Abstract
Objectives
Addiction to opioid narcotics represents a major public health challenge. Animal models of one component of addiction, physical dependence, show this trait to be highly heritable. The analysis of opioid dependence using contemporary in-silico techniques offers an approach to discover novel treatments for dependence and addiction.
Methods
In these experiments, opioid withdrawal behavior in 18 inbred strains of mice was assessed. Mice were treated for 4 days with escalating doses of morphine before the administration of naloxone allowing the quantification of opioid dependence. After haplotypic analysis, experiments were designed to evaluate the top gene candidate as a modulator of physical dependence. Behavioral studies as well as measurements of gene expression on the mRNA and protein levels were completed. Finally, a human model of opioid dependence was used to quantify the effects of the 5-HT3 antagonist ondansetron on signs and symptoms of withdrawal.
Results
The Htr3a gene corresponding to the 5-HT3 receptor emerged as the leading candidate. Pharmacological studies using the selective 5-HT3 antagonist ondansetron supported the link in mice. Morphine strongly regulated the expression of the Htr3a gene in various central nervous system regions including the amygdala, dorsal raphe, and periaqueductal gray nuclei, which have been linked to opioid dependence in previous studies. Using an acute morphine administration model, the role of 5-HT3 in controlling the objective signs of withdrawal in humans was confirmed.
Conclusion
These studies show the power of in-silico genetic mapping, and reveal a novel target for treating an important component of opioid addiction.
Keywords: addiction, hyperalgesia, morphine, ondansetron, opioid, serotonin
Introduction
Addiction to illicit and prescription opioid narcotic drugs (heroin, morphine, codeine, oxycodone and related agents) is a significant public health issue because of the number of people it affects and its cost to society. Each month in the United States, 4.9% of persons aged 12 years or older (>11 million) use prescription pain relievers for nonmedical purposes [1]. Young adults (age 18−25 years) are particularly hard hit by this problem, they have the highest rate of abuse of prescription pain relievers [1]. Opioid addiction has significant adverse consequences for personal health and society [2-4]. As many as 90% of patients in chronic pain management settings receive opioid pain relievers, and the prevalence of drug abuse is 9−41% among these patients [4]. Anxiety, increased pain sensitivity, poor concentration, tachycardia and flu-like symptoms develop during opioid withdrawal, a syndrome reflecting physical dependence on these drugs [5]. The severity of the dependence and resulting withdrawal symptoms is a major contributor to the addictive potential of opioid narcotics. Current strategies for treatment of opioid withdrawal are suboptimal; they rely on the administration of controlled substances (methadone and buprenorphine), or medications with significant hemodynamic side effects (clonidine). As it can lead to new approaches for prevention or treatment of addiction, identification of novel genetic factors affecting dependence on opioids is of great public health significance.
Susceptibility to opioid addiction is heritable in humans [6]. One dimension of addiction is physical dependence, which can be modeled in rodents. The jumping behavior displayed by morphine-dependent mice after administration of naloxone, a potent opioid receptor antagonist, is a commonly used measure of physical dependence. Naloxone-precipitated jumping is a highly heritable trait amongst inbred mouse strains [7], and the interstrain differences are largely independent of differences in the method of drug administration or the duration of treatment [8,9]. Furthermore, naloxone-precipitated withdrawal has been used to quantify opioid dependence in human volunteers [10]. Despite these facts, no specific genes linked to physical dependence have been identified. We, however, recently used a computational genetic mapping method [11,12] to successfully identify several genetic factors underlying the variability in morphine-induced alterations in pain sensitivity (hyperalgesia) and responsiveness to analgesic medications in mice [13-15]. We therefore investigated whether haplotype-based computational genetic mapping could identify genes affecting susceptibility to opioid dependence in mice, and whether a pharmacological agent targeting the human homolog of the computationally identified murine gene could alleviate the signs and symptoms of withdrawal in humans.
Methods
Animals
All animal experiments were conducted using protocols approved by our Institutional Animal Care and Use Committee. Protocols complied with the Guide for the Care and Use of Laboratory Animals available through the National Academy of Sciences. Male mice from the inbred mouse strains (129/SvlmJ, A/HeJ, A/J, AKR/J, B10.D2-H2/oSNJ, BALB/cByJ, BALB/cJ, BUB/BnJ, C3H/HeJ, C57BL/6J, DBA/2J, FVB/NJ, LP/J, LG/J, MRL/MpJ, NZB/BinJ, NZW/LaCJ, SM/J) were obtained from Jackson Labs (Bar Harbor, Maine, USA) at 7−8 weeks of age, and housed for 7−10 days in our animal care facility for acclimation before use in experiments. The mice were kept under pathogen-free conditions and were provided food and water ad libitum with a 12 : 12 h light:dark cycle.
Behavioral assays
Morphine treatment
After baseline nociceptive testing, morphine (Sigma Chemical, St. Louis, Missouri, USA) was administered to mice subcutaneously (s.c.) 10 mg/kg twice per day on day 1, 20 mg/kg on days 2−3, and 40 mg/kg twice per day on day 4 in 50−100 μl volumes of 0.9% NaCl similar to our previous protocols for generating opioid-induced hyperalgesia (OIH), tolerance and dependence [9,13,14].
Precipitated withdrawal
For dependence determinations, mice were assessed 18 h after the final dose of morphine when spontaneous dependence-related hyperalgesia was maximal [16]. Naloxone (Sigma Chemical) 10 mg/kg was injected s.c. in 50 μl of NaCl as described previously by our laboratory and others [8,9]. After naloxone administration, mice were placed in clear plastic cylinders (10 cm in diameter and 40 cm in height), and the number of jumps during the following 15 min was counted. Naloxone-precipitated jumping behavior is the most robust response reflecting physical dependence on opioids observed across strains of inbred mice [17].
In some experiments, the selective 5-HT3 antagonist ondansetron (Sigma Chemical) was administered. For systemic administration, ondansetron was injected s.c. in 100 μl volumes of 0.9% NaCl to some groups of mice. The drug was either given at a dose of 1 mg/kg along with each dose of morphine during the chronic dosing paradigm, or given once over a range of doses 30 min before dependence or nociceptive testing. For intracerebroventricular (i.c.v.) administration, mice were briefly anesthetized with inhaled isoflurane. A 30-gauge half-inch needle was used to pierce the skull and enter the ventricles using an approach described previously [18]. Once inserted, 5 μl of injectate was slowly administered using a microsyringe, and the animals were used within 20 min after the injection.
Opioid dependence-related hyperalgesia
Mechanical allodynia was assayed using nylon von Frey filaments according to the ‘up-down’ algorithm described by Chaplan et al. [19] as previously described [14,20]. In these experiments, mice were placed on wire mesh platforms in clear cylindrical plastic cylinders. After 15 min of acclimation, fibers of sequentially increasing stiffness were applied to the plantar surface of one hind paw, pressed upward to cause a slight bend in the fiber and left in place for 5 seconds. Withdrawal of the hind paw from the fiber was scored as a response. When no response was obtained the next stiffest fiber in the series was applied to the same paw; if a response was obtained a less stiff fiber was applied. Testing proceeded in this manner until four fibers had been applied after the first one causing a withdrawal response allowing the estimation of the mechanical withdrawal threshold [21]. This data-fitting algorithm allowed the use of parametric statistics for analysis. This assay is sufficiently sensitive to detect mechanical thresholds as low as 0.02 g [14].
Conditioned place preference
To assess the dependence liability of morphine and subsequent effects of ondansetron on such dependence, a counter-balanced conditioned place preference (CPP) paradigm was used [22,23]. The CPP experiments were carried out using Place Preference System (MED Associates Inc., St. Albans, Vermont, USA), which consists of three compartments; two outer compartments for active association and a middle neutral compartment. One association compartment is constructed of white opaque plastic walls with a floor made of metal rods, whereas the other compartment is made of black opaque plastic walls with a metal mesh floor. The smaller middle neutral compartment is made of gray opaque plastic walls and floor. Experiments were conducted in a dimly lit room with homogenous lighting (approximately 20 lux). The place preference apparatus is equipped with motion photosensors with data recorded through a computer running MED PC software (MED Associates Inc.). C57BL/6J mice (8−12 weeks old) were used in all experiments, which were carried out during the second half of the light phase between 13 : 00 and 18 : 00 h. On Day 0, the mice were acclimated to the test room and the time spent in each compartment was recorded (preconditioning report). Any mouse that spent more than 75% of the time in either of the association compartments was excluded. On the next day, each mouse was randomized to a control or drug group and assigned to either the white or black association compartments in a counter-balanced manner (n = 10−15 per group). On Days 1, 3, and 5 the drug group received intraperitoneal injections of the drug(s): vehicle (0.9% saline), morphine 5 mg/kg or ondansetron 1 mg/kg plus morphine 5 mg/kg (separate injections). Twenty-five minutes after injection(s), each mouse was placed into its assigned association compartment for 25 min without access to the other compartments. On Days 2, 4, and 6, the Mice received saline injections and were placed in the alternative compartment after 25 min. On Day 7, mice were placed in the middle neutral compartment of the place preference apparatus with full access to the other two compartments and assessed for the length of time spent in each compartment (postconditioning report). The percentage of time spent in each association compartment was calculated relative to the total time spent in both association compartments.
Computational haplotype-based genetic mapping
Haplotype-based computational genetic analysis of the phenotypic data was performed as previously described [11,14,24,25]. This technique has been used recently to identify genes associated with a number of different murine phenotypic traits including opioid narcotic drug responses [9,13-15]. In brief, allelic data from multiple inbred strains were analyzed and a haplotype block map of the mouse genome was constructed. Only a limited number of haplotypes – typically 2, 3, or 4 – are present within a haplotype block. This analysis identifies haplotype blocks in which the haplotypic strain grouping within a block correlates with the distribution of phenotypic data among the inbred strains analyzed. To do this, a P value that assesses the likelihood that genetic variation within each block could underlie the observed distribution of phenotypes among the inbred strains is calculated as described using analysis of variance [11,12]. The phenotypic data were evaluated using the average value for each strain, obtained by assessing eight mice per strain. The haplotype blocks are then ranked based upon the calculated P value. When this computational analysis was performed, the haplotype map had 5694 haplotype blocks generated from 215 155 single nucleotide polymorphisms (SNPs) characterized across 19 inbred strains covering 2609 genes. From this analysis, the candidate haplotype blocks empirically selected for further analysis had the best P values.
Gene expression analysis
Mice were killed at specific time points by CO2 asphyxiation. Whole brains were dissected in block from the skull, and spinal cords were harvested by extrusion. Using low-power binocular magnification, tissues were dissected on a chilled surface. Brain tissue was separated into cortex, cerebellum, and brainstem. Dissected tissue was then quick frozen in liquid nitrogen and stored at −80°C until use. Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Valencia, California, USA) according to the manufacturer's instructions, its purity and concentration were determined spectrophotometrically as described previously for brain and spinal cord samples [26]. cDNA was synthesized from total RNA using random hexamer priming and a First Strand cDNA Synthesis Kit (Invitrogen, Carlsbad, California, USA). Briefly, 1 μg of total RNA was mixed with 4 μl of 10x reverse transcription buffer, 8 μl of 25 mmol/l MgCl2, 4 μl 0.1mol/l dithiothreitol, 1 μl RNasin, 2 μl SSII (50 u/μl), 5 μl hexomers and RNase-free water to 40 μl. Incubation was then carried out at 42°C for 60 min followed by heat inactivation at 70°C. Finally, 1 μl of RNase H was added to each reaction and incubated at 37°C for 20 min to degrade the RNA. For real-time quantitative PCR, reactions were conducted in a volume of 4 μl using the Sybr Green I master kit (PE applied Biosystems, Foster City, California, USA). Briefly, 2 μl of a mixture of 2x Sybr Green and primers were loaded with 2 μl diluted cDNA template in each well. Following this, 8 μl of mineral oil was loaded in each well to prevent loss of solution. Using an ABI prism 7900HT system (Applied Biosystems, Foster City, California, USA), PCR was carried out using the parameters 52°C, 5 min→95°C, 10 min then (95°C, 30s→60°C, 60s) for 40 cycles. Samples were analyzed in triplicate. Melting curves were performed to document single product formation, and agarose electrophoresis confirmed appropriate product size. For internal control 18s RNA was used. The 18s primers were purchased from Ambion (Austin, Texas, USA). The expression of Htr3a in morphine-treated versus control samples was analyzed using the ΔΔCt method [27].
We used our previously described technique for laser capture microdissection (LCM) of central nervous system (CNS) tissue [26]. Mice underwent intracardiac perfusion with 10 cm3 ice-cold 0.9% NaCl after CO2 asphyxiation followed by brain harvest. Brainstems were rapidly dissected, embedded in optimal cutting temperature medium, and stored at −80°C until sectioning. Later, 15-μmol/l sections were cut using a cryostat, placed on slides and rapidly dehydrated through ethanol and xylene. Each slide contained about six sections. The slides were then brought directly to the PixCell LCM instrument (Arcturus, Mountain View, California, USA), with a 15-μm laser spot diameter, power of 40 mW and 500-μs pulse duration to transfer tissue to the CapSure matrix (Arcturus). Each cap was used until 80−90% of the surface contained transferred tissue. Tissue was harvested from the amygdala, dorsal raphe, and periquaductal gray nuclei using standard brain atlases to guide the process. The RNA was extracted using the PicoPure (Arcturus) RNA spin column purification kit according to manufacturer's directions followed by mRNA amplification. It has been shown by our laboratory and others that amplification of laser-captured RNA clarifies rather than distorts differences between samples [28]. This process was performed using the RiboAmp RNA amplification kit (Arcturus) according to the manufacturer's directions for a single round of amplification, and the amount of material was quantified by absorbance spectrophotometry and then subjected to reverse transcription using random hexomer primers as described above. Amplified mRNA was then used for real-time PCR-based quantification as described.
Western blotting for 5-HT3 receptor protein was performed as described [29]. Briefly, tissues from specific CNS regions were homogenized in protease containing buffer and separated on acrylamide gels. After transfer, membranes were probed using rabbit polyclonal anti-5-HT3 antibody (Abcam, Cambridge, Massachusetts, USA) at 1 : 500 dilution. Membranes were then stripped and reprobed for actin abundance thus allowing normalization.
Human volunteers, study design, and procedures
Eight healthy male volunteers underwent an acute precipitated narcotic drug withdrawal protocol as previously described [30], with or without ondansetron pretreatment using a randomized double-blinded placebo-controlled crossover study design on two separate occasions. Test sessions for each individual volunteer were separated by at least 7 days. Female volunteers were excluded from the study because of modulation of opioid response by menstruation cycles [31]. The human experimental protocol was approved by the Institutional Review Board (Stanford University, USA). Each volunteer provided written informed consent before study enrolment, and the study was registered in the clinicaltrials.- gov database (identifier NCT00661674).
All study sessions were conducted by a blinded research assistant (N.D.) and supervised by an unblinded physician (L.C.) who administered the study medication and monitored heart rate, blood pressure, and arterial oxygenation throughout the study. The Subjective Opioid Withdrawal scale (SOWS) and Objective Opioid Withdrawal scale (OOWS) were completed after establishing intravenous (i.v.) access and before administration of study medications, as originally described by Handelsman et al. [5]. All OOWS measurements were obtained by the same blinded research assistant (N.D.). Ondansetron (Bedford Laboratories, Bedford, Ohio, USA) 8 mg or placebo (0.9% saline solution, Hospira Inc., Lake Forest, Illinois, USA) was administered in a double-blinded manner as an i.v. bolus. Thirty minutes later, morphine (Baxter Healthcare Corporation, Deerfield, Illinois, USA) 10 mg/70 kg was administered over 10 min. Patients remained in the lab under observation for 105 min and were offered music or video entertainment and caffeine-free meals or snacks ad lib during this period of time. After 105 min, the patient's vital signs and OOWS and SOWS were reassessed. One hundred and twenty minutes after i.v. morphine administration, 10 mg/70 kg naloxone (Hospira Inc.) was administered to the volunteer as an i.v. bolus. Vital signs, SOWS and OOWS were administered 5 and 15 min after the naloxone administration.
Statistical analysis
All data are displayed as the means ± SEM unless otherwise noted. Animal behavioral data were analyzed using one- or two-way analysis of variance with post-hoc Tukey testing or, for CPP data with t-testing. The outcome measures of interest between the placebo-treated and ondansetron-treated groups were compared using paired t-test and the Wilcoxon's signed-rank test where appropriate. Normal distribution was determined using QQ plots and the Kolmogorov–Smirnov test. Analyses were performed with SAS 9.1 statistical package (Cary, Illinois, USA) with P value of less than 0.05 considered statistically significant.
Results
Genetic variation within Htr3a affects morphine dependence
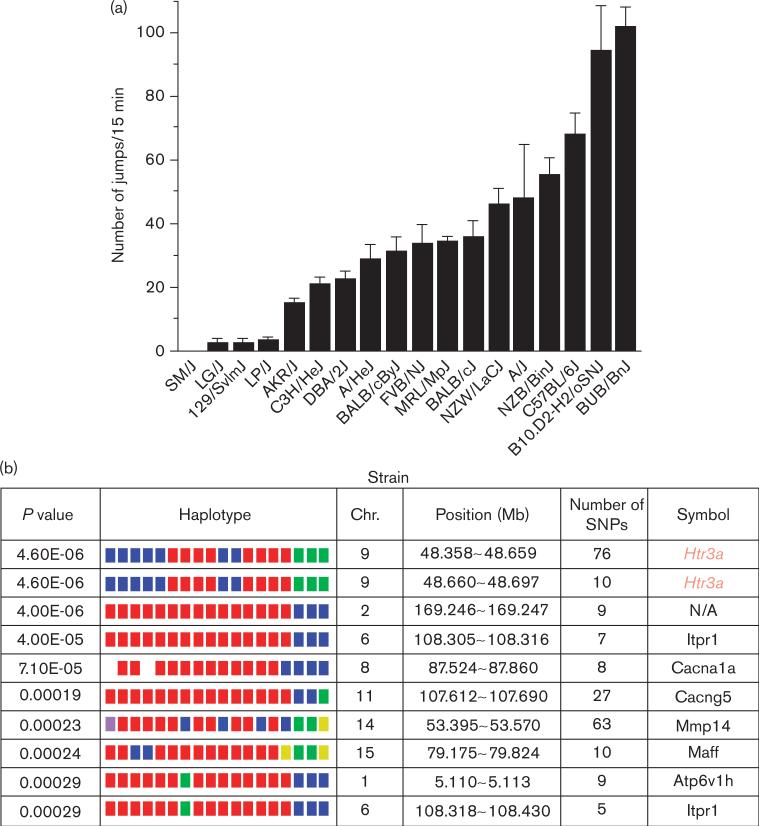
Eighteen inbred strains were made physically dependent on morphine, an archetypical opioid narcotic, by administration over a 4-day period. Then, the jumping behavior precipitated by the administration of an opioid receptor antagonist (naloxone) to the dependent mice was measured. There were very large interstrain differences in the withdrawal-induced jumping behavior; SM/J mice averaged only one jump in the 15-min period following naloxone administration, whereas BUB/BnJ mice jumped over 100 times under the same conditions (Fig. 1). Other investigators [8] evaluated eight of these strains by a similar method, and a comparative Pearson's correlation analysis of the data for the eight common strains indicated that two studies yielded similar results (coefficient of 0.87, P = 0.05).
Fig. 1.
Computational genetic analysis of interstrain differences in physical dependence on morphine. (a) Eighteen strains (eight mice per strain) were treated for 4 days with morphine to establish physical dependence. On the fifth day, the number of jumps made during the 15-min period after naloxone injection was measured as an indicator of the degree of opioid dependence. The data represent the mean number of jumps for each indicated strain ± SEM. (b) The morphine physical dependence data (mean number of jumps for each strain) was analyzed by computational genetic mapping. The 10 most strongly correlated haplotype blocks are shown. For each block, the chromosomal location (chr.), number of single nucleotide polymorphisms (SNPs) within a block and its gene symbol are listed. For each gene, the haplotypes are represented by a colored block, and the blocks are presented in the same rank order as the phenotypic data. Strains sharing the same haplotype have the same colored block. The calculated P value measures the probability that the strain groupings within a block would have the same degree of association with the phenotypic data by random chance. The genetic effect indicates the fraction of the interstrain variance that is potentially attributable to the haplotype. The letter ‘E’ is appropriate as an indicator of ‘exponent’.
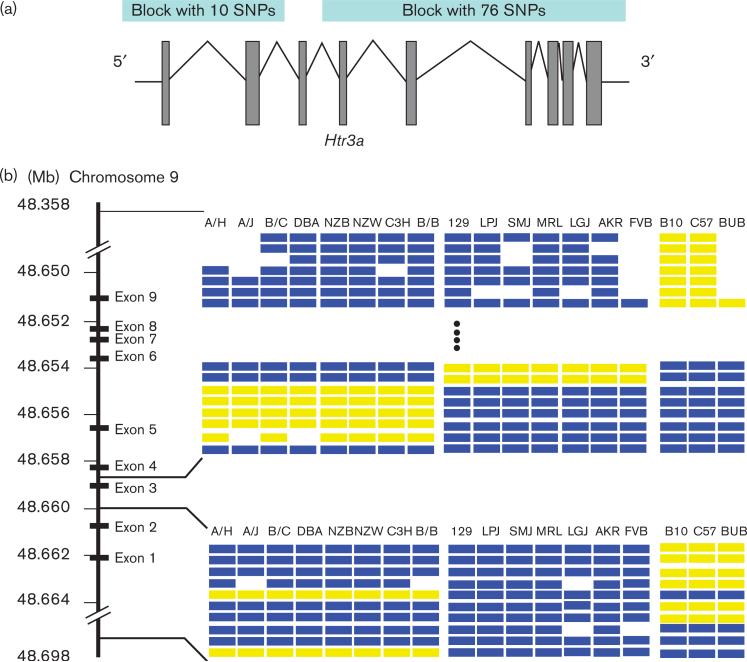
Haplotype-based computational genetic mapping was used to analyze data from this study [11,12]. The distribution of the naloxone-induced withdrawal behavior was compared with the pattern of genetic variation across the 18 strains analyzed. Interestingly, the two most highly correlated haplotype blocks (P value < 5 × 10−6) were in close proximity on chromosome 9 and corresponded to the 5′ and 3′ regions of the Htr3a gene (Figs 1 and 2). There are seven haplotype blocks covering this gene though the dependence-associated blocks account for the majority of SNPs. The remaining small noncorrelated blocks are located between the second and forth exons. None of the SNPs in the associated blocks alter the predicted amino acid sequence of the protein. This gene encodes the 5-HT3a receptor, which has well-established roles in modulating nausea, anxiety, and pain [32]. Although more speculative, it has also been postulated that this receptor could also affect opioid tolerance and dependence [33].
Fig. 2.
Diagram of the Htr3a gene and associated haplotypic blocks. (a) The intron/exon structure of the Htr3a gene along with the relative positions of the two associated haplotypic blocks are displayed. The two haplotype blocks whose pattern of genetic correlation best correlated with the severity of the naloxone-precipitated jumping response are located at the 5′ and 3′ regions of the Htr3a gene. Each of these haplotype blocks has three different haplotypes, and none of the 86 single nucleotide polymorphisms (SNPs) within these two blocks alters the predicted amino acid sequence of the 5-HT3a receptor (http://mouseSNP.roche.com). (b) There are seven haplotype blocks across the Htr3a gene. The two major blocks are the dependence correlated 3′ and 5′ blocks, each with 76 and 10 SNPs, respectively, covering most of Htr3a gene. The other five small blocks (not shown in the figure) are between the 5′ and 3′ blocks (between exons 2 and 4) and contain total 15 SNPs. The 5′ and 3′ blocks, which exhibited the highest correlation with physical dependence on morphine, had the same pattern of genetic variation across the 18 strains of mice used in our studies.
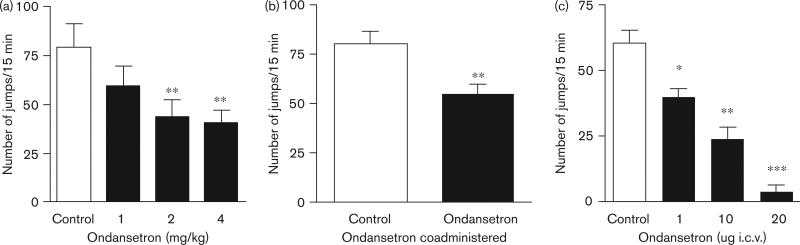
To determine whether 5-HT3 receptor function affects opioid withdrawal, the effect of a selective 5-HT3 receptor antagonist (ondansetron) on withdrawal-associated jumping was assessed. Administration of ondansetron before measurement of naloxone-induced jumping significantly reduced this response in morphine-dependent C57BL/6J mice in a dose-dependent manner (Fig. 3a). In addition, simultaneous administration of ondansetron with each morphine dose during the 4-day protocol for establishing dependence diminished the naloxone-precipitated withdrawal response (Fig. 3b). The latter effect was unlikely to be because of the presence of residual ondansetron at the time of the dependence measurement as a 1-mg/kg dose was not able to effectively inhibit withdrawal when given acutely, and approximately 5 half-lives of the drug had passed in the time between the last dose of ondansetron and the naloxone-precipitated withdrawal procedure. We next investigated whether 5-HT3 receptors expressed within the CNS were capable of altering the severity of withdrawal. In these experiments, ondansetron (or saline) was injected i.c.v. before naloxone was administered to morphine-dependent C57BL/6J mice. The i.c.v. administration of ondansetron profoundly blocked the naloxone-precipitated jumping behavior in a dose-dependent manner (Fig. 3c).
Fig. 3.
Administration of a selective 5-HT3 receptor antagonist (ondansetron) decreases naloxone-precipitated withdrawal behavior. (a) Mice (C57BL/6J) were treated with morphine over a 4-day period, and then were treated with saline or the indicated dose of ondansetron on the fifth day before assessment of naloxone-precipitated jumping behavior. Ondansetron treatment induced a statistically significant and dose-dependent reduction in jumping behavior. (b) Mice (C57BL/6J) were treated with saline or ondansetron (1 mg/kg) at the time of each of the bi-daily morphine injections during the 4-day dependence building protocol. Eighteen hours after the final dose, naloxone-precipitated jumping behavior was assessed. (c) Mice (C57BL/6J) were treated with saline or the indicated dose of ondansetron intracerebroventricular (i.c.v.) before assessment of naloxone-precipitated jumping behavior. Ondansetron treatment induced a statistically significant and dose-dependent reduction in jumping behavior. For all experiments six to eight mice were used per group. Data represent mean values ± SEM. *P<0.05, **P<0.01, ***P<0.001.
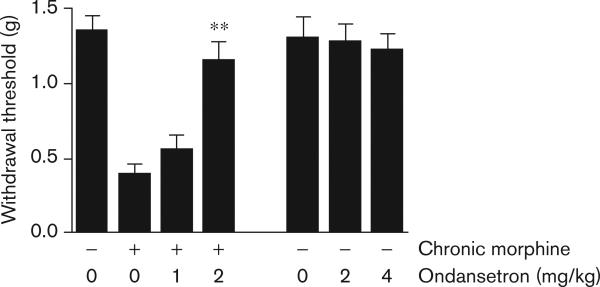
The spontaneous hyperalgesia observed in humans and mice after chronic exposure to opioids, which is referred to as OIH [34], is another measure of physical dependence [35-37]. In our experiments, chronic morphine treatment caused hyperalgesia in C57BL/6J mice. We chose to measure sensitization to a mechanical stimulus as this provides a robust degree of sensitization [14]. Ondansetron administration had no effect on pain sensitivity in control mice that were not treated with morphine, but reversed the hyperalgesia that developed during morphine abstinence (Fig. 4). Taken together, these experiments show that a 5-HT3 receptor antagonist markedly reduced the severity of two distinct measures of physical dependence on morphine.
Fig. 4.
Ondansetron treatment reduces morphine dependence-related hyperalgesia. The effect of the selective 5-HT3 antagonist ondansetron was evaluated in mice that were made dependent on morphine over a 4-day period. As a measure of physical dependence on morphine, mechanical nociceptive sensitization was measured 18 h after administration of the last dose of morphine, the point of maximal nociceptive sensitization. Mice were treated with saline (control) or the indicated dose of ondansetron 30 min before threshold assessment. Ondansetron dose dependently reversed the mechanical nociceptive sensitization caused by morphine withdrawal, but did not alter baseline nociceptive thresholds in control animals, even when doses of ondansetron higher than those effectively reversing hyperalgesia were administered. Six mice per group were used in these experiments, and the data represent mean values ± SEM. **P<0.01.
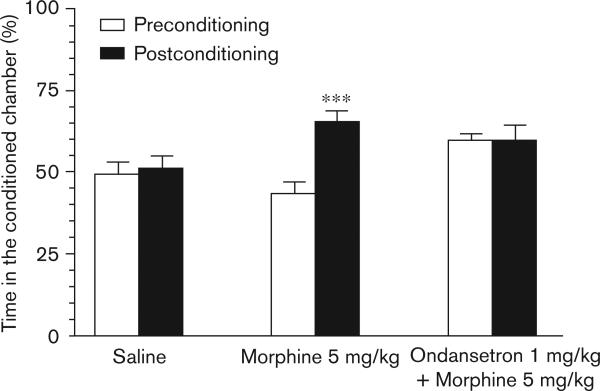
On account of the positive findings with respect to 5-HT3 blockade and reduction in signs of physical dependence, we extended our testing to a paradigm probing the reinforcing properties of opioid administration by using CPP. The group of mice that were administered morphine 5 mg/kg and placed in the conditioning chamber 25 min after injection had a mean difference between pre and postconditioning percentage of time spent in the compartment associated with drug of 21.29 percent (95% confidence interval: 16.82−25.75; P < 0.001). The group of mice that received ondansetron 1 mg/kg in addition to morphine 5 mg/kg displayed a mean difference of 0.40 percent (95% confidence interval: −9.666−10.47; P = 0.93) (Fig. 5). Thus, ondansetron completely blocked the place preference associated with morphine administration.
Fig. 5.
Ondansetron blocks the conditioned place preference associated with morphine administration. In this figure the mean percent time spent in the assigned drug associated chamber in the conditioned place preference assessments before and after conditioning is displayed. Three groups of mice were used: vehicle (n = 15); morphine 5 mg/kg (n = 14); ondansetron 1 mg/kg and morphine 5 mg/kg (n = 10). ***P<0.001.
Chronic morphine exposure reduces Htr3a mRNA expression within key brainstem nuclei
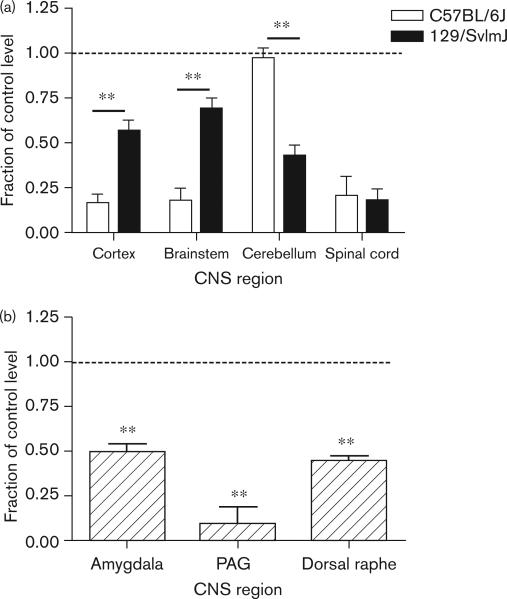
The i.c.v. injection experiments showed that ondansetron modulated naloxone-induced jumping, by acting within the CNS. Furthermore, none of the SNPs within either Htr3a-associated haplotype blocks altered its predicted amino acid sequence. Many SNPs were located within 3′ and 5′ regulatory regions, suggesting that they may alter mRNA transcription or stability. Thus, differences in CNS Htr3a mRNA expression could be responsible for interstrain differences in the severity of naloxone-precipitated withdrawal. To investigate this possibility, we evaluated Htr3a mRNA expression in several brain regions in the presence or absence of chronic morphine treatment. Measurements were made in strains with high (C57BL/6J) or low (129/SvlmJ) morphine dependence, which possess different Htr3a haplotypes. Chronic morphine treatment induced strain and brain-region-specific changes in Htr3a expression (Fig. 6); the C57BL/6J strain exhibited a more drastic decrease in Htr3a mRNA in cortical and brainstem tissue, whereas the 129/SvlmJ strain exhibited a larger decrease in the cerebellum. An approximately equal change in the spinal cord Htr3a expression was observed in both strains.
Fig. 6.
Morphine treatment has a differential and brain region-specific effect on Htr3a mRNA expression. (a) Mice with a high (C57BL/6J) or low propensity (129/SvlmJ) to develop morphine dependence were exposed to saline or morphine for 4 days. On the fifth day the mice were killed, the indicated brain regions were dissected, and level of Htr3a mRNA expression was measured using real-time quantitative PCR. Data represent mean values±SEM from duplicate measurements made on at least six mice per group. (b) Morphine induces changes in Htr3a mRNA expression in selected brainstem nuclei. Mice (C57BL/6J) were treated with saline or morphine as above. The mice were then killed, the indicated brainstem nuclei were isolated by laser capture microdissection, and Htr3a mRNA expression in the brainstem nuclei was analyzed. The Htr3a mRNA levels in morphine-treated mice were normalized relative to those in saline-treated animals. The data are displayed as the mean normalized value for tissues from n = 6 mice per group ± SEM. CNS, central nervous system; PAG, periaqueductal gray. **P<0.01.
We also investigated whether chronic morphine exposure would alter Htr3a mRNA levels within specific brainstem nuclei that were previously associated with physical dependence on opioids. The amygdala [38,39], dorsal raphe [38,40], and periaqueductal gray [41] are brainstem nuclei and are known to modulate signs of opioid dependence and withdrawal. Tissue from these three brainstem regions was harvested from dependence-developing C57BL/6J mice by LCM. Htr3a mRNA expression in all three of these brainstem nuclei was markedly reduced (two-fold to five-fold) after chronic morphine treatment (Fig. 6). Consistent with the computational prediction, therefore, there are strain-specific differences in the effect of chronic morphine exposure on CNS Htr3a mRNA expression, and chronic morphine exposure reduced Htr3a mRNA in three brainstem nuclei associated with opioid dependence.
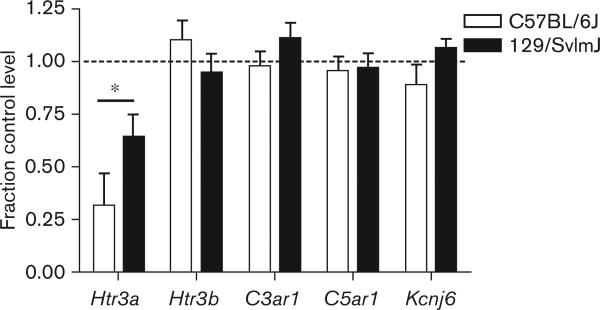
The expression levels of several additional genes were measured as a specificity control in C57BL/6J and 129/SvlmJ mice. There were no interstrain differences after chronic morphine treatment in the brain levels of expression of Kcnj6, C3ar1, C5ar1 or Htr3b mRNA between these two strains (Fig. 7). Importantly, the Htr3b gene is located adjacent to Htr3a on chromosome 9, and encodes a protein that forms heteromultimeric 5-HT3 receptors, with biophysical properties that are distinct from Htr3a homomultimers [42].
Fig. 7.
The regulation of central nervous system expression for several genes by morphine. Brain tissue was harvested after 4 days of saline versus morphine treatment for both high (C57BL/6J) and low (129/SvlmJ) dependence developing strains. In this survey of genes only expression of the Htr3a gene coding for the 5-HT3 serotonin receptor was opioid regulated (P < 0.05). Importantly, expression of the adjacent Htr3b gene coding for an alternate form of the 5-HT3 receptor was not altered by morphine treatment. Five mice per group were used in these experiments, and the displayed data represent mean values ± SEM. *P<0.05 (difference between strains).
Chronic morphine exposure reduces 5-HT3 protein expression
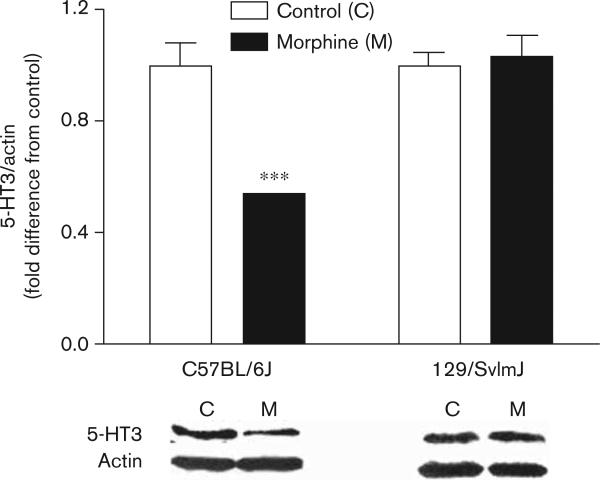
Western blot analysis was conducted to determine whether the changes in 5-HT3 protein followed the observed changes in Htr3a mRNA. This analysis showed that protein preparations from the brainstems of C57BL/6J mice had statistically significant decreases in 5-HT3 content after morphine treatment, whereas no such changes were observed in preparations made from 129/SvlmJ mice (Fig. 8).
Fig. 8.
The regulation of brainstem 5-HT3 protein levels by morphine. Brain tissue was harvested after 4 days of saline versus morphine treatment for both high (C57BL/6J) and low (129/SvlmJ) dependence developing strains. Only in tissue from the C57BL/6J strain showed morphine-induced 5-HT3 changes. Five mice per group were used in these experiments, and the displayed data represent mean values±SEM. Average control mouse expression was set to 1. ***P<0.001 (difference between control and morphine-treated groups).
Pretreatment with ondansetron reduces signs of opioid withdrawal in humans
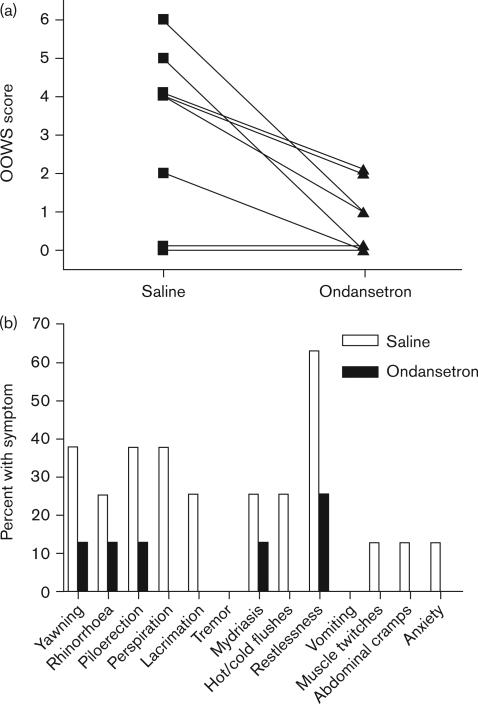
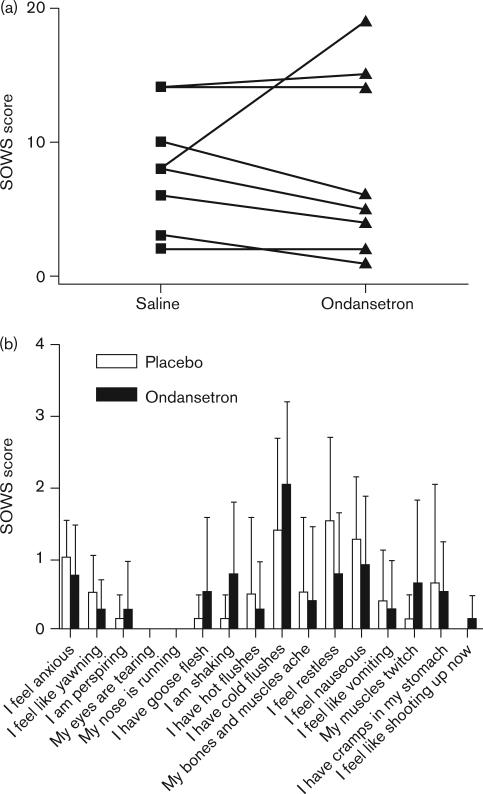
On the basis of the murine results, the efficacy of the 5-HT3 receptor antagonist ondansetron was tested in human volunteers using an experimental protocol for inducing opioid withdrawal [30]. Eight healthy male volunteers were pretreated with placebo or ondansetron (8 mg) before i.v. administration of morphine and subsequent naloxone-precipitated withdrawal. The effect of the pretreatment drug was assessed using well-established objective (OOWS, primary outcome) and subjective (SOWS) measures of opioid withdrawal [5]. Ondansetron pretreatment caused a substantial (76.4%±22.6) and statistically significant (P=0.0313) decrease in mean OOWS score (Fig. 9). Seven of the eight volunteers developed objective signs of opioid withdrawal, and ondansetron pretreatment reduced these signs in all seven affected individuals. The OOWS score is a composite measure of 13 physically observable signs. The volunteers manifested 12 of the 13 measured signs, and ondansetron pretreatment decreased all 12 of these individual signs indicating a broad-spectrum effect (Fig. 9). In contrast, there was only a very small mean decrease in the subjective symptoms (SOWS score) that did not reach statistical significance (4.1%±62.5, P>0.05, Fig. 10).
Fig. 9.
The effect of ondansetron pretreatment on the acute, naloxone-precipitated withdrawal response in human volunteers. Ondansetron 8 mg intravenous (i.v.) or placebo (normal saline) i.v. was administered 30 min before morphine (10 mg/70 kg) i.v. administration in eight volunteers. Naloxone-precipitated (10 mg/70 kg) withdrawal was then induced 120 min after morphine administration. (a) The composite OOWS scores for each volunteer after naloxone-precipitated opioid withdrawal after saline or ondansetron pretreatment are displayed. A P value=0.0313 was calculated based on signed-rank test of the difference in OOWS score after pretreatment with ondansetron and placebo. (b) The Objective Opioid Withdrawal scale (OOWS) subcategory responses to ondansetron pretreatment are displayed. The OOWS scale is composed of thirteen physically observable signs, which are rated as present (1) or absent (0) during the observation period. The percent of the volunteers who experienced each indicated naloxone-precipitated withdrawal sign after ondansetron or placebo pretreatment is shown.
Fig. 10.
The effect of ondansetron pretreatment on the acute, naloxone-precipitated Subjective Opiate Withdrawal scale (SOWS) response in human volunteers. Ondansetron 8 mg intravenous (i.v.) or placebo (normal saline) i.v. was administered 30 min before morphine (10 mg/70 kg) i.v. administration in eight volunteers. Naloxone-precipitated (10 mg/70 kg) opioid withdrawal was then induced 120 min after morphine administration. (a) The composite SOWS scores for each volunteer after naloxone-precipitated opioid withdrawal for volunteers receiving saline or ondansetron pretreatment are displayed. A P value=0.5625 was calculated based on signed-rank test of the difference in SOWS score after pretreatment with ondansetron and placebo. (b) The SOWS subcategory responses to ondansetron pretreatment in humans are displayed. The SOWS score is composed of 16 subjective symptoms rated on a scale of 0−4 (0=not at all, 1=a little, 2=moderately, 3=quite a bit, 4=extremely) based on what volunteers were experiencing at the time of testing. The mean score for volunteers who experienced each indicated naloxone-precipitated withdrawal symptom after ondansetron or placebo pretreatment is shown.
Discussion
Computational haplotype-based genetic analysis indicated that genetic variation within the Htr3a gene could affect interstrain differences in the degree of physical dependence developing after chronic morphine exposure. This computational prediction was confirmed in pharmacological experiments; a 5-HT3 receptor antagonist reduced two distinct manifestations of opioid withdrawal in mice. Similarly, the CPP paradigm commonly used to estimate the addictive properties of drugs showed that blockade of the 5-HT3 receptor completely abolished the reinforcing properties of morphine. Furthermore, Htr3a mRNA expression within three brainstem nuclei associated with opioid dependence was strongly down-regulated after chronic morphine exposure. Alterations in brainstem 5-HT3 protein followed the same pattern. The strain-dependent and brain-region-specific differences in Htr3a mRNA and 5-HT3 protein expression within the CNS provide a potential mechanism that could partially explain how genetic differences within Htr3a affect susceptibility to opioid dependence. On the basis of these results, a widely used antiemetic drug (ondansetron) was tested in human volunteers, and these 5-HT3 receptor antagonists were shown to significantly reduce the objective signs of morphine withdrawal in humans. Therefore, this report provides the first demonstration that a 5-HT3 antagonist, which is a readily available medication, can reduce the severity of narcotic drug withdrawal symptoms in humans.
The molecular biology, genetics, expression pattern, and function of the 5-HT3 receptor have been extensively characterized. 5-HT3 receptors are transmitter-gated, cation selective ion channels that mediate neuronal depolarization and neurotransmitter release in both the CNS and peripheral nervous system. This receptor is targeted by antiemetic drugs during chemotherapy and anesthesia, and is also considered a potential target for treatment of gastrointestinal disorders, anxiety, and pain [32]. Although the data are far less clear and compelling, 5-HT3 receptor antagonists have also been speculatively considered for treatment of addiction [43,44]. 5-HT3 receptors modulate the activity of the mesolimbic system, which are heavily implicated in addiction to multiple substances [45]. Animal studies provide data indicating that 5-HT3 receptors could be involved in addiction and withdrawal. Mice deficient in the Htr3a gene display diminished sensitization to cocaine, a commonly used laboratory model for cocaine addiction [46]. Ondansetron reduced the CPP induced by morphine in rodents, which is a measure of the use-reinforcing properties of a drug [47], and reduced place aversion, a measure of unpleasantness during naloxone-precipitated opioid withdrawal [48]. Ondansetron reduced naloxone-precipitated withdrawal behaviors in mice and rats in some [33,49,50] but not in all studies [48]. However, three analyses [51-53] of place conditioning and related models, found that 5-HT3-blocking agents were ineffective. In one of these studies, ondansetron was not effective at lower doses in rats, and was only variably effective at higher doses when the drug itself had an effect on place conditioning. In addition, this antagonist had no effect on heroin self-administration [54]. In humans, one-time low-dose oral ondansetron was not effective in reducing opioid cravings in addicts exposed to video materials containing drug-related cues [55]. On the basis of the results of the mouse genetic model, we designed a completely different type of clinical study in human volunteers. The results presented here represent the first indication that 5-HT3 antagonists can effectively ameliorate narcotic withdrawal symptoms in humans.
The role of the amygdala in modulating opioid withdrawal is of particular interest; this was one of the nuclei showing prominent opioid modulation of Htr3a expression in our studies. The expression of Htr3a is particularly high in the amygdala [56]. It has long been recognized that this structure is involved in various physiological aspects of the opioid withdrawal syndrome in rodents. For example, microinjection of naloxone and its derivatives into the amygdala precipitates jumping behavior in morphine-dependent rats [57,58]. Additional experiments have identified the periaqueductal gray matter as a key center mediating the signs of opioid withdrawal [57]. This was the area showing the greatest degree of Htr3a modulation of opioids in our experiments. Moreover, the amygdala is one of the structures at the center of the neuroadaptive changes supporting the larger syndrome of addiction to opioids and other substances of abuse [59]. Although not tested for opioids specifically, ondansetron microinjection into the mouse amygdala (and dorsal raphe) blocked an aversive behavior (light/dark exploration) exhibited by rodents during withdrawal from nicotine, cocaine, benzodiazepines, and ethanol [38].
Painful symptoms are also part of the opioid withdrawal syndrome, and increased pain sensitivity can be measured in chronic pain and addict populations when the serum drug concentrations are near their nadirs [35,60]. Rodents display nociceptive sensitization after chronic morphine administration, even without antagonist-precipitated withdrawal. This sensitization is used as a measure of opioid dependence [36,37]. In addition to blocking naloxone-induced withdrawal behaviors, ondansetron effectively reversed the spontaneous hyperalgesia in our morphine-dependent mice. Other investigators have reported that spinal 5-HT3 receptors may modulate OIH as well [61]. These findings emphasize that the 5-HT3 is involved in at least two objectively quantifiable manifestations of opioid dependence.
Although the murine Htr3a gene was the focus of the present studies, many additional studies indicate important differences between naturally occurring human Htr3a and Htr3b variants. For example, five naturally occurring human Htr3a variants cause changes in the cell surface expression of 5-HT3 receptors [62]. More recently four naturally occurring variants of the human Htr3b gene were found to alter the expression and function of heteromeric 5-HT3A/B receptors [63]. Many gene association studies linking variants of these genes with depression, bipolar affective disorder, nausea, and other phenotypes are available. One functional variant of the Htr3b gene has particularly strong linkage to depression in women and nausea after antidepressant administration [64,65].
Opioid addiction is a complex syndrome involving a maladaptive pattern of drug use that is characterized by tolerance, physical dependence (withdrawal during abstinence), continued drug use despite acknowledged harm from the drug, excessive preoccupation with use of the drug, and social isolation. A medication that reduces the physiological manifestations could be an important part of addiction prevention and treatment, but would not address all of the complex neuroplastic events that underlie the overall symptom complex [66,67]. In contrast, the increased use of opioid narcotics for control of chronic pain has focused attention on the physical dependence associated with chronic opioid use. Although a patient receiving chronic opioid medications may not develop addiction, the physical dependence, tolerance and hyperalgesia that can develop may limit the value of chronic narcotic therapy and can complicate ongoing patient management [34,68]. Therefore, treatment with 5-HT3 antagonists may provide part of the solution to significant public health problems associated with opioid use.
Acknowledgements
This study was supported by NIH/NIDA grant DA021332 (J.D.C.) and NIH/NIGMS grant GM071400 (L.F.C.).
Footnotes
Larry F. Chu and De-Yong Liang have contributed equally to this study.
References
- 1.Results from the 2006 National Survey on Drug Use and Health: National Findings Substance Abuse and Mental Health Services Administration. Department of Health and Human Services. 2006 [Google Scholar]
- 2.Birnbaum HG, White AG, Reynolds JL, Greenberg PE, Zhang M, Vallow S, et al. Estimated costs of prescription opioid analgesic abuse in the United States in 2001: a societal perspective. Clin J Pain. 2006;22:667–676. doi: 10.1097/01.ajp.0000210915.80417.cf. [DOI] [PubMed] [Google Scholar]
- 3.Gruber SA, Silveri MM, Yurgelun-Todd DA. Neuropsychological consequences of opiate use. Neuropsychol Rev. 2007;17:299–315. doi: 10.1007/s11065-007-9041-y. [DOI] [PubMed] [Google Scholar]
- 4.Manchikanti L. Prescription drug abuse: what is being done to address this new drug epidemic? Testimony before the Subcommittee on Criminal Justice, Drug Policy and Human Resources. Pain Physician. 2006;9:287–321. [PubMed] [Google Scholar]
- 5.Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- 6.Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- 7.Kest B, Palmese CA, Juni A, Chesler EJ, Mogil JS. Mapping of a quantitative trait locus for morphine withdrawal severity. Mamm Genome. 2004;15:610–617. doi: 10.1007/s00335-004-2367-3. [DOI] [PubMed] [Google Scholar]
- 8.Kest B, Hopkins E, Palmese CA, Adler M, Mogil JS. Genetic variation in morphine analgesic tolerance: a survey of 11 inbred mouse strains. Pharmacol Biochem Behav. 2002;73:821–828. doi: 10.1016/s0091-3057(02)00908-5. [DOI] [PubMed] [Google Scholar]
- 9.Liang DY, Guo T, Liao G, Kingery WS, Peltz G, Clark JD. Chronic pain and genetic background interact and influence opioid analgesia, tolerance, and physical dependence. Pain. 2006;121:232–240. doi: 10.1016/j.pain.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Bickel WK, Stitzer ML, Wazlavek BE, Liebson IA. Naloxone-precipitated withdrawal in humans after acute morphine administration. NIDA Res Monogr. 1986;67:349–354. [PubMed] [Google Scholar]
- 11.Wang J, Liao G, Usuka J, Peltz G. Computational genetics: from mouse to human? Trends Genet. 2005;21:526–532. doi: 10.1016/j.tig.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Liao G, Wang J, Guo J, Allard J, Cheng J, Ng A, et al. In silico genetics: identification of a functional element regulating H2-Ealpha gene expression. Science. 2004;306:690–695. doi: 10.1126/science.1100636. [DOI] [PubMed] [Google Scholar]
- 13.Liang DY, Liao G, Lighthall GK, Peltz G, Clark DJ. Genetic variants of the P-glycoprotein gene Abcb1b modulate opioid-induced hyperalgesia, tolerance and dependence. Pharmacogenet Genomics. 2006;16:825–835. doi: 10.1097/01.fpc.0000236321.94271.f8. [DOI] [PubMed] [Google Scholar]
- 14.Liang DY, Liao G, Wang J, Usuka J, Guo Y, Peltz G, et al. A genetic analysis of opioid-induced hyperalgesia in mice. Anesthesiology. 2006;104:1054–1062. doi: 10.1097/00000542-200605000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith SB, Marker CL, Perry C, Liao G, Sotocinal SG, Austin JS, et al. Quantitative trait locus and computational mapping identifies Kcnj9 (GIRK3) as a candidate gene affecting analgesia from multiple drug classes. Pharmacogenet Genomics. 2008;18:231–241. doi: 10.1097/FPC.0b013e3282f55ab2. [DOI] [PubMed] [Google Scholar]
- 16.Lin RJ, Sternsdorf T, Tini M, Evans RM. Transcriptional regulation in acute promyelocytic leukemia. Oncogene. 2001;20:7204–7215. doi: 10.1038/sj.onc.1204853. [DOI] [PubMed] [Google Scholar]
- 17.Kest B, Palmese CA, Hopkins E, Adler M, Juni A, Mogil JS. Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic morphine physical dependence. Neuroscience. 2002;115:463–469. doi: 10.1016/s0306-4522(02)00458-x. [DOI] [PubMed] [Google Scholar]
- 18.Pedigo NW, Dewey WL, Harris LS. Determination and characterization of the antinociceptive activity of intraventricularly administered acetylcholine in mice. J Pharmacol Exp Ther. 1975;193:845–852. [PubMed] [Google Scholar]
- 19.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Angst MS, Clark JD. A murine model of opioid-induced hyperalgesia. Brain Res Mol Brain Res. 2001;86:56–62. doi: 10.1016/s0169-328x(00)00260-6. [DOI] [PubMed] [Google Scholar]
- 21.Poree LR, Guo TZ, Kingery WS, Maze M. The analgesic potency of dexmedetomidine is enhanced after nerve injury: a possible role for peripheral alpha2-adrenoceptors. Anesth Analg. 1998;87:941–948. doi: 10.1097/00000539-199810000-00037. [DOI] [PubMed] [Google Scholar]
- 22.Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- 23.Reid LD, Marglin SH, Mattie ME, Hubbell CL. Measuring morphine's capacity to establish a place preference. Pharmacol Biochem Behav. 1989;33:765–775. doi: 10.1016/0091-3057(89)90468-1. [DOI] [PubMed] [Google Scholar]
- 24.Guo Y, Weller P, Farrell E, Cheung P, Fitch B, Clark D, et al. In silico pharmacogenetics of warfarin metabolism. Nat Biotechnol. 2006;24:531–536. doi: 10.1038/nbt1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang DY, Shi X, Li X, Li J, Clark JD. The beta2 adrenergic receptor regulates morphine tolerance and physical dependence. Behav Brain Res. 2007;181:118–126. doi: 10.1016/j.bbr.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Lighthall G, Liang DY, Clark JD. Alterations in spinal cord gene expression after hindpaw formalin injection. J Neurosci Res. 2004;78:533–541. doi: 10.1002/jnr.20274. [DOI] [PubMed] [Google Scholar]
- 27.Liang D, Li X, Lighthall G, Clark JD. Heme oxygenase type 2 modulates behavioral and molecular changes during chronic exposure to morphine. Neuroscience. 2003;121:999–1005. doi: 10.1016/s0306-4522(03)00483-4. [DOI] [PubMed] [Google Scholar]
- 28.Feldman AL, Costouros NG, Wang E, Qian M, Marincola FM, Alexander HR, et al. Advantages of mRNA amplification for microarray analysis. Biotechniques. 2002;33:906–914. doi: 10.2144/02334mt04. [DOI] [PubMed] [Google Scholar]
- 29.Liang DY, Clark JD. Modulation of the NO/CO-cGMP signaling cascade during chronic morphine exposure in mice. Neurosci Lett. 2004;365:73–77. doi: 10.1016/j.neulet.2004.04.054. [DOI] [PubMed] [Google Scholar]
- 30.Compton P, Miotto K, Elashoff D. Precipitated opioid withdrawal across acute physical dependence induction methods. Pharmacol Biochem Behav. 2004;77:263–268. doi: 10.1016/j.pbb.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Hoehe M. Influence of the menstrual cycle on neuroendocrine and behavioral responses to an opiate agonist in humans: preliminary results. Psychoneuroendocrinology. 1988;13:339–344. doi: 10.1016/0306-4530(88)90059-5. [DOI] [PubMed] [Google Scholar]
- 32.Costall B, Naylor RJ. 5-HT3 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:27–37. doi: 10.2174/1568007043482624. [DOI] [PubMed] [Google Scholar]
- 33.Roychoudhury M, Kulkarni SK. Prevention of morphine discontinuation phenomenon in mice by ondansetron, a selective 5-HT3 antagonist. Methods Find Exp Clin Pharmacol. 1996;18:677–683. [PubMed] [Google Scholar]
- 34.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 35.Chu LF, Clark DJ, Angst MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: a preliminary prospective study. J Pain. 2006;7:43–48.. doi: 10.1016/j.jpain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Kayan S, Woods LA, Mitchell CL. Morphine-induced hyperalgesia in rats tested on the hot plate. J Pharmacol Exp Ther. 1971;177:509–513. [PubMed] [Google Scholar]
- 37.VonVoigtlander PF, Lewis RA. A withdrawal hyperalgesia test for physical dependence: evaluation of mu and mixed-partial opioid agonists. J Pharmacol Methods. 1983;10:277–282. doi: 10.1016/0160-5402(83)90022-0. [DOI] [PubMed] [Google Scholar]
- 38.Costall B, Jones BJ, Kelly ME, Naylor RJ, Onaivi ES, Tyers MB. Sites of action of ondansetron to inhibit withdrawal from drugs of abuse. Pharmacol Biochem Behav. 1990;36:97–104. doi: 10.1016/0091-3057(90)90132-2. [DOI] [PubMed] [Google Scholar]
- 39.Gulati A, Bhargava HN. Brain and spinal cord 5-HT2 receptors of morphine-tolerant-dependent and -abstinent rats. Eur J Pharmacol. 1989;167:185–192. doi: 10.1016/0014-2999(89)90578-5. [DOI] [PubMed] [Google Scholar]
- 40.Tao R, Ma Z, Auerbach SB. Alteration in regulation of serotonin release in rat dorsal raphe nucleus after prolonged exposure to morphine. J Pharmacol Exp Ther. 1998;286:481–488. [PubMed] [Google Scholar]
- 41.Ingram SL, Vaughan CW, Bagley EE, Connor M, Christie MJ. Enhanced opioid efficacy in opioid dependence is caused by an altered signal transduction pathway. J Neurosci. 1998;18:10269–10276. doi: 10.1523/JNEUROSCI.18-24-10269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brady CA, Stanford IM, Ali I, Lin L, Williams JM, Dubin AE, et al. Pharmacological comparison of human homomeric 5-HT3A receptors versus heteromeric 5-HT3A/3B receptors. Neuropharmacology. 2001;41:282–284. doi: 10.1016/s0028-3908(01)00074-0. [DOI] [PubMed] [Google Scholar]
- 43.Gyermek L. 5-HT3 receptors: pharmacologic and therapeutic aspects. J Clin Pharmacol. 1995;35:845–855. doi: 10.1002/j.1552-4604.1995.tb04129.x. [DOI] [PubMed] [Google Scholar]
- 44.Tricklebank MD. Interactions between dopamine and 5-HT3 receptors suggest new treatments for psychosis and drug addiction. Trends Pharmacol Sci. 1989;10:127–129. doi: 10.1016/0165-6147(89)90157-0. [DOI] [PubMed] [Google Scholar]
- 45.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 46.Hodge CW, Bratt AM, Kelley SP. Deletion of the 5-HT(3A)-receptor subunit blunts the induction of cocaine sensitization. Genes Brain Behav. 2008;7:96–102. doi: 10.1111/j.1601-183X.2007.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carboni E, Acquas E, Leone P, Perezzani L, Di Chiara G. 5-HT3 receptor antagonists block morphine- and nicotine-induced place-preference conditioning. Eur J Pharmacol. 1988;151:159–160. doi: 10.1016/0014-2999(88)90710-8. [DOI] [PubMed] [Google Scholar]
- 48.Higgins GA, Nguyen P, Joharchi N, Sellers EM. Effects of 5-HT3 receptor antagonists on behavioural measures of naloxone-precipitated opioid withdrawal. Psychopharmacology (Berl) 1991;105:322–328. doi: 10.1007/BF02244425. [DOI] [PubMed] [Google Scholar]
- 49.Hui SC, Sevilla EL, Ogle CW. Prevention by the 5-HT3 receptor antagonist, ondansetron, of morphine-dependence and tolerance in the rat. Br J Pharmacol. 1996;118:1044–1050. doi: 10.1111/j.1476-5381.1996.tb15504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinelli A, Trivulzio S, Tomasoni L. Effects of ondansetron administration on opioid withdrawal syndrome observed in rats. Eur J Pharmacol. 1997;340:111–119. doi: 10.1016/s0014-2999(97)01349-6. [DOI] [PubMed] [Google Scholar]
- 51.Acquas E, Carboni E, Leone P, Di Chiara G. 5-HT3 receptors antagonists block morphine- and nicotine- but not amphetamine-induced place-preference conditioning. Pharmacol Res Commun. 1988;20:1113–1114. doi: 10.1016/s0031-6989(88)80752-5. [DOI] [PubMed] [Google Scholar]
- 52.Borg PJ, Taylor DA. Voluntary oral morphine self-administration in rats: effect of haloperidol or ondansetron. Pharmacol Biochem Behav. 1994;47:633–646. doi: 10.1016/0091-3057(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 53.Joharchi N, Sellers EM, Higgins GA. Effect of 5-HT3 receptor antagonists on the discriminative stimulus properties of morphine in rats. Psychopharmacology (Berl) 1993;112:111–115. doi: 10.1007/BF02247370. [DOI] [PubMed] [Google Scholar]
- 54.Higgins GA, Wang Y, Corrigall WA, Sellers EM. Influence of 5-HT3 receptor antagonists and the indirect 5-HT agonist, dexfenfluramine, on heroin self-administration in rats. Psychopharmacology (Berl) 1994;114:611–619. doi: 10.1007/BF02244992. [DOI] [PubMed] [Google Scholar]
- 55.Sell LA, Cowen PJ, Robson PJ. Ondansetron and opiate craving. A novel pharmacological approach to addiction. Br J Psychiatry. 1995;166:511–514. doi: 10.1192/bjp.166.4.511. [DOI] [PubMed] [Google Scholar]
- 56.Tecott LH, Maricq AV, Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc Natl Acad Sci U S A. 1993;90:1430–1434. doi: 10.1073/pnas.90.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maldonado R, Stinus L, Gold LH, Koob GF. Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J Pharmacol Exp Ther. 1992;261:669–677. [PubMed] [Google Scholar]
- 58.Tremblay EC, Charton G. Anatomical correlates of morphine-withdrawal syndrome: differential participation of structures located within the limbic system and striatum. Neurosci Lett. 1981;23:137–142. doi: 10.1016/0304-3940(81)90030-6. [DOI] [PubMed] [Google Scholar]
- 59.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 60.Doverty M, Somogyi AA, White JM, Bochner F, Beare CH, Menelaou A, et al. Methadone maintenance patients are cross-tolerant to the antinociceptive effects of morphine. Pain. 2001;93:155–163. doi: 10.1016/S0304-3959(01)00306-2. [DOI] [PubMed] [Google Scholar]
- 61.Vera-Portocarrero LP, Zhang ET, King T, Ossipov MH, Vanderah TW, Lai J, et al. Spinal NK-1 receptor expressing neurons mediate opioid-induced hyperalgesia and antinociceptive tolerance via activation of descending pathways. Pain. 2007;129:35–45. doi: 10.1016/j.pain.2006.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krzywkowski K, Jensen AA, Connolly CN, Brauner-Osborne H. Naturally occurring variations in the human 5-HT3A gene profoundly impact 5-HT3 receptor function and expression. Pharmacogenet Genomics. 2007;17:255–266. doi: 10.1097/FPC.0b013e3280117269. [DOI] [PubMed] [Google Scholar]
- 63.Walstab J, Hammer C, Bonisch H, Rappold G, Niesler B. Naturally occurring variants in the HTR3B gene significantly alter properties of human heteromeric 5-hydroxytryptamine-3A/B receptors. Pharmacogenet Genomics. 2008;18:793–802. doi: 10.1097/FPC.0b013e3283050117. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka M, Kobayashi D, Murakami Y, Ozaki N, Suzuki T, Iwata N, et al. Genetic polymorphisms in the 5-hydroxytryptamine type 3B receptor gene and paroxetine-induced nausea. Int J Neuropsychopharmacol. 2008;11:261–267. doi: 10.1017/S1461145707007985. [DOI] [PubMed] [Google Scholar]
- 65.Yamada K, Hattori E, Iwayama Y, Ohnishi T, Ohba H, Toyota T, et al. Distinguishable haplotype blocks in the HTR3A and HTR3B region in the Japanese reveal evidence of association of HTR3B with female major depression. Biol Psychiatry. 2006;60:192–201. doi: 10.1016/j.biopsych.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 66.Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101(Suppl 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- 67.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 68.Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 2002;100:213–217. doi: 10.1016/S0304-3959(02)00422-0. [DOI] [PubMed] [Google Scholar]