Summary
Enhancers are often located many tens of kilobases away from the promoter they regulate, sometimes residing closer to the promoter of a neighboring gene. How do they know which gene to activate? We have used homing P[en] constructs to study the enhancer-promoter communication at the engrailed locus. Here we show that engrailed enhancers can act over large distances, even skipping over other transcription units, choosing the engrailed promoter over those of neighboring genes. This specificity is achieved in at least three ways. First, early acting engrailed stripe enhancers exhibit promoter specificity. Second, a proximal promoter-tethering element is required for the action of the imaginal disc enhancer(s). Our data suggest that there are two partially redundant promoter-tethering elements. Third, the long-distance action of engrailed enhancers requires a combination of the engrailed promoter and sequences within or closely linked to the promoter proximal Polycomb-group response elements. These data show that multiple mechanisms ensure proper enhancer-promoter communication at the Drosophila engrailed locus.
Keywords: Promoter specificity, Regulatory DNA, Transcriptional control, Drosophila
INTRODUCTION
Enhancers are often located many tens of kilobases away from the promoter they regulate, sometimes residing closer to the promoter of a neighboring gene. How do they know which gene to activate? Some enhancers preferentially activate one type of core promoter (promoter specificity) (reviewed by Smale, 2001). In other cases, sequences near the promoter, called promoter-tethering elements, are required for transcriptional activation by a particular enhancer. These elements have been found near the Scr, Abd-B, string and white promoters (Calhoun et al., 2002; Akbari et al., 2008; Qian et al., 1992; Lehman et al., 1999). Promoter-tethering elements might bind proteins that specifically interact with proteins bound to distant enhancers, facilitating their ability to activate an associated promoter. In addition, insulator elements, which block the action of an enhancer in a directional way, provide directionality to enhancer action at some genomic locations (reviewed by Gaszner and Felsenfeld, 2006).
The engrailed (en) gene exists in a gene complex with the coregulated invected (inv) gene (Coleman et al., 1987; Gustavson et al., 1996). en and inv are co-expressed in a complex manner throughout development. Early in development, they are required for segmentation, and are expressed in a series of stripes continually throughout embryogenesis. Although the location of En stripes does not change throughout embryonic development, the enhancers and the trans-acting proteins that regulate their expression do change. For example, separate fragments of regulatory DNA act as enhancers for activation by the pair-rule genes, for activation by Wingless signaling and for regulation by the trithorax and Polycomb group genes (DiNardo et al., 1988; Kassis, 1990; DeVido et al., 2008). En and inv are also expressed in the hindgut, clypeolabrum, central nervous system (CNS), peripheral nervous system (PNS), fat body and the posterior compartments of the imaginal discs (DiNardo et al., 1985). The regulatory sequences of engrailed are distributed throughout a 70 kb region (Kuner et al., 1985). Interestingly, the en and inv promoters are separated by ∼54 kb, yet they appear to be regulated by the same enhancers (Goldsborough and Kornberg, 1994; Gustavson et al., 1996), suggesting that en/inv enhancers must be able to act over long distances. What ensures they activate only en/inv and not flanking genes? We have used homing P-transgenes to address this question.
Most P-based constructs insert in the genome in a non-selective manner. However, a few pieces of regulatory DNA have been found to alter the insertional specificity of P-constructs, causing the P-construct to insert near the gene that the regulatory DNA came from. This phenomenon is called P-element homing and was first observed with DNA from the en gene (Hama et al., 1990). DNA fragments from the bithorax complex, linotte gene and, most recently, even skipped, have also been shown to mediate homing (Bender and Hudson, 2000; Taillebourg and Dura, 1999; Fujioka et al., 2009). For en, P-constructs containing a DNA fragment including the engrailed promoter and 2.4 kb of upstream sequences (P[en-lacZ]) cause homing to the en region of the chromosome (Kassis et al., 1992). Insertions are not site specific, but occur over a region of ∼300 kb, including en and inv and flanking genes (Hama et al., 1990; Whiteley and Kassis, 1997; DeVido et al., 2008). P[en-lacZ] has no enhancer activity on its own, but acts as an enhancer detector; that is, its expression is directed by flanking genomic enhancers (Kassis, 1990; Kassis et al., 1992). We have recently shown that P[en-lacZ] can be stimulated by en enhancers even when it is inserted into neighboring genes (DeVido et al., 2008). Furthermore, this long-distance enhancer activity was dependent upon en DNA fragments that also act as Polycomb-group response elements (PREs). PREs are DNA elements that bind and mediate the action of the Polycomb group of transcriptional repressors (for reviews, see Müller and Kassis, 2006; Ringrose and Paro, 2007). We do not know whether the PRE activity can be separated from the enhancer-detection activity of these DNA fragments.
Here we show that, in addition to the PRE fragments, the en promoter is necessary for long-distance interactions with en/inv enhancers. Our data suggest that enhancer-promoter specificity at the en locus is complex, using different mechanisms for different enhancers. First, the early stripe enhancers, which respond to the pair-rule transcriptional activators, exhibit promoter specificity. Second, a promoter-tethering element is required for interactions with the imaginal disc enhancer(s). Finally, both the promoter and the DNA fragment that includes the promoter-proximal PREs are important for the long-range action of en enhancers.
MATERIALS AND METHODS
Construction of plasmids
P[enHSP] constructs were generated by cutting the vector pUZ (Lyko et al., 1997) with restriction enzymes SpeI and NotI (New England Biolabs). Inserts were generated by PCR with the following en primers. P[enHSP1] (-2.407 to -0.395 kb): P1, GGGGCGGCCGCGAATTCCGTTGATATGAT and P2, GCGACTAGTGCATGCTGGAGCTGTCAG; P[enHSP2] (-1.945 to -0.395 kb): P3, GCGGCCGCGAAAGTGTGTAGGGGAAT and P2; P[enHSP3] (-2.407 to -0.579 kb): P1 and GCGACTAGTCCACAGACACTTTTC. These PCR-amplified fragments were also cut with both SpeI and NotI and ligated into pUZ. The resulting clones were sequenced in and around the cloned PCR fragment to ensure sequence fidelity.
Transgenic lines
P[enHSP] transgenic lines were generated by injections into w1118 embryos by Genetic Services (Sudbury, MA, USA). Chromosomal insertion sites were localized by inverse PCR. Genomic DNA was extracted from transgenic flies and separately digested with MboI, RsaI and HpaII. T4 ligase was added to the digested DNA to create circular DNA fragments that were amplified using P-end primers GATTAACCCTTAGCATGTCCGTGG and AAGCATACGTTAAGTGGATGT or ATACTTCGGTAAGCTTCGGCTATCGAC and GCAGCCTTGG TA AAACTCCC. PCR fragments were then sequenced (Macrogen, USA) and the resulting sequence was entered into a BLAST search to localize insertion sites.
Whole-mount in situ hybridization of embryos
Digoxigenin (DIG)-labeled RNA antisense probe synthesis and whole mount in situ hybridization was carried out as previously described (Langlais et al., 2004), with the following modifications. Selected gene fragments ranging in size from 300 bp to 1000 bp were cloned for use as templates for probe synthesis. Probes were not fragmented with carbonate buffer. Probe template primer sequences are available upon request.
Immunohistochemistry and X-gal staining
Preparation of embryos for immunocytochemistry was performed as in DiNardo et al. (DiNardo et al., 1985). Primary antibodies used were: rabbit polyclonal anti-β-galactosidase (1:15,000, Cappel), for immunoperoxidase staining; mouse monoclonal anti-β-galactosidase (1:500, Promega), for fluorescence staining; and rabbit polyclonal anti-En (1:200). The antibody used was raised against the N-terminus of En and does not recognize Inv (DiNardo et al., 1985). An ABC Elite Kit (Vector Labs) was used for immunoperoxidase staining. For immunofluorescence, anti-rabbit Alexa Fluor 555 (1:600) and anti-mouse Alexa Fluor 488 (1:400) (Invitrogen) were used. Embryos were mounted in Vectashield (Vector Labs).
For imaginal disc staining, third instar larvae were cut in half and inverted, fixed with either 1% glutaraldehyde or 3.2% formaldehyde (Polysciences, EM grade) in PBS for 15 minutes at room temperature, then washed three times with PBS and once with X-gal buffer (1 mM MgCl2, 150 mM NaCl, 10 mM NaPO4, pH 7.2) and stained at 37°C in X-gal buffer containing 1.6% X-gal, 5 mM K3Fe(CN)6 and 5 mM K4Fe(CN)6 for the times noted in the figure legends.
Generation and characterization of new en mutants
enJ86 and enΔ139 were generated by excision of P[en3R]-134B as described in DeVido et al. (DeVido et al., 2008). enΔ139 is a deletion of 139 bp extending from -412 to -273 bp upstream of the en transcription start site. In enJ86, a 339 bp deletion, extending from -412 to -73 bp, is present. In addition, enJ86 has retained 1.8 kb of the P-construct, including the 3′ P end and 1.58 kb of rosy sequences.
RESULTS
Expression patterns of genes flanking en and inv
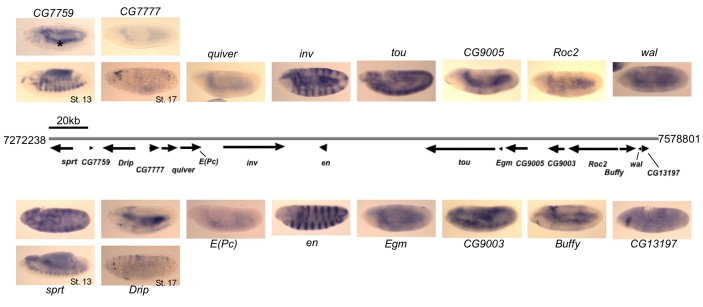
en and inv are contained within a 100 kb region. These two genes are co-expressed and share regulatory DNA (Goldsborough and Kornberg, 1994; Gustavson et al., 1996). We have previously shown that an enhancer-trapping P-construct with en sequences from -2.407 kb to +188 bp fused to lacZ that is inserted in tou is expressed like en (DeVido et al., 2008). Therefore we asked whether any other genes in the vicinity of en/inv have similar expression patterns. We performed in situ hybridization on embryos with RNA probes for transcripts flanking en and inv (Fig. 1). We compared our results with those in the literature and also to results in the Berkeley Drosophila Genome Project gene expression database (http://www.fruitfly.org/cgi-bin/ex/insitu.pl). No other genes in the region are expressed in patterns similar to en/inv. Fig. 1 shows in situ hybridization on stage 11 embryos. An additional embryo is shown for those probes that gave a pattern at another embryonic stage. E(Pc), the gene adjacent to the 5′ end of inv, is ubiquitously expressed at a low level. tou, the gene immediately adjacent to the 5′ end of en, is expressed ubiquitously, with somewhat stronger expression in the nervous system. CG13197 is expressed in the progenitors of the salivary glands. sprt and CG7759 are both expressed in the somatic mesoderm. Drip and CG7777 are both expressed at late stages in a few cells. We also examined the expression of E(Pc), tou, sprt and CG7759 in imaginal discs to determine whether these genes had the same expression pattern as en/inv in larval tissues. Whereas en and inv are expressed in the posterior compartment of the imaginal discs, E(Pc), tou, sprt and CG7759 all had light ubiquitous expression in this tissue (data not shown).
Fig. 1.
Expression patterns of en, inv and flanking genes in Drosophila embryos. Gray line indicates genomic DNA, with the coordinates listed at both ends (genome version R5.1, FlyBase). Arrows below indicate the transcription units and the direction of transcription. In situ hybridization was performed on embryos using DIG-labeled RNA antisense probes and alkaline phosphatase staining. All embryos are stage 11 unless otherwise noted. For some genes, an additional image of a later stage embryo is shown to demonstrate a specific staining pattern if no pattern was observed at stage 11. Several genes show weak ubiquitous expression or do not show specific staining at any stage in embryos. Non-specific yolk sac staining is observed in many embryos, indicated with an asterisk in CG7759. All embryos are oriented anterior to the left and dorsal up.
engrailed enhancers can act over long distances
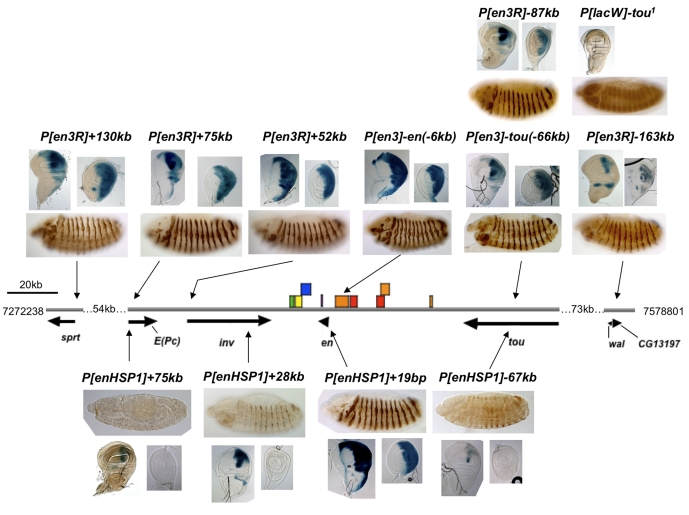
We examined the expression pattern of 17 lines with P[en-lacZ] insertions into en/inv and flanking genes (Table 1). Two constructs were used; each had the same en-lacZ transgene with different markers {either the mini-white (P[en3]) or rosy marker gene (P[en3R]) (Fig. 2)}. Both of these constructs act as enhancer traps; that is, their expression patterns are totally dependent on flanking genomic enhancers (Kassis et al., 1992; DeVido et al., 2008). In the region of en and inv, P[en3] and P[en3R] insertions occurred over a 293 kb region, from 130 kb downstream to 163 kb upstream of the major transcription start site of en (Table 1; Fig. 3). We examined the β-gal expression patterns of these lines in both embryos and imaginal discs (Fig. 3, top). As expected, insertions of P[en3R] or P[en3] in the immediate vicinity of en and inv perfectly mimic en/inv expression (Fig. 3). Likewise, P[en3R] inserted near the E(Pc) promoter, ∼23 kb upstream of inv, was expressed just like en/inv, even though E(Pc) itself is ubiquitously expressed (Stankunas et al., 1998) (Fig. 1). More remarkable is that an insertion of P[en3R] just upstream of the sprt gene, located five transcription units and ∼78 kb upstream of inv, is also expressed like en/inv in both embryos and imaginal discs (Fig. 3, top). For transgenes inserted upstream of en, we have already reported that an insertion of P[en3] into tou (P[en3]-tou) is expressed in late embryonic stripes and in imaginal discs in a manner similar to en (Fig. 3) (DeVido et al., 2008). We assayed the expression of five additional P[en3R] transgenes in tou and found they all exhibited similar patterns (a representative line is shown in Fig. 3). Finally, P[en3R] inserted into the wal gene, 110 kb upstream of en, shows some aspects of en expression (Fig. 3). By contrast, β-gal expression from a P[lacW] insertion into tou is not expressed like en. P[lacW] is an enhancer-trapping transgene in which lacZ is transcribed from the P-element promoter (Bier et al., 1989). Since P[lacW] is not expressed like en, these data suggest that the en fragment is necessary for the en-like expression pattern of the P[en3] and P[en3R] lacZ transgenes inserted in tou.
Table 1.
Insertion site of transgenes studied
| Construct | Line | Gene* | Location† |
|---|---|---|---|
| P[en3R] | +130kb | sprt | 7,285,340 |
| P[enHSP1] | +75kb | E(Pc) | 7,340,264 |
| P[en3R] | +75kb | E(Pc) | 7,340,298 |
| P[en3R] | +75kb-2 | E(Pc) | 7,340,671 |
| P[en3R] | +52kb | inv | 7,363,188 |
| P[en3R] | +52kb-2 | inv | 7,363,197 |
| P[en3R] | +52kb-3 | inv | 7,363,221 |
| enGAL4 | (+42kb) | inv | 7,373,480 |
| P[enHSP1] | +28kb | inv | 7,387,233 |
| P[enHSP1] | +28kb+19bp | inv | 7,387,340 |
| P[enHSP1] | +19bp | en | 7,415,369 |
| P[enHSP1] | +28kb+19bp | en | 7,415,369 |
| P[en3R] | −204bp | en | 7,415,592 |
| P[en3R] | −210bp | en | 7,415,598 |
| P[enHSP3] | −250bp | en | 7,415,638 |
| P[enHSP2] | −250bp | en | 7,415,638 |
| P[en3R] | −412bp | en | 7,415,800 |
| P[enHSP2] | −1kb | en | 7,416,417 |
| P[en3] | en (−6kb) | en | 7,421,628 |
| P[en3Δboth] | en (−6kb) | en | 7,421,628 |
| P[en3] | tou (−66kb) | tou | 7,481,428 |
| P[enHSP1] | −67kb | tou | 7,482,731 |
| P[enHSP1] | −67kb-2 | tou | 7,482,786 |
| P[lacW] | tou1 (−73kb) | tou | 7,488,577 |
| P[enHSP1] | −74kb | tou | 7,489,278 |
| P[en3R] | −77kb | tou | 7,492,176 |
| P[en3R] | −77kb-1 | tou | 7,492,223 |
| P[en3R] | −77kb-2 | tou | 7,492,778 |
| P[en3R] | −86kb | tou | 7,501,843 |
| P[en3R] | −87kb | tou | 7,502,613 |
| P[en3R] | −163kb | wal | 7,578,342 |
Line names indicate the number of kilobases or bases upstream (negative numbers) or downstream (positive numbers) of the major en transcription start site (at 7,415,388) (Soeller et al., 1988). A few lines were named previously. For these, the number of kilobases from the en transcription start site is shown in parentheses.
Gene inserted in or next to.
Nucleotide insertion site (genome version R5.1, FlyBase).
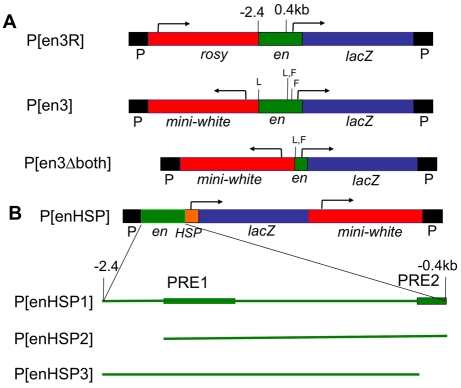
Fig. 2.
P-constructs used in these experiments. (A) Constructs with the en promoter. P[en3R] {P[en1] from Kassis et al. (Kassis et al., 1992)} and P[en3] (Devido et al., 2008) contain en sequences (green) extending from -2.407 kb through to the start site of en transcription (indicated by the arrow) and 188 bp of the en untranslated leader fused to Adh-lacZ (blue). P[en3Δboth] contains en sequences from -395 bp through to +188 bp (Devido et al., 2008). L, loxP site; F, FRT site; P, P-element ends. (B) Constructs with the heat shock promoter (HSP). The pUZ vector is shown along with the insertion site for the en fragments (green) (Lyko et al., 1997). The locations of two Polycomb response elements (PREs) are shown as green boxes (DeVido et al., 2008; Kassis, 1994). The extent of the en fragment in each construct is shown by the green line.
Fig. 3.
Expression patterns of enhancer traps in the en/inv genomic region. Gray line indicates genomic DNA. Arrows under the genomic DNA line show the position and direction of transcription units. Vertical arrows indicate the position of insertion of the transgene indicated. The exact insertion site of each P-construct is listed in Table 1. Transgenes with the en promoter are above the genomic DNA line, those with the heat shock promoter are below the genomic DNA line. The position of all known en stripe enhancers and other enhancers important for these experiments are shown as colored boxes: green, clypeolabrum; yellow, posterior spiracles; blue, hindgut; orange, stripe in every segment; red, even or odd stripes (pair-rule enhancers); purple, en intron enhancer: hindgut, posterior spiracles, fat body, even stripes and every segment (Kassis, 1990) (our unpublished data). The imaginal disc enhancer is upstream of en but the exact location is not known (unpublished data). β-Galactosidase protein is visualized by immunoperoxidase staining in embryos and X-gal staining in imaginal discs. A lateral view of a germ-band-shortened embryo (stage 13), ∼10 hours after egg laying, with anterior to the right and dorsal up, is shown for each transgene. A wing (left) and leg (right) imaginal disc (posterior compartment to the right) is also shown for each transgene (except P[lacW]-tou). Discs were fixed in formaldehyde and stained for varying amounts of time to equalize staining intensity. Lines with P[en3] or P[en3R] insertions in en or inv stained within 30 minutes; E(Pc),1 hour; tou, 2.5 hours; sprt and wal, 5 hours. Note that the expression in the posterior compartment of the P[en3R]-131 line is variegated, suggesting that activation by en enhancers in some cells is stronger than in others. This variegation is not evident when the discs are stained for a longer period of time. For imaginal disc staining, lines P[enHSP1]+28kb, +19bp and -67kb were fixed with formaldehyde and stained for varying times: P[enHSP1]+19bp, 30 minutes; P[enHSP1]+28kb, 2.5 hours; P[enHSP1]-67kb, 48 hours. Line P[enHSP1]+75kb was fixed with glutaraldehyde and stained for >24 hours. We were not able to see β-gal activity in this line when it was fixed with formaldehyde.
The location of known en enhancers for stripes and other patterns relevant to this paper are shown in Fig. 3 (Kassis, 1990; Hama et al., 1990; DeVido et al., 2008) (our unpublished data). Seventy kilobases of DNA between the 3′ end of inv and the 3′ end of tou have been assayed for enhancer activity (our unpublished data). The location of the imaginal disc en enhancer(s) is currently unknown, although genetic evidence suggests that it is located upstream of en (our unpublished data). Although the DNA of the inv gene has not been tested for enhancer activity, genetic data suggest that inv and en share regulatory DNA and data show that a breakpoint mutation in en, enCX1 (which breaks either in the first exon or the first intron), separates stripe enhancers from those that cause expression in the clypeolabrum and hindgut (Gustavson et al., 1996). This suggests that there are no stripe enhancers near or in the inv gene. Thus, our data suggest a remarkable ability of en enhancers to act over long distances, activating the en and inv promoters and ignoring other promoters in the region.
We also examined whether P[en3R] altered the expression of sprt, E(Pc) and tou when inserted near those genes in lines P[en3R]+130kb, +75kb and -87kb, respectively. It was possible that insertion of P[en3R] would cause flanking genes to be expressed in en-like patterns. However, we saw no en-like expression of these genes in either embryos or imaginal discs (data not shown), again suggesting the specificity of en enhancers for the en promoter fragment.
There is a possibility that additional, distantly located `shadow enhancers' exist for en and that these transgenes are being activated by en shadow enhancers, not the enhancers shown in Fig. 3 (Hong et al., 2008). However, even if en shadow enhancers do exist, they would have to have specificity for the en and inv promoters, as no other gene in the region is expressed like en and inv (Fig. 1). We favor a model, supported below, in which the en enhancers are able to activate expression over large distances, activating their own promoter over other promoters in the vicinity.
In addition to en/inv-like patterns discussed above, there are some local effects that influence the expression of P[en3R] transgenes. For instance, in P[en3R]+130kb embryos, β-gal is detected in the presumptive mesoderm in addition to the expression shown, reflecting the expression pattern of sprt (Tomancak et al., 2002) and CG7759. Also, some of the inserts in tou are ubiquitously expressed at a low level in discs. This is evident only when the staining time is extended. Finally, not all en enhancers are able to act on all P[en3] or P[en3R] en-lacZ transgenes at all locations. For example, the enhancers responsible for early en stripes are not able to activate P[en3] and P[en3R] when they are inserted in tou (DeVido et al., 2008) (data not shown). The reason for this is not clear, as the insertions in E(Pc), sprt and wal are expressed in early stripes (not shown). In addition, enhancers for expression in the hindgut and clypeolabrum, which are located downstream of the en promoter (Gustavson et al., 1996) (Fig. 3), more efficiently activate P[en3R] transgenes inserted in inv or E(Pc) than those inserted in en and upstream of it (data not shown). These enhancers might not be able to act over as great a distance as other enhancers, or the endogenous en promoter might `trap' them, attenuating their ability to activate transcription of upstream transgenes (see below).
The en promoter is necessary for long-distance enhancer action
We have previously shown that en DNA from -2.4 to -0.4 kb is necessary for the long-distance action of en enhancers on P[en3] inserted in tou (DeVido et al., 2008). We used the construct P[enHSP1] (Fig. 2) to ask whether the en promoter fragment is also necessary for long-distance enhancer action. P[enHSP1] contains en sequences -2.4 to -0.4 kb cloned upstream of GAL4-binding sites and the heat shock promoter (hsp) driving lacZ and a mini-white reporter gene (the pUZ vector) (Lyko et al., 1997). We examined the expression pattern of β-gal from P[enHSP1] inserted in E(Pc), inv, en and tou (Fig. 3). Like the expression of β-gal from P[en3] and P[en3R], β-gal was expressed in an en-like manner in embryos and imaginal discs when P[enHSP1] is inserted into the en promoter (Fig. 3, bottom) (although not in early embryos, see below). This was in contrast to an insertion in tou, in which β-gal expression from P[enHSP1] was very weak, with en-like stripes only evident in the abdominal segments late in embryogenesis. Furthermore, β-gal expression in the imaginal discs was only evident in a small portion of the posterior compartment in the wing disc; no other discs showed any β-gal expression (Fig. 3, bottom). P[enHSP1] inserted in E(Pc) gave no expression in embryos, and only very light expression in a portion of the posterior compartment in just the wing disc (Fig. 3). Finally, most surprising to us, even when P[enHSP1] was inserted into the inv gene, β-gal was expressed in only a subset of en-expressing cells. Stripes were activated late and remained weak throughout development. Furthermore, β-gal expression in imaginal discs was confined to a small portion of the wing disc. We were concerned that the location in the inv intron might disrupt the expression of P[enHSP1]-inv. However, we do not believe that this is the case because enGAL4 (which contains en sequences -2.4 kb through to the en promoter fused to GAL4; FlyBase) is also in the inv intron and is expressed like en (Table 1).
Our results show that, although the heat shock promoter is able to act with many en enhancers when P[enHSP1] is inserted near the en promoter, it is not able to interact with those same enhancers when it is located at a distance. We suggest that when P[enHSP1] is inserted in the immediate vicinity of the en promoter it is able to form the correct chromatin confirmation, or interact with nearby regulatory fragments that allow it to respond to many en enhancers. However, when the P[enHSP1] is not in the immediate vicinity of the en promoter, it cannot access most en enhancers.
The 2 kb fragment of DNA that mediates homing of P[enHSP1] contains two subfragments that mediate mini-white silencing and also act as PREs in embryos (Fig. 2) (DeVido et al., 2008; Kassis, 1994). Chromatin immunoprecipitation experiments have shown that this entire 2 kb fragment is bound by Polycomb group proteins in both embryos and SL-2 cells (Nègre et al., 2006; Strutt and Paro, 1997). As stated above, this fragment has also been shown to be required for the ability of en enhancers to stimulate expression from P[en3] inserted in tou (DeVido et al., 2008). Taken together, our data clearly show that both the 2 kb promoter-proximal PRE-containing fragment and the en promoter are required for the long-distance action of en enhancers.
Early stripe enhancers exhibit promoter specificity
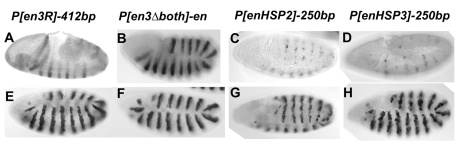
We made two other versions of P[enHSP] that contain smaller fragments of en DNA that also homed to the en region, P[enHSP2] and P[enHSP3] (Fig. 2). In all, we obtained six lines with P-constructs inserted within 1 kb upstream of the transcription start site of en (Table 1). This allowed us to compare the expression pattern of transgenes with the en promoter to those without it, which were inserted at nearly the same location. We also examined the expression from P[en3Δboth]-en that is inserted 6 kb upstream of en. Our data show that the early stripe enhancers, those activated by the pair-rule genes, act preferentially on the en promoter (Fig. 4; data not shown). Although lacZ expression from P[en3R]-210bp, -412bp and P[en3Δboth]-en(-6kb) occurred in early embryos, expression from P[enHSP] constructs did not occur until approximately late stage 10, when it turns on in a stochastic manner, only visible in just a few cells per stripe (Fig. 4C,D). The stripes fill in a little later in development, after which the embryos are almost indistinguishable from P-en-promoter-containing lines (Fig. 4E-H). These data suggest that the pair-rule proteins, which directly activate early en expression, exhibit promoter specificity.
Fig. 4.
Promoter specificity of early en enhancers. (A-H) Immunoperoxidase staining showing β-gal protein in embryos. The name of the line is shown on top, with embryos at two stages shown beneath. (A,B) Stage 8; (C,D) stage 10; (E-H) stage 11. All embryos are oriented anterior to the left and dorsal up. Images are lateral views, except C and G, which are dorsal-lateral views.
Sequences within PRE2 act as a promoter-tethering element
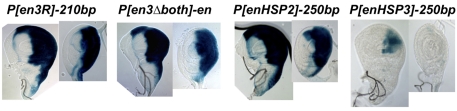
We also examined the expression of lacZ in imaginal discs from the lines with P-constructs inserted just upstream of en. Expression of β-gal in imaginal discs from P[en3R]-210bp and -412bp, P[enHSP1]+19bp, and P[enHSP2]-250bp and -1kb was nearly identical (Figs 3 and 5; data not shown). By contrast, to our surprise, there was almost no β-gal expression in imaginal discs from P[enHSP3]-250bp (Fig. 5), the construct that lacks PRE2. In this case, β-gal is evident only in a small portion of the posterior compartment in the wing disc. Note that P[enHSP2]-250bp and P[enHSP3]-250bp are inserted in the same orientation and at the same exact position, 250 bp upstream of the major transcription start site of en (Table 1; Fig. 6B). We suggest that sequences within PRE2 are required for the imaginal disc enhancer(s) to activate the heat shock promoter, even when it is inserted very near the en transcription start site. Thus, PRE2 fulfills the role of a promoter-tethering element for the heat shock promoter, a proximal-promoter fragment of DNA necessary for enhancer activity.
Fig. 5.
A promoter-tethering element is contained within PRE2. The name of the line is on top, with a wing disc (left) and a leg disc (right) shown underneath each line. Discs were fixed with formaldehyde and stained for varying times: P[en3R]-210bp and P[en3Δboth]-en(-6kb), 45 minutes; P[enHSP2]-250bp, 2 hours; P[enHSP]-250bp, 29 hours.
Fig. 6.
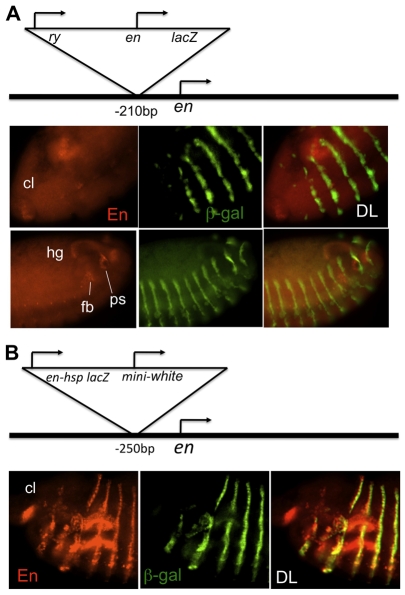
The en promoter, but not the heat shock promoter, captures en stripe enhancers. (A) Top: diagram of P-construct inserted in line P[en3R]-210bp. Thick line indicates genomic DNA. Arrows indicate the position and direction of transcription of the genomic en gene and the genes within P[en3R]. Bottom: double-label (DL) for En (red) and β-gal (green) shows that P[en3R]-210bp interferes with the transcription of the endogenous en gene in stripes. β-gal is expressed in stripes. By contrast, En, but not β-gal, is expressed in the clypeolabrum (cl), hindgut (hg), PNS, fat body (fb) and posterior spiracles (ps). Top row of images: ventral-lateral view of the anterior part of a stage 11 embryo is shown, with dorsal up and anterior to the left. Bottom row of images: lateral view of the posterior part of a stage 13 embryo, with dorsal up and anterior to the left. (B) Top: the position and orientation of the P[enHSP]-250bp transgene. Images show that En and β-gal are both expressed in stripes in these embryos. As in A, En, but not β-gal, is expressed in the clypeolabrum (cl). Lateral view of the anterior part of a stage 13 embryo, with anterior to the left and dorsal up, is shown. The En antibody also stains the salivary glands non-specifically, and these are evident as horizontal line-like structures in the En and DL images.
We previously found that PRE2 could mediate weak long-range activation of lacZ from P[en3] when inserted in tou, but that a fragment containing PRE1 could substitute for PRE2. Here, PRE1 is still present in P[enHSP3], and thus PRE1 cannot substitute for the activity of PRE2 in this assay. This leads to the conclusion that the promoter-tethering activity is distinct from the previously described activity of PRE2. Finally, we note that there appears to be another, perhaps redundant, promoter-tethering element present in the en promoter fragment from -395 to +188 bp. P[en3Δboth] contains sequences from -395 to +188 bp but no PRE1 or PRE2 sequences (Fig. 2). β-gal expression from P[en3Δboth]-en(-6kb) in both embryos and imaginal discs mimics en, showing that PRE2 is not required for β-gal expression when the en promoter is present. These data suggest that promoter-tethering sequences are present in both PRE2 and within the en promoter fragment we used, perhaps fulfilling redundant roles (see below).
The en promoter `traps' en enhancers, whereas the heat shock promoter does not
In embryos homozygous for P[en3R]-210bp or P[en3R]-412bp, En is not expressed in stripes, but can be detected in the PNS, hindgut (hg), clypeolabrum (cl), fat body (fb) and posterior spiracles (ps) (Fig. 6A). β-gal, however, is detected in stripes but not in the hg, cl, PNS, fb, or ps (Fig. 6A). The enhancers for hg, cl, fb and ps are located downstream of the en transcription unit, whereas most of the stripe enhancers are located upstream (except those located in the intron) (Fig. 1) (the location of the PNS enhancer is not known). We suggest that the stripe enhancers, first encountering the en promoter within P[en3R], drive lacZ expression, but are precluded from activating the endogenous en promoter. However, the hg, cl, fb and ps enhancers first encounter the endogenous en promoter and do not activate the expression of lacZ. By contrast, when P[enHSP2] or P[enHSP3] is inserted just upstream of en, En is expressed in stripes in addition to its expression in the hg, cl, fb, PNS and ps (Fig. 6B; data not shown). Thus, en stripe enhancers are not `trapped' by the heat shock promoter. β-gal expression from P[enHSP2] and P[enHSP3] is not seen in the hg, PNS, cl, fb or ps (Fig. 6B; data not shown). The differential effects of P[en3R] and P[enHSP2] and P[enHSP3] on en stripe expression is reflected in the phenotype of these lines. P[en3R]-210bp and -412bp lines die as embryos with severe segmentation defects (data not shown). By contrast, P[enHSP2]-250bp homozygous embryos do not have segmentation defects; however, this construct is a lethal en allele, with lines dying at third instar larval or pupal stages. We suggest that P[enHSP2]-250bp disrupts en expression later in development, perhaps in the imaginal discs. Finally, P[enHSP3]-250bp flies survive over en null mutants, with only a slight wing defect, reflecting minimal interference with en expression.
Note that the promoter competition we observe does not occur to the same extent for P[en3] and P[en3R] insertions further upstream of en. There is no disruption of en expression from insertions into flanking genes; that is, P[en3] or P[en3R] inserted in inv, tou, E(Pc), sprt or wal do not cause en mutations. Even when P[en3] is inserted ∼6 kb upstream of en, it does not disrupt embryonic en stripes, and survives weakly over en null alleles, with only minor defects in the posterior compartment in the wing. Our data suggest that the en promoter present in a P[en] construct only strongly competes with the endogenous en promoter when it is inserted into the immediate vicinity of the endogenous en promoter.
More evidence for two partially redundant promoter-tethering elements at en
We previously reported that a 530 bp deletion in the en locus, including the 181 bp PRE and sequences upstream, caused a slight defect in the posterior region of the wings, suggesting a decrease in the activity of the wing imaginal disc enhancer (DeVido et al., 2008). Using the same method, we generated another line, enJ86B, that contains a 339 bp deletion, removing sequences from -412 to -73 bp upstream of the en transcription start site (see Materials and methods for details). Embryonic en expression is normal in this line (data not shown). enJ86B/enIM99 (enIM99 is a lethal en allele) flies survive, but have wings with slight defects in the posterior compartment, suggesting a disruption in en expression in the wing imaginal disc. A smaller deletion, from -412 to -273 bp upstream of the en transcription start site (line enΔ139), causes no discernible phenotypic defect. We suggest that there is a promoter-tethering element in the 200 bp fragment extending from -273 to -73 bp upstream of the en transcription start site that facilitates expression in the imaginal discs.
DISCUSSION
Comparison of the inv and en promoters
en and inv exist in a gene complex, encode related proteins with redundant functions and share regulatory DNA (Goldsborough and Kornberg, 1994; Gustavson et al., 1996). Thus, en enhancers must be able to activate both the en and inv promoters, which are separated by 54 kb. What properties do these two promoters share? First, en and inv are both TATA-less promoters. Both en and inv have the initiator promoter element (Inr) and the downstream promoter element (DPE). The inv promoter has a perfect match to the Inr consensus sequence for Drosophila (at nucleotide 7,363,212), and a near match to the DPE 28 bp after the initiating adenine. The en promoter has a near match to the Inr consensus and a perfect match to the DPE located 30 bp downstream of the third nucleotide of the Inr sequence (Burke and Kadonaga, 1996). Second, both promoters have binding sites for the transcription factor GAGA, which are located just upstream of the transcription start site. GAGA-binding sites greatly increase the activity of the en promoter (Orihara et al., 1999). Third, both have Polycomb response elements (PREs) located very close to the promoters (Nègre et al., 2006; DeVido et al., 2008) (Cunningham, Brown and Kassis, unpublished). Finally, we compared the DNA sequences from 600 bp upstream to 400 bp downstream of the inv promoter with the 588 bp en promoter fragment we used and found a few stretches of sequence identity. The longest was a 14/15 bp match located from -57 to -42 upstream of the en transcription start site and from -40 to -25 bp upstream of the inv transcription start site. The functional significance of this is unknown.
We examined the sequences around the presumed transcription start sites for all of the transcripts shown in Fig. 1 (we assumed that the transcripts for these genes in FlyBase and GenBank are full length). Strikingly, aside from sprt, none of these genes had sequences that matched the TATA, Inr or DPE consensus sequences [consensus sequences from Juven-Gershon et al. (Juven-Gershon et al., 2008)]. Like en and inv, the sprt gene has Inr and DPE elements. Unlike en and inv, no PREs were found at the sprt gene [as judged by the binding of PcG proteins (Oktaba et al., 2008; Schwartz et al., 2006)]. We suggest that sprt is not activated by en enhancers because it lacks the PREs (or associated sequences) that are necessary for the long-distance action of the en enhancers.
The minimal heat shock promoter present in P[enHSP] contains sequences -44 to +204 bp of the HSP70 promoter. It contains the TATA element but does not have any of the GAGA sites that are located further upstream. We previously found that a slightly different version of this promoter (from -73 to +70 bp) would not function in a reporter construct with the en stripe enhancer present in the intron, although it was able to function with enhancers that drive expression in the hindgut, fat body and posterior spiracles (Kassis, 1990). Those data, combined with our current results, clearly show that different en enhancers have different promoter requirements. The ability of different types of core promoters to recognize different enhancers has been reported by many other investigators and may be a common mechanism to achieve enhancer specificity in Drosophila (Merli et al., 1996; Ohtsuki et al., 1998; Butler and Kadonaga, 2001; Butler and Kadonaga, 2002; Juven-Gershon et al., 2008).
Promoter-enhancer communication at engrailed
At least three distinct processes mediate promoter specificity at en. First, the early stripe enhancers, those activated by the pair-rule transcription activators, require the en promoter; they are not able to stimulate the heat shock promoter. We suggest this could be due to the type of core promoter present at en, or to sequences very near the transcription start site. The en allele enJ86 contains a deletion from -412 to -73 bp upstream of the en transcription start site and shows no disruption of early en expression. Thus, sequences within 73 bp of the transcription start site are sufficient for interaction with early stripe enhancers. Caudal, an early acting developmental transcription factor, was recently found to specifically activate DPE-containing promoters (Juven-Gershon et al., 2008). It would be interesting to test whether pair-rule proteins also exhibit promoter specificity.
Second, we propose there are two promoter-tethering elements that mediate interactions with the imaginal disc enhancers. One of them is located in the 181 bp element, PRE2, and another is located between -273 and -73 bp. en joins a growing list of Drosophila genes that have promoter-tethering elements, including the homeotic genes Scr and Abd-B, as well as the white and string genes (Calhoun et al., 2002; Akbari et al., 2008; Qian et al., 1992; Lehman et al., 1999). It is likely that many other genes with extensive regulatory regions have promoter-tethering elements.
Finally, we have previously shown that the 2 kb PRE fragment, from -2.4 to -0.4 kb, is required for distantly located transgenes to interact with the en enhancers (DeVido et al., 2008). Here we show that the en promoter is also required for long-range enhancer-promoter interactions. We suggest that both the promoter and the PRE fragment are necessary to form the correct chromatin structure to allow interactions with distant en enhancers. In conclusion, our data suggest that multiple mechanisms exist to ensure that en enhancers activate the correct promoters.
We thank Renato Paro for the pUZ vector; Pat O'Farrell and Nikita Yakubovich for the anti-En antibody; Miki Fujioka, Jim Jaynes, Karl Pfeifer, Mark Mortin and the Kassis lab members for comments on this manuscript. Diane Mucci was supported by a grant from the Cystic Fibrosis Foundation and by internal funds from the Food and Drug Administration. This research was supported by the Intramural Research Program of the NIH, NICHD. Deposited in PMC for release after 12 months.
References
- Akbari, O. S., Bae, E., Johnsen, H., Villaluz, A., Wong, D. and Drewell, R. A. (2008). A novel promoter-tethering element regulates enhancer-driven gene expression at the bithorax complex in the Drosophila embryo. Development 135, 123-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender, W. and Hudson, A. (2000). P element homing to the Drosophila bithorax complex. Development 127, 3981-3992. [DOI] [PubMed] [Google Scholar]
- Bier, E., Vaessin, H., Shepherd, S., Lee, K., McCall, K., Barbel, S., Ackerman, L., Carretto, R., Uemura, T., Grell, E. et al. (1989). Searching for pattern and mutation in the Drosophila genome with a P-lacZ vector. Genes Dev. 3, 1273-1287. [DOI] [PubMed] [Google Scholar]
- Burke, T. W. and Kadonaga, J. T. (1996). Drosophila TFIID binds to a conserved downstream basal promoter element that is present in many TATA-box-deficient promoters. Genes Dev. 10, 711-724. [DOI] [PubMed] [Google Scholar]
- Butler, J. E. F. and Kadonaga, J. T. (2001). Enhancer-promoter specificity mediated by DPE or TATA core promoter motifs. Genes Dev. 15, 2515-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, J. E. F. and Kadonaga, J. T. (2002). The RNA polymerase II core promoter: a key component in the regulation of gene expression. Genes Dev. 16, 2583-2592. [DOI] [PubMed] [Google Scholar]
- Calhoun, V. C., Stathopoulos, A. and Levine, M. (2002). Promoter-proximal tethering elements regulate enhancer-promoter specificity in the Drosophila Antennapedia complex. Proc. Natl. Acad. Sci. USA 99, 9243-9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, K. G., Poole, S. J., Weir, M. P., Soeller, W. C. and Kornberg, T. (1987). The invected gene of Drosophila: sequence analysis and expression studies reveal a close kinship to the engrailed gene. Genes Dev. 1, 19-28. [DOI] [PubMed] [Google Scholar]
- DeVido, S. K., Kwon, D., Brown, J. L. and Kassis, J. A. (2008). The role of Polycomb-group response elements in regulation of engrailed transcription in Drosophila. Development 135, 669-676. [DOI] [PubMed] [Google Scholar]
- DiNardo, S., Kuner, J. M., Theis, J. and O'Farrell, P. H. (1985). Development of embryonic pattern in D. melanogaster as revealed by accumulation of the nuclear engrailed protein. Cell 43, 59-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo, S., Sher, E., Heemskerk-Jongens, J., Kassis, J. A. and O'Farrell, P. H. (1988). Two-tiered regulation of spatially patterned engrailed gene expression during Drosophila embryogenesis. Nature 332, 604-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka, M., Wu, X. and Jaynes, J. B. (2009). A chromatin insulator mediates transgene homing and very long-range enhancer-promoter communication. Development 136, 3077-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaszner, M. and Felsenfeld, G. (2006). Insulators: exploiting transcriptional and epigenetic mechanisms. Nat. Rev. Genet. 7, 703-713. [DOI] [PubMed] [Google Scholar]
- Goldsborough, A. S. and Kornberg, T. B. (1994). Allele-specific quantification of Drosophila engrailed and invected transcripts. Proc. Natl. Acad. Sci. USA 91, 12696-12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson, E., Goldsborough, A. S., Ali, Z. and Kornberg, T. B. (1996). The Drosophila engrailed and invected genes: Partners in regulation, expression, and function. Genetics 142, 893-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama, C., Ali, Z. and Kornberg, T. B. (1990). Region-specific recombination and expression are directed by portions of the Drosophila engrailed promoter. Genes Dev. 4, 1079-1093. [DOI] [PubMed] [Google Scholar]
- Hong, J. W., Hendrix, D. A. and Levine, M. S. (2008). Shadow enhancers as a source of evolutionary novelty. Science 321, 1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juven-Gershon, T., Hsu, J. Y. and Kadonaga, J. T. (2008). Caudal, a key developmental regulator, is a DPE-specific transcriptional factor. Genes Dev. 22, 2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juven-Gershon, T., Hsu, J. Y., Theisen, J. W. M. and Kadonaga, J. T. (2008). The RNA polymerase II core promoter: the gateway to transcription. Curr. Opin. Cell Biol. 20, 253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis, J. A. (1990). Spatial and temporal control elements of the Drosophila engrailed gene. Genes Dev. 4, 433-443. [DOI] [PubMed] [Google Scholar]
- Kassis, J. A. (1994). Unusual properties of regulatory DNA from the Drosophila engrailed gene: Three “pairing-sensitive” sites within a 1.6-kb region. Genetics 136, 1025-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis, J. A., VanSickle, E. P. and Sensabaugh, S. M. (1991). A fragment of engrailed regulatory DNA can mediate transvection of the white gene in Drosophila. Genetics 128, 751-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis, J. A., Noll, E., VanSickle, E. P., Odenwald, W. F. and Perrimon, N. (1992). Altering the insertional specificity of a Drosophila transposable element. Proc. Natl. Acad. Sci. USA 89, 1919-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner, J. M., Nakanishi, M., Ali, Z., Drees, B., Gustavson, E., Theis, J., Kauvar, L., Kornberg, T. and O'Farrell, P. H. (1985). Molecular cloning of engrailed: a gene involved in the development of pattern in Drosophila melanogaster. Cell 42, 309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutach, A. K. and Kadonaga, J. T. (2000). The downstream promoter element DPE appears to be as widely used as the TATA box in Drosophila core promoters. Mol. Cell. Biol. 20, 4754-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlais, K. K., Stewart, J. A. and Morton, D. B. (2004). Preliminary characterization of two atypical sGC in the central and peripheral nervous system of Drosophila melanogaster. J. Exp. Biol. 207, 2323-2338. [DOI] [PubMed] [Google Scholar]
- Lehman, D. A., Patterson, B., Johnston, L. A., Balzer, T., Britton, J. S., Saint, R. and Edgar, B. A. (1999). Cis-regulatory elements of the mitotic regulator, string/Cdc25. Development 126, 1793-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyko, F., Brenton, J. D., Surani, M. A. and Paro, R. (1997). An imprinting element from the mouse H19 locus functions as a silencer in Drosophila. Nat. Genet. 16, 171-173. [DOI] [PubMed] [Google Scholar]
- Merli, C., Bergstrom, D. E., Cygan, J. A. and Blackman, R. K. (1996). Promoter specificity mediates the independent regulation of neighboring genes. Genes Dev. 10, 1260-1270. [DOI] [PubMed] [Google Scholar]
- Müller, J. and Kassis, J. A. (2006). Polycomb response elements and targeting of Polycomb group proteins in Drosophila. Curr. Opin. Genet. Dev. 16, 476-484. [DOI] [PubMed] [Google Scholar]
- Nègre, N., Hennetin, J., Sun, L. V., Lavrov, S., Bellis, M., White, K. P. and Cavalli, G. (2006). Chromosomal distribution of PcG proteins during Drosophila development. PLoS Biol. 4, e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki, S., Levine, M. and Cai, H. N. (1998). Different core promoters possess distinct regulatory activities in the Drosophila embryo. Genes Dev. 12, 547-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktaba, K., Gutierrez, L., Gagneur, J., Girardot, C., Sengupta, A. K., Furlong, E. E. M. and Müller, J. (2008). Dynamic regulation of Polycomb group protein complexes controls pattern formation and the cell cycle in Drosophila. Dev. Cell 15, 1-13. [DOI] [PubMed] [Google Scholar]
- Orihara, M., Hosono, C., Kojima, T. and Saigo, K. (1999). Identification of engrailed promoter elements essential for interactions with a stripe enhancer in Drosophila embryos. Genes Cells 4, 205-218. [DOI] [PubMed] [Google Scholar]
- Qian, S., Varjavand, B. and Pirrotta, V. (1992). Molecular analysis of the zeste-white interaction reveals a promoter-proximal element essential for distant enhancer-promoter communication. Genetics 131, 79-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringrose, L. and Paro, R. (2007). Polycomb/Trithorax response elements and epigenetic memory of cell identity. Development 134, 223-232. [DOI] [PubMed] [Google Scholar]
- Schwartz, Y. B., Kahn, T. G., Nix, D. A., Li, X. Y., Bourgon, R., Biggin, M. and Pirrotta, V. (2006). Genome-wide analysis of Polycomb targets in Drosophila melanogaster. Nat. Genet. 38, 700-705. [DOI] [PubMed] [Google Scholar]
- Smale, S. T. (2001). Core promoters: active contributors to combinatorial gene regulation. Genes Dev. 15, 2503-2508. [DOI] [PubMed] [Google Scholar]
- Soeller, W. C., Poole, S. J. and Kornberg, T. (1988). In vitro transcription of the Drosophila engrailed gene. Genes Dev. 2, 68-81. [DOI] [PubMed] [Google Scholar]
- Stankunas, K., Berger, J., Ruse, C., Sinclair, D. A. R., Randazzo, F. and Brock, H. W. (1998). The Enhancer of Polycomb gene of Drosophila encodes a chromatin protein conserved in yeast and mammals. Development 125, 4055-4066. [DOI] [PubMed] [Google Scholar]
- Strutt, H. and Paro, R. (1997). The Polycomb group protein complex of Drosophila melanogaster has different compositions at different target genes. Mol. Cell. Biol. 17, 6773-6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taillebourg, E. and Dura, J. M. (1999). A novel mechanism for P element homing in Drosophila. Proc. Natl. Acad. Sci USA 96, 6856-6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomancak, P., Beaton, A., Weiszmann, R., Kwan, E., Shu, S., Lewis, S. E., Richards, S., Ashburner, M., Hartenstein, V., Celniker, S. E. et al. (2002). Systematic determination of patterns of gene expression during Drosophila embryogenesis. Genome Biol. 3, 0088.1-0088.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley, M. and Kassis, J. A. (1997). Rescue of Drosophila engrailed mutants with a highly divergent mosquito engrailed cDNA using a homing, enhancer-trapping transposon. Development 124, 1531-1541. [DOI] [PubMed] [Google Scholar]