Abstract
Context: A significant proportion of the first-degree female relatives of women with polycystic ovary syndrome (PCOS) may be at risk for developing PCOS. However, it is not known at which stage of pubertal development the hormonal and metabolic abnormalities ensue in PCOS.
Objective: The aim of the study was to assess the reproductive and metabolic profiles of daughters of women with PCOS (PCOSd) during the peripubertal period, a stage during which the gonadal axis is activated and PCOS may become clinically manifest.
Design: Ninety-nine PCOSd [30 prepubertal and 69 pubertal (Tanner II-V)] and 84 daughters of control women (Cd) (20 prepubertal and 64 pubertal) were studied. An oral glucose tolerance test, a GnRH agonist test (leuprolide acetate, 10 μg/kg sc), and a transabdominal ultrasound were performed. Gonadotropins, sex steroids, SHBG, glucose, insulin, and lipids were determined.
Results: Both groups had similar chronological ages and body mass index sd scores according to Tanner stage distribution. Ovarian volume and 2-h insulin were significantly higher in PCOSd compared to Cd at all Tanner stages. In Tanner stages IV and V, basal testosterone and poststimulated LH, testosterone, and 17-hydroxyprogesterone concentrations were significantly higher in PCOSd compared to Cd.
Conclusions: Hyperinsulinemia and an increased ovarian volume are present in PCOSd before the onset of puberty and persist during pubertal development. The biochemical abnormalities of PCOS appear during late puberty. Considering the early onset and the nature of the alterations, PCOSd constitute a high-risk group for metabolic and reproductive derangements.
Hyperinsulinemia and increased ovarian volume are present in daughters of PCOS women before the onset of puberty, while ovarian hyperandrogenism appears to be present only during late puberty.
Polycystic ovary syndrome (PCOS) is a highly prevalent (5–10%) endocrine-metabolic dysfunction in adult women, characterized by chronic anovulation and hyperandrogenism. In addition, most women with PCOS also have peripheral insulin resistance which plays a key role in the pathogenesis of this syndrome (1,2,3) and the long-term health consequences, such as type 2 diabetes mellitus and cardiovascular (4,5,6) disease. Therefore, the diagnosis of PCOS should be established as soon as possible, preferably during adolescence when this dysfunction may become clinically manifested (7).
In most girls, PCOS usually manifests after adolescence with weight gain, clinical hyperandrogenism, oligoamenorrhea and insulin resistance (8), although early clinical manifestations of PCOS may emerge in some girls during the peripubertal years with premature pubarche. Nevertheless, the diagnosis of PCOS in adolescence can be difficult because some features of the syndrome may be viewed as “physiological” at this age (9). Therefore, PCOS usually is not recognized until adulthood, resulting in delayed treatment.
It has been proposed that PCOS is the result of an abnormal regulation of steroidogenesis, specifically of androgen secretion by the ovary. Provocative tests using GnRH agonists have shown that the hyperandrogenism has an ovarian source in most cases (10). Such alteration may precede the development of clinical manifestations of PCOS and has been termed functional ovarian hyperandrogenism (FOH) (10). However, in reproductive age women with PCOS, insulin has also been proposed as a candidate factor responsible for FOH (11).
In girls with premature pubarche, Ibañez et al. (12,13) observed that the metabolic abnormalities were present before the onset of hyperandrogenism. We have also noted that some of the metabolic features of PCOS were present in daughters of PCOS women before the onset of hyperandrogenism (14). However, in that study we were unable to establish precisely the dynamics of the onset of different features of this syndrome during various pubertal stages.
It has been proposed that an important proportion of the first-degree female relatives of a PCOS woman may be at risk for developing PCOS (15,16). Therefore, the aim of the present study was to assess the reproductive and metabolic profiles in the daughters of women with PCOS during the peripubertal period, a stage during which the gonadal axis is activated and PCOS may become clinically manifest. To study this question, we performed an oral glucose tolerance test and evaluated the hormonal response to a GnRH agonist in a group of peripubertal girls born to women with PCOS, and compared their responses with a carefully matched group of girls born to healthy women in a cross-sectional study.
Subjects and Methods
Subjects
We studied 30 prepubertal girls (7–10 yr old) and 69 pubertal girls (Tanner II-V; ages 8–15 yr) born to PCOS mothers [PCOS daughters (PCOSd)]. The control group consisted of 20 prepubertal girls and 64 pubertal girls born to mothers with regular menstrual cycles and without hyperandrogenism [control daughters (Cd)].
Both groups of girls were matched for Tanner stage score for breast development at the beginning of the study. No girls were taking oral contraceptives or any other medication. Girls with any chronic diseases were excluded.
PCOS mothers were recruited from patients attending the Unit of Endocrinology and Reproductive Medicine, University of Chile. This group of PCOS mothers is part of a group of patients who have attended our polyclinic since they were diagnosed with PCOS, in some cases for more than 20 yr. The diagnosis of PCOS was made according to the National Institutes of Health consensus criteria (17). PCOS mothers were evaluated before pregnancy, and they exhibited chronic oligomenorrhea or amenorrhea, hirsutism, serum testosterone concentration greater than 0.6 ng/ml, free androgen index (FAI) greater than 5.0, and/or androstenedione concentration greater than 3.0 ng/ml. In addition, PCOS women showed the characteristic ovarian morphology of polycystic ovaries on ultrasound, based on the criteria described by Adams et al. (18). Before pregnancy, in PCOS mothers the glucose response to an oral glucose tolerance test was normal, but they had different degrees of hyperinsulinemia after oral glucose load. All patients had a waist-to-hip ratio (WHR) greater than 0.85. We excluded patients with hyperprolactinemia, androgen-secreting neoplasm, Cushing’s syndrome, late onset 21-hydroxylase deficiency, and thyroid disease.
All PCOSd were born at term from singleton pregnancies. The incidence of gestational diabetes for PCOS mothers, according to the World Health Organization (WHO) criteria (1999) (19), was 28% and the incidence of pregnancy-induced hypertension was 10%. Moreover, 56% of the PCOS patients were primiparous.
As control mothers, we selected women of socioeconomic level similar to the PCOS patients, with history of singleton pregnancies and regular 28- to 32-d menstrual cycles and without hirsutism and other clinical manifestations of hyperandrogenism, infertility, or pregnancy complications. Control mothers were comparable in age with PCOS mothers (36.6 ± 6.1 vs. 38.2 ± 7.6 yr). However, PCOS mothers had a higher body mass index (BMI) than control mothers at the moment that the girls were evaluated (29.3 ± 4.3 vs. 27.0 ± 4.9 kg/m2; P < 0.001). No siblings were included in either group.
Study protocol
The girls were admitted with their mothers to the Pediatric Unit of our Clinical Research Center at approximately 0830 h. We performed a complete physical examination, including anthropometric measurements [weight, height, waist, hip, BMI, and BMI sd score (SDS) calculated by using the Growth Analyser Program and the U.S. growth charts BMI for age] (20). Obesity was defined as body weight above the 95th percentile. A single observer (V.P.) assessed the pubertal development according to the criteria of Tanner. Hirsutism was evaluated by determining the presence of terminal hair using the modified Ferriman-Gallway score (21). The Chilean population is less hirsute than other populations, so a score of 6 or greater was employed to determine the presence of hirsutism (22) because such a score has been suggested to be pathological in young adolescents (23). The presence of acne and acanthosis nigricans was also determined. Menstrual regularity was not considered in the analysis of the data because irregular menses are common in the first years after the onset of menarche. Prepubertal and pubertal girls with a history of early puberty and precocious pubarche were excluded.
In both groups of girls, we performed an oral glucose tolerance test (1.75 g/kg, up to a maximum of 75 g glucose in 250 ml water) after a 12-h overnight fast. Blood samples (5 ml) were drawn for the measurement of glucose and insulin concentrations before and 30, 60, 90, and 120 min after the glucose load. Glucose tolerance was evaluated by using the criteria of the American Diabetes Association (ADA). Impaired glucose tolerance (IGT) was defined as 2-h postload glucose of at least 140 mg/dl and less than 200 mg/dl (24). Insulin resistance was estimated by the homeostasis model assessment for insulin resistance (HOMA-IR) as described previously (25). Moreover, whole-body insulin sensitivity index (ISI) composite was calculated (26). Circulating concentrations of SHBG and serum lipids [total cholesterol, triglycerides, low-density lipoprotein-cholesterol (LDL-C), and high-density lipoprotein-cholesterol (HDL-C)] were determined in the fasting sample.
On the following day, transabdominal ultrasound was performed and analyzed by a single observer (F.S.), who was blinded to the condition of the subject. The examination was performed with a 5-MHz abdominal probe, using Medison Sonoace 6000C equipment (Medison Co., Ltd., Seoul, Korea). Ovarian volume was calculated using the simplified formula for a prolate ellipsoid (27). The larger ovary was used to evaluate the ovarian size.
Subsequently, all subjects underwent a GnRH agonist test with 10 μg/kg sc leuprolide acetate (Lupron; Abbott Laboratories, North Chicago, IL) as described (28,29). The test was started between 0800 and 0900 h; serum LH and FSH levels were measured before and 3 and 24 h after leuprolide injection. Serum testosterone, androstenedione, 17 α-hydroxyprogesterone (17-OHP), and estradiol concentrations were determined at baseline and 24 h after leuprolide administration. Basal serum SHBG and testosterone were used to calculate the FAI as the ratio of serum testosterone: SHBG × 100. Maximal values after leuprolide testing were defined as the peak value for gonadotropins at 3 h and for steroids at 24 h.
Postmenarchal girls were studied during the early follicular phase of the menstrual cycle (d 3–7). In the premenarchal girls, the study was performed whenever feasible.
The protocol was approved by the institutional review boards of the San Juan de Dios and San Borja Arriarán Hospitals and the University of Chile. All parents signed informed consents, and girls gave their assent before entering the study.
Assays
Serum glucose was determined by the glucose oxidase method (Photometric Instrument 4010; Roche, Basel, Switzerland). The intraassay coefficient of variation of this method was less than 2.0%. Serum insulin was assayed by RIA (Diagnostic Systems Laboratories, Inc., Webster, Texas). The intra- and interassay coefficients of variation were 5 and 8%, respectively. The lipid profile was determined by standard colorimetric assays (Photometric Instrument 4010; Roche). Serum LDL-C concentration was calculated by using the Friedewald’s formula: LDL-C = total cholesterol − HDL-C − (triglycerides/5).
Serum LH, FSH, and estradiol were determined by electrochemiluminescence assays (Roche). Assay sensitivities for LH, FSH, and estradiol assays were 0.1 U/liter, 0.1 U/liter, and 5.0 pg/ml, respectively. Intra- and interassay coefficients of variation were 1.8 and 5.2% for LH, 1.8 and 5.3% for FSH, and 5.7 and 6.2% for estradiol, respectively.
Serum androstenedione (Diagnostic Systems Laboratories) and 17-OHP (Diagnostic Products Corp., Los Angeles, CA) were assayed by RIA. SHBG was determined by radioimmunometric assay (Diagnostic Products Corp.). For androstenedione, 17-OHP, and SHBG assays, the assay sensitivities were 0.1 ng/ml, 0.1 ng/ml, and 0.04 nmol/liter, respectively, and intra- and interassay coefficients of variation 4.3 and 6.0%, 5.0 and 5.0%, and 3.8 and 7.9%, respectively.
Serum testosterone was assayed by RIA (Diagnostic Systems Laboratories) in our laboratory. The limit of detection was 10 ng/dl. The intra- and interassay coefficients of variation were 7.0 and 11.0%. This RIA was validated against the liquid chromatography, tandem mass spectrometry (LC-MS/MS) method performed (by S.B.) in the Laboratory of Steroid Hormone Assay, Boston Medical Center (Boston, MA) according to a modification of the technique by Choi et al. (30). In brief, after addition of 200 pg d3-testosterone, extraction was performed using acetonitrile. The samples were first separated by an online C18 HTLC precolumn using a binary gradient consisting of water (A) and methanol (B) at a flow rate of 2.0 ml/min. The fraction containing the analyte was separated further by a C18 analytical column, using a binary gradient consisting of water and acetonitrile/water.
Mass spectrometry was performed using TSQ Thermo-Finnigan Quantum Ultra with APCI (Thermo Fisher Scientific Inc., Waltham, MA) source, using multiple reaction monitoring and the following parameters: discharge current, 4.0 uA; vaporization temperature, 430 C; sheath gas, 35 Arb; capillary temperature, 270 C; and collision energy, 1.2 mTorr. Mass transitions monitored for testosterone were m/z 289.210 to 97.15 and 109.12 m/z for testosterone and from 292.38 to 97.15 and 109.11 m/z for the deuterated testosterone (internal standard). A quantitative calibration was performed with every batch of samples, with calibration standards prepared at concentrations of 2, 5, 10, 50, 100, 150, and 250 ng/dl. The standard curve is linear at testosterone concentrations ranging from 2 to 2000 ng/dl. The cross-reactivity of dehydroepiandrosterone, dehydroepiandrosterone sulfate, dihydrotestosterone, androstenedione, and estradiol in the testosterone assay is negligible at 10 times the circulating concentrations of these hormones. The limit of detection, defined as the lowest concentration which could be detected with a coefficient of variation of less than 20% using a serum volume of 60 μl was determined to be 2 ng/dl.
At all time points (baseline and poststimulated) where sufficient volume of serum was available, we performed measurements of total testosterone concentrations by both methods. Because of the limited sample volume, it was not always possible to perform LC-MS/MS and RIA in all the same samples (less than 10%).
Statistical evaluation
Data are expressed as mean and sd. Normal distribution was assessed by the Kolmogorov-Smirnov test. Comparisons of means between the two groups (PCOSd and Cd) for each Tanner stage were performed using the Student t test when data were normally distributed, or the Mann-Whitney test when not normally distributed. Categorical data were analyzed using χ2 or Fisher’s exact test. Spearman correlations were used to evaluate the relationship between the variables of interest. Statistical analysis was performed using STATA 7.0 package (StataCorp LP, College Station, TX). A P value of less than 0.05 was considered to be statistically significant.
Results
Table 1 shows the clinical characteristics and ovarian volume of Cd and PCOSd during each Tanner stage. Age, BMI, BMI SDS, waist circumference, WHR, and birth weight did not differ significantly between Cd and PCOSd during any Tanner stage. In Tanner stage V, 5 of 15 (33.3%) of the PCOSd and 2 of 14 (14.3%) of the Cd (P = 0.389) were obese, with a body weight above the 95th percentile. At the same Tanner stage, hirsutism was more prevalent in PCOSd than in Cd, and 40.0% of PCOSd exhibited a score of more than 6. No girl in the control group had a Ferriman-Galloway score above 6 (P = 0.05). Acne was observed in a similar proportion of girls in both groups, and acanthosis nigricans was more frequently observed in PCOSd than in Cd (25.0 vs. 12.5%). The proportion of postmenarchal girls in each group was similar. Ovarian volume was significantly higher in PCOSd compared with Cd in all Tanner stages.
Table 1.
Clinical characteristics of Cd and PCOSd during different Tanner stages
| Tanner I
|
Tanner II
|
Tanner III
|
Tanner IV
|
Tanner V
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cd (n = 20) | PCOSd (n = 30) | Cd (n = 15) | PCOSd (n = 13) | Cd (n = 19) | PCOSd (n = 21) | Cd (n = 16) | PCOSd (n = 20) | Cd (n = 14) | PCOSd (n = 15) | |
| Age (yr) | 8.5 ± 1.2 | 8.2 ± 0.9 | 9.7 ± 0.7 | 9.6 ± 1.0 | 10.7 ± 1.0 | 10.8 ± 1.5 | 12.0 ± 1.4 | 12.1 ± 1.5 | 13.2 ± 1.1 | 13.1 ± 1.7 |
| Weight (kg) | 31 ± 6 | 30 ± 7 | 39 ± 8 | 35 ± 7 | 44 ± 8 | 45 ± 14 | 51 ± 5 | 50 ± 11 | 55 ± 8 | 60 ± 17 |
| Weight SDS | 0.6 ± 0.9 | 0.8 ± 0.9 | 0.9 ± 0.8 | 0.4 ± 1.1 | 0.8 ± 0.8 | 0.8 ± 1.1 | 0.9 ± 0.6 | 0.7 ± 0.8 | 0.7 ± 0.8 | 1.1 ± 0.8 |
| Height (cm) | 130 ± 9 | 126 ± 8 | 139 ± 5 | 137 ± 8 | 145 ± 7 | 144 ± 9 | 153 ± 7 | 152 ± 7 | 154 ± 4 | 155 ± 7 |
| Height SDS | −0.1 ± 1.0 | 0.1 ± 1.2 | 0.4 ± 0.9 | 0.1 ± 1.0 | 0.5 ± 0.9 | 0.9 ± 0.9 | 0.4 ± 1.2 | 0.1 ± 0.8 | 0.3 ± 0.9 | 0.1 ± 0.1 |
| BMI (kg/m2) | 19 ± 3 | 19 ± 3 | 20 ± 3 | 19 ± 3 | 21 ± 3 | 21 ± 4 | 22 ± 2 | 22 ± 3 | 23 ± 3 | 25 ± 5 |
| BMI SDS | 0.8 ± 1.0 | 1.1 ± 0.8 | 1.0 ± 0.8 | 0.6 ± 1.0 | 0.9 ± 0.7 | 0.9 ± 0.9 | 1.0 ± 0.4 | 0.8 ± 0.7 | 1.0 ± 0.6 | 1.3 ± 0.6 |
| Waist circumference (cm) | 61 ± 6 | 62 ± 9 | 67 ± 8 | 64 ± 9 | 70 ± 8 | 70 ± 12 | 70 ± 4 | 70 ± 7 | 71 ± 6 | 74 ± 9 |
| WHR | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| Birth weight (kg) | 3.3 ± 0.4 | 3.2 ± 0.4 | 3.3 ± 0.5 | 3.3 ± 0.7 | 3.3 ± 0.4 | 3.3 ± 0.7 | 3.2 ± 0.3 | 3.3 ± 0.5 | 3.4 ± 0.3 | 3.2 ± 0.2 |
| % Postmenarcheal | 0 | 0 | 0 | 0 | 26.4 | 26.6 | 53.6 | 63.2 | 100 | 100 |
| Ovarian volume (cm3) | 2.6 ± 1.2 | 5.1 ± 4.9a | 2.9 ± 1.3 | 6.9 ± 5a | 3.1 ± 1.6 | 6.1 ± 2.2a | 6.1 ± 2.4 | 9.3 ± 4.2a | 6.9 ± 3.9 | 13.7 ± 4.4a |
Values are expressed as median ± sd.
P < 0.05 between Cd and PCOSd.
Table 2 shows the metabolic characteristics of Cd and PCOSd during different Tanner stages. In Tanner stages I, II, and III, fasting glucose, insulin, lipids, and HOMA-IR were not significantly different between the groups. Two-hour glucose was not significantly different between the groups. However, 2-h insulin was significantly higher in the PCOSd group than the Cd group at each of three Tanner stages.
Table 2.
Metabolic parameters in Cd and PCOSd during different Tanner stages
| Tanner I
|
Tanner II
|
Tanner III
|
Tanner IV
|
Tanner V
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cd (n = 20) | PCOSd (n = 30) | Cd (n = 15) | PCOSd (n = 13) | Cd (n = 19) | PCOSd (n = 21) | Cd (n = 16) | PCOSd (n = 20) | Cd (n = 14) | PCOSd (n = 15) | |
| Fasting | ||||||||||
| Glucose (mg/dl) | 83 + 10 | 88 ± 10 | 80 ± 7 | 78 ± 13 | 86 ± 6 | 80 ± 13 | 85 ± 9 | 84 ± 12 | 85 ± 11 | 86 ± 14 |
| Insulin (μIU/ml) | 8.5 ± 2.6 | 9.1 ± 6.6 | 8.9 ± 3.0 | 12 ± 8 | 15 ± 10 | 24 ± 23 | 13 ± 8 | 18 ± 8a | 15 ± 10 | 18 ± 9 |
| Triglycerides (mg/dl) | 116 ± 25 | 130 ± 64 | 124 ± 24 | 122 ± 37 | 137 ± 53 | 125 ± 49 | 101 ± 20 | 126 ± 43a | 97 ± 21 | 135 ± 28a |
| Cholesterol (mg/dl) | 178 ± 34 | 172 ± 34 | 165 ± 17 | 170 ± 27 | 167 ± 29 | 158 ± 24 | 165 ± 20 | 164 ± 27 | 161 ± 31 | 142 ± 34 |
| HDL-C (mg/dl) | 44 ± 8 | 45 ± 11 | 38 ± 6 | 41 ± 8 | 39 ± 10 | 44 ± 11 | 45 ± 6 | 48 ± 12 | 41 ± 8 | 42 ± 9 |
| LDL-C (mg/dl) | 111 ± 32 | 101 ± 32 | 103 ± 18 | 104 ± 27 | 101 ± 22 | 84 ± 32 | 100 ± 20 | 92 ± 30 | 95 ± 21 | 77 ± 28 |
| HOMA-IR | 1.7 ± 0.6 | 2.0 ± 1.7 | 1.7 ± 0.5 | 2.4 ± 1.7 | 3.2 ± 2.2 | 4.5 ± 5.5 | 2.6 ± 1.8 | 3.2 ± 1.7 | 3.2 ± 2.9 | 3.6 ± 2.3 |
| 2h | ||||||||||
| Glucose (mg/dl) | 92 ± 17 | 104 ± 16 | 89 ± 9 | 94 ± 18 | 100 ± 16 | 107 ± 18 | 92 ± 15 | 100 ± 27 | 92 ± 20 | 100 ± 32 |
| Insulin (μIU/ml) | 27 ± 12 | 51 ± 59a | 33 ± 11 | 53 ± 24a | 49 ± 24 | 96 ± 91a | 46 ± 17 | 77 ± 42a | 30 ± 30 | 72 ± 34a |
| ISI composite | 7.6 ± 1.9 | 7.2 ± 3.4 | 6.9 ± 1.3 | 6.3 ± 3.0 | 5.0 ± 2.9 | 4.8 ± 3.4 | 6.0 ± 3.2 | 3.9 ± 1.8a | 13.6 ± 8.4 | 3.5 ± 1.5a |
Values are expressed as median ± sd.
P < 0.05 between Cd and PCOSd.
In Tanner stages IV and V, fasting glucose and HOMA-IR were not significantly different between the groups. Two-hour glucose did not differ significantly between the groups; however, one PCOSd in Tanner stage IV and two in Tanner stage V showed IGT according to the ADA criteria. Fasting insulin and triglyceride concentrations were significantly higher in PCOSd compared with Cd. The 2-h insulin was significantly higher in PCOSd than Cd in Tanner stages IV and V. Moreover, in these Tanner stages, ISI composite was significantly lower in the PCOSd group than the Cd group.
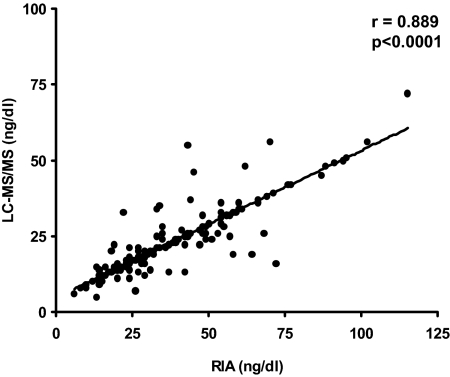
Basal gonadotropin and steroid levels are shown in Table 3. Both groups of girls had similar basal levels of gonadotropins and sex steroids in Tanner stages I, II, and III. In Tanner stage IV, basal LH, 17-OHP, and testosterone concentrations (RIA and LC-MS/MS) and, LH/FSH ratio were significantly higher in PCOSd compared with Cd. In Tanner stage V, basal 17-OHP and testosterone level concentrations (RIA and LC-MS/MS) were also significantly higher in PCOSd compared with Cd. In Tanner stages IV and V, SHBG concentrations were significantly lower, and the FAI was significantly higher in PCOSd compared with Cd. In a Spearman regression analysis, 2-h insulin concentrations were positively correlated with stimulated testosterone levels (LC-MS/MS) in Tanner IV and V in the PCOSd group (r = 0.660, P = 0.001; and r = 0.601, P = 0.045, respectively). There was a high correlation in testosterone measurements with RIA and LC-MS/MS methods (r = 0.889; P < 0.0001) (Fig. 1).
Table 3.
Basal gonadotropin and sex steroid concentrations in Cd and PCOSd during different Tanner stages
| Tanner I
|
Tanner II
|
Tanner III
|
Tanner IV
|
Tanner V
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cd (n = 20) | PCOSd (n = 30) | Cd (n = 15) | PCOSd (n = 13) | Cd (n = 19) | PCOSd (n = 21) | Cd (n = 16) | PCOSd (n = 20) | Cd (n = 14) | PCOSd (n = 15) | |
| LH (IU/liter) | 0.2 ± 0.2 | 0.4 ± 0.9 | 0.5 ± 0.7 | 0.4 ± 0.6 | 2.7 ± 2.6 | 3.0 ± 3.3 | 5.0 ± 4.7 | 6.3 ± 5.5a | 2.7 ± 2.0 | 3.7 ± 2.4 |
| FSH (IU/liter) | 2.4 ± 1.3 | 1.9 ± 0.7 | 4.2 ± 2.3 | 3.3 ± 1.9 | 6.1 ± 2.4 | 5.0 ± 2.4 | 5.8 ± 3.0 | 6.9 ± 2.1 | 5.6 ± 2.0 | 5.9 ± 1.8 |
| LH/FSH ratio | 0.1 ± 0.2 | 0.1 ± 0.1 | 0.1 ± 0.2 | 0.1 ± 0.1 | 0.4 ± 0.3 | 0.5 ± 0.4 | 0.7 ± 0.4 | 1.0 ± 0.7a | 0.6 ± 0.7 | 0.7 ± 0.4 |
| 17-OHP (ng/ml) | 0.8 ± 0.6 | 0.9 ± 0.5 | 0.6 ± 0.3 | 0.6 ± 0.2 | 0.8 ± 0.4 | 0.8 ± 0.3 | 1.0 ± 0.3 | 1.4 ± 0.7a | 1.1 ± 0.6 | 1.7 ± 0.9a |
| Androstenedione (ng/ml) | 0.4 ± 0.2 | 0.5 ± 0.3 | 0.8 ± 0.4 | 0.6 ± 0.2 | 1.2 ± 0.8 | 1.2 ± 0.5 | 1.6 ± 0.5 | 1.8 ± 1.2 | 1.6 ± 0.4 | 2.0 ± 2.1 |
| Testosterone RIA (ng/dl) | 23 ± 16 | 25 ± 14 | 29 ± 14 | 27 ± 9 | 40 ± 20 | 48 ± 19 | 41 ± 14 | 72 ± 49a | 47 ± 10 | 74 ± 36a |
| Testosterone LC-MS/MS (ng/dl) | 16 ± 8 | 15 ± 6 | 18 ± 7 | 18 ± 3 | 25 ± 13 | 28 ± 12 | 25 ± 6 | 37 ± 2a | 29 ± 5 | 40 ± 8a |
| Estradiol (pg/ml) | 9.0 ± 7.3 | 6.5 ± 2.1 | 17 ± 16 | 16 ± 13 | 30 ± 11 | 30 ± 25 | 48 ± 25 | 48 ± 48 | 41 ± 26 | 40 ± 21 |
| SHBG (nmol/liter) | 65 ± 35 | 68 ± 39 | 57 ± 44 | 71 ± 25 | 52 ± 23 | 56 ± 19 | 50 ± 22 | 31 ± 18a | 41 ± 17 | 16 ± 11a |
| FAI RIA | 2.1 ± 2.6 | 2.3 ± 3.3 | 4.3 ± 6.2 | 1.7 ± 1.5 | 3.2 ± 2.2 | 3.6 ± 2.2 | 3.2 ± 1.8 | 10 ± 8a | 4.6 ± 2.2 | 19 ± 12a |
| FAI LC-MS/MS | 1.4 ± 1.5 | 1.4 ± 1.9 | 2.6 ± 3.5 | 1.1 ± 0.9 | 2.0 ± 1.2 | 2.0 ± 1.1 | 2.0 ± 1.1 | 5.7 ± 4.5a | 2.9 ± 1.2 | 11 ± 7a |
Values are expressed as median ± sd.
P < 0.05 between Cd and PCOSd.
Figure 1.
Correlation between basal testosterone serum concentrations measured by RIA and LC-MS/MS.
Basal and stimulated estradiol concentrations were similar in PCOSd and Cd in Tanner stages I, II, III, and IV. However, in Tanner stage V, stimulated estradiol concentration was significantly higher in the PCOSd group compared with the Cd group (288.9 ± 174.4 vs. 141.2 ± 103.0 pg/ml; P = 0.019).
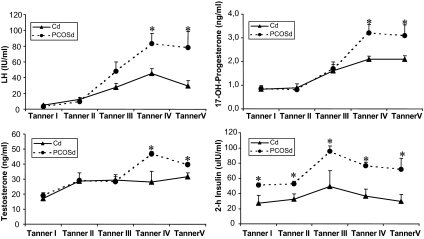
Figure 2 shows serum LH, 17-OHP, and testosterone (LC-MS/MS) levels after stimulation with leuprolide acetate and the insulin levels after the glucose load. In general, both groups of girls showed similar dynamics of hormone secretion. In Tanner stages I, II, and III, stimulated levels of LH, 17-OHP, and testosterone were not significantly different between Cd and PCOSd. In Tanner stages IV and V, stimulated concentrations of these hormones were significantly higher in PCOSd compared with Cd. In contrast, 2-h insulin concentrations were significantly higher in PCOSd compared with Cd in all Tanner stages, with both groups of girls showing the highest values in Tanner stage III.
Figure 2.
LH, 17-OHP, and testosterone (LC-MS/MS) levels after stimulation with leuprolide acetate and the insulin levels after the glucose load (2-h) in PCOSd and Cd. Values are expressed as mean ± sem. *, P < 0.05.
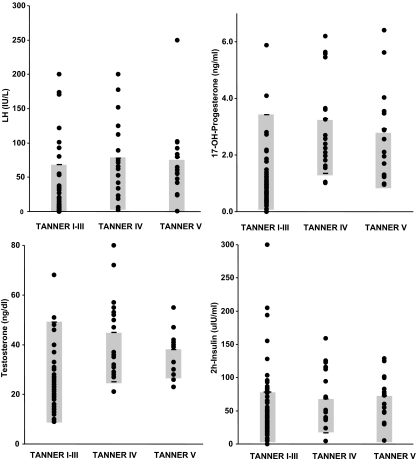
Figure 3 shows the individual concentrations of stimulated LH, 17-OHP, testosterone (LC-MS/MS) and insulin in PCOSd during Tanner I-II, IV, and V. The Cd group is shown as the shaded area. Considering stimulated testosterone levels as the main biochemical feature in Tanner stage IV, 9 of 20 (45.0%) of the PCOSd had testosterone levels above the highest concentrations observed in Cd. Of these “hyperandrogenic” girls, five showed concomitantly elevated 17-OHP concentrations, five showed high LH levels, and eight had high insulin levels. However, only two showed an increase in all these parameters. In Tanner stage V, 9 of 15 (60.0%) presented high testosterone levels, seven of them showed concomitantly elevated 17-OHP, three elevated LH, and six elevated insulin. Two showed an increase in all these parameters.
Figure 3.
Individual concentrations of stimulated LH, 17-OHP, testosterone (LC-MS/MS) and insulin (2-h) in PCOSd during Tanner I-III, IV and V. The Cd group is shown as the shaded area.
Discussion
In this study, we evaluated metabolic and reproductive parameters in peripubertal PCOSd. Peripubertal PCOSd showed an increased ovarian volume and significantly higher levels of 2-h insulin compared with control daughters at all Tanner stages. In contrast, in Tanner stages IV and V, basal and stimulated LH, testosterone, and 17-OHP concentrations were higher in PCOSd compared with Cd. These data indicate that the metabolic abnormalities and increased ovarian volume are present in PCOSd even before puberty and persist during pubertal development. In contrast, the biochemical reproductive features of PCOS appear during late puberty.
In the present study, elevated 2-h insulin levels were observed in PCOSd before the onset of puberty, and ISI composite, which is considered a useful index of insulin sensitivity in subjects with normal glucose tolerance and girls with PCOS (31), was reduced in PCOSd in late puberty. This observation is in accordance with the concept that insulin resistance and hyperinsulinemia are early features of PCOS (12,13,32,33,34,35). In a study of PCOS adolescents, Lewy et al. (33) found similar basal insulin and glucose concentrations in PCOS and control adolescents, but PCOS girls had significantly higher first- and second-phase insulin secretion during hyperglycemic clamp studies. Recently, Kent et al. (36) reported that hyperinsulinism does not appear until the later stages of puberty in children of women with PCOS, which is in disagreement with the results of the present study probably because salivary insulin levels, which were employed in that study, were not able to detect hyperinsulinemia in early stages of puberty. Thus, the bulk of the evidence is consistent with our observations that insulin resistance is an early feature during the ontogeny of PCOS.
Furthermore, as observed in adult women with PCOS, IGT and type 2 diabetes are also highly prevalent among adolescents with PCOS (34). In the present study, we used 2-h postchallenge glucose values to screen for abnormal glucose tolerance and found that 9.0% of PCOSd exhibited glucose intolerance during late puberty that is higher than that observed in our Chilean population of obese girls (2.2%) (37), suggesting a high prevalence of glucose intolerance in PCOSd. Nevertheless, no PCOSd had type 2 diabetes. In these girls, fasting serum glucose, fasting insulin, and HOMA-IR index were poor predictors of insulin resistance and glucose intolerance. In contrast, SHBG concentrations may reflect changes in insulin resistance more accurately than the HOMA-IR index, as previously described (14,31,38).
Although the presence of obesity may amplify the functional abnormalities that are associated with PCOS (39,40), in the present study, however, only 22.5% of PCOSd were over the 95th percentile during late puberty. Therefore, the biochemical features observed in our PCOSd population are not always attributable to obesity, and some PCOSd with normal weight also exhibit PCOS-like features.
Insulin resistance plays a pivotal role in the pathogenesis of PCOS (41). Hyperinsulinemia stimulates the development of antral follicles, increasing the sensitivity of granulosa cells to FSH, thus increasing the number of follicles and ovarian volume (42). In addition, insulin acts synergically with LH to stimulate the synthesis of testosterone by ovarian theca cells from normal and PCOS women (43,44,45). Thus, hyperinsulinemia may contribute to the development of FOH in PCOS women through the overactivation of the CYP17 enzyme pathway (11). Interestingly, in the present study, stimulated levels of insulin were higher in PCOSd compared with control girls from the beginning of puberty and even before the onset of hyperandrogenism, suggesting that insulin may play an early and pivotal role in the pathogenesis of this syndrome.
To the best of our knowledge, no data regarding clinical and metabolic features, gonadal function, or ultrasound studies have been reported in PCOSd before and during different stages of puberty. In the present study, we identified a subgroup of girls within the PCOSd who exhibit PCOS-like features, such as elevated testosterone concentrations and increased ovarian volume. Increased ovarian volume has been proposed as a diagnostic criterion for PCOS (46) and may represent an early sign of this syndrome (47). Enlarged ovaries in perimenarchal girls may persist over time and may be associated with subsequent hyperandrogenism (47). In addition, using the leuprolide acetate test, most but not all girls with elevated testosterone concentrations showed during the later stages of puberty higher 17-OHP levels, which may indicate the development of functional ovarian hyperandrogenism (48,49).
Establishing a diagnosis of PCOS in adolescent girls is difficult because some features of the syndrome are physiological at this age. During the first years after menarche, 60% of the menstrual cycles are anovulatory, and enlarged ovaries are often observed as a consequence of the physiological processes of maturation (50,51). Moreover, during this time, there is a physiological increase in insulin resistance and androgen levels due to the increase of GH (52). Recently, Mortensen et al. (53) showed that polycystic-size ovary in asymptomatic adolescents may be a normal variant. However, half of these girls exhibited a subclinical PCOS type of ovarian dysfunction. These findings are in agreement with the concept that in reproductive-aged unselected women, approximately 6% suffer from PCOS, which is even higher in women with enlarged ovaries on ultrasound (27). Nevertheless, in our study, we did not observe a subclinical PCOS type of ovarian dysfunction in the control girls.
Clinical evidence of hyperandrogenism may not be apparent during early puberty because it is the reflection of the degree and duration of androgen exposure, which at this time may still be low (7). Therefore, androgen measurements, and in particular determination of testosterone, are very important for the evaluation of PCOS in these girls (54). Unfortunately, there are many challenges in the measurement and interpretation of low testosterone levels prevalent in peripubertal girls. It has been suggested that most commercial RIAs and immunoassay kits that are commonly used to measure total testosterone levels lack the sensitivity, precision, and accuracy required to measure the low total testosterone concentrations in women and prepubertal children (55,56). Therefore, in our study, total testosterone was assessed by RIA as well as LC-MS/MS, widely considered the reference method against which all other methods are compared (56). We observed a high correlation between both methods. This approach allowed us to establish the onset of biochemical hyperandrogenism more accurately.
In conclusion, the present cross-sectional study indicates that during puberty, PCOSd start to show some features of PCOS, such as increased LH, testosterone, and 17-OHP concentrations, hyperinsulinemia, and increased ovarian volume. Obviously, not all PCOSd have all of these manifestations. However, there are girls who exhibit essentially all the features of PCOS, whereas others exhibit only one or two of them. On the whole, 45% of PCOSd are affected in Tanner IV and 60% in Tanner V, taking biochemical hyperandrogenism as the main criterion. We believe that this study represents significant progress in the effort to elucidate the ontogeny of reproductive and metabolic abnormalities in PCOS. Therefore, the clinical relevance of such findings needs to be evaluated during longitudinal follow-up of this cohort some years after menarche. This will allow us to establish how many PCOSd ultimately develop PCOS.
Acknowledgments
The authors are grateful to Anqi Zhang, Ph.D., M.D., for testosterone determination by chromatography, tandem mass spectrometry in the Steroid Hormone Assay Laboratory of the Endocrine Section, Boston Medical Center.
Footnotes
This work was supported by a grant from the National Fund for Scientific and Technological Development (FONDECYT) 1071007 and by the Alexander von Humboldt Foundation. The LC-MS/MS assay development has been supported by National Institutes of Health Grant 1RO1AG22356 (S.B., principal investigator).
This work was presented in part at the 90th Annual Meeting of The Endocrine Society, San Francisco, CA, June 15–18, 2008.
Disclosure Summary: T.S.-P., E.C., V.P., B.E., M.M., A.L.d.G., J.P., N.C., F.S., and F.C. have nothing to disclose. S.B. has received a research grant for National Institutes of Health-funded studies.
First Published Online February 17, 2009
For editorial see page 1883
Abbreviations: BMI, Body mass index; Cd, control daughters; FAI, free androgen index; FOH, functional ovarian hyperandrogenism; HDL-C, high-density lipoprotein-cholesterol; HOMA-IR, homeostasis model assessment for insulin resistance; IGT, impaired glucose tolerance; ISI, insulin sensitivity index; LC-MS/MS, liquid chromatography, tandem mass spectrometry; LDL-C, low-density lipoprotein-cholesterol; 17-OHP, 17 α-hydroxyprogesterone; PCOS, polycystic ovary syndrome; PCOSd, PCOS daughters; SDS, sd score; WHR, waist-to-hip ratio.
References
- Franks S 1995 Polycystic ovary syndrome. N Engl J Med 333:853–861 [DOI] [PubMed] [Google Scholar]
- Holte J 1996 Disturbances in insulin secretion and sensitivity in women with the polycystic ovary syndrome. Baillieres Clin Endocrinol Metab 10:221–247 [DOI] [PubMed] [Google Scholar]
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A 1989 Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 38:1165–1174 [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J 1999 Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 22:141–146 [DOI] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Dodson WC, Dunaif A 1999 Prevalence and predictors of risk for type II diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84:165–169 [DOI] [PubMed] [Google Scholar]
- O'Meara NM, Blackman JD, Ehrmann DA 1993 Defects in β-cell function in functional ovarian hyperandrogenism. J Clin Endocrinol Metab 76:1241–1247 [DOI] [PubMed] [Google Scholar]
- Rosenfield RL 2007 Identifying children at risk for polycystic ovary syndrome. J Clin Endocrinol Metab 92:787–796 [DOI] [PubMed] [Google Scholar]
- Buggs C, Rosenfield RL 2005 Polycystic ovary syndrome in adolescence. Endocrinol Metab Clin North Am 34:677–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witchel SF 2006 Puberty and polycystic ovary syndrome. Mol Cell Endocrinol 254–255:146–153 [DOI] [PubMed] [Google Scholar]
- Rosenfield RL, Ghai K, Ehrmann DA, Barnes RB 2000 Diagnosis of the polycystic ovary syndrome in adolescence: comparison of adolescent and adult hyperandrogenism. J Pediatr Endocrinol Metab 3(Suppl 5):1285–1289 [PubMed] [Google Scholar]
- Pasquali R, Patton L, Pocognoli P, Cognigni GE, Gambineri A 2007 17-Hydroxyprogesterone responses to gonadotropin-releasing hormone disclose distinct phenotypes of functional ovarian hyperandrogenism and polycystic ovary syndrome. J Clin Endocrinol Metab 92:4208–4217 [DOI] [PubMed] [Google Scholar]
- Ibañez L, Potau N, Zampolli M, Rique S, Saenger P, Carrascosa A 1997 Hyperinsulinemia and decreased insulin-like growth factor-binding protein-1 are common features in prepubertal and pubertal girls with a history of premature pubarche. J Clin Endocrinol Metab 82:2283–2288 [DOI] [PubMed] [Google Scholar]
- Ibañez L, Ferrer A, Ong K, Amin R, Dunger D, de Zegher F 2004 Insulin sensitization early after menarche prevents progression from precocious pubarche to polycystic ovary syndrome. J Pediatr 144:23–29 [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Maliqueo M, Codner E, Echiburú B, Crisosto N, Pérez V, Pérez-Bravo F, Cassorla F 2007 Early metabolic derangements in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 92:4637–4642 [DOI] [PubMed] [Google Scholar]
- Legro RS, Driscoll D, Strauss 3rd JF, Fox J, Dunaif A 1998 Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci USA 95:14956–14960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahsar-Miller MD, Nixon C, Boots LR, Go RC, Azziz R 2001 Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS. Fertil Steril 75:53–58 [DOI] [PubMed] [Google Scholar]
- Zawadzky JK, Dunaif A 1992 Diagnosis criteria: towards a rational approach. In: Hershmann JM, ed. Current issues in endocrinology and metabolism. Boston: Blackwell Scientific Publications; 377–384 [Google Scholar]
- Adams J, Polson DW, Franks S 1986 Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Br Med J (Clin Res Ed) 293:355–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1999 Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: Diagnosis and classification of diabetes mellitus. Geneva: World Health Organization, Department of Noncommunicable Disease Surveillance [Google Scholar]
- Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL 2002 Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 109:45–46 [DOI] [PubMed] [Google Scholar]
- Hatch R, Rosenfield RL, Kim MH, Tredway D 1981 Hirsutism: implications, etiology, and management. Am J Obstet Gynecol 140:815–830 [DOI] [PubMed] [Google Scholar]
- Tellez R, Frenkel J 1995 Clinical evaluation of body hair in healthy women. Rev Med Chil 123:1349–1354 [PubMed] [Google Scholar]
- Gordon CM 1999 Menstrual disorders in adolescents. Excess androgens and the polycystic ovary syndrome. Pediatr Clin North Am 46:519–543 [DOI] [PubMed] [Google Scholar]
- 2000 Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 23(Suppl 1):S4–S19 [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA 1999 Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470 [DOI] [PubMed] [Google Scholar]
- Swanson M, Sauerbrei EE, Cooperberg PL 1981 Medical implications of ultrasonically detected polycystic ovaries. J Clin Ultrasound 9:219–222 [DOI] [PubMed] [Google Scholar]
- Ibáñez L, Potau N, Zampolli M, Street ME, Carrascosa A 1997 Girls diagnosed with premature pubarche show an exaggerated ovarian androgen synthesis from the early stages of puberty: evidence from gonadotropin-releasing hormone agonist testing. Fertil Steril 67:849–855 [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Hitchsfeld C, Codner E, Maliqueo M, Iñiguez G, Echiburú B, Sánchez F, Crisosto N, Cassorla F 2007 Gonadal function in low birth weight infants: a pilot study. J Pediatr Endocrinol Metab 20:405–414 [DOI] [PubMed] [Google Scholar]
- Choi HH, Gray PB, Storer TW, Calof OM, Woodhouse L, Singh AB, Padero C, Mac RP, Sinha-Hikim I, Shen R, Dzekov J, Dzekov C, Kushnir MM, Rockwood AL, Meikle AW, Lee ML, Hays RD, Bhasin S 2005 Effects of testosterone replacement in human immunodeficiency virus-infected women with weight loss. J Clin Endocrinol Metab 90:1531–1541 [DOI] [PubMed] [Google Scholar]
- Fruzzetti F, Perini D, Lazzarini V, Parrini D, Genazzani AR 15 August 2008 Adolescent girls with polycystic ovary syndrome showing different phenotypes have a different metabolic profile associated with increasing androgen levels. Fertil Steril 10.1016/j.fertnstert.2008.06.004 [DOI] [PubMed] [Google Scholar]
- Arslanian SA, Lewy VD, Danadian K 2001 Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and β-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab 86:66–71 [DOI] [PubMed] [Google Scholar]
- Lewy VD, Danadian K, Witchel SF, Arslanian S 2001 Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr 138:38–44 [DOI] [PubMed] [Google Scholar]
- Palmert MR, Gordon CM, Kartashov AI, Legro RS, Emans SJ, Dunaif A 2002 Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab 87:1017–1023 [DOI] [PubMed] [Google Scholar]
- Coviello AD, Legro RS, Dunaif A 2006 Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab 91:492–497 [DOI] [PubMed] [Google Scholar]
- Kent SC, Gnatuk CL, Kunselman AR, Demers LM, Lee PA, Legro RS 2008 Hyperandrogenism and hyperinsulinism in children of women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab 93:1662–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo TV, Avila AA, Unuane MN, Codner E 2006 Fasting glucose versus oral glucose tolerance test for detection of glucose intolerance in obese children. Rev Med Chil 134:1146–1152 [DOI] [PubMed] [Google Scholar]
- Jayagopal V, Kilpatrick ES, Jennings PE, Hepburn DA, Atkin SL 2003 The biological variation of testosterone and sex hormone-binding globulin (SHBG) in polycystic ovarian syndrome: implications for SHBG as a surrogate marker of insulin resistance. J Clin Endocrinol Metab 88:1528–1533 [DOI] [PubMed] [Google Scholar]
- Luque-Ramírez M, Alvarez-Blasco F, Mendieta-Azcona C, Botella-Carretero JI, Escobar-Morreale HF 2007 Obesity is the major determinant of the abnormalities in blood pressure found in young women with the polycystic ovary syndrome. J Clin Endocrinol Metab 92:2141–2148 [DOI] [PubMed] [Google Scholar]
- Yildiz BO, Knochenhauer ES, Azziz R 2008 Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab 93:162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaif A 1997 Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 18:774–800 [DOI] [PubMed] [Google Scholar]
- Fulghesu AM, Villa P, Pavone V, Guido M, Apa R, Caruso A, Lanzone A, Rossodivita A, Mancuso S 1997 The impact of insulin secretion on the ovarian response to exogenous gonadotropins in polycystic ovary syndrome. J Clin Endocrinol Metab 82:644–648 [DOI] [PubMed] [Google Scholar]
- Cara JF, Rosenfield RL 1988 Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal-interstitial cells. Endocrinology 123:733–739 [DOI] [PubMed] [Google Scholar]
- Willis D, Mason H, Gilling-Smith C, Franks S 1996 Modulation by insulin of follicle-stimulating hormone and luteinizing hormone actions in human granulosa cells of normal and polycystic ovaries. J Clin Endocrinol Metab 81:302–309 [DOI] [PubMed] [Google Scholar]
- Nestler JE, Jakubowicz DJ, de Vargas AF, Brik C, Quintero N, Medina F 1998 Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab 83:2001–2005 [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group 2004 Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81:19–25 [DOI] [PubMed] [Google Scholar]
- Venturoli S, Porcu E, Fabbri R, Pluchinotta V, Ruggeri S, Macrelli S, Paradisi R, Flamigni C 1995 Longitudinal change of sonographic ovarian aspects and endocrine parameters in irregular cycles of adolescence. Pediatr Res 38:974–980 [DOI] [PubMed] [Google Scholar]
- Barnes RB, Rosenfield RL, Burstein S, Ehrmann DA 1989 Pituitary-ovarian responses to nafarelin testing in the polycystic ovary syndrome. N Engl J Med 320:559–565 [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Rosenfield RL, Barnes RB, Brigell DF, Sheikh Z 1992 Detection of functional ovarian hyperandrogenism in women with androgen excess. N Engl J Med 327:157–162 [DOI] [PubMed] [Google Scholar]
- Apter D, Vihko R 1985 Premenarcheal endocrine changes in relation to age at menarche. Clin Endocrinol (Oxf) 22:753–760 [DOI] [PubMed] [Google Scholar]
- Venturoli S, Porcu E, Fabbri R, Magrini O, Paradisi R, Pallotti G, Gammi L, Flamigni C 1987 Postmenarchal evolution of endocrine pattern and ovarian aspects in adolescents with menstrual irregularities. Fertil Steril 48:78–85 [DOI] [PubMed] [Google Scholar]
- Hannon TS, Janosky J, Arslanian SA 2006 Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res 60:759–763 [DOI] [PubMed] [Google Scholar]
- Mortensen M, Rosenfield RL, Littlejohn E 2006 Functional significance of polycystic-size ovaries in healthy adolescents. J Clin Endocrinol Metab 91:3786–3790 [DOI] [PubMed] [Google Scholar]
- Rieder J, Santoro N, Cohen HW, Marantz P, Coupey SM 2008 Body shape and size and insulin resistance as early clinical predictors of hyperandrogenic anovulation in ethnic minority adolescent girls. J Adolesc Health 43:115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H 2007 Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab 92:405–413 [DOI] [PubMed] [Google Scholar]
- Matsumoto AM, Bremner WJ 2004 Serum testosterone assays—accuracy matters. J Clin Endocrinol Metab 89:520–524 [DOI] [PubMed] [Google Scholar]