Abstract
Olfaction represents an ideal model system for the study of mammalian habituation given that it is an anatomically relatively simple system with strong reciprocal connections to the limbic system, driving both reflexive and non-reflexive (motivated) behaviors that are easily quantifiable. Data are reviewed here demonstrating short-term habituation of the odor-evoked heart-rate orienting reflex described according to the criteria for habituation outlined by Thompson and Spencer in 1966. A necessary and sufficient mechanism of short-term habituation is then described, which involves a metabotropic glutamate receptor mediated depression of afferent input to the piriform (primary olfactory) cortex. Finally, evidence for, and a mechanisms of, dishabituation of the orienting reflex and cortical adaptation are described.
Non-associative memory allows organisms to adjust their behavioral responses to sensory input based on recent stimulation history. Habituation is a reduction in responsiveness to repeated or prolonged stimulation and allows animals to filter biologically less relevant input, and in turn devote more attention and processing energy toward more relevant or dynamic stimuli. Dishabituation and sensitization both involve an increase in stimulus evoked responses following a change in stimulation, such as introduction of an intense stimulus, and allow a return of responsiveness (dishabituation) or heightened responsiveness (sensitization) following an unexpected disturbance. Together, these three processes, habituation, dishabituation and sensitization, allow rapid changes in neural activity and behavioral expression, and are thus fundamental to normal neural circuit function.
The neural mechanisms of non-associative memory have been most thoroughly examined in invertebrate preparations (see other articles in this issue). For example, changes in efficacy of sensorimotor and interneuron synapses have been demonstrated to play necessary and sufficient roles in habituation, dishabituation and sensitization in Aplysia (e.g., (Castellucci et al. 1970; Ezzeddine et al. 2003; Kupfermann et al. 1970; Li et al. 2005)). The systems level neurobiology of these processes in mammalian systems has also received considerable attention (e.g., (Bespalov et al. 2007; Glanzman et al. 1972; Groves et al. 1972; Weber et al. 2002)), although direct causal connections between specific synaptic mechanisms and behavioral habituation have been less evident.
We have developed a model of habituation in the rat using odor-evoked heart rate orienting responses that provides a tractable system for studying both circuit and synaptic mechanisms of habituation in rodents. The mammalian olfactory system, as described in more detail below, is a relatively simple circuit wherein second order neurons (mitral cells of the olfactory bulb) receive direct input from olfactory receptor neurons and in turn project directly to the olfactory (piriform) cortex. Both of these synapses are glutamatergic, and the synaptic physiology and neural circuitry of the piriform cortex are well described (Neville et al. 2004). Importantly, the olfactory system has strong interconnections with the limbic system and hypothalamus, and the circuitry underlying odor-evoked cardiac orienting reflexes has been described (Kapp et al. 1992). Furthermore, the olfactory system is the target of many neuromodulatory systems, including the noradrenergic nucleus locus coeruleus, the serotonergic raphe nucleus and the cholinergic nucleus of the diagonal band (Shipley et al. 1996).
Olfactory system
Compared to mammalian thalamocortical sensory systems, the olfactory pathway is relatively simple. Odorant features are selectively transduced within the receptor sheet by a large family of receptor proteins (Buck et al. 1991), and the features are refined by spatial and temporal coding within olfactory bulb local circuits (Friedrich et al. 2004; Laurent 2002; Leon et al. 2003; Lin et al. 2006). The olfactory system second-order neurons, mitral cells are also the output neurons of the olfactory bulb and project directly to the olfactory cortex. The olfactory cortex is a 3-layered paleocortical structure, and composed of several anatomically distinct subregions, the largest of which is the piriform cortex. Mitral cells form glutamatergic synapses on piriform cortex pyramidal cells.
Phenomenally precise projections of olfactory receptor neurons to the olfactory bulb glomerular layer, combined with restricted receptive fields of receptor neurons, produce odor-specific spatial patterns of olfactory bulb activity (Johnson et al. 2004; Rubin et al. 1999; Sharp et al. 1977). That is, a given chemical stimulus will evoke a unique spatial pattern of activity across the olfactory bulb, while a different stimulus, activating different receptors, will induce a different, though perhaps overlapping spatial activity pattern. Mitral cells, through direct excitatory input from receptor neurons and extensive excitatory and inhibitory interconnections within the bulb, translate this spatial pattern into a spatiotemporal code that is broadly transmitted to the piriform cortex through extensive axonal projections (Neville & Haberly 2004). Mitral cell axons, forming the lateral olfactory tract (LOT), terminate in superficial layer I of the piriform cortex onto the apical dendrites of layer II and III pyramidal cells. Individual mitral cells terminate in broad patches or clusters in the piriform cortex (Buonviso et al. 1991; Zou et al. 2001). Piriform cortex pyramidal cells, in turn, make extensive associational connections throughout the piriform cortex, back to the olfactory bulb and even to other cortical structures (Chen et al. 2003; Johnson et al. 2000). The relatively diffuse afferent input combined with a broad, extensive intra-cortical association fiber system creates a highly combinatorial network, ideal for synthetic processing of complex feature ensembles (Haberly 2001). The diffuse, combinatorial network array also results in a relatively weak spatial odorant representation in cortex (Cattarelli et al. 1988; Illig et al. 2003; Rennaker et al. 2007; Zou et al. 2005), in strong contrast to the spatial patterning in olfactory bulb. Both the olfactory bulb and piriform cortex are targets of modulatory inputs including NE, ACh and 5-HT (Shipley & Ennis 1996).
Behavioral assays of odor habituation
Several different assays of odor habituation have been developed. In humans, changes in odor detection threshold or intensity ratings after repeated or prolonged odor exposure are generally used (Dalton 2000). In animals, the most commonly used metric is the duration of investigation of a scented object (Cleland et al. 2002; Yadon et al. 2005). With prolonged continuous exposure, or with repeated brief exposures, the length of time spent investigating the exposed odor decreases. Introduction of a new odor initiates investigation of that new odor, demonstrating stimulus specificity of the original habituation (Cleland et al. 2002; Mandairon et al. 2006). A similar task measures the rearing or “head-up” response to odors presented from above (King et al. 1990).
Both of these odor investigation-based tasks evoke a variety of differently motivated behaviors in animals. The reduction in investigation over time, therefore, may be partly due to habituation to the sensory stimulus, but also due to changes in motivational state unrelated to the perceived stimulus per se. In attempting to identify neural correlates of odor habituation, therefore, it may be most parsimonous to utilize odor-evoked behaviors that are more closely, or exclusively driven by the odor, and based on relatively simple neural circuitry. Odor-evoked heart-rate orienting reflexes (HROR) satisfies these two criteria and has been used in our laboratory to explore the neural basis of short-term odor habituation (Fig. 1).
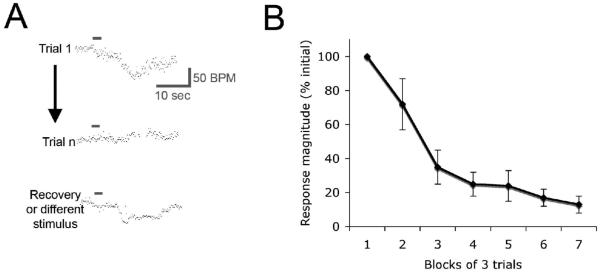
Fig. 1.
(A) Representative example of an odor-evoked HROR recorded from a freely moving rat using telemetry. A two-second odor pulse is presented at each horizontal bar. The initial HROR habituates after repeated stimulus presentations and subsequently recovers. The habituation is odor specific. (B) Habituation of the HROR over repeated stimulus presentations. Data from (Fletcher & Wilson 2001)).
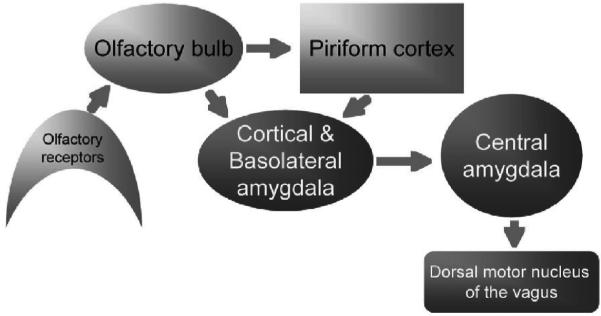
HROR are bradycardia responses to novel stimuli, and have been described for most sensory modalities in a variety of species including humans (Sokolov et al. 1975). These responses have a final common pathway involving the dorsal motor nucleus of the vagus and ACh release onto cardiac pacemaker cells. Odor-evoked HROR are driven by direct olfactory bulb and piriform cortex inputs to the amygdala, with intra-amygdaloid connections between the basolateral and the central nucleus, which in turn projects to the brainstem (Fig. 2; (Kapp et al. 1992)). We monitor HROR using surgically implanted electrocardiogram recording telemetry devices (Data Sciences, International). This allows the rats to move freely without any wires or restraint which could interfere with expression of the HROR. The rats are placed in a darkened recording chamber and allowed to acclimate for several minutes, before an odor is delivered from above. The odor pulse lasts 2-5 sec (in different experiments), and can induce large (>30 beats/minute) drops in heart rate within 5-10 sec. Response duration is generally 20-30 sec (Fig. 1). Odorants we have tested range from complex mixtures to monomolecular odorants such as ethyl esters.
Fig. 2.
Hypothesized minimal circuitry required for odor-evoked heart-rate orienting reflexes.
Habituation of HROR occurs following repeated odor stimulation (Fig. 1)(Fletcher et al. 2001), spontaneously recovers with time (Sokolov & Vinogradova 1975), and shows stimulus specificity, with very little cross-habituation between molecularly dissimilar odorants (e.g., (Fletcher & Wilson 2001)). The degree of cross-habituation is odor experience-dependent, i.e., cross-habituation is reduced between familiar odors and enhanced between unfamiliar odors (Fletcher et al. 2002). Importantly, preliminary data demonstrate that odor-evoked HROR can also demonstrate dishabituation, with nearly complete recovery of the habituated response following a loud auditory stimulus (Smith, Shionoya & Wilson, in prep.).
Neurobiology of odor habituation
Prolonged exposure to an odorant can cause olfactory receptor adaptation (Kurahashi et al. 1997), as well as reductions in glomerular and second-order neuron responses (Scott 1977; Verhagen et al. 2007; Wilson 1998a). However, piriform cortex responses show rapid, pronounced response decrement (adaptation), exceeding that of more peripheral neurons and often leading to complete cessation of responding (Wilson 1998a). Importantly, odor-evoked HROR magnitude is significantly correlated with the magnitude of piriform cortex odor-evoked activity recorded simultaneously (Best et al. 2005), and as described below, pharmacological prevention of piriform cortex adaptation prevents odor-evoked HROR habituation (Best et al. 2005). What follows is a description of piriform cortical correlates of odor habituation placed in the context of Thompson and Spencer’s (1966) outline of critical characteristics needed to define habituation and its neural mechanisms. We focus solely on short-term habituation and cortical adaptation, and thus those characteristics relevant only to long-term effects are not included.
1) Repeated applications of a stimulus result in decreased responses
Odor stimulation induces single-unit responses in olfactory bulb mitral cells and in piriform cortex pyramidal cells. With repeated stimulation at 30 sec ISI, responses in both areas adapt (Fig. 3), although responses in piriform cortex show the most adaptation, often with complete loss of evoked activity (Wilson 1998a).
Fig. 3.
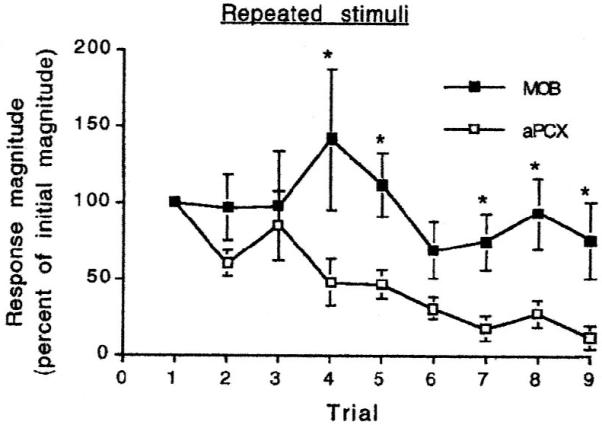
Single-unit piriform cortex (aPCX) responses adapt rapidly to repeated odor pulses despite relatively maintained responses in mitral cells of the main olfactory bulb (MOB), the source of cortical afferents. Data from (Wilson 1998a).
The strong cortical adaptation despite relatively maintained responses in mitral cells is of interest given that mitral cells form glutamatergic synapses on cortical pyramidal cells (Haberly, 2001). In vivo intracellular recordings in our lab have demonstrated that cortical odor adaptation is associated with depression of the mitral cell-pyramidal cell synapse (Wilson 1998b). Recordings in in vitro piriform cortex from our lab have demonstrated a similar depression can be evoked with mitral cell axon stimulation patterned to mimic odor evoked mitral cell activity (Best et al. 2004). This depression is homosynaptic and can be blocked by the group III metabotropic glutamate receptor mGluRIII antagonist CPPG, as can adaptation of cortical odor responses (Best & Wilson 2004). Pre-synaptic mGluR’s mediate synaptic depression in several central circuits (Krieger et al. 2002; Weber et al. 2002), and are implicated in habituation of other, potentially non-olfactory driven behaviors in rodents (e.g., (Bespalov et al. 2007)). No changes in membrane potential (e.g., hyperpolarization) were observed in vivo or in vitro, suggesting enhanced synaptic inhibition of pyramidal cells (e.g. from inhibitory interneurons) does significantly contribute to cortical adaptation (Best & Wilson 2004; Wilson 1998b).
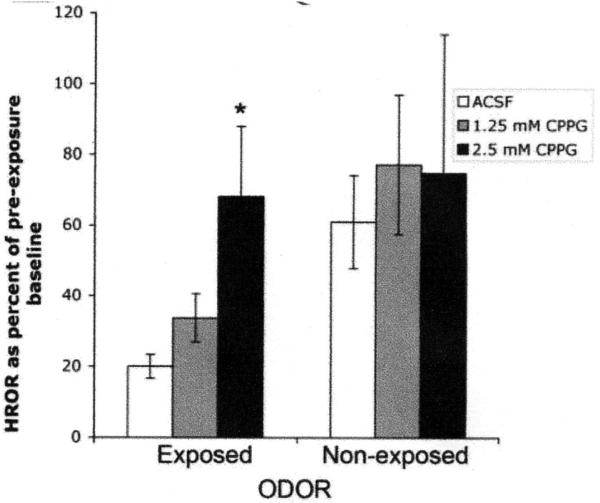
Importantly, cortical infusion of CPPG in awake rats also prevents adaptation of odor-evoked piriform cortex local field potentials and odor-evoked HROR habituation (Fig. 4 (Best et al. 2005). Similar infusions also retard habituation of investigation of scented objects (Yadon & Wilson 2005). Furthermore, preliminary data from our lab suggest that bilateral piriform cortex infusions of the mGluRIII agonist AP4, induce a depression of odor-evoked HROR’s, similar to the reduction seen after habituation.
Fig. 4.
Intra-cortical blockade of mGluRIII receptors prevents habituation of the HROR response. The same compound blocks cortical afferent synaptic depression and cortical odor adaptation. From (Best et al. 2005).
Together, these results demonstrate that the odor-evoked HROR habituates, that this habituation is correlated with adaptation of piriform cortex odor responses, that both HROR habituation and cortical adaptation involve a pre-synaptic mGluRIII receptor-induced depression of cortical afferent synapses, and that mGluRIII mediated changes within the piriform cortex may be both necessary and sufficient for habituation of the odor-evoked HROR.
2) Withholding the stimulus produces recovery
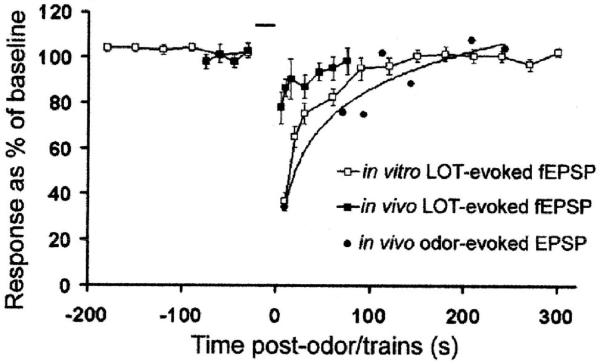
Odor-evoked HROR responses spontaneously recover from habituation, as do odor-evoked piriform cortex responses and piriform cortex afferent synaptic depression. The in vitro and in vivo synaptic depression recovers with a similar time course as the odor-evoked cortical responses (Best & Wilson 2004; Wilson 1998b). Recovery of all responses occurs over a time course of a few minutes (Fig. 5).
Fig. 5.
Adapted cortical responses and the associated synaptic depression of cortical afferent synapses recover within a few minutes. Habituated HROR responses also recover with a roughly similar time course (not shown). Data from (Best & Wilson 2004).
3) Increased frequency of stimulation increases the rate of habituation induction
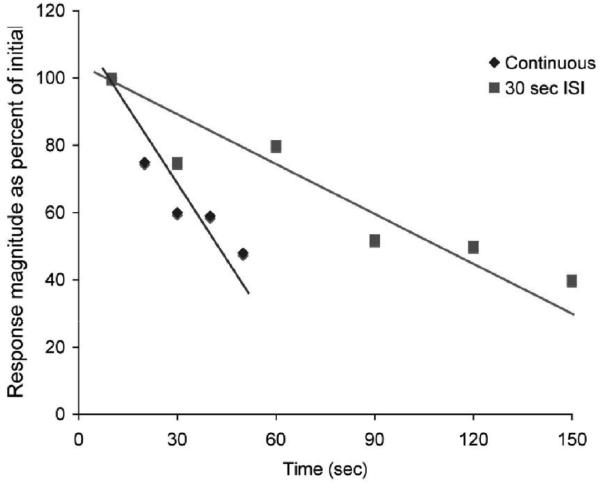
While we have not examined the effects of varying stimulation rate on HROR habituation, a re-examination of our cortical odor response data (Wilson 1998a) suggests that continuous odor exposure (actually repeated inhalations at 0.5 Hz) induces more rapid cortical adaptation compared to that induced by short odor pulses separated by a 30 sec ISI (Fig. 6). Linear regression on raw single-unit odor response magnitude data shows a 300% increase in adaptation slope when stimuli are presented continuously (m = -1.175) compared to when presented at 30 sec ISI (m = -0.411). Thus, increasing the frequency of stimulation increases the rate of cortical adaptation.
Fig. 6.
Effect of ISI on adaptation rate of single-unit cortical odor responses. Odor-evoked response magnitude in response to continuous stimulation and to short odor pulses at 30 sec ISI are shown. Data derived from (Wilson 1998a).
4) The rate or extent of habituation is inversely related to the stimulus intensity
To our knowledge, the effect of stimulus intensity has not been examined in either the HROR habituation or cortical odor adaptation paradigms. We hypothesize that, as in other paradigms, lower stimulus intensities will result in more rapid and/or complete habituation and cortical adaptation. As odor intensity increases, olfactory receptor neuron tuning becomes less selective (Malnic et al. 1999). Thus, a given odor may activate more neurons in the olfactory bulb and piriform cortex at high intensity than at low intensity. Homosynaptic depression of a few activated synapses during low intensity exposure, therefore, may be expected to have a more pronounced effect on cortical and behavioral responses. In vitro, varying the intensity of electrical stimulation of mitral cell axons did not influence the magnitude or recovery of afferent synaptic depression (Best & Wilson 2004). It should be noted however, that olfactory bulb mitral cell responses do not vary linearly with intensity (Hamilton et al. 1989; Meredith 1986; Wellis et al. 1989), thus intensity effects on cortical adaptation and habituation may not be as simple as hypothesized. In humans, exposure to higher intensity odors induces more adaptation to low intensity test odors than visa versa (Dalton 2000), as might be expected if the extent of habituation is inversely related to stimulus intensity. Further work is needed on this issue.
5) Habituation to one stimulus may generalize to other similar stimuli
Habituation to one odor induces cross-habituation to molecularly similar odors, as demonstrated in a variety of behavioral assays in many species (Cain 1970; Cleland et al. 2002; Fletcher & Wilson 2001). However, the extent of cross-habituation is dependent on past experience - familiar odors show less cross-habituation than novel odors (Cleland et al. 2002; Fletcher & Wilson 2002). Similarly, piriform cortical responses show cross-adaptation to molecular similar odors, though with familiarity this cross-adaptation can be markedly reduced (Wilson 2003; Wilson 2001). The extent of cortical cross adaptation may in part be due to that fact that afferent depression is homosynaptic (Best & Wilson 2004; Linster et al. 2007; Wilson 1998b). However, the nature of piriform cortical circuitry allows the system to learn previous patterns of input, and use these stored templates to complete degraded patterns on subsequent stimulation (Haberly 2001; Hasselmo et al. 1990). This cortical sensory memory is dependent on plasticity of intracortical association fibers, and pharmacological disruption of normal association fiber synaptic plasticity results in enhanced cross adaptation at both the cortical and behavioral levels (Fletcher & Wilson 2002; Wilson 2001).
6) Presentation of a strong stimulus may induce dishabituation
In humans, the extent of odor habituation is influenced by the expectations of the subject. For example, being told that a novel odor may be hazardous delays/reduces habituation to that odorant, compared to the level of habituation expressed by other subjects to the same stimulus that were told the odor was beneficial or neutral (Dalton 2000; Kobayashi et al. 2007). This suggests that central factors related to expectation, arousal and/or attention may influence odor habituation.
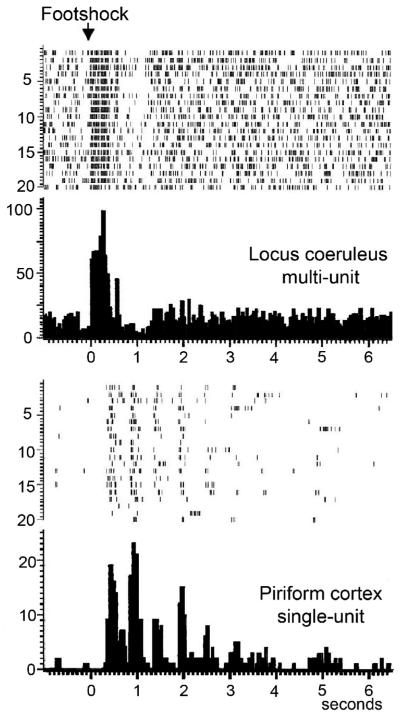
In invertebrates, dishabituation (and short-term sensitization) can be induced by a stimulus that causes release of the monoamine 5-HT onto sensory and motor neurons, as well as local interneurons. Similarly, in the mammalian olfactory system an intense stimulus such as footshock can facilitate respiration-entrained activity in PCX neurons (Bouret et al. 2002)(Fig. 7), suggestive of a dishabituation (or sensitization) effect. This cortical activation is correlated with a footshock induced activation of the noradrenergic locus coeruleus, which heavily innervates the olfactory bulb and cortex (Shipley & Ennis 1996), and preliminary microdialysis data in our laboratory suggest that footshock or a loud acoustic stimulus increases NE release within the PCX (Smith, Shionoya & Wilson, in prep.). Furthermore, electrical stimulation of the medial forebrain bundle can induce a similar effect in olfactory bulb mitral cells, an effect blocked by NE ß-receptor antagonists (Wilson et al. 1991). Finally, medial forebrain bundle stimulation can reduce mitral cell odor adaptation (Wilson et al. 1992), and tailpinch can cause dishabituation of odor evoked unit responses in the rat olfactory system (Scott 1977).
Fig. 7.
(TOP) Footshock in an anesthetized rat produces transient activation of the locus coeruleus followed by prolonged activation of piriform cortical cells (raster plots of single trials and cumulative peri-stimulus histogram). Importantly, the activation of PCX occurred as a heightened phasic response to the respiratory cycle, as might be expected if the system was dishabituated by the footshock to background room odors (Wilson and Sara, unpublished observation).
Preliminary work in our lab (Smith, Shionoya & Wilson, in prep.) shows that the odor-evoked HROR dishabituates following a loud acoustic stimulus. Specifically, the same acoustic stimulus that elevated NE release within the PCX described above, causes nearly complete recovery of the odor-evoked HROR after habituation to approximately 20% of initial magnitude.
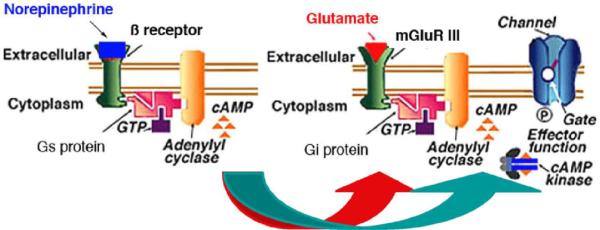
Together, these results suggest that intense stimuli (or cognitive factors such as implied danger) may evoke dishabituation by activation of monoaminergic NE input to the olfactory system. In fact, we have previously demonstrated that the NE ß-receptor agonist isoproterenol blocks the mGluRIII mediated cortical afferent synaptic depression underlying cortical adaptation in vitro, suggesting a possible mechanism for direct reversal of habituation by a dishabituating stimulus (Best & Wilson 2004). Group III mGluR action has been shown to be inhibited by activation of pre-synaptic ß-adrenergic receptors (see Fig. 8) (Cai et al. 2001). Furthermore, NE input to the olfactory bulb can increase mitral cell responsiveness to receptor input (Jiang et al. 1996), thus providing an upstream alternative or additive mechanism for odor dishabituation. Interestingly, the locus coeruleus is most strongly responsive to novel stimuli (Vankov et al. 1995). Thus, as the dishabituating stimulus is repeated, dishabituation effects should also habituate, as proposed by Thompson and Spencer (Thompson et al. 1966). Work ongoing in our lab is currently examining the behavioral neurobiology of odor dishabituation and sensitization.
Fig. 8.
Hypothesized interaction between NE and mGluRIII signaling cascades leading to dishabituation.
Summary
The work described here places short-term odor habituation within the framework described by Thompson and Spencer in 1966. Those defining characteristics of habituation perfectly overlay odor habituation at both the behavioral (autonomic reflex) and neural (cortical adaptation) levels. Odor-evoked behavioral and cortical responses show decrement with repeated stimulation, spontaneous recovery with time, stimulus frequency and intensity dependence, stimulus specificity, and dishabituation. Importantly, pharmacological blockade of cortical adaptation prevents behavioral habituation, and preliminary data suggest that induction of cortical adaptation induces behavioral response decrement, providing both a necessary and sufficient link between cortical adaptation and habituation in olfaction. Furthermore, the mGluRIII mechanism described for short-term odor habituation includes a mechanism for dishabituation, namely norepinephrine release within the olfactory system, though further work is necessary in this area.
The mechanisms of both habituation and dishabituation described here in rats are notably similar to that described in invertebrates such as Aplysia. A necessary and sufficient decrease in pre-synaptic neurotransmitter release in the circuit linking sensory input to motor output underlies habituation in both systems, while a monoamine mediated increase in transmitter release mediates dishabituation in Aplysia and preliminary data suggest similarly in rats. While there may be differences in the identity of specific components (e.g, serotonin involvement in Aplysia dishabituation versus norepinephrine in rat), these two very divergent systems seem to share similar basic tools for adapting sensory evoked responses to repetitive or redundant sensory streams.
Finally, while this review has emphasized mechanisms of short-term habituation, recent behavioral pharmacology work has demonstrated a double-dissociation between short- and long-term odor habituation, with long-term habituation of odor investigation relying on an NMDA receptor-dependent mechanism, and as described here, short-term habituation relying on an mGluRIII mechanism (McNamara et al. 2008). Together with the known relative simplicity of the olfactory sensory pathway, these findings place olfaction as an ideal model system for the study of the neurobiology of mammalian habituation.
Acknowledgement
The author would like to thank Dr. Rankin for organizing this workshop, and NIDCD and NSF for funding of the research in the author’s lab described here.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bespalov A, Jongen-Relo AL, van Gaalen M, et al. Habituation deficits induced by metabotropic glutamate receptors 2/3 receptor blockade in mice: reversal by antipsychotic drugs. J Pharmacol Exp Ther. 2007;320(2):944–50. doi: 10.1124/jpet.106.110684. [DOI] [PubMed] [Google Scholar]
- Best AR, Thompson JV, Fletcher ML, et al. Cortical metabotropic glutamate receptors contribute to habituation of a simple odor-evoked behavior. J Neurosci. 2005;25(10):2513–7. doi: 10.1523/JNEUROSCI.5298-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best AR, Wilson DA. Coordinate synaptic mechanisms contributing to olfactory cortical adaptation. J Neurosci. 2004;24(3):652–60. doi: 10.1523/JNEUROSCI.4220-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Locus coeruleus activation modulates firing rate and temporal organization of odour-induced single-cell responses in rat piriform cortex. Eur J Neurosci. 2002;16(12):2371–82. doi: 10.1046/j.1460-9568.2002.02413.x. [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65(1):175–87. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Buonviso N, Revial MF, Jourdan F. The Projections of Mitral Cells from Small Local Regions of the Olfactory Bulb: An Anterograde Tracing Study Using PHA-L (Phaseolus vulgaris Leucoagglutinin) Eur J Neurosci. 1991;3(6):493–500. doi: 10.1111/j.1460-9568.1991.tb00836.x. [DOI] [PubMed] [Google Scholar]
- Cai Z, Saugstad JA, Sorensen SD, et al. Cyclic AMP-dependent protein kinase phosphorylates group III metabotropic glutamate receptors and inhibits their function as presynaptic receptors. J Neurochem. 2001;78(4):756–66. doi: 10.1046/j.1471-4159.2001.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain WS. Odor intensity after self-adaptation and cross-adaptation. Perception & Pyschophysics. 1970;7(5):271–5. [Google Scholar]
- Castellucci V, Pinsker H, Kupfermann I, et al. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167(926):1745–8. doi: 10.1126/science.167.3926.1745. [DOI] [PubMed] [Google Scholar]
- Cattarelli M, Astic L, Kauer JS. Metabolic mapping of 2-deoxyglucose uptake in the rat piriform cortex using computerized image processing. Brain Res. 1988;442(1):180–4. doi: 10.1016/0006-8993(88)91449-7. [DOI] [PubMed] [Google Scholar]
- Chen S, Murakami K, Oda S, et al. Quantitative analysis of axon collaterals of single cells in layer III of the piriform cortex of the guinea pig. J Comp Neurol. 2003;465(3):455–65. doi: 10.1002/cne.10844. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, et al. Behavioral models of odor similarity. Behav Neurosci. 2002;116(2):222–31. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Dalton P. Psychophysical and behavioral characteristics of olfactory adaptation. Chem Senses. 2000;25(4):487–92. doi: 10.1093/chemse/25.4.487. [DOI] [PubMed] [Google Scholar]
- Ezzeddine Y, Glanzman DL. Prolonged habituation of the gill-withdrawal reflex in Aplysia depends on protein synthesis, protein phosphatase activity, and postsynaptic glutamate receptors. J Neurosci. 2003;23(29):9585–94. doi: 10.1523/JNEUROSCI.23-29-09585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M, Wilson DA. Ontogeny of odor discrimination: a method to assess novel odor discrimination in neonatal rats. Physiol Behav. 2001;74(45):589–93. doi: 10.1016/s0031-9384(01)00602-3. [DOI] [PubMed] [Google Scholar]
- Fletcher ML, Wilson DA. Experience modifies olfactory acuity: acetylcholine-dependent learning decreases behavioral generalization between similar odorants. J Neurosci. 2002;22(2):RC201. doi: 10.1523/JNEUROSCI.22-02-j0005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich RW, Laurent G. Dynamics of olfactory bulb input and output activity during odor stimulation in zebrafish. J Neurophysiol. 2004;91(6):2658–69. doi: 10.1152/jn.01143.2003. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Groves PM, Thompson RF. Stimulus generalization of habituation in spinal interneurons. Physiol Behav. 1972;8(1):155–8. doi: 10.1016/0031-9384(72)90145-x. [DOI] [PubMed] [Google Scholar]
- Groves PM, Lynch GS. Mechanisms of habituation in the brain stem. Psychol Rev. 1972;79(3):237–44. doi: 10.1037/h0032689. [DOI] [PubMed] [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26(5):551–76. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Hamilton KA, Kauer JS. Patterns of intracellular potentials in salamander mitral/tufted cells in response to odor stimulation. J Neurophysiol. 1989;62(3):609–25. doi: 10.1152/jn.1989.62.3.609. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Wilson MA, Anderson BP, et al. Associative memory function in piriform (olfactory) cortex: computational modeling and neuropharmacology. Cold Spring Harb Symp Quant Biol. 1990;55:599–610. doi: 10.1101/sqb.1990.055.01.057. [DOI] [PubMed] [Google Scholar]
- Illig KR, Haberly LB. Odor-evoked activity is spatially distributed in piriform cortex. J Comp Neurol. 2003;457(4):361–73. doi: 10.1002/cne.10557. [DOI] [PubMed] [Google Scholar]
- Jiang M, Griff ER, Ennis M, et al. Activation of locus coeruleus enhances the responses of olfactory bulb mitral cells to weak olfactory nerve input. J Neurosci. 1996;16(19):6319–29. doi: 10.1523/JNEUROSCI.16-19-06319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Xu Z, et al. Local and global chemotopic organization: general features of the glomerular representations of aliphatic odorants differing in carbon number. J Comp Neurol. 2004;480(2):234–49. doi: 10.1002/cne.20335. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Illig KR, Behan M, et al. New features of connectivity in piriform cortex visualized by intracellular injection of pyramidal cells suggest that “primary” olfactory cortex functions like “association” cortex in other sensory systems. J Neurosci. 2000;20(18):6974–82. doi: 10.1523/JNEUROSCI.20-18-06974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp BS, Whalen PJ, Supple WF, et al. Amygdaloid contributions to conditioned arousal and sensory information processing. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory and mental dysfunction. Wiley-Liss, Inc; New York: 1992. pp. 229–54. [Google Scholar]
- King C, Hall WG. Developmental change in unilateral olfactory habituation is mediated by anterior commissure maturation. Behav Neurosci. 1990;104(5):796–807. doi: 10.1037//0735-7044.104.5.796. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Sakai N, Kobayakawa T, et al. Effects of Cognitive Factors on Perceived Odor Intensity in Adaptation/Habituation Processes: from 2 Different Odor Presentation Methods. Chem Senses. 2007 doi: 10.1093/chemse/bjm075. [DOI] [PubMed] [Google Scholar]
- Krieger P, El Manira A. Group III mGluR-mediated depression of sensory synaptic transmission. Brain Res. 2002;937(12):41–4. doi: 10.1016/s0006-8993(02)02462-9. [DOI] [PubMed] [Google Scholar]
- Kupfermann I, Castellucci V, Pinsker H, et al. Neuronal correlates of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167(926):1743–5. doi: 10.1126/science.167.3926.1743. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 1997;385(6618):725–9. doi: 10.1038/385725a0. [DOI] [PubMed] [Google Scholar]
- Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci. 2002;3(11):884–95. doi: 10.1038/nrn964. [DOI] [PubMed] [Google Scholar]
- Leon M, Johnson BA. Olfactory coding in the mammalian olfactory bulb. Brain Res Brain Res Rev. 2003;42(1):23–32. doi: 10.1016/s0165-0173(03)00142-5. [DOI] [PubMed] [Google Scholar]
- Li Q, Roberts AC, Glanzman DL. Synaptic facilitation and behavioral dishabituation in Aplysia: dependence on release of Ca2+ from postsynaptic intracellular stores, postsynaptic exocytosis, and modulation of postsynaptic AMPA receptor efficacy. J Neurosci. 2005;25(23):5623–37. doi: 10.1523/JNEUROSCI.5305-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DY, Shea SD, Katz LC. Representation of natural stimuli in the rodent main olfactory bulb. Neuron. 2006;50(6):937–49. doi: 10.1016/j.neuron.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Linster C, Henry L, Kadohisa M, et al. Synaptic adaptation and odor-background segmentation. Neurobiol Learn Mem. 2007;87(3):352–60. doi: 10.1016/j.nlm.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, et al. Combinatorial receptor codes for odors. Cell. 1999;96(5):713–23. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Ferretti CJ, Stack CM, et al. Cholinergic modulation in the olfactory bulb influences spontaneous olfactory discrimination in adult rats. Eur J Neurosci. 2006;24(11):3234–44. doi: 10.1111/j.1460-9568.2006.05212.x. [DOI] [PubMed] [Google Scholar]
- McNamara AM, Magidson PD, Linster C, et al. Behavioral evidence of two separate glutamatergic systems involved in olfactory habituation learning. Learn Mem. 2008 in press. [Google Scholar]
- Meredith M. Patterned response to odor in mammalian olfactory bulb: the influence of intensity. J Neurophysiol. 1986;56(3):572–97. doi: 10.1152/jn.1986.56.3.572. [DOI] [PubMed] [Google Scholar]
- Neville KR, Haberly L. Olfactory cortex. In: Shepherd GM, editor. The synaptic organization of the brain. 5th edn. Oxford University Press; New York: 2004. pp. 415–54. [Google Scholar]
- Rennaker RL, Chen CF, Ruyle AM, et al. Spatial and temporal distribution of odorant-evoked activity in the piriform cortex. J Neurosci. 2007;27(7):1534–42. doi: 10.1523/JNEUROSCI.4072-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23(3):499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- Scott JW. A measure of extracellular unit responses to repeated stimulation applied to observations of the time course of olfactory responses. Brain Res. 1977;132(2):247–58. doi: 10.1016/0006-8993(77)90419-x. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Kauer JS, Shepherd GM. Laminar analysis of 2-deoxyglucose uptake in olfactory bulb and olfactory cortex of rabbit and rat. J Neurophysiol. 1977;40(4):800–13. doi: 10.1152/jn.1977.40.4.800. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Ennis M. Functional organization of olfactory system. J Neurobiol. 1996;30(1):123–76. doi: 10.1002/(SICI)1097-4695(199605)30:1<123::AID-NEU11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Sokolov EN, Vinogradova OS. Neuronal mechanisms of the orienting reflex. L. Erlbaum Associates; Hillsdale, N.J., New York: 1975. p. viii.p. 302. [Google Scholar]
- Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73(1):16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Vankov A, Herve-Minvielle A, Sara SJ. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci. 1995;7(6):1180–7. doi: 10.1111/j.1460-9568.1995.tb01108.x. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Wesson DW, Netoff TI, et al. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat Neurosci. 2007;10(5):631–9. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- Weber M, Schnitzler HU, Schmid S. Synaptic plasticity in the acoustic startle pathway: the neuronal basis for short-term habituation? Eur J Neurosci. 2002;16(7):1325–32. doi: 10.1046/j.1460-9568.2002.02194.x. [DOI] [PubMed] [Google Scholar]
- Wellis DP, Scott JW, Harrison TA. Discrimination among odorants by single neurons of the rat olfactory bulb. J Neurophysiol. 1989;61(6):1161–77. doi: 10.1152/jn.1989.61.6.1161. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Habituation of odor responses in the rat anterior piriform cortex. J Neurophysiol. 1998a;79(3):1425–40. doi: 10.1152/jn.1998.79.3.1425. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Rapid, experience-induced enhancement in odorant discrimination by anterior piriform cortex neurons. J Neurophysiol. 2003;90(1):65–72. doi: 10.1152/jn.00133.2003. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Scopolamine enhances generalization between odor representations in rat olfactory cortex. Learn Mem. 2001;8(5):279–85. doi: 10.1101/lm.42601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA. Synaptic correlates of odor habituation in the rat anterior piriform cortex. J Neurophysiol. 1998b;80(2):998–1001. doi: 10.1152/jn.1998.80.2.998. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Blockade of mitral/tufted cell habituation to odors by association with reward: a preliminary note. Brain Res. 1992;594(1):143–5. doi: 10.1016/0006-8993(92)91039-h. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Olfactory associative conditioning in infant rats with brain stimulation as reward: II. Norepinephrine mediates a specific component of the bulb response to reward. Behav Neurosci. 1991;105(6):843–9. doi: 10.1037//0735-7044.105.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadon CA, Wilson DA. The role of metabotropic glutamate receptors and cortical adaptation in habituation of odor-guided behavior. Learn Mem. 2005;12(6):601–5. doi: 10.1101/lm.41405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Fusheng L, Buck LB. Odor maps in the olfactory cortex. Proc Natl Acad Sci U S A. 2005;102:7724–9. doi: 10.1073/pnas.0503027102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zou Z, Horowitz LF, Montmayeur JP, et al. Genetic tracing reveals a stereotyped sensory map in the olfactory cortex. Nature. 2001;414(6860):173–9. doi: 10.1038/35102506. [DOI] [PubMed] [Google Scholar]