Abstract
Context: Dopamine receptor (DR) and somatostatin receptor subtype expression in pituitary adenomas may predict the response to postsurgical therapies.
Objectives: Our objectives were to assess and compare the mRNA levels of DR1-5 and somatostatin receptors 1–5 in normal pituitaries (NPs), nonfunctioning pituitary adenomas (NFPAs), and somatotropinomas. In addition, we determined whether the level of DR expression correlates with the in vivo response to octreotide-LAR in acromegalic patients.
Design and Patients: Eight NPs, 30 NFPAs, and 39 somatotropinomas were analyzed for receptor mRNA levels by real-time RT-PCR. The DR2 short variant was estimated as the DR2 long/DR2 total (DR2T). The relationship between DR expression and the postsurgical response to octreotide-LAR was assessed in 19 of the acromegalic patients.
Results: DR3 was not detected. The relationship between expression levels of DR subtypes in NPs and somatotropinomas was DR2T⋙DR4≫DR5>DR1, whereas in NFPAs, DR2T⋙DR4≫DR1>DR5. The DR2 short variant was the predominant DR2 variant in the majority of samples. In acromegalics treated with octreotide-LAR, DR1 was negatively correlated with percent GH reduction (3 months: r = −0.67, P = 0.002; and 6 months: r = −0.58, P = 0.009), and DR5 was positively correlated with percent IGF-I reduction (3 months: r = 0.55, P = 0.01; and 6 months: r = 0.47, P = 0.04).
Conclusions: DR2 is the predominant DR subtype in NPs, NFPAs, and somatotropinomas. The fact that DR1, DR4, and DR5 are also expressed in many adenomas tested suggests that these receptors might also play a role in the therapeutic impact of postsurgical medical therapies in patients with NFPA and acromegaly. This was supported by the finding that the in vivo response to octreotide-LAR was negatively associated with DR1 and positively associated with DR5.
Dopamine receptor (DR) subtype 2 is the predominant DR in normal pituitaries and adenomas and DR1 and DR5 are negatively and positively associated with octreotide-LAR response, respectively.
Dopamine receptor (DR) agonists (DAs) can reduce hormone release and tumor mass in some but not all patients with nonfunctioning pituitary adenomas (NFPAs) and somatotropinomas (1,2,3). A lack of response may be due to an inappropriate expression of DR. However, assessment of the relationship between therapeutic response and DR expression is complicated by the fact that DRs are encoded by five separate genes (DR1-5), in which the DR2 subtype exists as two variants [DR2 long (DR2L) and DR2 short (DR2S)], generated by alternative splicing. In addition, the DR subtypes have been shown to differentially activate intracellular signal transduction pathways depending on the cellular context (4,5). To date, DR2 and DR4 have been shown to be expressed in normal human pituitaries (NPs) (6,7). In rat pituitaries, DR2 is strongly expressed in lactotrophs, but is also found in other pituitary cell types (8,9,10,11). Although cell-specific expression of DR2 has not been directly tested in human NP, a broad expression pattern is supported by the observations that DR2 expression was positively correlated with DA-induced suppression of both prolactin (PRL) and GH release in primary cultures of somatotropinomas and with NFPA tumor shrinkage (12,13,14). In addition, both the DR2L and DR2S have been identified in all pituitary adenoma subtypes studied thus far, where their relative expression differs between individual samples and studies (6,13,15,16,17). Expression levels of the DR2 variants appear to impact the response to DA based on the observation that NFPA shrinkage was more pronounced in tumors with high DR2S (13). In contrast to the role DR2 plays in DA-mediated regulation of pituitary adenoma function, limited information is available regarding the expression pattern and function of the other DR subtypes in the context of pituitary function (13).
There is also evidence that human DR2 can form heterodimers with somatostatin receptor (SSTR) subtypes SSTR2 and SSTR5, and the composition of these receptor complexes can alter the response to both DA or somatostatin analogs (SAs) in heterologous cell systems (18,19). The functional interaction of DR and SSTR is further supported by the observation that the SSTR5-mediated GH suppression in adenoma cell cultures taken from acromegalic patients was greater in DR2 negative, compared with DR2 positive tumors (20). In addition, a recent report revealed that the in vitro response to octreotide was enhanced in adenomas that expressed higher levels of DR2 (12). The potential for a functional relationship between DR and SSTR has prompted the development of chimeric DR2/SSTR2/5 agonists for therapeutic use (1,3,21). Together, these observations suggest that the therapeutic response of pituitary adenomas to DA and/or SA may be dependent on the relative pattern of expression of both DR and SSTR subtypes.
In the current study, quantitative real-time RT-PCR (qrtRT-PCR) was used to assess and compare DR1-5 mRNA content in NPs, NFPAs, and somatotropinomas. In addition, we sought to determine whether there is a relationship between the expression levels of DR2 and SSTR2 or SSTR5, previously shown to heterodimerize. In the patient population studied, it was not possible to evaluate the therapeutic efficacy of DA and correlate it with pituitary tumor DR subtype expression pattern because DA is not supplied by the Brazilian public health system for treatment of NFPA, and, for acromegaly, it is supplied irregularly. However, as previously described in detail by our laboratory (22,23), we have followed a subset of acromegalic patients that began postsurgical treatment with octreotide-LAR, and reported that SSTR2 expression positively correlated percent GH, IGF-I, and tumor volume reduction, in which a higher SSTR2 to SSTR5 ratio was observed among patients who obtained hormonal control. Therefore, in the current study, it was possible to determine whether therapeutic efficacy of SA correlated with somatotropinoma DR subtype expression pattern.
Patients and Methods
Patients
NPs (n = 8) were obtained during autopsy, after accidental death, with no clinical or pathological evidence of endocrine disorders or premortal trauma. Time between death and sample collection was 6–12 h. Pathological examination excluded the presence of pituitary adenomas and metastasis from nonpituitary tumors.
Diagnosis of acromegaly and NFPA was made according to the criteria previously described (22). Pituitary tumor specimens were obtained during transsphenoidal surgery (n = 39 acromegalic; n = 30 NFPA). Details regarding postsurgical treatment of acromegalics (n = 19) with octreotide-LAR and assessment of SA clinical efficacy (percent GH, IGF-I, and tumor volume reduction) after 3 and 6 months of treatment have been previously described (22,23).
This study was approved by the Ethics Committee of the Hospital Universitário Clementino Fraga Filho, and the Institutional Review Board of the University of Illinois at Chicago and Jesse Brown Veterans Affairs Medical Center. Informed consent was obtained from each patient or relative, in case of autopsy, before study entry.
Methods
Assessment of DR and SSTR by qrtRT-PCR
Details of sample collection, RNA extraction, quantification, reverse transcription (RT), in addition to the development, validation and application of qrtRT-PCR to assess mRNA levels have been previously reported by our group (22). Quality of total RNA was confirmed by standard agarose gel electrophoresis with ethidium bromide staining (when sample size allowed). In addition, the expression level (copy number) of three housekeeping genes, hypoxanthine-guanine phosphoribosyltransferase (HPRT), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and β-actin, was determined for each sample to control for variations in the quality and amount of RNA used in the RT reaction and the efficiency of the RT reaction, as previously reported (22). The sensitivity of the qrtRT-PCR was 10 copies/0.05 μg total RNA (i.e. the lowest copy number on the cDNA standard curve that was within the linear range of amplification). Transcripts were considered nondetectable if fluorescence levels did not increase above no-DNA controls after 40 cycles of amplification.
Primer sets for the DR subtypes (supplemental Table S1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org) were selected using Primer 3 software with human mRNA sequences as templates. Primer set selection and validation for the SSTR subtypes and all housekeeping genes were previously reported (22).
It was not possible to design a specific and efficient set of primers that only amplified DR2S because it required one of the primers to span the splice site (Ex4/Ex6, refer to Entrez Gene, Gene identification no. 1813), where the distribution of this region is GC rich (76%; see NM_016574 bases 861–918). Therefore, we chose to design two separate sets of primers to amplify DR2 total (DR2T) and DR2L, where these primer sets met stringent criteria to maximize specificity and efficiency, including: 1) similar annealing temperature; 2) no primer dimer formation; 3) 45–55% GC content; 4) amplification of a product of 100–200 bp; 5) no homology to sequences other than the designated target; 6) amplification of a single PCR product (by conventional PCR and dissociation curves in qrtRT-PCR), and identity verified by sequencing; and 7) amplification of standard curves with approximately 100% efficiency (supplemental Table S1). Therefore, the technique as applied can be used to accurately quantify copy numbers for both DR2T and DR2L transcript, and the ratio of DR2L to DR2T reflects the expression level of DR2S.
In the current study, we assessed DR expression in 14 NFPAs previously assessed for SSTR1-5 expression (22) (supplemental Table S3), in addition to performing DR1-5 expression analysis on 16 newly acquired NFPA samples. SSTR expression analysis was not performed on the new NFPA samples. Our laboratory has also reported the expression pattern of SSTR in 19 somatotropinomas (22,23), and since that time, 20 additional acromegalic patients were enrolled in the study. In the case of the somatotropinomas, assessment of SSTR subtype expression was repeated using cDNA generated from new RT reactions performed on all samples (old and new) at the same time; these same RT samples were used to assess the pattern of DR subtypes.
Statistical analysis
Analyses were done using SPSS 11.0 version for Windows (SPSS, Inc., Chicago, IL). Results were reported as median (minimum − maximum). Mann-Whitney’s nonparametric test was used to compare numerical variables between groups. Correlations between numerical variables were studied using Spearman’s correlation test. P values less than 0.05 were considered significant.
Results
Individual patient/sample characteristics
Individual demographical data and SSTR and/or DR subtype expression patterns are provided as supplemental data files, which are published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org, for NPs (supplemental Table S2), NFPAs (supplemental Table S3), and somatotropinomas (supplemental Table S4).
DR subtype expression in NPs, NFPAs, and somatotropinomas
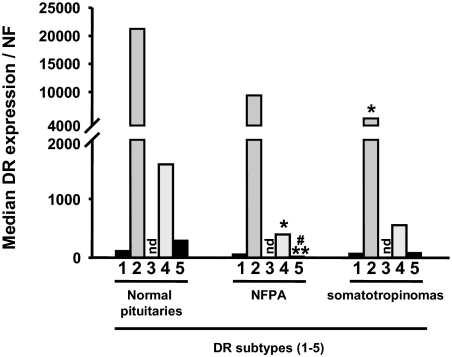
In NPs (n = 8, supplemental Table S2), DR2 and DR5 were expressed in 100% of the samples, whereas DR1 and DR4 were less than 10 copies (the sensitivity of the qrtRT-PCR) in 37.5 and 12.5% of the samples, respectively. In NFPAs (n = 30, supplemental Table S3), DR2 and DR4 were expressed in all samples. DR1 and DR5 were less than 10 copies in 16.7 and 36.7% NFPA samples, respectively. In somatotropinomas (n = 39, supplemental Table S4), DR2 and DR4 were detected in all samples, whereas DR1 and DR5 were less than 10 copies in 2.5 and 12.8% samples, respectively. In contrast, DR3 mRNA was not detectable in any pituitary sample analyzed.
As summarized in Fig. 1 and Table 1, DR2 was the dominant DR subtype in NPs and somatotropinomas, followed by DR4, DR5, and DR1. In NFPAs, DR2 was also dominant, followed by DR4, whereas the median expression of DR1 was higher than DR5. No correlations were found between DR mRNA levels and sex or age within sample types. When comparing expression levels of DR subtypes between NPs and tumors, DR2 was significantly more expressed in NPs compared with somatotropinomas (P = 0.02), and a similar relationship was found in NFPAs, but this did not reach statistical significance. In addition, DR4 and DR5 were more expressed in NPs compared with adenomas, although it only reached statistical significance in NFPAs (P = 0.04 and 0.001, respectively). Between adenoma types the expression level of the DR subtypes was similar, with the exception of DR5, which was higher in somatotropinomas compared with NFPAs (P = 0.002). The median DR2L/DR2T value was 0.36 for NPs, NFPAs, and somatotropinomas (Table 1).
Figure 1.
Median DR subtype (DR1-5) mRNA levels in NPs, NFPAs, and somatotropinomas. nd, Not detected; NF, normalization factor calculated from the level of HPRT, GAPDH, and β-actin using the GeNorm 3.3 visual basic application for Microsoft Excel (http://medgen.ugent.be/∼jvdesomp/genorm/) as previously developed and validated by Vandesompele et al. (46). *, Values that differ between NPs and adenomas (*, P < 0.05; **, P < 0.01); #, Values that differ between NFPAs and somatotropinomas.
Table 1.
Median (minimum/maximum) DR mRNA expression levels in normal human pituitaries, NFPAs, and somatotropinomas
| DR1/NF | DR2T/NF | DR2L/NF | DR2L/DR2Ta | DR4/NF | DR5/NF | |
|---|---|---|---|---|---|---|
| Normal human pituitaries | 114 (0–478) | 21,212 (3,343–57,954) | 8,943 (1,248–17,902) | 0.36 (0.23–0.49) | 1,587 (0–5,846) | 289 (35–2,367) |
| NFPAs | 54 (0–645) | 9,396 (78–131,394) | 4,070 (0–20,135) | 0.36 (0–1.0) | 394 (39–8,368) | 20 (0–498) |
| Somatotropinomas | 68 (3–13,198) | 5,276 (190–40,245) | 1,709 (47–32,975) | 0.36 (0.11–0.82) | 552 (70–7,942) | 77 (4–734) |
NF, Normalization factor calculated from the level of HPRT, GAPDH, and β-actin using the GeNorm 3.3 visual basic application for Microsoft Excel (http://medgen.ugent.be/∼jvdesomp/genorm/) as previously developed and validated by Vandesompele et al. (46).
DR2L/DR2T is used as an estimate of DR2S expression.
Relationship between DR subtype expression and the in vivo response of acromegalic patients to octreotide-LAR
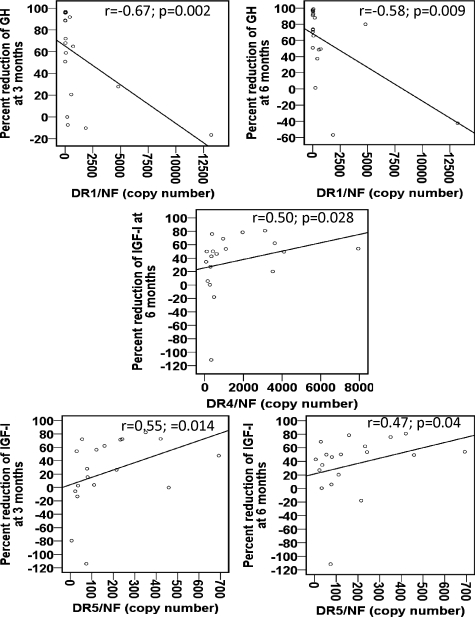
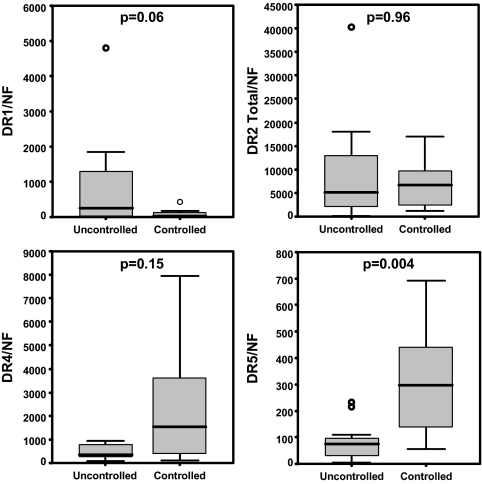
In the subset of acromegalic patients in which detailed clinical data were available (n = 19, supplemental Table S4, and Ref. 23), no correlations were found between DR subtype expression and GH, IGF-I, or PRL levels at diagnosis. However, a negative correlation was found between DR1 expression and hormone suppression, which was significant for the percent GH reduction after 3 and 6 months of octreotide-LAR treatment (r = −0.67 and −0.58; P = 0.002 and 0.009, respectively; Fig. 2). On the other hand, a positive correlation between DR5 expression and hormone suppression was observed, which was significant for the percent IGF-I reduction after 3 and 6 months of treatment (r = 0.55 and 0.47; P = 0.01 and 0.04, respectively). DR5 expression was higher (P = 0.004), and the level of DR1 expression was lower (P = 0.06) in biochemically controlled (GH <2.5 μg/liter and normal IGF-I for age), compared with uncontrolled patients (Fig. 3). In addition, DR4 expression and percent IGF-I reduction after 6 months of treatment were positively correlated (r = 0.5; P = 0.03; Fig. 2). However, a similar trend was not observed at 3 months, or for GH response at both time points, and no differences was observed between biochemically controlled and uncontrolled patients in DR4 expression levels (Fig. 3). Finally, no correlations were found between DR subtype expression and percent tumor volume reduction.
Figure 2.
Correlations between DR subtype (DR1-5) mRNA content and the percent reduction of GH and IGF-I at 3 and 6 months of treatment with octreotide-LAR. Correlations between numerical variables were studied using Spearman’s correlation test. NF, Normalization factor calculated from the level of HPRT, GAPDH, and β-actin using the GeNorm 3.3 visual basic application for Microsoft Excel (http://medgen.ugent.be/∼jvdesomp/genorm/) as previously developed and validated by Vandesompele et al. (46).
Figure 3.
Comparison of DR subtype (DR1-5) levels between patients with and without hormonal control of disease with octreotide-LAR. Statistical significance was determined by the Mann-Whitney U test. The lower and upper bars represent the first and third quartiles, respectively. The lines across the box represent median value. The lines above and below the box represent the highest and lowest values, excluding outliers. Outliers are shown by circles. NF, Normalization factor calculated from the level of HPRT, GAPDH, and β-actin using the GeNorm 3.3 visual basic application for Microsoft Excel (http://medgen.ugent.be/∼jvdesomp/genorm/) as previously developed and validated by Vandesompele et al. (46).
Relationship between the expression of DR and SSTR in NPs, NFPAs, and somatotropinomas
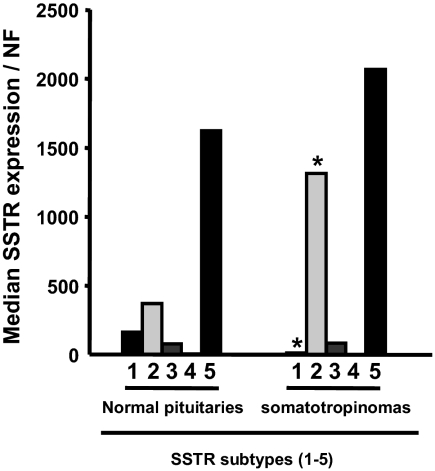
We reported the expression levels of SSTR subtypes by qrtRT-PCR in patients for whom we have data regarding clinical response to octreotide-LAR (23). In the present study, we have expanded the number of somatotropinomas samples to include an additional 20 samples in which patients were lost to follow-up or not treated with octreotide-LAR (supplemental Table S4). The present work confirms our previous findings that SSTR5 is the dominant SSTR subtype expressed in GH-secreting adenomas, followed by SSTR2≫SSTR3≫SSTR1≫SSTR4 (Fig. 4). Similarly, SSTR5 was the most expressed SSTR subtype in NPs (supplemental Table S2 and Fig. 4), followed by SSTR2≫SSTR1≫SSTR3≫SSTR4. NPs expressed more SSTR1 (P = 0.04) and less SSTR2 (P = 0.02), as compared with somatotropinomas, whereas there were no significant differences in the expression of SSTR5, SSTR3, and SSTR4.
Figure 4.
Median SSTR subtype (SSTR1-5) mRNA levels in normal and somatotropinomas. NF, Normalization factor calculated from the level of HPRT, GAPDH, and β-actin using the GeNorm 3.3 visual basic application for Microsoft Excel (http://medgen.ugent.be/∼jvdesomp/genorm/) as previously developed and validated by Vandesompele et al. (46). *, Values that differ between NPs and somatotropinomas (P < 0.05).
Although positive correlations were seen between DR2T and SSTR2 (r = 0.83; P = 0.01), between DR2L and SSTR2 (r = 0.88; P = 0.004) and SSTR5 (r = 0.74; P = 0.04) in NPs, there were no significant correlations between the expression of DR2 (DR2T, DR2L, or DR2L/DR2T) and SSTR2 or SSTR5 in somatotropinomas or in NFPAs, in which SSTR expression profiles were available (supplemental Tables S2 and S3).
Discussion
Several reports have examined the expression of DR2 in NFPAs and somatotropinomas using in situ hybridization and conventional RT-PCR (6,13,15,24,25). However, only a single study using conventional RT-PCR has reported relative expression levels of all DR subtypes in NFPAs (n = 18), in which DR2 and DR4 were detected in 67 and 17%, respectively, whereas expression of DR1, DR3, and DR5 was not detectable (13). To the best of our knowledge, the current report is the first study to measure mRNA copy numbers for all DR subtypes and compare their expression levels among NPs, NFPAs, and somatotropinomas. In the majority of samples, all DR subtypes were expressed, with the exception of DR3, which was below the detection limit of our assay system in all samples. DR2 was clearly the dominant DR subtype in NPs, NFPAs, and somatotropinomas, however, NPs expressed higher levels of DR2 compared with adenomas. This finding is consistent with the observation that DR2 expression is highest in lactotrophs (8), a cell population that is absent in NFPAs and somatotropinomas. Although it has been previously reported that estrogen enhances the expression of DR2, in a rat PRL-producing pituitary cell line (26), where the effect was greater for the DR2L spliced variant compared with the DR2S, we did not observe any correlation between sex and DR2 expression. The lack of relationship between sex and DR2 expression in NPs may be more related to the small sample set available, in which levels could vary in female pituitaries depending on the physiological status (premenopausal vs. postmenopausal). Also in the female patients with adenomas (particularly those with NFPAs), many were advanced in years and were likely postmenopausal. However, it could also be argued that only lactotrophs are sensitive to estrogen-mediated regulation of DR2 expression, and this population is absent in the adenomas studied.
Splice variants of the DR2 transcript (DR2L and DR2S) were originally identified in the brains and pituitaries of humans and rats (27). Specifically, the coding sequence of the DR2S differs from the DR2L only by the absence of exon 5 (87 bases). Although expression levels of these variants have been extensively studied in the rat brain, far fewer studies have focused on the pituitary (27,28,29,30,31,32). Early data generated using in situ hybridization (27,28), ribonuclease protection assays (30), and conventional RT-PCR (27,29,31,32) indicated that DR2L is approximately five to 10 times greater than DR2S in the rat pituitary; however, a recent report using real-time RT-PCR with TaqMan probes suggests that the expression level of DR2L is only 10% greater than DR2S (33). These discrepancies likely occur due to challenges in designing primers and probes with appropriate specificity and efficiency to amplify only the DR2S variant, as well as the inability of ethidium bromide, used in conventional RT-PCR, to accurately quantify gene expression levels (34). Although it is often stated that the mRNA expression levels of DR2L are far greater that DR2S in human pituitaries, similar technical challenges are faced. Despite these challenges, it is clear that the relative expression of DR2 variants in pituitary adenomas show heterogeneity of expression. Specifically, collective examination of two separate reports (one using in situ and one using conventional RT-PCR) that examined the relative expression of DR2L and DR2S in NFPAs (6,13) revealed that 22% did not express DR2 (eight of 36), whereas those that did express DR2, 21.5% expressed more DR2S than DR2L (six of 28), 57% expressed more DR2L than DR2S (16/28), and 21.5% expressed similar levels of DR2S and DR2L (six of 28). In addition, a one-to-one relationship between DR2L and DR2S expression in three somatotropinomas was reported (15), similar to the results of some (24,25) but not all studies (6). Heterogeneity in the expression levels of the DR2 variants in NFPAs and somatotropinomas was also observed in the present study, however, the majority show more DR2S expression than DR2L (79 and 77%, respectively).
Although both DR2L and DR2S have activated the MAPK and ERK pathways, which are associated with cell cycle regulation, growth, differentiation, and apoptosis (35), DR2S has been more potent than DR2L in inhibiting hormone secretion, cAMP accumulation, and cell growth (36). Moreover, DR2S was observed to activate phospholipase D, which may be related to its more potent antiproliferative effect (37). The differential coupling of the DR2 splice variants to intracellular signal transduction pathways may explain the observation that DR2 expression level was positively correlated to DA-induced shrinkage of NFPAs, and this effect was more pronounced in tumors with high DR2S (13). Although in this report we could not examine the relationship between DR2 variant expression and DA-induced response, it is interesting to note that the two somatotropinomas that also stained positive for PRL (nos. 3 and 20, supplemental Table S4) had DR2L to DR2T ratios (0.11 and 0.23, respectively), well below the median (0.36). These results raise the possibility that GH/PRL-secreting tumors have higher DR2S, which may explain the report that patients harboring this tumor subtype tended to respond better than patients with pure GH-secreting tumors to DA treatment (38).
In contrast to the information available regarding the inhibitory role of DA/DR2 in pituitary function, far less is known about the expression and function of the other DR subtypes. In this report we observed that the majority of samples (NPs and adenomas), in addition to expressing high levels of DR2, also expressed DR1, DR4, and DR5, however, their relative expression levels varied between individual samples. In studies largely performed in brain and/or nonpituitary cells, DR4 (like DR2) has been shown to couple with Gi/Go to down-regulate cAMP, whereas DR1 and DR5 couple with Gsα to up-regulate cAMP (5,39,40,41,42). Although caution should be exercised in directly extrapolating data from one tissue to another, it is tempting to speculate that NFPAs and GH-secreting adenomas that express higher levels of DR1 and DR5 may be resistant to DA-mediated reduction in hormone release and/or proliferation. This notion is supported by the observation that expression of human DR1 in a rat GH/PRL pituitary cell line (GH4C1) resulted in cAMP activation and potentiation of Ca2+ channel opening (43).
There is also evidence that human DR2 forms heterodimers with SSTR2 and SSTR5, and the composition of these receptor complexes can alter the response to both DA and SA in heterologous cell systems (18,19). The functional interaction of DR and SSTR is supported by the observations that the SSTR5-mediated GH suppression in adenoma cell cultures taken from acromegalic patients was greater in DR2 negative, compared with DR2 positive tumors (20), and the in vitro response to octreotide was enhanced in adenomas that expressed higher levels of DR2 (12), but this latter relationship was not observed with respect to the in vivo response to octreotide. Given these associations, we compared the expression levels of DR2 with SSTR2 and SSTR5 in NPs, NFPAs, and somatotropinomas. Despite the positive relationship between DR2 and SSTR2/5 in NPs, there were no significant correlations between the level of expression of DR2 and SSTR2/5 in NFPAs and somatotropinomas. In addition, similar to that reported by Ferone et al. (12), in this study there was no relationship between expression of DR2 in somatotropinomas and the in vivo response to octreotide-LAR. However, we did observe that DR1 expression was negatively correlated, whereas DR5 expression was positively correlated with in vivo octreotide-LAR response. It should be noted that a significant correlation was only observed between DR1 expression and GH suppression, and not IGF-I suppression, which might be explained by clinical observations that normalization of IGF-I by SA can lag behind GH. However, our observation that DR5 expression was correlated with IGF-I suppression, and not GH, is more difficult to explain. Perhaps this discrepancy may be related to the combined variations due to small sample size, low DR5 copy numbers, and the pulsatile nature of GH release, and, therefore, further studies are required to confirm these results. Despite these discrepancies, we did find that patients who were controlled by octreotide-LAR therapy (GH <2.5 μg/liter and normal IGF-I for age) had lower DR1 (P = 0.06) and higher DR5 (P = 0.004) expression compared with uncontrolled patients. Therefore, we might speculate that the ability of DR1 to couple to Gsα (5,40,41) may antagonize the actions of SAs. In contrast, the positive relationship between DR5 was unexpected given the fact that this DR subtype has coupled to Gsα in nonpituitary cell systems (5,41). However, it remains to be determined whether DR5 would couple to Gsα in pituitary cells because it is well documented that the G protein-coupled receptors, including DR subtypes, can be differentially linked to intracellular signal transduction pathways depending on the cell type (44,45).
In conclusion, these results confirm that DR2 is the predominant DR subtype in NPs, NFPAs, and somatotropinomas. The expression level of DR2S, thought to be the most bioactive DR2 variant, appears to be greater than DR2L in NPs and in the majority of tumors tested. The fact that DR1, DR4, and DR5 are also expressed in many NFPAs and somatotropinomas tested suggests that these DR subtypes might also play a role in the therapeutic impact of DA. Although a limitation of this study is the fact that mRNA levels may not translate into functional protein levels, this report provides preliminary evidence that the pattern of DR subtype expression in somatotropinomas may aid in predicting the in vivo response to SAs, consistent with reports demonstrating that DRs and SSTRs can heterodimerize and alter the response to their respective ligands.
Supplementary Material
Footnotes
This work was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (to L.V.N.), Programa Ramon y Cajal del Ministerio de Educación y Ciencia (RYC-2007-00186), Spain (to R.M.L.), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (to M.R.G.), National Institute of Diabetes and Digestive and Kidney Diseases Grant 30677, and Veteran Affairs Merit (to R.D.K.) and Novartis Pharmaceuticals (to G.F.T. and M.R.G.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 17, 2009
Abbreviations: DA, Dopamine agonist; DR, dopamine receptor; DR2L, dopamine receptor 2 long variant; DR2S, dopamine receptor 2 short variant; DR2T, dopamine receptor 2 total; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HPRT, hypoxanthine-guanine phosphoribosyltransferase; NFPA, nonfunctioning pituitary adenoma; NP, normal pituitary; PRL, prolactin; qrtRT-PCR, quantitative real-time RT-PCR; RT, reverse transcription; SA, somatostatin analog; SSTR, somatostatin receptor.
References
- Colao A, Filippella M, Pivonello R, Di Somma C, Faggiano A, Lombardi G 2007 Combined therapy of somatostatin analogues and dopamine agonists in the treatment of pituitary tumours. Eur J Endocrinol 156(Suppl 1):S57–S63 [DOI] [PubMed] [Google Scholar]
- Ferone D, Pivonello R, Resmini E, Boschetti M, Rebora A, Albertelli M, Albanese V, Colao A, Culler MD, Minuto F 2007 Preclinical and clinical experiences with the role of dopamine receptors in the treatment of pituitary adenomas. Eur J Endocrinol 156(Suppl 1):S37–S43 [DOI] [PubMed] [Google Scholar]
- Saveanu A, Jaquet P 24 July 2008 Somatostatin-dopamine ligands in the treatment of pituitary adenomas. Rev Endocr Metab Disord 10.1007/s11154-008-9086-0 [DOI] [PubMed] [Google Scholar]
- Civelli O, Bunzow JR, Grandy DK 1993 Molecular diversity of the dopamine receptors. Annu Rev Pharmacol Toxicol 33:281–307 [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG 1998 Dopamine receptors: from structure to function. Physiol Rev 78:189–225 [DOI] [PubMed] [Google Scholar]
- Renner U, Arzberger T, Pagotto U, Leimgruber S, Uhl E, Müller A, Lange M, Weindl A, Stalla GK 1998 Heterogeneous dopamine D2 receptor subtype messenger ribonucleic acid expression in clinically nonfunctioning pituitary adenomas. J Clin Endocrinol Metab 83:1368–1375 [DOI] [PubMed] [Google Scholar]
- Van Tol HH, Wu CM, Guan HC, Ohara K, Bunzow JR, Civelli O, Kennedy J, Seeman P, Niznik HB, Jovanovic V 1992 Multiple dopamine D4 receptor variants in the human population. Nature 358:149–152 [DOI] [PubMed] [Google Scholar]
- Caron MG, Beaulieu M, Raymond V, Gagné B, Drouin J, Lefkowitz RJ, Labrie F 1978 Dopaminergic receptors in the anterior pituitary gland. Correlation of [3H]dihydroergocryptine binding with the dopaminergic control of prolactin release. J Biol Chem 253:2244–2253 [PubMed] [Google Scholar]
- Cronin MJ, Thorner MO, Hellmann P, Rogol AD 1984 Bromocriptine inhibits growth hormone release from rat pituitary cells in primary culture. Proc Soc Exp Biol Med 175:191–195 [DOI] [PubMed] [Google Scholar]
- Foord SM, Peters JR, Dieguez C, Scanlon MF, Hall R 1983 Dopamine receptors on intact anterior pituitary cells in culture: functional association with the inhibition of prolactin and thyrotropin. Endocrinology 112:1567–1577 [DOI] [PubMed] [Google Scholar]
- Goldsmith PC, Cronin MJ, Weiner RI 1979 Dopamine receptor sites in the anterior pituitary. J Histochem Cytochem 27:1205–1207 [DOI] [PubMed] [Google Scholar]
- Ferone D, de Herder WW, Pivonello R, Kros JM, van Koetsveld PM, de Jong T, Minuto F, Colao A, Lamberts SW, Hofland LJ 2008 Correlation of in vitro and in vivo somatotropic adenoma responsiveness to somatostatin analogs and dopamine agonists with immunohistochemical evaluation of somatostatin and dopamine receptors and electron microscopy. J Clin Endocrinol Metab 93:1412–1417 [DOI] [PubMed] [Google Scholar]
- Pivonello R, Matrone C, Filippella M, Cavallo LM, Di Somma C, Cappabianca P, Colao A, Annunziato L, Lombardi G 2004 Dopamine receptor expression and function in clinically nonfunctioning pituitary tumors: comparison with the effectiveness of cabergoline treatment. J Clin Endocrinol Metab 89:1674–1683 [DOI] [PubMed] [Google Scholar]
- Saveanu A, Gunz G, Guillen S, Dufour H, Culler MD, Jaquet P 2006 Somatostatin and dopamine-somatostatin multiple ligands directed towards somatostatin and dopamine receptors in pituitary adenomas. Neuroendocrinology 83:258–263 [DOI] [PubMed] [Google Scholar]
- Caccavelli L, Feron F, Morange I, Rouer E, Benarous R, Dewailly D, Jaquet P, Kordon C, Enjalbert A 1994 Decreased expression of the two D2 dopamine receptor isoforms in bromocriptine-resistant prolactinomas. Neuroendocrinology 60:314–322 [DOI] [PubMed] [Google Scholar]
- Ferone D, Pivonello R, Lastoria S, Faggiano A, Del Basso de Caro ML, Cappabianca P, Lombardi G, Colao A 2001 In vivo and in vitro effects of octreotide, quinagolide and cabergoline in four hyperprolactinaemic acromegalics: correlation with somatostatin and dopamine D2 receptor scintigraphy. Clin Endocrinol (Oxf) 54:469–477 [DOI] [PubMed] [Google Scholar]
- Stefaneanu L, Kovacs K, Horvath E, Buchfelder M, Fahlbusch R, Lancranjan L 2001 Dopamine D2 receptor gene expression in human adenohypophysial adenomas. Endocrine 14:329–336 [DOI] [PubMed] [Google Scholar]
- Baragli A, Alturaihi H, Watt HL, Abdallah A, Kumar U 2007 Heterooligomerization of human dopamine receptor 2 and somatostatin receptor 2 co-immunoprecipitation and fluorescence resonance energy transfer analysis. Cell Signal 19:2304–2316 [DOI] [PubMed] [Google Scholar]
- Rocheville M, Lange DC, Kumar U, Patel SC, Patel RC, Patel YC 2000 Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science 288:154–157 [DOI] [PubMed] [Google Scholar]
- Zatelli MC, Piccin D, Tagliati F, Bottoni A, Ambrosio MR, Margutti A, Scanarini M, Bondanelli M, Culler MD, degli Uberti EC 2005 Dopamine receptor subtype 2 and somatostatin receptor subtype 5 expression influences somatostatin analogs effects on human somatotroph pituitary adenomas in vitro. J Mol Endocrinol 35:333–341 [DOI] [PubMed] [Google Scholar]
- Ferone D, Saveanu A, Culler MD, Arvigo M, Rebora A, Gatto F, Minuto F, Jaquet P 2007 Novel chimeric somatostatin analogs: facts and perspectives. Eur J Endocrinol 156(Suppl 1):S23–S28 [DOI] [PubMed] [Google Scholar]
- Taboada GF, Luque RM, Bastos W, Guimarães RF, Marcondes JB, Chimelli LM, Fontes R, Mata PJ, Filho PN, Carvalho DP, Kineman RD, Gadelha MR 2007 Quantitative analysis of somatostatin receptor subtype (SSTR1-5) gene expression levels in somatotropinomas and non-functioning pituitary adenomas. Eur J Endocrinol 156:65–74 [DOI] [PubMed] [Google Scholar]
- Taboada GF, Luque RM, Neto LV, Machado Ede O, Sbaffi BC, Domingues RC, Marcondes JB, Chimelli LM, Fontes R, Niemeyer P, de Carvalho DP, Kineman RD, Gadelha MR 2008 Quantitative analysis of somatostatin receptor subtypes (1–5) gene expression levels in somatotropinomas and correlation to in vivo hormonal and tumor volume responses to treatment with octreotide LAR. Eur J Endocrinol 158:295–303 [DOI] [PubMed] [Google Scholar]
- Gandelman KY, Harmon S, Todd RD, O'Malley KL 1991 Analysis of the structure and expression of the human dopamine D2A receptor gene. J Neurochem 56:1024–1029 [DOI] [PubMed] [Google Scholar]
- Mack KJ, Todd RD, O'Malley KL 1991 The mouse dopamine D2A receptor gene: sequence homology with the rat and human genes and expression of alternative transcripts. J Neurochem 57:795–801 [DOI] [PubMed] [Google Scholar]
- Guivarc'h D, Vincent JD, Vernier P 1998 Alternative splicing of the D2 dopamine receptor messenger ribonucleic acid is modulated by activated sex steroid receptors in the MMQ prolactin cell line. Endocrinology 139:4213–4221 [DOI] [PubMed] [Google Scholar]
- Dal Toso R, Sommer B, Ewert M, Herb A, Pritchett DB, Bach A, Shivers BD, Seeburg PH 1989 The dopamine D2 receptor: two molecular forms generated by alternative splicing. EMBO J 8:4025–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnauld E, Arsaut J, Demotes-Mainard J 1991 Differential plasticity of the dopaminergic D2 receptor mRNA isoforms under haloperidol treatment, as evidenced by in situ hybridization in rat anterior pituitary. Neurosci Lett 130:12–16 [DOI] [PubMed] [Google Scholar]
- Giros B, Sokoloff P, Martres MP, Riou JF, Emorine LJ, Schwartz JC 1989 Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature 342:923–926 [DOI] [PubMed] [Google Scholar]
- Inoue A, Seto M, Sugita S, Hide I, Hirose T, Koga N, Kikuchi T, Nakata Y 1998 Differential effects on D2 dopamine receptor and prolactin gene expression by haloperidol and aripiprazole in the rat pituitary. Brain Res Mol Brain Res 55:285–292 [DOI] [PubMed] [Google Scholar]
- O'Malley KL, Mack KJ, Gandelman KY, Todd RD 1990 Organization and expression of the rat D2A receptor gene: identification of alternative transcripts and a variant donor splice site. Biochemistry 29:1367–1371 [DOI] [PubMed] [Google Scholar]
- Rao DD, McKelvy J, Kebabian J, MacKenzie RG 1990 Two forms of the rat D2 dopamine receptor as revealed by the polymerase chain reaction. FEBS Lett 263:18–22 [DOI] [PubMed] [Google Scholar]
- Byrnes EM, Bridges RS 2007 Reproductive experience and expression of dopamine D(2) receptor mRNA: a possible mechanism for reduced prolactin secretion in primiparous rats. J Neuroendocrinol 19:773–778 [DOI] [PubMed] [Google Scholar]
- Dutton MD, Varhol RJ, Dixon DG 1995 Technical considerations for the use of ethidium bromide in the quantitative analysis of nucleic acids. Anal Biochem 230:353–355 [DOI] [PubMed] [Google Scholar]
- Choi EY, Jeong D, Park KW, Baik JH 1999 G protein-mediated mitogen-activated protein kinase activation by two dopamine D2 receptors. Biochem Biophys Res Commun 256:33–40 [DOI] [PubMed] [Google Scholar]
- Hayes G, Biden TJ, Selbie LA, Shine J 1992 Structural subtypes of the dopamine D2 receptor are functionally distinct: expression of the cloned D2A and D2B subtypes in a heterologous cell line. Mol Endocrinol 6:920–926 [DOI] [PubMed] [Google Scholar]
- Senogles SE 2000 The D2s dopamine receptor stimulates phospholipase D activity: a novel signaling pathway for dopamine. Mol Pharmacol 58:455–462 [DOI] [PubMed] [Google Scholar]
- Abs R, Verhelst J, Maiter D, Van Acker K, Nobels F, Coolens JL, Mahler C, Beckers A 1998 Cabergoline in the treatment of acromegaly: a study in 64 patients. J Clin Endocrinol Metab 83:374–378 [DOI] [PubMed] [Google Scholar]
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH 1995 Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem 65:1157–1165 [DOI] [PubMed] [Google Scholar]
- De Keyser J, Dierckx R, Vanderheyden P, Ebinger G, Vauquelin G 1988 D1 dopamine receptors in human putamen, frontal cortex and calf retina: differences in guanine nucleotide regulation of agonist binding and adenylate cyclase stimulation. Brain Res 443:77–84 [DOI] [PubMed] [Google Scholar]
- Lezcano N, Bergson C 2002 D1/D5 dopamine receptors stimulate intracellular calcium release in primary cultures of neocortical and hippocampal neurons. J Neurophysiol 87:2167–2175 [DOI] [PubMed] [Google Scholar]
- Sanyal S, Van Tol HH 1997 Dopamine D4 receptor-mediated inhibition of cyclic adenosine 3′,5′-monophosphate production does not affect prolactin regulation. Endocrinology 138:1871–1878 [DOI] [PubMed] [Google Scholar]
- Liu YF, Civelli O, Zhou QY, Albert PR 1992 Cholera toxin-sensitive 3′,5′-cyclic adenosine monophosphate and calcium signals of the human dopamine-D1 receptor: selective potentiation by protein kinase A. Mol Endocrinol 6:1815–1824 [DOI] [PubMed] [Google Scholar]
- Kostenis E, Waelbroeck M, Milligan G 2005 Techniques: promiscuous Gα proteins in basic research and drug discovery. Trends Pharmacol Sci 26:595–602 [DOI] [PubMed] [Google Scholar]
- Pivonello R, Ferone D, Lombardi G, Colao A, Lamberts SW, Hofland LJ 2007 Novel insights in dopamine receptor physiology. Eur J Endocrinol 156(Suppl 1):S13–S21 [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F 2002 Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.