Summary
Cilia have been implicated in Hedgehog (Hh) and Wnt signaling in mouse but not in Drosophila. To determine whether the role of cilia is conserved in zebrafish, we generated maternal-zygotic (MZ) oval (ovl; ift88) mutants that lack all cilia. MZovl mutants display normal canonical and non-canonical Wnt signaling but show defects in Hh signaling. As in mouse, zebrafish cilia are required to mediate the activities of Hh, Ptc, Smo and PKA. However, in contrast to mouse Ift88 mutants, which show a dramatic reduction in Hh signaling, zebrafish MZovl mutants display dampened, but expanded, Hh pathway activity. This activity is largely due to gli1, the expression of which is fully dependent on Hh signaling in mouse but not in zebrafish. These results reveal a conserved requirement for cilia in transducing the activity of upstream regulators of Hh signaling but distinct phenotypic effects due to differential regulation and differing roles of transcriptional mediators.
Keywords: Cilia, Hedgehog signaling, Wnt signaling, Gli, Spinal cord, Somite, Zebrafish
INTRODUCTION
The cilium, a microtubule-based organelle, is present on the surface of most vertebrate cells. The ciliary axoneme extends from a basal body, a modified centrosome, and is assembled and maintained by intraflagellar transport (IFT) proteins (Pedersen and Rosenbaum, 2008). Although cilia were first described over 100 years ago, their functions during animal development and physiology are only beginning to be unraveled (Eggenschwiler and Anderson, 2007; Sharma et al., 2008). Defective cilia have been implicated in numerous diseases termed ciliopathies, including situs inversus, sterility, respiratory dysfunction, polycystic kidney disease, defects in vision, smell and hearing, mental retardation and obesity (Sharma et al., 2008).
Genetic and in vitro studies have suggested that cilia are also involved in Hedgehog (Hh) (Haycraft et al., 2005; Huangfu and Anderson, 2005; Huangfu et al., 2003; Liu et al., 2005; May et al., 2005; Ocbina and Anderson, 2008), Wnt (Corbit et al., 2008; Ferrante et al., 2009; Gerdes et al., 2007; Ross et al., 2005), and platelet-derived growth factor (PDGF) signaling (Schneider et al., 2005). For example, it has been suggested that mutations that disrupt ciliogenesis, including Kif3a, Ift88 and Ofd1, lead to increased activity of the canonical Wnt pathway (Corbit et al., 2008). Moreover, disruption of basal body proteins implicated in Bardet-Biedl syndrome (BBS) results in phenotypes reminiscent of mutants with impaired non-canonical Wnt/planar cell polarity (PCP) signaling (Gerdes et al., 2007; Ross et al., 2005). Conversely, canonical Wnt signaling appears upregulated upon basal body disruption (Gerdes et al., 2007). However, cilia mutants do not fully phenocopy loss- or gain-of-function mutants that affect canonical or non-canonical Wnt signaling (Eggenschwiler and Anderson, 2007). Hence, it remains controversial whether cilia affect Wnt signaling.
A role for cilia in mammalian Hh signaling is well established (Eggenschwiler and Anderson, 2007). Hh signaling initiates when Shh ligand binds to its receptor, Patched (Ptc), releasing the repression of Smoothened (Smo) and leading to activation of the Gli family of transcription factors. Shh controls the balance between Gli activator and repressor activity: in the presence of Shh, Gli activator activity predominates, whereas in the absence of Shh, Gli proteins are proteolytically processed to generate C-terminally truncated Gli repressors, which function to repress Hh pathway activity. In the neural tube, Shh ligand forms a ventral-to-dorsal gradient and specifies cell fates in a concentration-dependent manner. Loss of Hh signaling in mice leads to loss of all ventral cell types in the neural tube. Similarly, mouse cilia mutants (that lack cilia, e.g. Ift88 mutants) do not specify ventral neural cell types such as the floor plate, V3 interneurons and motoneurons (Huangfu and Anderson, 2005; Huangfu et al., 2003; Liu et al., 2005). Analysis of expression of Ptc1 (Ptch1 - Mouse Genome informatics), a direct readout of Hh signaling, indicates that Hh pathway activity is strongly diminished in these mutants (Huangfu et al., 2003). In the absence of cilia, Shh, Ptc, Smo and dnPKA (dominant-negative PKA) can no longer modulate Hh pathway activity (Haycraft et al., 2005; Huangfu et al., 2003; Liu et al., 2005; Ocbina and Anderson, 2008). For instance, whereas loss of Ptc1 results in activation of the Hh pathway and a ventralized neural tube, Ptc1; Ift88 double mutants show little Hh pathway activity, resembling Ift88 single mutants (Huangfu et al., 2003).
Genetic and biochemical analyses suggest that cilia are required for the function or generation of both Gli activator and repressor activity (Haycraft et al., 2005; Huangfu and Anderson, 2005; Huangfu et al., 2003; Liu et al., 2005; May et al., 2005). In mouse, Gli2 functions as the main activator and Gli3 mainly functions as a transcriptional repressor (Bai et al., 2002; Litingtung and Chiang, 2000). By contrast, Gli1 expression is induced by Hh signaling and only plays a minor role during development by amplifying Hh signaling (Bai et al., 2002; Park et al., 2000). In the absence of cilia, Gli2 activator is no longer functional and Gli3 processing is compromised (Eggenschwiler and Anderson, 2007), whereas the activity of ectopically expressed Gli1 and of the truncated form of Gli3 repressor (Gli3R) is not affected, at least in tissue culture (Haycraft et al., 2005). Together, these results suggest that cilia mediate Hh signaling by modulating Gli activity. The requirement of cilia in Hh signaling is further supported by the demonstration that several key components of the Hh signaling pathway are enriched in cilia, including Ptc, Smo, all three Gli proteins and Su(fu), a negative regulator of the pathway (Corbit et al., 2005; Haycraft et al., 2005; Rohatgi et al., 2007). Since the initial identification of IFT mutants, additional mouse mutants that compromise cilia integrity have been isolated (Caspary et al., 2007; Cortellino et al., 2009; Ferrante et al., 2006; Hoover et al., 2008; Rana et al., 2004; Tran et al., 2008; Vierkotten et al., 2007), all of which link abnormal cilia with abnormal Hh signaling.
Intriguingly, cilia are not required for Drosophila Hh and Wnt signal transduction (Han et al., 2003), even though most components of these signaling pathways are conserved from fruit flies to humans (Huangfu and Anderson, 2006). It remains controversial whether cilia are required for Hh and Wnt signaling in non-mammalian vertebrates such as zebrafish. For example, although morpholino knockdown of some basal body and ciliary proteins in zebrafish results in defects in non-canonical Wnt signaling accompanied by an increased canonical Wnt response (Aanstad et al., 2009; Ferrante et al., 2009; Gerdes et al., 2007), none of the zebrafish mutations in ciliary components leads to clear Wnt signaling defects (Sun et al., 2004; Tsujikawa and Malicki, 2004). Similarly, although morpholino knockdown of some ciliary proteins, including ift80 and qilin (cluap1 - Zebrafish Information Network), leads to a reduction of Hh signaling (Aanstad et al., 2009; Beales et al., 2007), detailed analysis of IFT mutants, such as ift57, ift88 and ift172, does not reveal any Hh phenotype (Lunt et al., 2009). It is thus possible that, as in Drosophila, cilia are not required for zebrafish Hh or Wnt signaling. Precedent for this scenario comes from the observation that Fused is required for Hh signaling in Drosophila and zebrafish but not in mouse (Wilson et al., 2009). Moreover, the requirement for different Gli genes also differs between mouse and zebrafish. For example, zebrafish gli1 is expressed even in the absence of Hh signaling and is required for the activation of high-threshold target genes (Karlstrom et al., 2003; Ninkovic et al., 2008). Although current evidence suggests that cilia are not required for Hh and Wnt signaling in zebrafish, it is conceivable that the presence of maternally provided ciliary components in zygotic zebrafish cilia mutants (Bisgrove et al., 2005; Sun et al., 2004) might mask a role in signal transduction. Indeed, during early embryogenesis, cilia still form in zygotic cilia mutants (Tsujikawa and Malicki, 2004). It has therefore remained unclear whether cilia are required for Hh and Wnt signaling in zebrafish.
To conclusively address the role of cilia in zebrafish, we generated zebrafish mutants (MZovl) that lack all cilia. We found that cilia are not required for Wnt signaling but are indispensable for normal Hh signaling. Surprisingly, unlike mouse cilia mutants, MZovl mutants showed dampened, but expanded, Hh pathway activity that is dependent on Gli1 activity. Our results suggest that the divergent regulation of Gli genes accounts for the distinct phenotypic consequences of the absence of cilia in zebrafish and mouse.
MATERIALS AND METHODS
Zebrafish strains
Zebrafish strains were maintained and raised under standard conditions. hsp-GFP-Gli1 transgenic fish were generated by injecting hsp-GFP-Gli1 plasmid DNA into wild-type embryos, and transgenic lines were established by screening for GFP expression after heat shock.
Generation of maternal-zygotic ovl embryos by germline replacement
Donor embryos from ovltz288b/+ intercrosses were labeled by injecting GFP-nanos3′UTR RNA. Wild-type host embryos were injected with morpholino antisense oligonucleotide against dead end to block germ cell development. Cells were transplanted from the donors into the hosts at the dome stage. Transplanted host embryos were screened at 24 hpf for successful transfer of donor germ cells as indicated by GFP expression (Ciruna et al., 2002). The genotype of the donors was determined by nested PCR analysis, first with primers oval.5 (5′-GCATATAACGCCTCATTTGCTA-3′) and oval.6 (5′-CAACTCCAATGTTCTGCATGAT-3′), followed by PCR with oval.F (5′-CATTTTAGATGAGCCATGTTTGAA-3′) and oval.6, and DdeI digestion. This results in a single band of 367 bp in wild-type embryos, bands of 220 and 147 bp in homozygous ovl/ovl embryos, and all three bands in heterozygous ovl/+ embryos. Host embryos containing homozygous ovltz288b/tz288b germ cells were raised to adulthood. Most female adult fish with ovltz288b/tz288b germline were fertile, whereas all male fish were sterile owing to sperm immotility. Females with mutant germlines and male ovl/+ fish were crossed to obtain maternal-zygotic ovl embryos (50% of embryos).
mRNA, DNA and morpholino injections
Synthetic mRNA was generated with the mMessage mMachine Kit (Ambion). Embryos were injected at the one-cell stage with 1-2 nl of mRNA solution to achieve the appropriate concentration: 100 pg GFP-nanos3′UTR, 60 pg shh, 100 pg rSmoM2-EGFP, 120 pg dnPKA, 6 pg gli3R-EGFP, 60 pg m-mRFP (membrane-targeted monomeric red fluorescent protein). To induce expression from the heat-shock promoter, 15 pg of hsp-Gli3R-EGFP plasmid was injected together with Tol2 transposase mRNA (50 pg) into one-cell stage embryos, and injected embryos were heat shocked at appropriate stages at 37°C in a heat block for 30 minutes. All morpholino oligonucleotides (Gene Tools, LLC) were injected into embryos at the one-cell stage at appropriate concentrations: 0.4 pmol dead end MO (5′-GCTGGGCATCCATGTCTCCGACCAT-3′) (Ciruna et al., 2002); 0.6-2.4 pmol p53MO (5′-GCGCCATTGCTTTGCAAGAATTG-3′) (Robu et al., 2007); 0.3 pmol ptc1MO (5′-TCTCTGGGATCCGAGGCCATAGTCC-3′) (Wolff et al., 2003); 0.3 pmol ptc2MO (5′-AGGAGACATTAACAGCCGAGGCCAT-3′) (Wolff et al., 2003); 0.4-0.8 pmol gli1MO (5′-CCGACACACCCGCTACACCCACAGT-3′) (Karlstrom et al., 2003); 1-1.2 pmol gli2aMO (5′-GGATGATGTAAAGTTCGTCAGTTGC-3′) (Karlstrom et al., 2003); 0.6 pmol gli2bMO (5′-CGGGAGCTGGAACACCGGCCTCCAT-3′) (Ke et al., 2008); 1.2-1.6 pmol gli3MO (5′-ACAACTGGGCATTCCTCAGAGCATC-3′) (Tyurina et al., 2005). Previous studies have shown that these morpholinos can specifically and efficiently reduce the activity of the corresponding genes (Ciruna et al., 2002; Karlstrom et al., 2003; Ke et al., 2008; Robu et al., 2007; Tyurina et al., 2005; Wolff et al., 2003). Co-injection of p53MO reduces the cell death induced by non-specific off-target effects that result from morpholino injection (Robu et al., 2007).
In situ hybridization and immunohistochemistry
Whole-mount in situ hybridization and antibody staining were performed according to standard protocols. Probes were zebrafish axin2, eng2, gli1, hlx1, krox20 (egr2 - Zebrafish Information Network), lft2, myoD (myod1), nkx2.2a, nkx2.2b, olig2, pax3, ptc1, shh, sp5, sp5l and tal2. To obtain cross-sections, embryos were embedded in OCT compound (VWR) after whole-mount in situ hybridization and sectioned at 20 μm using a Leica cryostat. All sections shown represent the region of the neural tube above the yolk extension (between somites 10 and 16) in embryos at 24 hpf. For immunohistochemistry, the following antibodies were used: mouse monoclonal antibody to acetylated α-tubulin (1:500, Sigma), mouse monoclonal antibody to γ-tubulin (1:800, Sigma), rabbit polyclonal antibody to Prox1 (1:400, Abcam), and mouse monoclonal antibody to engrailed (4D9, 1:20, Developmental Studies Hybridoma Bank). For fluorescent detection of antibody labeling, appropriate Alexa Fluor-conjugated secondary antibodies (1:500, Molecular Probes) were used.
Cyclopamine treatment
Embryos at 50% epiboly were treated with cyclopamine (Toronto Chemical) at a final concentration of 100 μM in 1% DMSO. Control embryos were treated simultaneously with an equal concentration of DMSO. Treated embryos were grown to desired stages for analysis.
RESULTS
Generation of maternal-zygotic ovl mutants
To study the role of cilia in zebrafish, we used a putative null allele of oval (ovltz288b), a gene that codes for the IFT protein Ift88 (also known as Polaris) (Tsujikawa and Malicki, 2004). This protein is essential and is specifically required for cilia formation in every organism studied to date (Pedersen and Rosenbaum, 2008). Owing to the perdurance of maternally contributed ovl mRNA (Bisgrove et al., 2005), most cilia can still form normally during early stages in ovltz288b/tz288b mutants (Tsujikawa and Malicki, 2004). To completely eliminate ovl activity, we generated maternal-zygotic ovl mutants (MZovl) using the germline replacement technique (Ciruna et al., 2002). Homozygous ovl mutant donor germ cells were transplanted into wild-type hosts depleted of their germ cells. The resulting females were fertile, even though their germline was exclusively reconstituted by mutant germ cells. By contrast, germline-replaced males were sterile owing to a substantially shortened sperm flagellum and sperm immotility (data not shown). Crosses of females with mutant germlines and male ovl/+ fish generated 50% MZovl mutants and 50% heterozygous embryos that lacked maternal ovl contribution (Movl). By 24 hours post-fertilization (hpf), Movl embryos were indistinguishable from wild-type embryos by all analyses (see Fig. S1 in the supplementary material) and were used as controls in subsequent experiments.
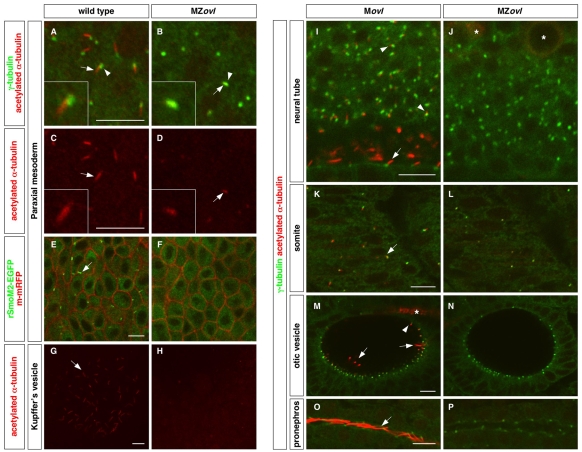
To analyze the cilia defects in MZovl mutants, we used immunohistochemistry to detect ciliary proteins. Acetylated α-tubulin antibodies recognize the stabilized microtubules in the ciliary axoneme, and γ-tubulin antibodies detect the basal body, which is the anchor of the cilium. In wild-type embryos, cilia were observed shortly after gastrulation as cells exit the cell cycle and a ciliary shaft extends from the basal body (Fig. 1A,C). Even though the basal body appeared to form normally, MZovl mutants failed to form the axoneme of cilia, and domains of stabilized microtubules were only found co-localizing with the basal body (Fig. 1B,D). To further test for the presence of cilia in MZovl mutants, we used rSmoM2 protein, a dominant-active rat (r) Smo mutant that constitutively localizes throughout the ciliary axoneme (Corbit et al., 2005). In wild-type embryos, EGFP-tagged rSmoM2 showed strong ciliary localization (Fig. 1E). By contrast, rSmoM2-EGFP protein was found exclusively in the cytoplasm in MZovl mutant cells (Fig. 1F), confirming the absence of cilia. Systematic analyses indicated that MZovl mutants lack all cilia in all tissues examined, including Kupffer's vesicle, the neural tube, somites, the otic vesicle and the pronephric duct (Fig. 1G-P).
Fig. 1.
Zebrafish MZovl mutants lack cilia. Cilia were visualized by staining with acetylated α-tubulin antibody (red) (A-D,G-P) and basal bodies were visualized by staining with γ-tubulin antibody (green) (A,B,I-P). (A-D) The ciliary axoneme (arrows) and the basal body (arrowheads) are formed in cells of the paraxial mesoderm in wild-type embryos (A,C), whereas cilia (arrows) are absent in MZovl mutants (B,D). Note that domains of stabilized microtubules (arrows in B,D) can only be found co-localizing with the basal body (arrowhead in B) in MZovl mutants. C and D correspond to the red channel of A and B, respectively. Insets show magnified views of regions of interest. (E,F) Cilia (arrow) labeled by rSmoM2-EGFP (green) are present in wild-type embryos but not in MZovl mutants. The cell membrane was labeled with m-mRFP (red). (G-P) Cilia (arrows and arrowheads) are absent in Kupffer's vesicle (G,H), the neural tube (I,J), somites (K,L), the otic vesicle (M,N), and the pronephric duct (O,P) in MZovl mutants as compared with control embryos. In the neural tube (I), both floor plate cilia (arrow) and cilia in neurons of the dorsal neural tube (arrowheads) are indicated. In the otic vesicle (M), both the longer tether cilia (arrows) and the short non-motile cilia (arrowhead) are indicated. Note that despite the absence of cilia, the basal body forms in MZovl mutants. Asterisks in J and M mark the staining of some neurons and axons, which also label with antibody to acetylated α-tubulin. Embryos are at the 10-somite stage (A-D), 1-somite stage (E,F), 8-somite stage (G,H) and at 24 hpf (I-P). A-H are dorsal views and I-P are lateral views. Scale bars: 10 μm.
Characterization of MZovl mutants
The complete lack of ciliogenesis in MZovl mutants allowed us to determine the specific roles of cilia during zebrafish embryogenesis. MZovl mutants can be easily distinguished from Movl siblings and wild-type embryos by their body curvature phenotype after 24 hpf (see Fig. S2 in the supplementary material), whereas zygotic ovl/ovl (Zovl) mutants do not display body axis curvature until 2 days post-fertilization (dpf) (Tsujikawa and Malicki, 2004). Similar to Zovl mutants (Tsujikawa and Malicki, 2004), MZovl fish displayed left-right defects (see Fig. S3 in the supplementary material) and developed pronephric cysts and pericardial edema at 3 dpf (data not shown). To determine the role of cilia in Wnt and Hh signaling, we characterized MZovl mutants using morphological and molecular criteria.
Canonical Wnt signaling
MZovl mutants displayed none of the characteristic phenotypes associated with gain or loss of canonical Wnt signaling. For example, loss of canonical Wnt signaling results in enlarged head structures and truncated tail and trunk, whereas gain of Wnt signaling leads to the opposite phenotype with anterior head truncation (Lekven et al., 2001). MZovl mutants did not display any morphological abnormalities resembling these Wnt signaling mutants (see Fig. S2 in the supplementary material). To directly determine whether canonical Wnt signaling is affected in the absence of cilia, we analyzed the expression of Wnt target genes, including axin2, sp5 and sp5l, in MZovl mutants. Despite the absence of cilia in all cells, MZovl embryos displayed normal expression of all Wnt target genes (Fig. 2A-F). These results indicate that cilia are not required for canonical Wnt signal transduction in zebrafish.
Fig. 2.
Characterization of MZovl mutants. (A-F)MZovl mutants (B,D,F) show similar expression of axin2 (A,B), sp5 (C,D) and sp5l (E,F) to wild-type embryos (A,C,E). (G,H)MZovl mutants (H) display similar anterior-posterior axis formation to wild-type embryos (G) as indicated by krox20 (rhombomeres 3 and 5, brackets) and myoD [somites (arrowheads) and adaxial cells (arrows)] expression. (I-L)MZovl mutants show reduced ptc1 and gli1 expression in the ventral neural plate (out of focus, asterisks) and adaxial muscle cells (arrows), and expanded expression in the paraxial mesoderm (brackets). (M-R)MZovl mutants (P-R) show reduced, but expanded, ptc1 and gli1 expression compared with control Movl embryos (M-O). N and Q are cross-sectional views of M and P (dashed lines indicate plane), respectively, with the notochord marked by an asterisk. In situ hybridization was carried out in embryos at the 15-somite stage (A-F), 9-somite stage (G,H), 6-somite stage (I-L) and at 24 hpf (M-R). A-F,M,O,P,R are lateral views and G-L are dorsal views. Scale bars: 100 μm in A-M,O,P,R; 10 μm in N,Q.
Non-canonical Wnt signaling
To test whether non-canonical Wnt/PCP signaling is affected by the lack of cilia, we characterized convergent extension movements. Loss of PCP signaling disrupts convergent extension movements, resulting in a shortened and widened embryo (Heisenberg et al., 2000). MZovl embryos underwent normal convergent extension movements and developed a normal body axis (Fig. 2G,H; see Fig. S2 in the supplementary material). This result suggests that cilia are not required for non-canonical Wnt signaling in zebrafish.
Hedgehog signaling
In mouse cilia mutants, Hh pathway activity is strongly diminished. To determine whether cilia are required for Hh signaling in zebrafish, we first analyzed the expression of two direct transcriptional targets of Hh signaling, ptc1 and gli1. During early somitogenesis, expression of both ptc1 and gli1 was reduced in the ventral neural plate and the adaxial muscle cells of MZovl mutants as compared with wild-type embryos (Fig. 2I-L). Strikingly, we also observed a substantial expansion of the ptc1 and gli1 expression domains (Fig. 2I-L). The expression pattern of ptc1 in MZovl mutants was distinctly different from that in embryos that lack Hh signaling, in which ptc1 expression was completely absent (see Fig. 4E). Similarly, at 24 hpf, ptc1 expression in MZovl mutants was dampened in cells close to the notochord, but expanded to more distant cells in the neural tube and somites (Fig. 2M,N,P,Q). gli1 expression along the ventral brain and spinal cord was markedly reduced, but expanded to cells that normally do not express gli1 (Fig. 2O,R). Thus, the absence of cilia has two effects on Hh signal transduction: a marked reduction in maximal signaling activity and a spatial expansion of low-level Hh pathway activity. These findings reveal a key difference in Hh pathway activity between mouse and zebrafish in the absence of cilia: mouse cilia mutants have little ptc1 expression (Huangfu et al., 2003), whereas cilia-deficient zebrafish mutants display a reduced, but spatially expanded, ptc1 domain.
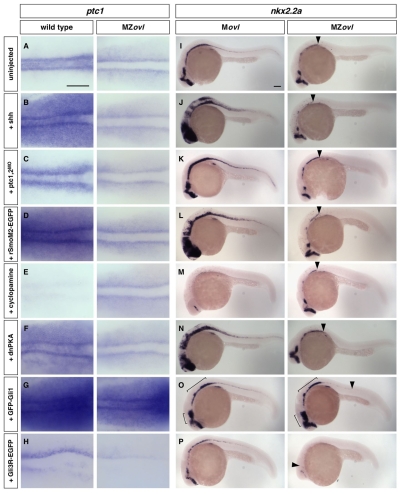
Fig. 4.
Epistasis analysis of cilia and the Hh signaling pathway. (A-P)MZovl and control zebrafish embryos were stained for the expression of ptc1 (A-H) and nkx2.2a (I-P) following different manipulations. MZovl embryos are insensitive to ectopic expression of shh mRNA (B,J), depletion of ptc1,2 using morpholinos (C,K), expression of rSmoM2-EGFP mRNA (D,L), treatment with cyclopamine at 100 μM (E,M), and expression of dnPKA mRNA (F,N). By contrast, expression of GFP-Gli1 (G,O) enhances, whereas expression of Gli3R-EGFP (H,P) reduces, ptc1 and nkx2.2a expression. Arrowheads in I-P indicate the posterior extent of nkx2.2a expression in MZovl mutants. Brackets in O indicate regions with expanded nkx2.2a expression. A-H are dorsal views of the presomitic mesoderm of 5-somite stage embryos, and I-P are lateral views of 24 hpf embryos. Scale bars: 100 μm.
Dampened and expanded Hh pathway activity in MZovl mutants
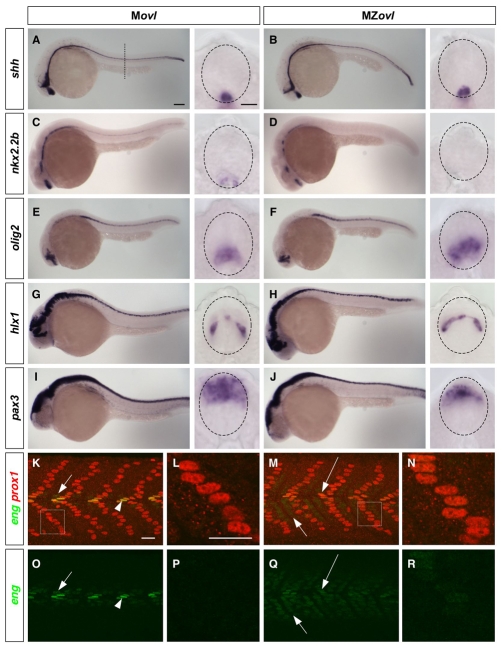
In zebrafish, Hh signaling is essential for patterning the ventral neural tube and somites. To determine whether Hh-dependent cell fate specification is perturbed in the absence of cilia, we carried out marker analysis of the neural tube (Guner and Karlstrom, 2007) and somites (Wolff et al., 2003). In zebrafish, the specification of medial floor plate (MFP) does not require Hh signaling, whereas the flanking lateral floor plate (LFP) and p3 neuronal precursors are induced by the highest level of Hh signaling (Odenthal et al., 2000). In MZovl mutants, shh was expressed normally in the MFP and underlying notochord (Fig. 3A,B), suggesting that defects in Hh signaling are not due to alterations in the expression of inductive signals. By contrast, MZovl mutants lacked expression of nkx2.2a and nkx2.2b (Fig. 3C,D; Fig. 4I), markers of the LFP and p3 neuronal precursors in the neural tube. Consistent with this, foxa2 expression was maintained in the MFP but lost in the LFP in MZovl mutants (data not shown), suggesting that maximal pathway activation is compromised in the absence of cilia. Strikingly, olig2+ motoneuron precursors, which are induced by low-level Hh signaling and are located just dorsal to the LFP/p3 domain, were specified and expanded in MZovl mutants (Fig. 3E,F). The neural patterning phenotype of MZovl mutants is markedly different from that of embryos that lack Hh signaling, in which neither LFP/p3 cells nor motoneurons are specified (Guner and Karlstrom, 2007), suggesting that low-level Hh pathway activity is still present in MZovl mutants. Consistent with these findings, MZovl mutants displayed a normal domain of V0 interneurons (hlx1+), although dorsally shifted, whereas pax3 expression in the dorsal neural tube was reduced and restricted dorsally (Fig. 3G,J). These phenotypes contrast with those of mouse cilia mutants, in which the motoneuron domain is lost and the dorsal neural tube domain remains normal (Huangfu and Anderson, 2005; Huangfu et al., 2003; Liu et al., 2005). Thus, the neural patterning defects in MZovl mutants reflect the altered Hh pathway activity: loss of high-level activity concomitant with expansion of low-level activity.
Fig. 3.
Neural and somite patterning in MZovl mutants. (A-J) Marker analysis of neural patterning. Movl control zebrafish embryos (A,C,E,G,I) and MZovl mutants (B,D,F,H,J) were stained at 24 hpf for the expression of shh (A,B), nkx2.2b (C,D), olig2 (E,F), hlx1 (G,H) and pax3 (I,J). Each panel contains a lateral view and a cross-sectional view (between somites 10 and 16; dashed line in A indicates plane) with the neural tube outlined (dashed lines). MZovl mutants show normal shh and hlx1 expression (B,H), an absence of nkx2.2b expression (D), expanded olig2 expression (F), and reduced pax3 expression (J) in the neural tube. (K-R) Marker analysis of somite patterning. Movl control embryos (K,L,O,P) and MZovl mutants (M,N,Q,R) were stained at 24 hpf with antibodies against eng (green) and prox1 (red). Muscle pioneers (prox1+, strong eng+, arrowheads in K,O) are replaced with prox1+, weak eng+ cells (long arrows in M,Q) in MZovl mutants. Medial fast fibers (prox1-, weak eng+) are expanded in MZovl mutants (short arrows in M,Q) as compared with Movl embryos (short arrows in K,O). Superficial slow fibers (prox1+) also express a low level of eng in MZovl mutants (N,R) as compared with Movl controls (L,P). K-N are merged confocal images with both green and red channels, and the corresponding green channel images are shown in O-R, respectively. L and N are higher magnification views of the boxed regions in K and M, respectively. Lateral views. Scale bars: 100 μm in lateral views in A-J; 10 μm in cross-sections in A-J; 20 μm in K-R.
A similar effect on Hh signaling was observed during somite patterning in MZovl mutants. Four different muscle cell types are specified by different levels of Hh signaling activity: superficial slow myofibers (SSFs), medial fast fibers (MFFs) and muscle pioneers (MPs) are induced by increasing levels of Hh activity, whereas fast myofibers are specified independently of Hh signaling (Wolff et al., 2003). Analysis of somite markers in MZovl mutants revealed that MPs (prox1+ and strong eng+) were lost, whereas MFFs were greatly expanded at the expense of fast myofibers (Fig. 3K,M,O,Q). Although SSFs were induced normally in MZovl mutants based on expression of prox1 and slow myosin heavy chain, weak eng expression was also detected in these cells (Fig. 3K-R; data not shown), suggesting an elevated Hh pathway activity. Hence, similar to the neural patterning phenotype, defects in somite patterning in MZovl mutants reflect a dampened, but expanded, Hh pathway activity.
Taken together, these results indicate that cell types that are normally specified by maximal Hh signaling activity, such as LFP/p3 cells in the neural tube and MPs in the somite, are lost in MZovl mutants, whereas cells that require intermediate level of Hh signaling activity, such as motoneurons in the neural tube and MFFs in the somites, are expanded. Intriguingly, the phenotype of MZovl mutants strongly resembles that of zebrafish iguana (dzip1 - Zebrafish Information Network) mutants (Sekimizu et al., 2004; Wolff et al., 2004) (see Fig. S4 in the supplementary material), suggesting that Iguana might be involved in ciliogenesis or cilia function. Indeed, we found that iguana homozygous mutants lack primary cilia markers and have a reduced number of motile cilia (see Fig. S5 in the supplementary material). Thus, iguana mutants provide additional support for the contention that the absence of cilia results in dampened, but expanded, Hh pathway activity in zebrafish.
Zebrafish cilia are required for Hh signal transduction
The different phenotypes of mouse and zebrafish cilia mutants raise the possibility that cilia have different roles in vertebrate Hh signaling. To address this question, we determined which components of the Hh signaling pathway depend on intact cilia to modulate pathway activity. In wild-type embryos, ectopic expression of Shh, rSmoM2-EGFP or dnPKA, or depletion of both Ptc1 and Ptc2 using antisense morpholino oligonucleotides, resulted in substantial upregulation and expansion of ptc1 expression in adaxial cells and the paraxial mesoderm (Fig. 4A-D,F). By contrast, similarly injected MZovl mutants remained indistinguishable from uninjected MZovl embryos (Fig. 4A-D,F), suggesting that the activity of Shh, Smo and PKA in transducing Hh signaling requires intact cilia. In converse experiments, we inhibited Hh signaling using cyclopamine, a potent antagonist of Smo. Whereas cyclopamine completely blocked Hh signaling in wild-type embryos, MZovl mutants were refractory to cyclopamine treatment (Fig. 4E). We observed similar epistatic relationships between cilia and components of the Hh pathway in neural tube and somite patterning (Fig. 4I-N; see Fig. S6 in the supplementary material). Whereas ectopic expression of Shh, rSmoM2-EGFP or dnPKA, or depletion of both Ptc1 and Ptc2, induced substantial expansion of nkx2.2a expression in control embryos, the same manipulations had little effect on nkx2.2a expression in MZovl embryos (Fig. 4I-L,N). Similarly, whereas cyclopamine largely abolished nkx2.2a expression in control embryos, nkx2.2a expression in MZovl mutants was insensitive to cyclopamine treatment (Fig. 4M). Together, these results strongly support the model that cilia are required to transduce the activities of Shh, Ptc, Smo and PKA (Haycraft et al., 2005; Huangfu et al., 2003; Liu et al., 2005; Ocbina and Anderson, 2008) and provide further evidence that cilia function is completely abolished in MZovl mutants.
Although mouse cilia mutants have strongly reduced endogenous Gli activator and repressor activity, misexpressed Gli1 and Gli3R are functional in primary limb bud cells that lack cilia (Haycraft et al., 2005). In zebrafish, Gli1 is the main activator in mediating Hh signaling, whereas Gli2a, Gli2b and Gli3 have repressor activity (Karlstrom et al., 1999; Karlstrom et al., 2003; Ke et al., 2008; Tyurina et al., 2005). To determine whether a similar relationship between Gli proteins and cilia is found in vivo and in zebrafish, we generated a GFP-Gli1 transgene under the control of a heat-shock-inducible promoter (hsp-GFP-Gli1). Expression of GFP-Gli1 in control embryos resulted in substantial upregulation of ptc1 and nkx2.2a expression (Fig. 4G,O). Induction of GFP-Gli1 expression in MZovl embryos greatly enhanced ptc1 expression and led to expansion of the nkx2.2a expression domain in the ventral brain and partial restoration of nkx2.2a expression in the spinal cord (Fig. 4G,O). Conversely, we generated a C-terminally truncated Gli3 construct (Gli3R-EGFP) to test whether a processed Gli repressor can function in the absence of cilia. Misexpression of Gli3R-EGFP in control embryos strongly suppressed Hh pathway activation, resulting in reduced ptc1 and nkx2.2a expression (Fig. 4H,P). Similarly, expression of Gli3R-EGFP almost completely abolished ptc1 and nkx2.2a expression in MZovl mutants (Fig. 4H,P), suggesting that the repressive activity of Gli3R does not require intact cilia. Taken together, our analyses indicate that zebrafish and mouse cilia have a similar epistatic relationship with components of the Hh signaling pathway: cilia are required for the activity of Shh, Ptc, Smo and PKA, whereas Gli1 and Gli3R are functional even in the absence of cilia.
Expanded Hh pathway activity depends on Gli1 activity
If the role of cilia in Hh signal transduction is shared between mouse and zebrafish, why do cilia-deficient mutants display distinct phenotypes? Unlike mouse cilia mutants that have minimal Hh pathway activity in the neural tube (Huangfu et al., 2003), MZovl mutants maintain dampened, but expanded, pathway activity. In the current mouse model, the absence of cilia leads to loss of Gli activator activity and to reduced Gli repressor activity. Since Hh pathway activity is determined by the balance of Gli activator and repressor activity, higher pathway activity in zebrafish cilia mutants might be caused by higher Gli activator activity and/or lower Gli repressor activity than that in mouse cilia mutants. In the first scenario, baseline Gli activator activity in the absence of cilia would be sufficient to induce low-threshold target genes (activation model). For example, it has been shown that zebrafish Gli1 is expressed even in the absence of Hh signaling (Karlstrom et al., 2003) and can have Hh-independent effects (Ninkovic et al., 2008). By contrast, mouse Gli1 expression is fully dependent on Hh signaling (Bai et al., 2002). It is therefore conceivable that higher pathway activity in zebrafish cilia mutants is due to a higher baseline activity of Gli1. In the second scenario, lower Gli repressor activity in the absence of cilia might de-repress low-threshold targets (de-repression model). For example, in the limb of mouse cilia mutants, reduced Gli3 repressor activity leads to de-repression of Hoxd genes and Gremlin, resulting in a polydactyly phenotype (Liu et al., 2005; May et al., 2005). The loss of Gli repressors in zebrafish might lead to a higher activation of downstream genes than occurs in mouse. To test these possibilities, we interfered with Gli activity in zebrafish cilia mutants.
The de-repression model suggests that low-threshold targets might be expressed upon removal of all Gli repressors, even when upstream signals can no longer be transduced owing to a lack of cilia. To test this model, we used cyclopamine to specifically block Smo-mediated upstream signaling, mimicking the loss of Smo activity in cilia mutants. In parallel, we knocked down gli2a, gli2b and gli3 activity using a morpholino cocktail, mimicking the loss of Gli repressors observed in mouse cilia mutants. As predicted by the de-repression model, embryos treated with cyclopamine together with gli2a/bMO and gli3MO showed a slight upregulation of ptc1 and substantial expansion of gli1 expression as compared with cyclopamine-treated control embryos (Fig. 5A-D). However, unlike MZovl mutants, specification of olig2+ motoneurons in the neural tube was not rescued and only a small patch of olig2 expression was restored in the brain (Fig. 5E,F). Similarly, eng2 expression in somites was not restored under the same conditions (Fig. 5G,H). These results suggest that loss of Gli repressors alone is not sufficient to explain downstream target expression in MZovl mutants. Consistent with this, we found that Gli repressor activity is still present in MZovl mutants. Morpholino knockdown of gli2a or gli3 in MZovl embryos further enhanced ptc1 expression (Fig. 6A,B; data not shown). Moreover, eng2 expression in somites was upregulated substantially in both gli2aMO-treated and gli3MO-treated MZovl embryos, and downregulation of gli3 greatly rescued nkx2.2a expression in MZovl mutants (Fig. 6C-H). These results reveal that, similar to mouse cilia mutants (Huangfu et al., 2003), some Gli repressor activity is still present in cilia-deficient zebrafish embryos, and, more importantly, that de-repression of the Hh signaling pathway due to loss of Gli repressor activity is not sufficient to confer the expanded pathway activity in MZovl mutants.
Fig. 5.
Loss of Gli repressors in the absence of Hh signaling is not sufficient to activate the Hh pathway. Wild-type control zebrafish embryos (A,C,E,G) and wild-type embryos injected with a cocktail of gli2a, gli2b and gli3 morpholinos (B,D,F,H) were treated with cyclopamine from 50% epiboly to 24 hpf, and stained for the expression of ptc1 (A,B), gli1 (C,D), olig2 (E,F) and eng2 (G,H). (A-D) Embryos treated with cyclopamine, gli2aMO, gli2bMO and gli3MO show a slight upregulation of ptc1 expression (arrowheads in B) and substantial upregulation of gli1 expression (D). Note that gli1 is expressed at a low level in the absence of Hh signaling (arrowhead in C). (E,F) Compared with control embryos (E), embryos treated with cyclopamine, gli2aMO, gli2bMO and gli3MO show a small patch of olig2 expression in the brain (arrowhead in F), but display no olig2 expression in the neural tube similar to controls. (G,H) Embryos treated with cyclopamine, gli2aMO, gli2bMO and gli3MO show no eng2 expression in somites similar to controls. Note that expression of eng2 in the midbrain-hindbrain boundary (arrowheads) does not require Hh signaling. Lateral views. Scale bar: 100 μm.
Fig. 6.
Gli repressor activity is present in MZovl mutants. MZovl control zebrafish embryos (A,C,E,G) and morpholino-injected MZovl mutants (B,D,F,H) were stained at 24 hpf for the expression of ptc1 (A,B), eng2 (C-F) and nkx2.2a (G,H). (A-D) gli2aMO-treated MZovl embryos show enhanced ptc1 and eng2 expression as compared with uninjected MZovl controls. (E-H) gli3MO-treated MZovl embryos show enhanced eng2 expression and partially rescued nkx2.2a expression in the neural tube compared with uninjected MZovl controls. Arrowheads in G and H indicate the posterior extent of nkx2.2a expression in the neural tube. Lateral views. Scale bar: 100 μm.
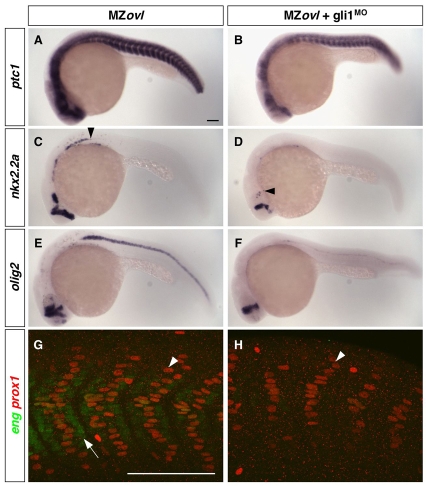
The activation model proposes that residual Gli activator activity in MZovl mutants might account for the expanded Hh pathway activity. To test this model, we determined whether Hh pathway activity in MZovl mutants depends on the activity of endogenous Gli1, the main activator in zebrafish (Karlstrom et al., 2003). Strikingly, depletion of Gli1 in MZovl mutants using gli1 morpholinos strongly reduced ptc1 and nkx2.2a expression, and the specification of olig2-expressing motoneurons was largely abolished (Fig. 7A-F). Similarly, MFFs were absent, accompanied by a substantial reduction of SSFs (Fig. 7G,H). These results show that Gli1 activity contributes to the expanded Hh pathway activity in MZovl mutants.
Fig. 7.
Expanded Hh pathway activity in MZovl mutants is dependent on Gli1 activity. (A-H) Uninjected MZovl contrls (A,C,E,G) and gli1MO-injected MZovl mutants (B,D,F,H) were stained at 24 hpf for the expression of ptc1 (A,B), nkx2.2a (C,D), olig2 (E,F), eng (green) and prox1 (red) (G,H). Note that gli1MO-treated MZovl embryos show reduced expression of ptc1 (B), nkx2.2a (D) and olig2 (F) as compared with corresponding untreated MZovl control embryos (A,C,E). Arrowheads in C and D indicate the posterior extent of nkx2.2a expression. In somites of gli1MO-treated MZovl embryos (H), medial fast fibers (prox1-, weak eng+, arrow in G) are abolished, and superficial slow fibers (prox1+, arrowheads in G,H) are substantially reduced in number. Lateral views. Scale bars: 100 μm.
DISCUSSION
The generation of maternal-zygotic oval mutants enabled us to perform the first analysis of the consequences of a complete lack of cilia in zebrafish. Previous studies of cilia mutants could only analyze the effects of partial or late loss of cilia, owing to the maternal contribution of ciliary components. Our study clarifies the role of cilia in vertebrate development and provides three major conclusions. First, cilia are not required for Wnt signaling. Second, mouse and zebrafish have similar epistatic requirements for cilia in Hh signaling. Third, the distinct roles and regulation of Gli genes in zebrafish and mouse result in different phenotypes caused by loss of cilia.
Wnt signaling
In contrast to previous suggestions that complete loss of cilia results in early lethality in zebrafish (Aanstad et al., 2009), MZovl mutants undergo normal gastrulation. Moreover, our morphological and marker analyses indicate that cilia are dispensable for canonical and non-canonical Wnt signaling in zebrafish. How can this conclusion be reconciled with studies that postulate a role for ciliary components in Wnt signaling? Since we observed that MZovl mutant cells only lack the ciliary axoneme but have intact basal bodies, it is possible that the basal body might mediate Wnt signaling in the absence of cilia. This model is supported by the observation that disruption of the basal body results in defects in Wnt signaling (Corbit et al., 2008; Ferrante et al., 2009; Gerdes et al., 2007; Ross et al., 2005), whereas loss of cilia components, including Ift88, results in abnormal Hh signaling (Haycraft et al., 2005; Huangfu and Anderson, 2005; Huangfu et al., 2003; Liu et al., 2005; May et al., 2005). It is thus conceivable that the ciliary axoneme and the basal body are two distinct signaling organelles with separable functions: the ciliary axoneme is required for Hh signal transduction, whereas the basal body might be essential for the normal Wnt response. Apparent contradictions in the field (Corbit et al., 2008; Eggenschwiler and Anderson, 2007) might be further resolved by the suggestion that some proteins have direct but independent roles in both cilia and Wnt signaling. For instance, proteins such as Kif3a in mouse (Corbit et al., 2008; Gerdes et al., 2007) and Lrrc6, Qilin and Kif3b in zebrafish (Aanstad et al., 2009; Kishimoto et al., 2008), might have direct roles in Wnt signaling, independent of their role in cilia. Thus, we propose that Wnt signaling does not require cilia but might be dependent on basal body integrity and the dual roles of some ciliary proteins.
Hh signaling
Our results reveal that cilia are required for normal Hh signal transduction not only in mouse, but also in zebrafish. In the absence of cilia, upstream regulators of the Hh pathway, such as Shh, Ptc, Smo and PKA, can no longer modulate Hh pathway activity, whereas Gli effectors such as Gli1 and Gli3R are functional upon misexpression. These results address a long-standing enigma in the field by providing the first genetic evidence that cilia function in Hh signaling is conserved from zebrafish to mouse. Together with recent reports that link cilia to Hh signaling in Xenopus and chick (Davey et al., 2006; Park et al., 2006; Yin et al., 2009), our results indicate that the ciliary requirement for Hh signaling is conserved in the entire vertebrate lineage.
Despite the conserved epistasis of cilia in Hh signal transduction, a phenotypic comparison of zebrafish MZovl mutants with mouse cilia mutants in nervous system patterning reveals surprising differences. Whereas the expression of Hh downstream targets is strongly reduced or absent in mouse cilia mutants (Huangfu et al., 2003), the absence of cilia results in dampened, but expanded, Hh pathway activity in zebrafish. Consequently, cell types whose specification requires the highest Hh signaling activity are lost in both zebrafish and mouse cilia mutants, whereas cell fates whose induction depends on intermediate Hh activity are expanded in zebrafish but absent in mouse. For example, the motoneuron domain is specified and expanded in MZovl mutants but is absent in mouse Ift88 mutants (Huangfu et al., 2003). Conversely, the dorsal pax3 domain is reduced in MZovl mutants but normal in mouse Ift88 mutants (Huangfu et al., 2003; Liu et al., 2005). A more detailed study of mouse cilia mutants, in particular at earlier stages of development, might reveal additional differences (or similarities) to MZovl zebrafish, but the current level of analysis indicates that Hh signaling activity in zebrafish cilia mutants is substantially higher than in mouse cilia mutants.
The dampening of Hh pathway activity in MZovl mutants and mouse cilia mutants is consistent with the idea that cilia have a conserved role in allowing maximal activation of the Hh pathway. But what is the basis for the expansion of pathway activity in MZovl mutants? It has been postulated that Smo might have cilia-independent activities in zebrafish in mediating the induction of low- and medium-threshold targets (Aanstad et al., 2009). This scenario is unlikely, however, because Shh, rSmoM2-EGFP, dnPKA, depletion of both Ptc1 and Ptc2, and treatment with cyclopamine did not affect the phenotype of MZovl embryos. The expansion of pathway activity in MZovl mutants must therefore occur downstream of these factors. Our results suggest that the balance between Gli activators and Gli repressors can tip towards activator activity in zebrafish cilia mutants. In particular, gli1MO-treated MZovl embryos show a much lower pathway output than untreated MZovl mutants. These results reveal that the expanded Hh pathway activity in MZovl mutants is largely dependent on Gli1. Although the phenotype of mouse Ift88; Gli1 mutants has not been reported, mouse Gli1 expression is strictly dependent on Hh signaling (Bai et al., 2002). It is therefore generally assumed that Gli1 is inactive in mouse cilia mutants (Eggenschwiler and Anderson, 2007). By contrast, our results suggest that Gli1 is a major contributor to the expanded downstream gene activation seen in zebrafish cilia mutants and explains, at least in part, the phenotypic differences between mouse and zebrafish cilia mutants. This model is also supported by previous studies that have shown a differential requirement and regulation of Gli genes in mouse and zebrafish. Mouse Gli1 has only minor roles in downstream gene activation and is activated by Hh signaling (Bai et al., 2002; Park et al., 2000). Mouse Gli2 is expressed independently of Hh and is the major activator of Hh target genes (Bai et al., 2002), but its activity depends on cilia (Eggenschwiler and Anderson, 2007; Haycraft et al., 2005). Thus, in the absence of cilia, mouse Gli2 is inactive, which results in little Hh pathway activity and no Gli1 expression. By contrast, zebrafish Gli2a has only minor roles in downstream gene activation, whereas zebrafish Gli1 is required for the induction of Hh target genes (Karlstrom et al., 2003). Importantly, zebrafish gli1 is induced by Hh signaling but is still expressed at low levels in the absence of Hh signaling (Karlstrom et al., 2003; Ninkovic et al., 2008). Thus, the differential requirement and regulation of mouse and zebrafish Gli1 contribute to the different phenotypes in the absence of cilia.
In addition to the role of Gli1 in expanded pathway activity, a reduction in Gli repressor activity might also contribute to the MZovl phenotype. In particular, significant levels of ptc1 expression were still observed in MZovl embryos depleted of Gli1 activity. A role for de-repression is consistent with the gain-of-function phenotype observed in the limb of mouse cilia mutants and attributed to the lack of Gli3 repressor (Liu et al., 2005; May et al., 2005). Although the lack of specific antibodies to zebrafish Gli proteins prevents us from directly analyzing the processing of endogenous Gli repressors in MZovl mutants, it is clear that some Gli repressor activity is still present in MZovl mutants. In particular, depletion of Gli2a and Gli3 in MZovl mutants enhanced the increase in Hh downstream gene activation. Moreover, we found that the removal of Gli repressors in cyclopamine-treated embryos results in an expansion of gli1 expression. Interestingly, however, these embryos, unlike MZovl mutants, did not express olig2 or eng2. This result raises the possibility that cilia might restrain Gli1 activity. The observation that Gli1 and Su(fu), a negative regulator of the pathway, are localized at the tip of cilia (Haycraft et al., 2005) is consistent with a role for cilia in limiting Gli1 activity.
Conclusions
In summary, our study clarifies the roles of cilia in vertebrate Wnt and Hh signaling and suggests that cilia are not involved in Wnt signaling but have conserved roles in transducing the activities of upstream components in the Hh pathway. We also provide an unusual example of how signaling pathways can diverge between species: the input (Hh), core components (Ptc, Smo, cilia, Gli) and output (ptc1, gli1, olig2, nkx2.2a expression) are highly conserved, but apparent changes in the regulation and role of transcriptional mediators (Gli) can contribute to phenotypic differences in specific mutant backgrounds (Gli mutants and cilia mutants). This scenario is reminiscent of the `re-wiring' observed in some bacterial species. For example, the polymyxin B resistance operon pbgP in enteric bacteria is regulated by the transcription factors PhoP and PmrA. Strikingly, the inputs (low Mg2+ and Fe3+), core components (PhoQ, PhoP, PmrB, PmrA) and output (pbgP expression) are highly conserved, but the regulatory architecture has diverged (Mitrophanov et al., 2008). It has been proposed that the different modes of regulation in enteric bacteria confer survival advantages in specific environments. It remains to be determined whether the differential requirements and regulation of Gli genes in zebrafish and mouse provide an advantage to either species or are simply a reflection of neutral evolution (Tuch et al., 2008).
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/18/3089/DC1
Supplementary Material
We thank the zebrafish community for providing probes and reagents; E. M. Jacobson for making hsp-GFP-Gli1 fish; J. Mao for the rSmoM2 plasmid; S. Zimmerman for fish care; members of the Schier laboratory, I. A. Drummond, A. Joyner and R. Losick for discussion; and A. P. McMahon, B. L. Allen and R. O. Karlstrom for critical comments on the manuscript. This work was supported by NIH grant R01 DE016779 to A.F.S. and A. Joyner. Deposited in PMC for release after 12 months.
References
- Aanstad, P., Santos, N., Corbit, K. C., Scherz, P. J., Trinh, le A., Salvenmoser, W., Huisken, J., Reiter, J. F. and Stainier, D. Y. (2009). The extracellular domain of Smoothened regulates ciliary localization and is required for high-level Hh signaling. Curr. Biol. 19, 1034-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, C. B., Auerbach, W., Lee, J. S., Stephen, D. and Joyner, A. L. (2002). Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development 129, 4753-4761. [DOI] [PubMed] [Google Scholar]
- Beales, P. L., Bland, E., Tobin, J. L., Bacchelli, C., Tuysuz, B., Hill, J., Rix, S., Pearson, C. G., Kai, M., Hartley, J. et al. (2007). IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat. Genet. 39, 727-729. [DOI] [PubMed] [Google Scholar]
- Bisgrove, B. W., Snarr, B. S., Emrazian, A. and Yost, H. J. (2005). Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer's vesicle are required for specification of the zebrafish left-right axis. Dev. Biol. 287, 274-288. [DOI] [PubMed] [Google Scholar]
- Caspary, T., Larkins, C. E. and Anderson, K. V. (2007). The graded response to Sonic Hedgehog depends on cilia architecture. Dev. Cell 12, 767-778. [DOI] [PubMed] [Google Scholar]
- Ciruna, B., Weidinger, G., Knaut, H., Thisse, B., Thisse, C., Raz, E. and Schier, A. F. (2002). Production of maternal-zygotic mutant zebrafish by germ-line replacement. Proc. Natl. Acad. Sci. USA 99, 14919-14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit, K. C., Aanstad, P., Singla, V., Norman, A. R., Stainier, D. Y. and Reiter, J. F. (2005). Vertebrate Smoothened functions at the primary cilium. Nature 437, 1018-1021. [DOI] [PubMed] [Google Scholar]
- Corbit, K. C., Shyer, A. E., Dowdle, W. E., Gaulden, J., Singla, V., Chen, M. H., Chuang, P. T. and Reiter, J. F. (2008). Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat. Cell Biol. 10, 70-76. [DOI] [PubMed] [Google Scholar]
- Cortellino, S., Wang, C., Wang, B., Bassi, M. R., Caretti, E., Champeval, D., Calmont, A., Jarnik, M., Burch, J., Zaret, K. S. et al. (2009). Defective ciliogenesis, embryonic lethality and severe impairment of the Sonic Hedgehog pathway caused by inactivation of the mouse complex A intraflagellar transport gene Ift122/Wdr10, partially overlapping with the DNA repair gene Med1/Mbd4. Dev. Biol. 325, 225-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey, M. G., Paton, I. R., Yin, Y., Schmidt, M., Bangs, F. K., Morrice, D. R., Smith, T. G., Buxton, P., Stamataki, D., Tanaka, M. et al. (2006). The chicken talpid3 gene encodes a novel protein essential for Hedgehog signaling. Genes Dev. 20, 1365-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggenschwiler, J. T. and Anderson, K. V. (2007). Cilia and developmental signaling. Annu. Rev. Cell Dev. Biol. 23, 345-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante, M. I., Zullo, A., Barra, A., Bimonte, S., Messaddeq, N., Studer, M., Dolle, P. and Franco, B. (2006). Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat. Genet. 38, 112-117. [DOI] [PubMed] [Google Scholar]
- Ferrante, M. I., Romio, L., Castro, S., Collins, J. E., Goulding, D. A., Stemple, D. L., Woolf, A. S. and Wilson, S. W. (2009). Convergent extension movements and ciliary function are mediated by ofd1, a zebrafish orthologue of the human oral-facial-digital type 1 syndrome gene. Hum. Mol. Genet. 18, 289-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes, J. M., Liu, Y., Zaghloul, N. A., Leitch, C. C., Lawson, S. S., Kato, M., Beachy, P. A., Beales, P. L., DeMartino, G. N., Fisher, S. et al. (2007). Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat. Genet. 39, 1350-1360. [DOI] [PubMed] [Google Scholar]
- Guner, B. and Karlstrom, R. O. (2007). Cloning of zebrafish nkx6.2 and a comprehensive analysis of the conserved transcriptional response to Hedgehog/Gli signaling in the zebrafish neural tube. Gene Expr. Patterns 7, 596-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Y. G., Kwok, B. H. and Kernan, M. J. (2003). Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr. Biol. 13, 1679-1686. [DOI] [PubMed] [Google Scholar]
- Haycraft, C. J., Banizs, B., Aydin-Son, Y., Zhang, Q., Michaud, E. J. and Yoder, B. K. (2005). Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 1, e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg, C. P., Tada, M., Rauch, G. J., Saude, L., Concha, M. L., Geisler, R., Stemple, D. L., Smith, J. C. and Wilson, S. W. (2000). Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405, 76-81. [DOI] [PubMed] [Google Scholar]
- Hoover, A. N., Wynkoop, A., Zeng, H., Jia, J., Niswander, L. A. and Liu, A. (2008). C2cd3 is required for cilia formation and Hedgehog signaling in mouse. Development 135, 4049-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu, D. and Anderson, K. V. (2005). Cilia and Hedgehog responsiveness in the mouse. Proc. Natl. Acad. Sci. USA 102, 11325-11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu, D. and Anderson, K. V. (2006). Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development 133, 3-14. [DOI] [PubMed] [Google Scholar]
- Huangfu, D., Liu, A., Rakeman, A. S., Murcia, N. S., Niswander, L. and Anderson, K. V. (2003). Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426, 83-87. [DOI] [PubMed] [Google Scholar]
- Karlstrom, R. O., Talbot, W. S. and Schier, A. F. (1999). Comparative synteny cloning of zebrafish you-too: mutations in the Hedgehog target gli2 affect ventral forebrain patterning. Genes Dev. 13, 388-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlstrom, R. O., Tyurina, O. V., Kawakami, A., Nishioka, N., Talbot, W. S., Sasaki, H. and Schier, A. F. (2003). Genetic analysis of zebrafish gli1 and gli2 reveals divergent requirements for gli genes in vertebrate development. Development 130, 1549-1564. [DOI] [PubMed] [Google Scholar]
- Ke, Z., Kondrichin, I., Gong, Z. and Korzh, V. (2008). Combined activity of the two Gli2 genes of zebrafish play a major role in Hedgehog signaling during zebrafish neurodevelopment. Mol. Cell. Neurosci. 37, 388-401. [DOI] [PubMed] [Google Scholar]
- Kishimoto, N., Cao, Y., Park, A. and Sun, Z. (2008). Cystic kidney gene seahorse regulates cilia-mediated processes and Wnt pathways. Dev. Cell 14, 954-961. [DOI] [PubMed] [Google Scholar]
- Lekven, A. C., Thorpe, C. J., Waxman, J. S. and Moon, R. T. (2001). Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev. Cell 1, 103-114. [DOI] [PubMed] [Google Scholar]
- Litingtung, Y. and Chiang, C. (2000). Specification of ventral neuron types is mediated by an antagonistic interaction between Shh and Gli3. Nat. Neurosci. 3, 979-985. [DOI] [PubMed] [Google Scholar]
- Liu, A., Wang, B. and Niswander, L. A. (2005). Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 132, 3103-3111. [DOI] [PubMed] [Google Scholar]
- Lunt, S. C., Haynes, T. and Perkins, B. D. (2009). Zebrafish ift57, ift88, and ift172 intraflagellar transport mutants disrupt cilia but do not affect hedgehog signaling. Dev. Dyn. 238, 1744-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, S. R., Ashique, A. M., Karlen, M., Wang, B., Shen, Y., Zarbalis, K., Reiter, J., Ericson, J. and Peterson, A. S. (2005). Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev. Biol. 287, 378-389. [DOI] [PubMed] [Google Scholar]
- Mitrophanov, A. Y., Jewett, M. W., Hadley, T. J. and Groisman, E. A. (2008). Evolution and dynamics of regulatory architectures controlling polymyxin B resistance in enteric bacteria. PLoS Genet. 4, e1000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic, J., Stigloher, C., Lillesaar, C. and Bally-Cuif, L. (2008). Gsk3beta/PKA and Gli1 regulate the maintenance of neural progenitors at the midbrain-hindbrain boundary in concert with E(Spl) factor activity. Development 135, 3137-3148. [DOI] [PubMed] [Google Scholar]
- Ocbina, P. J. and Anderson, K. V. (2008). Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts. Dev. Dyn. 237, 2030-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenthal, J., van Eeden, F. J., Haffter, P., Ingham, P. W. and Nusslein-Volhard, C. (2000). Two distinct cell populations in the floor plate of the zebrafish are induced by different pathways. Dev. Biol. 219, 350-363. [DOI] [PubMed] [Google Scholar]
- Park, H. L., Bai, C., Platt, K. A., Matise, M. P., Beeghly, A., Hui, C. C., Nakashima, M. and Joyner, A. L. (2000). Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development 127, 1593-1605. [DOI] [PubMed] [Google Scholar]
- Park, T. J., Haigo, S. L. and Wallingford, J. B. (2006). Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat. Genet. 38, 303-311. [DOI] [PubMed] [Google Scholar]
- Pedersen, L. B. and Rosenbaum, J. L. (2008). Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr. Top. Dev. Biol. 85, 23-61. [DOI] [PubMed] [Google Scholar]
- Rana, A. A., Barbera, J. P., Rodriguez, T. A., Lynch, D., Hirst, E., Smith, J. C. and Beddington, R. S. (2004). Targeted deletion of the novel cytoplasmic dynein mD2LIC disrupts the embryonic organiser, formation of the body axes and specification of ventral cell fates. Development 131, 4999-5007. [DOI] [PubMed] [Google Scholar]
- Robu, M. E., Larson, J. D., Nasevicius, A., Beiraghi, S., Brenner, C., Farber, S. A. and Ekker, S. C. (2007). p53 activation by knockdown technologies. PLoS Genet. 3, e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi, R., Milenkovic, L. and Scott, M. P. (2007). Patched1 regulates hedgehog signaling at the primary cilium. Science 317, 372-376. [DOI] [PubMed] [Google Scholar]
- Ross, A. J., May-Simera, H., Eichers, E. R., Kai, M., Hill, J., Jagger, D. J., Leitch, C. C., Chapple, J. P., Munro, P. M., Fisher, S. et al. (2005). Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat. Genet. 37, 1135-1140. [DOI] [PubMed] [Google Scholar]
- Schneider, L., Clement, C. A., Teilmann, S. C., Pazour, G. J., Hoffmann, E. K., Satir, P. and Christensen, S. T. (2005). PDGFRαα signaling is regulated through the primary cilium in fibroblasts. Curr. Biol. 15, 1861-1866. [DOI] [PubMed] [Google Scholar]
- Sekimizu, K., Nishioka, N., Sasaki, H., Takeda, H., Karlstrom, R. O. and Kawakami, A. (2004). The zebrafish iguana locus encodes Dzip1, a novel zinc-finger protein required for proper regulation of Hedgehog signaling. Development 131, 2521-2532. [DOI] [PubMed] [Google Scholar]
- Sharma, N., Berbari, N. F. and Yoder, B. K. (2008). Ciliary dysfunction in developmental abnormalities and diseases. Curr. Top. Dev. Biol. 85, 371-427. [DOI] [PubMed] [Google Scholar]
- Sun, Z., Amsterdam, A., Pazour, G. J., Cole, D. G., Miller, M. S. and Hopkins, N. (2004). A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131, 4085-4093. [DOI] [PubMed] [Google Scholar]
- Tran, P. V., Haycraft, C. J., Besschetnova, T. Y., Turbe-Doan, A., Stottmann, R. W., Herron, B. J., Chesebro, A. L., Qiu, H., Scherz, P. J., Shah, J. V. et al. (2008). THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat. Genet. 40, 403-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa, M. and Malicki, J. (2004). Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron 42, 703-716. [DOI] [PubMed] [Google Scholar]
- Tuch, B. B., Li, H. and Johnson, A. D. (2008). Evolution of eukaryotic transcription circuits. Science 319, 1797-1799. [DOI] [PubMed] [Google Scholar]
- Tyurina, O. V., Guner, B., Popova, E., Feng, J., Schier, A. F., Kohtz, J. D. and Karlstrom, R. O. (2005). Zebrafish Gli3 functions as both an activator and a repressor in Hedgehog signaling. Dev. Biol. 277, 537-556. [DOI] [PubMed] [Google Scholar]
- Vierkotten, J., Dildrop, R., Peters, T., Wang, B. and Ruther, U. (2007). Ftm is a novel basal body protein of cilia involved in Shh signalling. Development 134, 2569-2577. [DOI] [PubMed] [Google Scholar]
- Wilson, C. W., Nguyen, C. T., Chen, M. H., Yang, J. H., Gacayan, R., Huang, J., Chen, J. N. and Chuang, P. T. (2009). Fused has evolved divergent roles in vertebrate Hedgehog signalling and motile ciliogenesis. Nature 459, 98-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff, C., Roy, S. and Ingham, P. W. (2003). Multiple muscle cell identities induced by distinct levels and timing of hedgehog activity in the zebrafish embryo. Curr. Biol. 13, 1169-1181. [DOI] [PubMed] [Google Scholar]
- Wolff, C., Roy, S., Lewis, K. E., Schauerte, H., Joerg-Rauch, G., Kirn, A., Weiler, C., Geisler, R., Haffter, P. and Ingham, P. W. (2004). iguana encodes a novel zinc-finger protein with coiled-coil domains essential for Hedgehog signal transduction in the zebrafish embryo. Genes Dev. 18, 1565-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, Y., Bangs, F., Paton, I. R., Prescott, A., James, J., Davey, M. G., Whitley, P., Genikhovich, G., Technau, U., Burt, D. W. et al. (2009). The Talpid3 gene (KIAA0586) encodes a centrosomal protein that is essential for primary cilia formation. Development 136, 655-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.