/01-0167.htm
References
- 1.Wang JH, Liu YC, Lee SSJ, Yen MY, Chen YS, Wang JH, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 1998;26:1434–8. 10.1086/516369 [DOI] [PubMed] [Google Scholar]
- 2.Chang FY, Chou MY, Fan RL, Shaio MF. A clinical study of Klebsiella liver abscess. J Formos Med Assoc. 1988;87:282–7. [PubMed] [Google Scholar]
- 3.Yan JJ, Wu SM, Tsai SH, Wu JJ, Su IJ. Prevalence of SHV-12 among clinical isolate of Klebsiella pneumoniae producing extended-spectrum beta-lactamases and identification of a novel AmpC enzyme (CMY-8) in southern Taiwan. Antimicrob Agents Chemother. 2000;44:1438–42. 10.1128/AAC.44.6.1438-1442.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seeto RK, Rockey DC. Pyogenic liver abscess: change in etiology, management, and outcome. Medicine. 1996;75:99–113. 10.1097/00005792-199603000-00006 [DOI] [PubMed] [Google Scholar]
- 5.Nordmann P. Trends in beta-lactam resistance among Enterobacteriaceae. Clin Infect Dis. 1998;27(suppl 1):S100–6. 10.1086/514610 [DOI] [PubMed] [Google Scholar]
- 6.Jan IS, Hsueh PR, Teng LJ, Ho SW, Luh KT. Antimicrobial susceptibility testing for Klebsiella pneumoniae isolates resistant to extended-spectrum beta-lactam antibiotics. J Formos Med Assoc. 1998;97:661–6. [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing: 9th informational supplement M100-S10. Wayne (PA): National Committee for Clinical Laboratory Standards; 2000.
- 8.Hsueh PR, Teng LJ, Yang PC, Chen YC, Ho SW, Luh KT. Recurrent catheter-related infection caused by a single clone of Mycobacterium chelonae with two colonial morphotypes. J Clin Microbiol. 1998;36:1422–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macone AB, Pier GB, Pennington JE, Matthews WJ Jr, Goldmann DA. Mucoid Escherichia coli in cystic fibrosis. N Engl J Med. 1981;304:1445–9. [DOI] [PubMed] [Google Scholar]
- 10.Schurr MJ, Deretic V. Microbial pathogenesis in cystic fibrosis: co-ordinate regulation of heat-shock response and conversion to mucoid in Pseudomonas aeruginosa. Mol Microbiol. 1997;24:411–20. 10.1046/j.1365-2958.1997.3411711.x [DOI] [PubMed] [Google Scholar]