Abstract
Environmental tobacco smoke (ETS) is a key mediator of several diseases. Tobacco smoke contains a mixture of over 4700 chemical components many of which are toxic and have been implicated in the etiology of oxidative stress related diseases such as chronic obstructive pulmonary disease, Parkinson’s disease, asthma, cancer and cardiovascular disease. However, the mechanism of action of cigarette smoke in the onset of these diseases is still largely unknown. Previous studies have revealed that the free radicals generated by cigarette smoke may contribute to many of these chronic health problems and this study sought to address the role of environmental tobacco smoke in oxidative stress related damage in different regions of the mouse brain. In this study, male mice were exposed for 7 h/day, 7 days/week, for 6 months. Our results show that tobacco smoke led to increased generation of reactive oxygen species with an increase in NF-κB activation. Gel shift analysis also revealed the elevated level of the oxidative stress sensitive proinflammatory nuclear transcription factor-kappa B and activator protein-1 in different regions of the brain of cigarette smoke exposed mice. Tobacco smoke led to activation of COX-2 in all the regions of the brain. Activation of mitogen activated protein kinase and c-Jun N-terminal kinase were also observed in various regions of brain of ETS exposed mice. Overall our results indicate that exposure to long-term cigarette smoke induces oxidative stress leading to activation of stress induced kinases and activation of proinflammatory transcription factors.
Keywords: Cigarette smoke, ROS, NF-κB, AP-1, Signal transduction
1. Introduction
Over 6000 reports have examined the connection between cigarette smoking and disease. Smoking is now identified as a major cause of heart disease, stroke, several different forms of cancer, and a wide variety of other health problems [1]. Most of the smoking related deaths are attributed to heart disease and lung cancer, followed by chronic bronchitis, stroke, peripheral vascular disease and other circulatory diseases [2]. Although the effects of environmental tobacco smoke (ETS) related research has been reported in approximately 1000 articles in peer reviewed journals, however, the complexity of the effects of ETS is still not well understood and requires more research because of the presence of approximately 4000 individual compounds in the mixture and the duration of exposure seems detrimental to the outcome of the effects on health [3].
Tobacco smoke is a complex mixture containing at least 40 different carcinogens that mediate tumor initiation and promotion. These carcinogens include nicotine, nitrosamine, polycyclic aromatic hydrocarbons (PAH), aromatic amines, unsaturated aldehydes (e.g., crotonaldehyde), and some phenolic compounds (acrolein) [4]. Oxidative effects via free radical generation in smokers causes lipid peroxidation, oxidation of proteins and damage to tissues mainly that of lung. The antioxidant enzymes superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and melatonin are also severely affected by ETS resulting in deleterious effects [5]. In addition children who are exposed to passive smoking are exposed to oxidative stress that has been implicated in the etiopathogenesis of over 100 disorders [6, 7].
Nicotine alone has been shown to induce an increase dopamine and its metabolites in brain, an increase of norepinephrine in the cortex, norepinephrine metabolite 4-hydroxy-3-methoxy-phenylglycol in all areas [6], and apoptotic markers in the brain. Maternal nicotine administration has been shown to cause fetal brain stem damage [8] and an in utero exposure to N-nitroso compounds and their precursors may lead to brain tumor in adults [9, 10]. Whereas nicotine by itself can behave as a pharmacological agent, however, as an active component of ETS, its effects might be altered due to the presence of other compounds in ETS [7–9, 11]. Microarray analysis of brain exposed to ETS through inhalation have shown down-regulation of genes associated with synaptic function, neurotransmission, and neurotrophic support, and upregulated genes associated with stress responses, nitric oxide synthesis, antioxidant defenses, proteolysis, inflammatory response, and glial activation [12].
The gas phase of ETS contains free radicals such as superoxide radicals, hydroxyl radicals and H2O2 [13, 14] that are known to activate redox sensitive transcription factors, such as nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1), which lead to transcription of pro-inflammatory genes namely interleukin (IL)-8, tumor necrosis factor-alpha (TNF-α), and cycloxygenase (Cox)-2 [15–19]. In this report, we have examined the long-term effect of ETS-induced oxidative stress on activation of stress related transcription factors in different regions of the mice brain. We report that ETS induces the reactive oxygen species, lipid peroxidation, NF-κB activation, AP-1 activation, c-Jun N-terminal kinase (JNK), activation, MAPK activation and COX-2 activation in various regions of the brain of mice.
2. Materials and methods
2.1. Materials
Glycine, bovine serum albumin, leupeptin, PMSF, and pepstatin were obtained from Sigma (St. Louis, MO). NF-κB oligonucleotides were synthesized from Gibco-BRL. Antibodies against p65, p50, cyclin D1, c-Rel, JNK1, c-Jun, c-Fos, ERK and oligonucleotide for AP-1 and NF-κB were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibody against phosphorylated-MAPK and phosphorylated p38MAPK was obtained from cell signaling technology (Beverly, MA).
2.2. Animals and care
Mice were fed with AIN-76A diet and water ad libidum and housed under control conditions (23 ± 2° C; 12/12 h light/dark periods). All experimental protocols conducted in the mice were performed in accordance with the standards established by the US Animal Welfare Acts, set forth in NIH guidelines and the Policy and Procedures Manual (Johns Hopkins University School of Public Health Animal Care and Use Committee).
2.3. Exposure to ETS
Exposure ETS was performed as described by Rangaswamy et al., [20]. Briefly, male A/J mice (8 weeks old) were purchased from Jackson laboratories. Mice were divided in to two groups (n = 25). Group I was control mice; group II, experimental mice. Group I was kept in a filtered air environment and groups II was subjected to environmental tobacco smoke for 6 months (7 h/day, 7 days/week) generated by burning 2R4F reference cigarettes (2.45mg nicotine per cigarette; purchased from Tobacco Research Institute, University of Kentucky, Lexington, KY)using a smoking machine (ModelTE-10,Teague Enterprises, Davis, CA). Each smoldering cigarette is puffed for 2 s, once every minute for a total of eight puffs, at a flow rate of 1.05 l/min to provide a standard puff of 35 cm3. The smoking machine was adjusted to produce a mixture of sidestream smoke (89%) and mainstream smoke (11%), mimicking an exposure to (ETS), by burning five cigarettes at one time. Chamber atmosphere were monitored for total suspended particulates (TSP) and carbon monoxide (CO) every hour. The concentration of TSP and CO was an average of 90 mg/m3 and 350 ppm, respectively [21]. After 6 months of exposure the brain was dissected and different regions were stored at −80 °C for future use. For analysis a set of five animals were used and three such sets were performed as independent experiments. The data represents results from one set of experiment.
2.4. Assay of reactive oxygen species (ROS)
Oxidative species plays role in ETS -induced neuronal toxicity. The formation of ROS was measured by using the ROS sensitive dye, 2, 7-dichlorodihydrofluorescein diacetate (DCF), as an indicator [22]. The assay was performed on freshly dissected brain tissue samples. ETS treated and untreated brain tissue regions namely brain stem, cerebellum, frontal cortex, hippocampus and striatum tissue extracts (50 µg) were incubated with 10 µl of DCF-DA (10 µM) for 3 h at 37 °C. The fluorescent product formed was quantified by spectrofluorometer at the 485/525 nm. Changes in fluorescence were expressed as arbitrary unit.
2.5. Determination of lipid peroxidation
ETS induced lipidperoxidation was determined by detection of thiobarituric acid–reactive malondialdehyde (MDA), an end product of the peroxidation reaction of polyunsaturated fatty acids and related esters [23]. In brief tissue homogenates were made as described for ROS determination. Three-hundred micrograms of proteins was added to 800 µl of assay mixture containing 0.4% thiobarbituric acid, 0.5% Sodium dodeycl sulphate and 9.4% acetic acid. After 1 h incubation at room temperature at 95 °C, samples were cooled to room temperature, centrifuged at 14,000 × g for 10 min and the absorbance of the supernatant was measured at 532 nm. Results were normalized to MDA equivalent/mg protein and expressed as a percentage of thiobarbituric-reactive substances above control.
2.6. Preparation of nuclear extracts and assays for NF-κB and AP-1
To prepare nuclear extract 150 µg of each brain region was incubated on ice for 1 h in 0.4 ml of lysis buffer containing 20 mM Tris, pH 8, 2mM EDTA, 250 mM NaCl, 0.1% NP-40, 2 µg/ml leupeptin, 2 µg/ml aprotinin, 1mM PMSF, 0.5 µg/ml benzamidine, 1mM DTT, and 1mM sodium vanadate. The lysate was centrifuged, and the supernatant collected was used for electrophoretic mobility shift assays (EMSA).
To measure NF-κB activation, electrophoretic mobility shift assays (EMSA) was carried out essentially as described by Manna and Ramesh [19]. Briefly, 20 µg of nuclear extract from different regions of mice brain as indicated above was incubated with 32P-end-labeled 45-mer double-stranded NF-κB oligonucleotide (20 µg protein with 16 f moles DNA) from the HIV-LTR, 5′-TTGTTACAAGGGACTTTCCGCTGGG GACTTTCCAGGGA GGCGT GG-3′ (bold indicates NF-κB binding sites) for 15 min at 37 °C, in binding buffer. A double-stranded mutated oligonucleotide, 5′-TTGTTACAACTC ACTTTCCGCTGCTCACTTTCCAGGGA GGCGT GG-3′, was used to examine the specificity of binding of NF-κB to the DNA. To perform the supershift assays, specific antibodies were added to the nuclear extract in the binding buffer and incubated for 1h at 37 °C followed by the addition of labeled probe for further incubation. The DNA–protein complex formed was separated from free oligonucleotide on 4.5% native polyacrylamide gels. To determine the activation of AP-1, 20 µg of protein, extracted from different regions of mice brain as indicated above was incubated with 16 f mol of the 32P-end-labeled AP-1 consensus oligonucleotide 5′-CGCTTGATGACTCAGCCGGAA-3′ (bold indicates AP-1 binding sites) for 15 min at 37 °C in binding buffer followed by resolving the complex in 4.5% polyacralamide gels. The gels were visualized in a Phosphor Imager (Bio Rad, Hercules, CA) using ‘Quantity One’ software.
2.7. JNK assay
The c-Jun kinase assay was performed by a modified method as described earlier [24]. Briefly, brain extract proteins (250 µg/sample) from control and experimental mice were immunoprecipitated with 0.3 µg anti-JNK antibody for 60 min at 37 °C. Immune complexes were collected by incubation with protein A/G sepharose beads for 45 min at 37 °C. The beads were extensively washed with lysis buffer (4 µl × 400 µl) and kinase buffer (2 µl × 400 µl: 20 mM HEPES, pH 7.4, 1 mM DTT, 25 mM NaCl). Kinase assays were performed for 15 min at 30 °C with GST-Jun (1–79) as a substrate in 20 mM HEPES, pH 7.4, 10 mM MgCl2, 1 mM DTT, and 10 µCi [γ32P] ATP. Reactions were stopped with the addition of 2 × SDS sample buffer, boiled for 5 min, and subjected to SDS-PAGE (9%). GST-Jun (1–79) was visualized by staining with Comassie Blue, and the dried gel was analyzed in a Phospho Imager to observe the product of the kinase reaction.
2.8. Immunoblot analysis of MAP kinase and Cox-2
Phosphorylated MAP kinase,Cox-2 and p38MAP kinase was measured as described by Manna et al., [25]. In brief 50 µg aliquot of brain protein from different regions was resolved on 10% SDS-PAGE, electrotransferred onto nitrocellulose membrane, and probed with the phospho-specific anti-p44/42 MAP kinase (Thr 202/Tyr 204) antibody (New England Biolabs, Inc) raised in rabbit (1:3000 dilution) and Cox-2 antibody (1:250 dliution) (BD Biosciences, Inc). The membrane was then incubated with peroxidase-conjugated anti-rabbit IgG (1:3000 dilution), and bands were detected by chemiluminescence (ECL, Amersham).
3. Results
3.1. ETS induces ROS
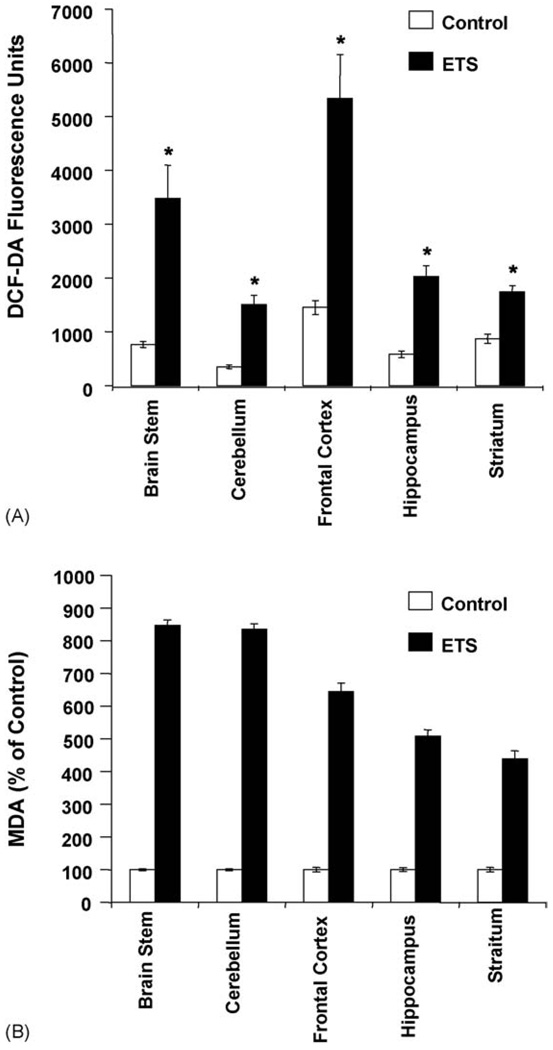
Oxidative stress is known to mediate apoptosis and previous studies have indicated tobacco smoke induced apoptosis in rat and human cell lines was due to ROS contained in tobacco smoke or generated by cells exposed to tobacco smoke [14]. In order to access the effects of environmental tobacco smoke on induction of reactive oxygen species, ETS exposed and control brain tissue from different regions were incubated with 10 µM of DCF-DA and then were assayed by spectrofluosremeter Results indicate that ETS causes a two- to three-fold increase in reactive oxygen species generation as compare to the control groups and were statistically significant (Fig. 1A). The maximum induction of ROS occurred in frontal cortex followed by cerebellum, hippocampus and striatum and all were significantly high in ETS brain as compared to control. The results indicate that long-term exposure to ETS induces ROS in brain and this induction varied across various regions of the brain.
Fig. 1.
(A) ETS induces ROS generation in mice brain. Fifty micrograms of protein from freshly prepared extracts from control and ETS treated brain tissue was incubated with DCF-DA (10 µM) for 3 h at 37 °C. The formation of ROS was measured by monitoring the change in fluorescence at wavelength of 485/525 nm. Values are mean ± S.D. (n = 5) and are significant at *P < 0.01 as compared to their respective controls. (B) ETS induces lipid peroxidation in mice brain. Control and ETS exposed mice brain were used to determine lipid peroxidation as described in Section 2. The results indicated are in percentage above control value and is representative of five independent experiments. Values are mean ± S.D. (n = 5) and are significant at *P < 0.01 as compared to their respective controls.
3.2. ETS up-regulates lipid peroxidation in different regions of the brain
As previously shown that exposure to ETS resulted in increased ROS in brain, which implicates that such an event could result in lipid peroxidation. Therefore we investigated the lipid peroxidation level in various regions of the brain. The level of MDA reflecting the measure of lipid peroxidation was significantly increased in different regions of ETS-exposed mice brain as compared to control (Fig. 1B). Thus, ETS exposure increased lipid peroxidation may result in brain tissue damage.
3.3. ETS induces NF-κB activation in different regions of the brain
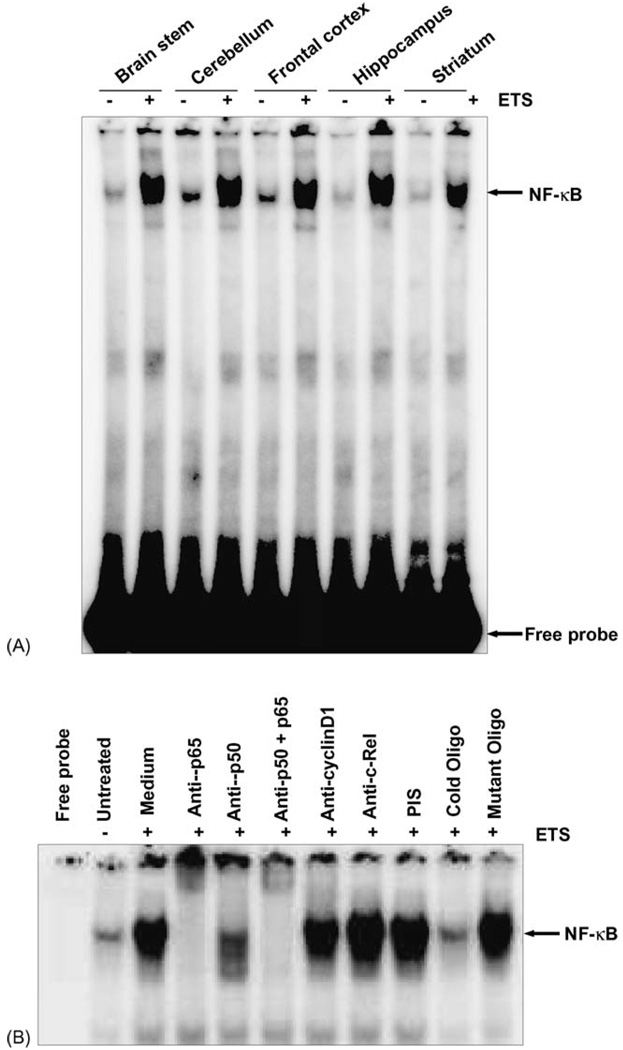
NF-κB activation is also dependent upon oxidative stress, which then can lead to cell death by apoptosis [26, 27]. To investigate this effect of ETS induced activation of NF-κB electrophoretic mobility shift assay (EMSA) was done in tissue extracts obtained from various regions of the brain. NF-κB binding was elevated significantly in all the regions of the brain exposed to ETS for 6 months 7 h/day as compared to control tissues (Fig. 2A). Since all the regions in the brain showed a similar profile of NF-κB activation it suggests a common signaling mechanism being responsible across the whole brain being induced by ETS.
Fig. 2.
(A) ETS induces NF-κB activation in mice brain. ETS increases NF-κB DNA binding in mice brain. Twenty micrograms nuclear proteins were analyzed in 7.4% native PAGE to detect NF-κB by electrophoretic mobility shift assay. The data shown is a representative of five independent experiments performed on extracts obtained from control and ETS exposed mice brain regions. (B) Supershift and specificity of NF-κB activation. Brain tissue extracts prepared from brain region of control and ETS exposed mice were incubated for 15 min with different antibodies, cold and mutant NF-κB oligonucleotides, and then assayed for NF-κB, as described in Section 2. The data shown is a representative of five independent experiments performed on extracts obtained from control and ETS exposed mice brain regions.
3.4. ETS activated NF-κB consists of p50 and p65 subunits
The classic NF-κB complex comprises of p50/p65 that are sequestered in the cytoplasm by interaction with a family of inhibitor proteins or IκBs [28]. Therefore to investigate whether or not the NF-κB complex is actually comprised of p50/p65 proteins we performed EMSA coupled to supershift assay in presence of specific antibodies. To show that the retarded band visualized by EMSA in ETS-exposed tissue was indeed NF-κB, we incubated the tissue extracts from control and ETS exposed brain tissue with antibody to either p50 or p65 subunits and then conducted EMSA. Antibodies to both the subunits of NF-κB shifted the band to a higher molecular weight (Fig. 2B), thus suggesting that the ETS-activated complex consisted of p50 and p65 subunits. Neither pre-immune serum nor any irrelevant antibodies as anti-c-Rel or anti-cyclinD1 had any effect on the mobility of NF-κB band. In addition of excess of unlabelled NF-κB (100-fold) oligonucleotide almost completely abolished the binding, indicating the specificity of NF-κB binding to the oligonucleotide. Mutant oligonucleotide did not suppress the NF-κB binding further indicating its specificity. These results suggest that the active NF-κB complex induced by ETS in the brain was composed of p50/p65 and represents the classical NF-κB complex as reported by us and elsewhere [14, 19].
3.5. ETS induces AP-1 activation in different regions of brain
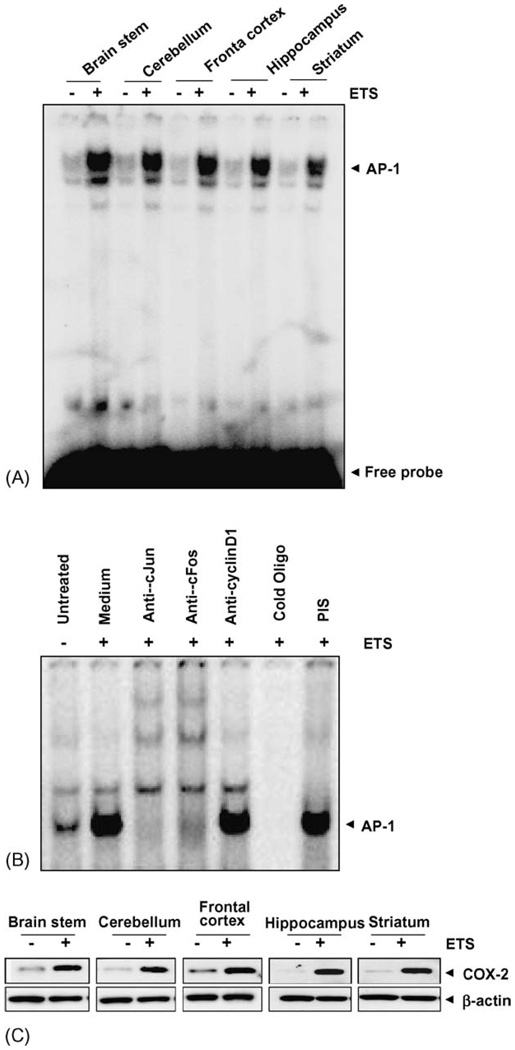
The gas phase of ETS contains free radicals such as superoxide radicals, hydroxyl radicals and H2O2, all of which can activate AP-1. AP-1 regulates multiple cellular functions, including proliferation, differentiation, and cell death [19]. In order to access the extent of this relationship we examined the effect of ETS on AP-1 activation. 20 µg of protein extract was incubated with 10 f mol of 32P-labeled AP-1 oligonucleotide and AP-1 binding was assayed by EMSA. The result indicated that AP-1 binding was increased in all different regions of the brain exposed to ETS as compared to its paired control (Fig. 3A). Further, we examined the specificity of AP-1 DNA binding by supershift assays. We observed that the protein- DNA complex was supershifted by both anti-c-Fos and anti-c-Jun antibodies during AP-1 activation. Neither pre-immune serum nor any irrelevant antibodies as anti-cyclinD1 had any effect on the mobility of AP-1 band (Fig. 3B).
Fig. 3.
(A) ETS induces AP-1 activation. ETS increases AP-1 DNA binding in mice brain. Twenty micrograms nuclear proteins were analyzed in 6.6% native PAGE to detect AP-1 by electrophoretic mobility shift assay. The data shown is a representative of five independent experiments performed on extracts obtained from control and ETS exposed mice brain regions. (B) Supershift and specificity of AP-1 activation. Brain tissue extracts prepared from brain region of control and ETS exposed mice were incubated for 15 min with different antibodies, cold AP-1 oligonucleotides, and then assayed for AP-1, as described in Section 2. (C) ETS induces COX-2 activation in mice brain. Tissue extracts from different regions of brain were collected, 100 µg of the protein was analyzed by 10% SDS-PAGE and detected for COX-2 using anti-COX-2 antibody by Western blot. The same blot was stripped off and re-probed with β-actin by Western blot.
3.6. ETS induces COX-2 expression in different regions of brain
Because COX-2 is known to be regulated by both NF-κB and AP-1, we next investigated whether ETS induced the expression of COX-2 in various regions of the brain [29].Whole-cell extracts were prepared from the brain tissue and analyzed for COX-2 expression by Western blotting. The results indicated that COX-2 protein expression was increased in all different regions of the brain exposed to ETS as compared to its paired control (Fig. 3C).
3.7. ETS induces JNK activation
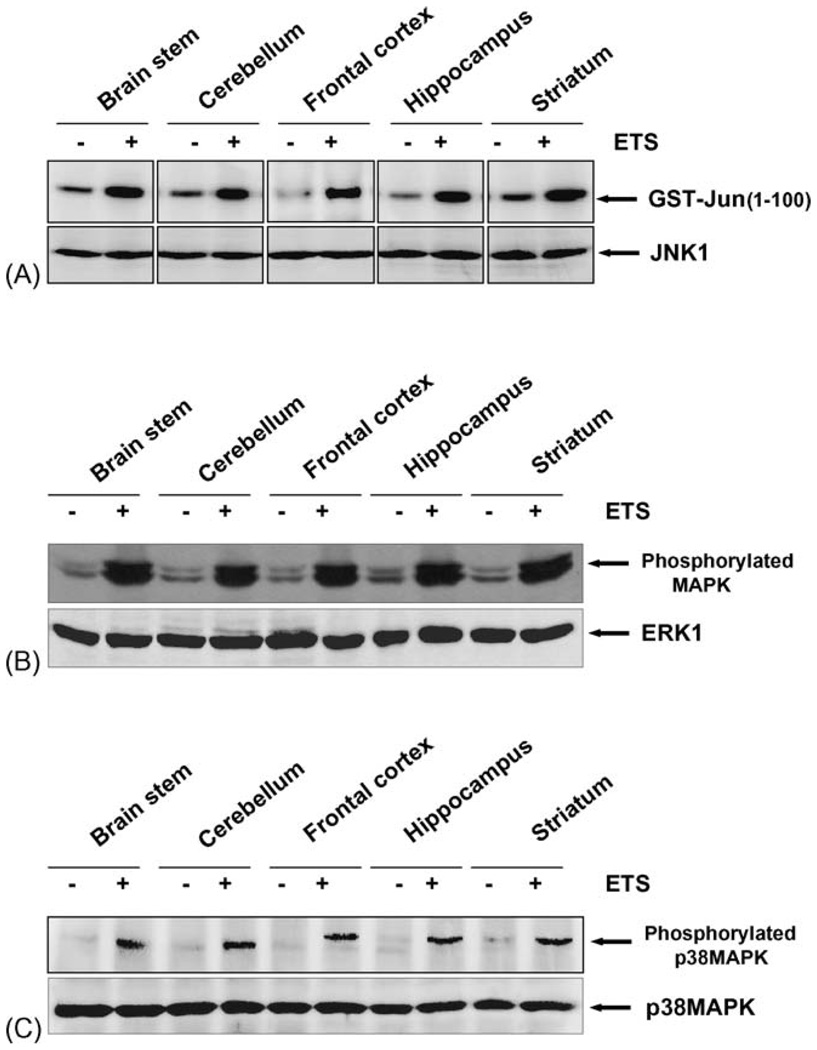
Activation of JNK is prior step of induction to AP-1 and NF-κB. The AP-1 transcription factor consists of homodimer of members from the Jun and hetrodimer of Jun and Fos family and these are regulated at least in part with JNK. It has also been suggested that the activation of NF-κB is regulated by upstream MAP kinase, which in turn regulate JNK activation [28, 30]. As ETS activates transcription factors like NF-κB and AP-1, we also assayed the JNK upon exposure to ETS. ETS activated JNK almost in all the regions of the brain that were exposed to ETS as compared to control (Fig. 4A). The results show that ETS exposure causes sustained activation of JNK. As a control, total levels of JNK protein were assayed using western blot in control and in ETS treated brain. The results indicate unchanged levels of total JNK protein.
Fig. 4.
(A) ETS induces JNK activation. Tissue extracts from different regions of brain were collected and 300 µg proteins were immunoprecipitated with anti-JNK (0.3 µg/sample) antibody, and the kinase reaction was performed using 32P-labeled ATP and GST-Jun as substrate protein. The radiolabeled GST-Jun was detected in 9% SDS-PAGE. The level of JNK was detected using 50 µg of same extract proteins and analyzed in 10% SDS-PAGE by Western blot. (B) ETS induces activation of MAPK. Tissue extracts from different regions of brain were collected, (100 µg) of the protein was analyzed by 10% SDS-PAGE and detected phosphorylated MAPK using anti-phospho MAPK antibody by Western blot. The same blot was stripped off and re-probed with anti-ERK1 antibody to detect the ERK1 by Western blot. (C) ETS induces activation of p38MAPK. Tissue extracts from different regions of brain were collected, (100 µg) of the protein was analyzed by 10% SDS-PAGE and detected phosphorylated p38MAPK using anti-phospho p38MAPK antibody by Western blot. The same blot was stripped off and re-probed with anti-p38MAPK antibody to detect the levels of p38MAPK by Western blot.
3.8. ETS induces MAPK activation
Activation of mitogen activated protein kinase is the prior step to JNK activation. Several lines of evidence suggest that phosphorylated MAPKs can participate in the regulation of NF-κB transcriptional activity [30]. To see the phosphorylation of MAPK by ETS, the control and ETS-exposed brain tissues were extracted and 100 µg proteins were analyzed by 10% SDS-PAGE and phosphorylated form of MAPK was detected by Western blot using anti-phospho-MAPK antibody. The result shown in Fig. 4B indicates that phosphorylated MAPK was increased in all the regions of the brain exposed to ETS as compared to control brain tissue. The same extracts were probed with anti-ERK antibody and the level of ERK was unchanged in all the lanes indicating equal loading of proteins as well as specificity of the phosphorylation of the MAPK because ERK also undergoes phoshorylation but through different signaling pathway [31]. These results therefore strongly suggest that ETS induces MAPK phosphorylation and that the level of phosphorylation was similar across all the regions of the brain. We next examined the effect of ETS on p38MAP kinase in various regions of the brain. We found that ETS induced the phosphorylation of the p38MAP kinase in all the regions of the brain examined without altering the levels of the p38 MAP kinase protein expression (Fig. 4C).
4. Discussion
The present study was designed to investigate the effect of long-term exposure of ETS on various regions of the brain of mice. We report that 6 months ETS exposure led to an increased ROS in cerebellum, frontal cortex, hippocampus and striatum with concomitant increase in lipid peroxidation. Long term exposure of ETS also led to an increase in the levels of the proinflammatory transcription factors NF-κB and AP-1 and a concomitant increase in COX-2 in all the regions of the brain examined. ETS also activated JNK and ERK signaling pathways in the brain of mice.
The effect of long-term exposure to ETS, affects brain regions by inducing oxidative stress leading to activation of redox sensitive transcription factor NF-κB, activator protein-1 via activation of signal responsive kinases namely JNK and MAP Kinase. Our first observation shows that brain tissues exposed to ETS showed increased ROS as compared to the control tissue. Since there is no report suggesting the extent of ROS induced by ETS in different regions of the brain, this data carries significant importance to the effect of ETS in brain. Frontal Cortex showed maximum ROS induction followed by brain stem, indicating thereby the sensitive response of these regions to ETS. Increased induction of ROS leads to significantly high level of lipid peroxidation in all the regions of the brain exposed to ETS. It has been reported that acute smoking can result in tissue damage due to increased production of lipid peroxidation and degradation products of extracellular matrix proteins [32]. There are also reports suggesting that oxidative stress leads to the induction of inflammatory signals as a defense to remove the damaged cells from the tissue via apoptosis [27, 33]. Our study indicates that ETS leads to an increase in ROS production in different regions of the brain and activates the redox-sensitive transcription factor NF-κB in all the regions of the brain. Therefore the results are in agreement with previous studies where ETS induced stimulation of inflammatory signals has been reported through the involvement of NF-κB resulting from the decrease in HDAC2 activity in rat lung exposed to cigarette smoke [33].
Tobacco smoke has been shown to induce inflammatory cytokines and NF-κB activation [34, 35]. Therefore, ROS induced NF-κB activation may be one of the initial event for the expression of cytokines involved in the inflammatory response. NF-κB regulates the expression of many genes involved in immune and inflammatory responses [30]. Our study shows that long-term exposure to ETS induces NF-κB activation in different regions of the brain. However, our results are different from a recent report where no apparent change in NF-κB activation was observed in cigarette smoke condensate treated lung carcinoma (A549) cells exposed to cigarette smoke condensate for 24 h [32]. This difference could be attributed to the duration of exposure and the nature of the cell type. Thus we conclude that the length of exposure is potentially an important factor in determining the activation of NF-κB by ETS. Since, we do not have any data co-relating the exposure to this measured effect, it is not feasible to comment, which region(s) of the brain is/are sensitive to the ETS at short duration of exposure. The 6 months period of 7 h/days exposures to ETS might have saturated the brain tissue across all regions and therefore we do not see any obvious differences in NF-κB activation in the various regions of the brain.
It has been also been reported that cigarette smoke activates another proinflammatory transcription factor, AP-1 [30, 35]. Since it is known that most agents that activates NF-κB also activates AP-1 so we examined the effect of long term exposure to ETS on AP-1 expression in various regions of the brain. We show that ETS activated AP-1, as observed by strong binding to DNA in brain tissues exposed to ETS as compared to their respective controls. This observation is in agreement with Marwick et al., who also have reported AP-1 activation in rat lung after 8 weeks of exposure to cigarette smoke [34]. Activation of AP-1 requires JNK activation, which is an early event in this signaling pathway [19]. We assayed for JNK activation and observed activated JNK in almost all the regions of the brain in STE exposed animals. Cigarette smoke has been shown to induce MAPKand this activation was co-related with concomitant increase in histone 3 phospho-acetylation, histone 4 acetylation, and increased DNA binding of the redox-sensitive transcription factor NF-κB in rat lung [34]. Activated MAPK was observed in all the regions of the brain of ETS-exposed animals. These results indicated that the brain is susceptible to MAPK activation by ETS. p38MAP kinase was also found to be phoshorylated by ETS in various regions of the brain tested. These results are in agreement with a study where nicotine has been shown to stimulate the phosphorylation of p38MAPK in rat aortic vascular smooth muscle cells [36]. As both MAPK as well as NF-κB are activated by oxidative stress, therefore, the results showed a co-relation between NF-κB and MAPK in the brain tissues as compared to control.
We also report that COX-2 was overexpressed in all the regions of the brain of ETS-treated animals. COX-2 is a proinflammatory enzyme whose expression is regulated by both NF-κB and AP-1. Our results are in agreement with previous reports where cigarette smoke condensate has been shown to induce the expression of both COX-2 mRNA and protein in lung cancer cell lines [37].
In summary, this is the first report showing the toxicological effect and the signaling mechanism of long-term exposure of ETS in mice brain. A significantly increased lipid peroxidation across all the regions of the brain did indicate tissue damage as a result of long term ETS exposure. The activation of proapoptotic molecules such as different kinases and transcription factors through up-regulation of oxidants coupled with possible down regulation of natural anti-oxidants indicates the probable mechanism of ETS-mediated toxicity. This study further contributes to the understanding of the deleterious effects of long-term exposure of ETS in brain.
Acknowledgment
This work was supported by grant: RCMI/NIH #G12RR03045-19. HL081205(SB.)
REFERENCES
- 1.DHHS Publication No (CDC) 89-8411. Rockville, Maryland: US Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health; 1989. Services DoHaH, Reducing the health consequences of smoking: 25 years of progress. A report of the Surgeon General. [Google Scholar]
- 2.English DR, Holman CDJ, Milne E, Winter MG, Hulse GK, Codde JP, et al. The quantification of drug caused morbidity and mortality in Australia. 1995 ed. Canberra: Commonwealth Department of Human Services and Health; 1995. [Google Scholar]
- 3.Smoke NRCET. Measuring exposures and assessing health effects. Washington DC: National Academy Press; 1986. [PubMed] [Google Scholar]
- 4.Palmer RM, Wilson RF, Hasan AS, Scott DA. Mechanisms of action of environmental factors—tobacco smoking. J Clin Periodontol. 2005;32 Suppl. 6:180–195. doi: 10.1111/j.1600-051X.2005.00786.x. [DOI] [PubMed] [Google Scholar]
- 5.Ozguner F, Koyu A, Cesur G. Active smoking causes oxidative stress and decreases blood melatonin levels. Toxicol Ind Health. 2005;21(12):21–26. doi: 10.1191/0748233705th211oa. [DOI] [PubMed] [Google Scholar]
- 6.Kosecik M, Erel O, Sevinc E, Selek S. Increased oxidative stress in children exposed to passive smoking. Int J Cardiol. 2005;100(1):61–64. doi: 10.1016/j.ijcard.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 7.Machaalani R, Waters KA, Tinworth KD. Effects of postnatal nicotine exposure on apoptotic markers in the developing piglet brain. Neuroscience. 2005;132(2):325–333. doi: 10.1016/j.neuroscience.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 8.Krous HF, Campbell GA, Fowler MW, Catron AC, Farber JP. Maternal nicotine administration and fetal brain stem damage: a rat model with implications for sudden infant death syndrome. Am J Obstet Gynecol. 1981;140(7):743–746. doi: 10.1016/0002-9378(81)90733-x. [DOI] [PubMed] [Google Scholar]
- 9.Preston-Martin S, Yu MC, Benton B, Henderson BE. N-Nitroso compounds and childhood brain tumors: a case-control study. Cancer Res. 1982;42(12):5240–5245. [PubMed] [Google Scholar]
- 10.Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H] nicotine binding sites in human brain. J Neurochem. 1988;50(4):1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- 11.Fuxe K, Andersson K, Eneroth P, Harfstrand A, Agnati LF. Neuroendocrine actions of nicotine and of exposure to cigarette smoke: medical implications. Psychoneuroendocrinology. 1989;14(12):19–41. doi: 10.1016/0306-4530(89)90054-1. [DOI] [PubMed] [Google Scholar]
- 12.Lee HM, Greeley GH, Herndon DN, Sinha M, Luxon BA, Englander EW. A rat model of smoke inhalation injury: influence of combustion smoke on gene expression in the brain. Toxicol Appl Pharmacol. 2005;208(3):255–265. doi: 10.1016/j.taap.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Leone A. Relationship between cigarette smoking and other coronary risk factors in atherosclerosis: risk of cardiovascular disease and preventive measures. Curr Pharm Des. 2003;9(29):2417–2423. doi: 10.2174/1381612033453802. [DOI] [PubMed] [Google Scholar]
- 14.Vayssier M, Banzet N, Francois D, Bellmann K, Polla BS. Tobacco smoke induces both apoptosis and necrosis in mammalian cells: differential effects of HSP70. Am J Physiol. 1998;275(4 Pt 1):L771–L779. doi: 10.1152/ajplung.1998.275.4.L771. [DOI] [PubMed] [Google Scholar]
- 15.Stein B, Baldwin AS, Jr, Ballard DW, Greene WC, Angel P, Herrlich P. Cross-coupling of the NF-kappa B p65 and Fos/Jun transcription factors produces potentiated biological function. Embo J. 1993;12(10):3879–3891. doi: 10.1002/j.1460-2075.1993.tb06066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahman I, Gilmour PS, Jimenez LA, MacNee W. Oxidative stress and TNF-alpha induce histone acetylation and NF-kappa B/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Mol Cell Biochem. 2002;234/235(12):239–248. [PubMed] [Google Scholar]
- 17.Nishikawa M, Kakemizu N, Ito T, Kudo M, Kaneko T, Suzuki M, et al. Superoxide mediates cigarette smoke-induced infiltration of neutrophils into the airways through nuclear factor-kappa B activation and IL-8 mRNA expression in guinea pigs in vivo. Am J Respir Cell Mol Biol. 1999;20(2):189–198. doi: 10.1165/ajrcmb.20.2.3305. [DOI] [PubMed] [Google Scholar]
- 18.Felix K, Manna SK, Wise K, Barr J, Ramesh GT. Low levels of arsenite activates nuclear factor-kappa B and activator protein-1 in immortalized mesencephalic cells. J Biochem Mol Toxicol. 2005;19(2):67–77. doi: 10.1002/jbt.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manna SK, Ramesh GT. Interleukin-8 induces nuclear transcription factor-kappa B through a TRAF6-dependent pathway. J Biol Chem. 2005;280(8):7010–7021. doi: 10.1074/jbc.M410994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114(9):1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teague SV, Pinkerton KE, Goldsmith M, Gebremichael A, Chang S, Jenkins RA, et al. Sidestream cigarette smoke generation and exposure system for environmental tobacco smoke studies. Inhal Toxicol. 1994;6:79–93. [Google Scholar]
- 22.Jadhav AL, Ramesh GT, Gunasekar PG. Contribution of protein kinase C and glutamate in Pb(2+)-induced cytotoxicity. Toxicol Lett. 2000;115(2):89–98. doi: 10.1016/s0378-4274(00)00177-6. [DOI] [PubMed] [Google Scholar]
- 23.Wise KC, Manna SK, Yamauchi K, Ramesh V, Wilson BL, Thomas RL, et al. Activation of nuclear transcription factor-kappa B in mouse brain induced by a simulated microgravity environment. In Vitro Cell Dev Biol Anim. 2005;41(34):118–123. doi: 10.1290/0501006.1. [DOI] [PubMed] [Google Scholar]
- 24.Ramesh GT, Manna SK, Aggarwal BB, Jadhav AL. Lead activates nuclear transcription factor-kappa B, activator protein-1, and amino-terminal c-Jun kinase in pheochromocytoma cells. Toxicol Appl Pharmacol. 1999;155(3):280–286. doi: 10.1006/taap.1999.8624. [DOI] [PubMed] [Google Scholar]
- 25.Manna SK, Sarkar S, Barr J, Wise K, Barrera EV, Jejelowo O, et al. Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-kappa B in human keratinocytes. Nano Lett. 2005;5(9):1676–1684. doi: 10.1021/nl0507966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aggarwal BB. Nuclear factor-kappa B: the enemy within. Cancer Cell. 2004;6(3):203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Storz P, Toker A. NF-kappa B signaling—an alternate pathway for oxidative stress responses. Cell Cycle. 2003;2(1):9–10. doi: 10.4161/cc.2.1.234. [DOI] [PubMed] [Google Scholar]
- 28.Lee FS, Hagler J, Chen ZJ, Maniatis T. Activation of the Ikappa B alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88(2):213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 29.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12(12):1063–1073. [PubMed] [Google Scholar]
- 30.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270(28):16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 31.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68(2):320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, et al. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappa B activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J. 2004;18(15):1897–1899. doi: 10.1096/fj.04-1506fje. [DOI] [PubMed] [Google Scholar]
- 33.Higuchi Y. Glutathione depletion-induced chromosomal DNA fragmentation associated with apoptosis and necrosis. J Cell Mol Med. 2004;8(4):455–464. doi: 10.1111/j.1582-4934.2004.tb00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marwick JA, Kirkham PA, Stevenson CS, Danahay H, Giddings J, Butler K, et al. Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol. 2004;31(6):633–642. doi: 10.1165/rcmb.2004-0006OC. [DOI] [PubMed] [Google Scholar]
- 35.Gensch E, Gallup M, Sucher A, Li D, Gebremichael A, Lemjabbar H, et al. Tobacco smoke control of mucin production in lung cells requires oxygen radicals AP-1 and JNK. J Biol Chem. 2004;279(37):39085–39093. doi: 10.1074/jbc.M406866200. [DOI] [PubMed] [Google Scholar]
- 36.Li JM, Cui TX, Shiuchi T, Liu HW, Min LJ, Okumura M, et al. Nicotine enhances angiotensin II-induced mitogenic response in vascular smooth muscle cells and fibroblasts. Arterioscler Thromb Vasc Biol. 2004;24(1):80–84. doi: 10.1161/01.ATV.0000104007.17365.1c. [DOI] [PubMed] [Google Scholar]
- 37.Shishodia S, Potdar P, Gairola CG, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappa B activation through inhibition of Ikappa Balpha kinase in human lung epithelial cells: correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis. 2003;24(7):1269–1279. doi: 10.1093/carcin/bgg078. [DOI] [PubMed] [Google Scholar]