Abstract
Objective
To better characterize the cumulus-oocyte interactions during oocyte maturation and fertilization using the cat model.
Design
Experimental in vitro study.
Setting
Smithsonian Institution.
Animal(s)
Domestic Shorthair cats.
Intervention(s)
Groups of denuded oocytes (DOs) and cumulus-oocyte complexes (COCs) were subjected to in vitro maturation (IVM; with or without FSH/LH, with or without the gap junction disruptor 1-heptanol, in separated groups or in co-culture) and inseminated in vitro (IVF; in separated groups or in co-culture).
Main Outcome Measure(s)
Nuclear maturation, pronuclear formation, kinetics of early embryo cleavage, and blastocyst formation/quality after different in vitro conditions were compared between DOs cultured separately and DOs co-cultured with COCs.
Result(s)
Without FSH/LH, the removal of cumulus cells prevented spontaneous meiotic resumption in DOs. With FSH/LH, groups of DOs progressed to the metaphase I stage, but fully advanced to metaphase II only in co-culture with intact (non-disrupted) COCs. Groups of DOs cultured separately were poorly fertilized and exhibited no blastocyst formation. In contrast, DOs co-cultured with intact COCs during IVM and IVF recovered fertilizability and ~ 35% formed blastocysts.
Conclusion(s)
Paracrine factors produced by cumulus-enclosed oocytes in the cat model will help to develop synthetic media for successful in vitro culture of DOs.
Keywords: Immature oocyte, cat model, cumulus cells, co-culture in vitro, gap junctions, meiosis, fertilization
INTRODUCTION
A network of gap junctions within the cumulus-oocyte complex (COC) enables cumulus cells to communicate with each other and with the oocyte. Cumulus-oocyte communications have been found to be essential in substrate transfer (ions, nucleotides, amino acids, metabolites, and regulatory molecules) during intrafollicular oocyte growth and for final nuclear/cytoplasmic maturation in the mouse (1, 2, 3), bovine (4, 5, 6), porcine models (7), as well as in human (6). After maturation, the expansion of the encompassing cumulus cells also creates the microenvironment that permits the spermatozoa to penetrate the oocyte (8, 9).
Even when COC physical integrity is intact during in vitro maturation (IVM) and fertilization (IVF), the developmental competence of such oocytes is suboptimal compared to counterparts matured in vivo (6, 10, 11, 12). Part of this compromise is related to a non-physiological, spontaneous resumption in meiosis that occurs in vitro in COC prematurely removed from the meiotic-inhibiting environment of the follicle. Additionally, the molecular pathways provoked in vitro by gonadotropins (FSH/LH) that contribute to the oocyte progression from the germinal vesicle (GV) to metaphase II (MII) are distinctive from cumulus-oocyte signaling induced by the LH peak that occurs naturally in vivo (6, 11). In the domestic cat model, in vitro matured oocytes also have impaired developmental competence (13). Although functional cumulus-oocytes communication appears important during IVM (14, 15, 16), the cellular and molecular basis of this phenomenon remains unclear and requires further investigations.
To decipher the role of cumulus cells during maturation and fertilization, previous studies have focused on the in vitro culture of immature oocytes denuded of their cells. Removing cumulus cells from the mouse, bovine and porcine oocyte before IVM do not prevent spontaneous resumption of meiosis in vitro, further impairs oocyte nuclear and cytoplasmic maturation, and also causes poor sperm-oocyte interaction (17, 18, 19, 20, 21, 22). Interestingly, denuded oocytes (DOs) co-cultured with isolated cumulus cells can partially recover meiotic and developmental competence as revealed in human (23), mouse (22, 24), and cow (8, 21, 25, 26) studies. More recently using another approach, a beneficial ‘cross-talk’ has been observed between DOs co-cultured with COCs, including between COCs originating from different follicle sizes (6, 21, 22, 24, 27). In the bovine model, there is benefit to meiotic maturation and fertilization of DOs during co-culture with intact COCs in the same micro-drop (21). However, there currently is no information available on the influence of cumulus cell removal on IVM and IVF events in any carnivore species, including the domestic cat, a significant research model in our laboratory. Besides filling a knowledge gap in the role of cumulus cells and mechanisms associated with meiotic resumption and fertilization capacity in a species being a valid model for human fertility (28, 29), such findings also have several applications. For example, our laboratory examines practical approaches for rescuing and/or cryopreserving germplasm from cats used as models for human disease where an individual can die abruptly or undergo ovariectomy for medical reasons (29, 30). It is common that the recovered COC does not have functional cumulus-oocyte communication to allow high levels of maturation, fertilization and embryo development to proceed in vitro at the time of oocyte collection or even after disruption by exposure to hyperosmotic cryoprotectant solutions (14, 15, 16). Thus, an improved understanding of cumulus-oocytes interactions would increase the ability to recover, preserve and use any intraovarian oocytes. There also are pragmatic reasons for exploring the potential of in vitro culture of DOs (rather than COCs), most having to do with convenience. More specifically, cumulus cells can (i) accelerate oocyte aging, (ii) be detrimental during cryopreservation and (iii) be an obstacle for conducting micromanipulations (7, 31, 32, 33, 34).
Our objective was to better understand the role of cumulus cells and cumulus-oocyte communications during IVM and IVF in the domestic cat model to ultimately be able to successfully culture DOs in vitro. Tactics included artificially removing cumulus cells, chemically disrupting cumulus-oocyte communications, and co-culturing DOs with COCs. This allowed generating new fundamental information about the control of mechanisms associated with (i) spontaneous meiotic resumption, (ii) nuclear maturation induced by FSH/LH, (iii) cytoplasmic maturation, and (iv) sperm-oocyte interaction.
MATERIALS AND METHODS
Oocyte collection and preparation
Ovaries from adult domestic cats were collected after routine ovario-hysterectomy from local veterinary clinics and transported to the laboratory within 6 h in PBS at 4°C. Immature oocytes then were recovered by repeatedly slicing the ovaries with a scalpel blade in Hepes-buffered Minimum Essential Medium (H-MEM; Gibco Laboratories, Grand Island, NY) supplemented with 1 mM pyruvate, 2 mM L-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 4 mg/ml bovine serum albumin (BSA; Sigma Chemical Co, St. Louis, MO). Only grade I immature oocytes (with homogenous dark cytoplasm, surrounded by several layers of compacted cumulus; 14) were selected and pooled from different ovaries collected on a given day. A proportion of immature oocytes (experimental design below) was denuded from the accompanying cumulus cells by gentle pipetting in 0.2% hyaluronidase (Sigma) before IVM.
In vitro maturation (IVM), fertilization (IVF), and culture of embryos
Basic IVM medium without FSH/LH consisted of MEM (Gibco) supplemented with 1 mM L-glutamine, 1 mM pyruvate, 100 IU/ml penicillin, 100 μg/ml streptomycin, 4 mg/ml BSA, and 1 μg/ml estradiol (Sigma). According to the experimental design (below and Fig. 1), groups of oocytes were exposed to basic IVM medium containing 0 or 4 mM 1-heptanol, a gap junction disruptor (Sigma) with or without 1 μg/ml FSH (1.64 IU/ml; NIDDK-ovine FSH-18; National Hormone and Pituitary Program, Rockville, MD) and 1 μg/ml LH (1.06 IU/ml; NIDDK-oLH-25; National Hormone and Pituitary Program). In vitro culture was performed in 50-μl microdrops under mineral oil (28 h; at 38.5°C in air with 5% CO2) with no more than 14 total oocytes per microdrop. For co-culture, groups of COCs and DOs were placed in the same microdrop (ratio 1:1; experimental design below and Fig. 1). After IVM, oocytes were extensively washed and percentage of COCs with expanded cumulus was recorded in each treatment group.
Fig. 1.
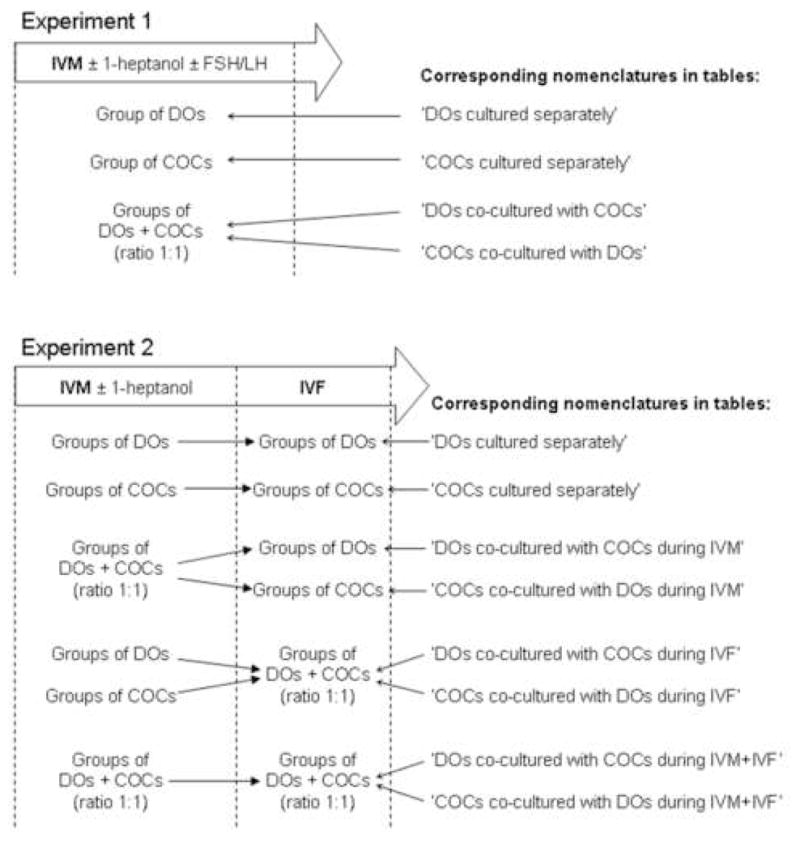
Culture conditions (and nomenclature used in tables) for groups of denuded oocytes (DOs) and cumulus-oocyte complexes (COCs) in Experiments 1 and 2 during in vitro maturation (IVM) and in vitro fertilization (IVF).
IVF then was performed using a standard protocol routinely used in our laboratory (35). Briefly, frozen/thawed, motile spermatozoa from a single sperm donor (3 males were alternatively used) were selected by swim-up processing in Ham’s F10 medium (Irvine Scientific, Santa Ana, CA) supplemented with 25 mM Hepes, 1 mM pyruvate, 2 mM glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 5% (v/v) fetal calf serum (Irvine Scientific; complete Ham’s with Hepes). Groups of oocytes were inseminated with 5 × 105 motile spermatozoa/ml in 50-μl microdrops of complete Ham’s without Hepes under equilibrated mineral oil at 38.5°C in air with 5% CO2. Approximately 10% of total oocytes in each treatment were incubated without spermatozoa to assess the incidence of spontaneous activation (parthenogenetic control). For co-culture, groups of COCs and DOs were placed in the same microdrop (no more than 14 total oocytes, ratio 1:1; experimental design below and Fig. 1). At 18 h post-insemination (hpi), oocytes were cleaned by gentle pipetting. Presumptive zygotes obtained from COCs and DOs were separately cultured in vitro for 7 days in complete Ham’s F10 (38.5°C, in air with 5% CO2). Proportions of the different embryo stages were recorded at 48 hpi and after 7 days of in vitro culture (16)..
Assessment of oocyte nuclear status and embryo stages
Oocytes or embryos were fixed at different time points (experimental design below) by air-drying on a microscope slide followed by exposure to 100% ethanol for at least 6 h at room temperature (35). Chromatin was stained with a PBS solution containing 10 μg/ml Hoechst 33342 (Sigma) and then examined by epifluorescence microscopy (Olympus BX 41; Olympus Corporation, Melville, NY). Incidence of nuclear maturation was defined as the number of oocytes at the telophase I (TI) or the MII stage relative to the total number of oocytes cultured in vitro. Immature oocytes were those arrested at the germinal vesicle breakdown (GVBD) stage or only progressing to metaphase I (MI) after culture (35). Oocytes with fragmented cytoplasm or without chromatin were considered degenerated. Fertilization was defined as two pronuclei present in the cytoplasm at 18 hpi. Oocytes with more than two pronuclei were considered polyspermic, whereas those with a single pronucleus were classified as parthenogenetically-activated. Number of oocytes with two pronuclei relative to the total number of oocytes placed in IVM represented overall fertilization success for a given treatment. After 7 days of in vitro culture, embryo stages were determined by the number of blastomere nuclei (after fixation and Hoechst staining). An embryo with 25 to 45 blastomeres without a blastocoele was defined as a morula (35). An embryo with a visible blastocoele before fixation and comprised of more than 50 blastomeres was classified as a blastocyst. Blastocyst quality was evaluated by the total number of blastomeres (the higher being the better). Percentage of cleaved embryos was calculated relative to the total number of oocytes cultured in vitro. However, cytoplasmic maturation in vitro (or developmental competence) was defined as the proportion of each embryo stage relative to the total number of cleaved embryos (35).
Experimental design and statistical analysis
Experiment 1 evaluated the impact of cumulus cell removal, disruption of cumulus-oocyte communications, and co-culture on subsequent oocyte nuclear maturation in vitro. In Experiment 1a (n = 848 total oocytes; 12 replicates), groups of DOs and COCs were allocated in equal numbers to IVM with or without FSH/LH and either separately or in co-culture (ratio 1:1; Fig. 1). In Experiment 1b, groups of DOs and COCs were equally allocated to IVM (with or without FSH/LH, and in the presence or absence of 4 mM 1-heptanol) and either separately or in co-culture (ratio 1:1; Fig. 1). After IVM (and observations of cumulus cell expansions in COCs), oocytes were fixed and stained to determine the incidence of nuclear maturation.
Experiment 2 examined the impact of cumulus cell removal and disruption of cumulus-oocyte communications on oocyte fertilizability and subsequent developmental competence in vitro. In Experiment 2a (n = 1601 total oocytes; 12 replicates) groups of DOs and COCs were allocated to different treatments that included presence or absence of 1-heptanol during IVM, and IVM and/or IVF separately or in co-culture (ratio 1:1; Fig. 1). At 18 hpi, DOs and COCs were fixed and stained to assess the presence of pronuclei. Ten oocytes in each treatment group also were fixed after incubation in IVF medium without spermatozoa to assess incidence of spontaneous activation. In Experiment 2b (n = 1703 total oocytes; 12 replicates), groups of DOs and COCs were subjected to IVM and IVF as described in Experiment 2a (Fig. 1). At 48 hpi, numbers of cleaved oocytes and visible blastomere numbers were recorded (but not fixed). After 7 days of in vitro culture, embryos were fixed and stained to determine number of blastomeres and proportion of different embryo stages per treatment group.
In each experiment, proportions were calculated by pooling the different replicates and then compared pair-wise using Chi-square testing. Mean numbers of blastomeres per blastocyst were compared using the Student t-test (SigmaStat; SPSS, Chicago, IL).
RESULTS
Impact of cumulus cell removal or cumulus-oocyte communication disruption on oocyte nuclear maturation in vitro
In Experiment 1a, groups of DOs cultured separately or co-cultured with COCs remained at the GV stage in the absence of FSH/LH, whereas COCs without gonadotropin spontaneously resumed meiosis, but only to the MI stage (Table 1) and without showing any cumulus expansion. In the presence of FSH/LH, groups of DOs and COCs cultured separately reached more advanced meiotic stages (P < 0.05) than in the absence of gonadotropins (Table 1). Proportions of COCs with expanded cumulus cells were not different (P > 0.05) between COCs cultured separately (97.1%) or co-cultured with DOs (85.7%). Percentage of MII in FSH/LH stimulated DOs co-cultured with COCs was similar (P > 0.05) to the value for similarly treated COCs and higher (P < 0.05) than that for groups of DOs cultured separately (Table 1). Proportions of COCs nuclear stages were not different (P > 0.05) when cultured separately or co-cultured with DOs (Table 1). Regardless of treatment, there were no differences (P > 0.05) in the proportions of degenerate oocytes after IVM culture (range, 2.8 to 6.4%).
TABLE 1.
Proportions of nuclear stages in groups of denuded oocytes (DOs) and cumulus-oocytes complexes (COCs) after 28 h of culture with or without FSH/LH and either separately or in co-culture.
| FSH/LH | n | % GV* | % GVBD* | % MI* | % MII* | |
|---|---|---|---|---|---|---|
| DOs cultured separately | − | 110 | 96.4a | 0.0a | 0.0a | 0.0a |
| DOs cultured separately | + | 102 | 0.0b | 29.4b | 40.2b | 24.5b |
| COCs cultured separately | − | 110 | 0.0b | 36.4b | 57.3c | 0.0a |
| COCs cultured separately | + | 103 | 0.0b | 0.0a | 10.7d | 86.4c |
| DOs co-cultured with COCs | − | 107 | 97.2a | 0.0a | 0.0a | 0.0a |
| COCs co-cultured with DOs | − | 105 | 0.0b | 38.1b | 58.1c | 0.0a |
| DOs co-cultured with COCs | + | 106 | 0.0b | 6.6c | 12.3d | 78.3c |
| COCs co-cultured with DOs | + | 105 | 0.0b | 0.0a | 11.4d | 83.8c |
Germinal Vesicle (GV), germinal vesicle breakdown (GVBD), metaphase I (MI), metaphase II (MII).
Within the same column, values with different superscripts differ (P < 0.05; Chi-square test).
In Experiment 1b, meiotic resumption in the groups of DOs was unaffected (P > 0.05) by presence or absence of 1-heptanol when these oocytes were (1) cultured separately with or without FSH/LH or (2) co-cultured with COCs without gonadotropins (Table 2). Additionally, the presence of 1-heptanol did not impair (P < 0.05) the incidence of cumulus cell expansions when COCs were cultured with FSH/LH (range, 83.3 to 97.9%). However, the presence of 1-heptanol negatively influenced (P < 0.05) the proportion of COCs undergoing meiotic resumption regardless of culture condition (Table 2). More specifically, the incidence of disrupted COCs nuclear stages in the presence of FSH/LH were similar (P > 0.05) to nuclear stage percentages observed in the FSH/LH stimulated DOs cultured separately (Table 2). However, spontaneous meiotic resumption of COCs (Table 2) as well as cumulus cell expansion was consistently prevented in the absence of FSH/LH. There also was no benefit (P > 0.05) to meiotic resumption when DOs were co-cultured with COCs in the simultaneous presence of FSH/LH and 1-heptanol (Table 2). As in Experiment 1a, percentages of degenerated oocytes were not different among treatments (range, 1.4 to 6.5%; P > 0.05).
TABLE 2.
Proportions of nuclear stages in groups of denuded oocytes (DOs) and cumulus-oocytes complexes (COCs) after 28 h of culture with or without FSH/LH, in the presence or absence of 1-heptanol, and either separately or in co-culture.
| FSH/LH | 1-heptanol | n | % GV* | % GVBD* | % MI* | % MII* | |
|---|---|---|---|---|---|---|---|
| DOs cultured separately | − | − | 69 | 94.2a | 0.0a | 0.0a | 0.0a |
| DOs cultured separately | − | + | 77 | 93.5a | 0.0a | 0.0a | 0.0a |
| DOs cultured separately | + | − | 78 | 0.0b | 32.1b | 41.0b | 21.8b |
| DOs cultured separately | + | + | 80 | 0.0b | 30.0b | 37.5b | 27.5b |
| COCs cultured separately | − | − | 77 | 0.0b | 39.0b | 58.4c | 0.0a |
| COCs cultured separately | − | + | 74 | 95.9a | 0.0a | 0.0a | 0.0a |
| COCs cultured separately | + | − | 70 | 0.0b | 2.9ac | 14.3d | 78.6c |
| COCs cultured separately | + | + | 80 | 0.0b | 27.5b | 42.5b | 25.0b |
| DOs co-cultured with COCs | − | − | 71 | 97.2a | 0.0a | 0.0a | 0.0a |
| COCs co-cultured with DOs | − | − | 80 | 0.0b | 37.5b | 60.0c | 0.0a |
| DOs co-cultured with COCs | − | + | 73 | 98.6a | 0.0a | 0.0a | 0.0a |
| COCs co-cultured with DOs | − | + | 75 | 96.0a | 0.0a | 0.0a | 0.0a |
| DOs co-cultured with COCs | + | − | 85 | 0.0b | 5.9c | 11.8d | 78.8c |
| COCs co-cultured with DOs | + | − | 83 | 0.0b | 6.0c | 15.7d | 74.7c |
| DOs co-cultured with COCs | + | + | 81 | 0.0b | 30.9b | 38.3b | 29.6b |
| COCs co-cultured with DOs | + | + | 80 | 0.0b | 31.3b | 40.0b | 23.8b |
Germinal Vesicle (GV), germinal vesicle breakdown (GVBD), metaphase I (MI), metaphase II (MII).
Within the same column, values with different superscripts differ (P < 0.05; Chi-square test).
Impact of cumulus cell removal or cumulus-oocyte communication disruption on oocyte cytoplasmic maturation in vitro and sperm-oocyte interaction
In the absence of 1-heptanol during IVM, percentages of fertilized DOs were the lowest (P < 0.05) when groups of DOs were cultured separately compared to COCs cultured in any conditions or DOs co-cultured with COCs during IVM, IVF, or IVM+IVF (Experiment 2a; Table 3). DOs reached optimal and similar (P > 0.05) fertilization success to COCs only when co-cultured with COCs during the combined IVM+IVF period (Table 3). Percentages of fertilized COCs were negatively affected (P < 0.05) after IVM with 1-heptanol, but remained higher (P < 0.05) than DOs cultured separately (Table 3). Although having no impact (P > 0.05) on fertilization success of DOs cultured separately, presence of 1-heptanol during IVM impaired (P < 0.05) fertilization percentage for DOs co-cultured with COCs during IVM or IVM+IVF (Table 3). As a result, DOs co-cultured with disrupted COCs during IVM only had the same fertilization success (P > 0.05) as DOs cultured separately after IVM with or without 1-heptanol (Table 3). Interestingly, DOs and COCs in co-culture during IVF or IVM+IVF reached similar (P > 0.05) fertilization success when exposed to 1-heptanol during IVM (Table 3). Fertilization success of COCs was not influenced (P > 0.05) by co-culture with DOs during IVM and/or IVF regardless of the presence or absence of 1-heptanol (Table 3). No spontaneous activation was observed when groups of DOs and COCs were incubated in the absence of spermatozoa regardless of the culture conditions. At 18 hpi, incidence of polyspermy was not different (range, 0 to 3%; P > 0.05) across treatment groups.
TABLE 3.
Percentages of fertilized oocytes, cleaved embryos, and proportions of embryo stages from groups of denuded oocytes (DOs) and cumulus-oocytes complexes (COCs) cultured separately or co-cultured during in vitro maturation (IVM), in vitro fertilization (IVF), or IVM+IVF in the presence or absence of 1-heptanol during IVM.
| 18 h post- insemination |
48 h post-insemination |
7 days of culture in vitro |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-heptanol during IVM |
n | % fertilized |
n | % cleaved |
% 4 cells /cleaved |
n embryos |
% < 8 cells |
% 8–16 cells |
% morulae |
% blastocysts |
|
| DOs cultured separately | − | 98 | 7.1a | 110 | 6.4a | 14.3a | 7 | 57.1a | 42.9a | 0.0a | 0.0a |
| DOs cultured separately | + | 97 | 7.2a | 109 | 6.4a | 0.0a | 7 | 71.4a | 28.6a | 0.0a | 0.0a |
| COCs cultured separately | − | 99 | 62.6b | 105 | 61.0b | 78.1b | 64 | 12.5b | 17.2b | 34.4b | 35.9b |
| COCs cultured separately | + | 101 | 17.8c | 107 | 16.8c | 16.7a | 18 | 44.4a | 27.8b | 27.8b | 0.0a |
| DOs co-cultured with COCs during IVM | − | 101 | 34.7d | 102 | 33.3d | 76.5b | 34 | 11.8b | 17.6b | 41.2b | 29.4b |
| COCs co-cultured with DOs during IVM | − | 99 | 63.6b | 105 | 62.9b | 75.8b | 66 | 13.6b | 18.2b | 33.3b | 34.8b |
| DOs co-cultured with COCs during IVM | + | 98 | 7.1a | 110 | 8.2a | 22.2a | 9 | 44.4a | 55.6a | 0.0a | 0.0a |
| COCs co-cultured with DOs during IVM | + | 101 | 16.8c | 107 | 17.8c | 10.5a | 19 | 42.1a | 26.3b | 31.6b | 0.0a |
| DOs co-cultured with COCs during IVF | − | 99 | 17.2c | 108 | 16.7c | 11.1a | 18 | 44.4a | 50.0a | 5.6c | 0.0a |
| COCs co-cultured with DOs during IVF | − | 100 | 63.0b | 106 | 61.3b | 80.0b | 65 | 12.3b | 20.0b | 32.3b | 35.4b |
| DOs co-cultured with COCs during IVF | + | 102 | 19.6c | 105 | 17.1c | 16.7a | 18 | 44.4a | 55.6a | 0.0a | 0.0a |
| COCs co-cultured with DOs during IVF | + | 101 | 18.8c | 107 | 17.8c | 21.1a | 19 | 42.1a | 36.8ab | 21.1b | 0.0a |
| DOs co-culture with COCs during IVM+IVF | − | 101 | 58.4b | 108 | 58.3b | 79.4b | 63 | 14.3b | 17.5b | 33.3b | 34.9b |
| COCs co-culture with DOs during IVM+IVF | − | 102 | 61.8b | 107 | 62.6b | 77.6b | 67 | 13.4b | 17.9b | 32.8b | 35.8b |
| DOs co-culture with COCs during IVM+IVF | + | 99 | 17.2c | 102 | 16.7c | 11.8a | 17 | 52.9a | 41.2a | 5.9c | 0.0a |
| COCs co-culture with DOs during IVM+IVF | + | 103 | 17.5c | 105 | 17.1c | 11.1a | 18 | 44.4a | 33.3ab | 22.2b | 0.0a |
Within the same column, values with different superscripts differ (P < 0.05; Chi-square test).
In Experiment 2b, percentages of cleavage were comparable to fertilization data (Table 3) with similar variations according to culture treatments. Observations at 48 hpi revealed that most embryos (range, 75.8 to 80.0%) were at the 4-cell stage when DOs were co-cultured with COCs during IVM or IVM+IVF in the absence of 1-heptanol (Table 3). Results were similar for groups of COCs cultured in any condition in the absence of the gap junction disruptor during IVM (Table 3). In contrast, fewer (P < 0.05) embryos were at the 4-cell stage at 48 hpi in all other conditions when 1-heptanol was present during IVM, DOs were cultured separately, or DOs were co-cultured with COCs only during IVF (Table 3). Proportions of blastocysts from DOs co-cultured with COCs during IVM or IVM+IVF were higher (P < 0.05) than when co-cultured during IVF or cultured separately (Table 3). Proportions of morulae and blastocysts from DOs co-cultured during IVM+IVF were optimal (~35%) and similar (P > 0.05) to proportions achieved with control COCs (Table 3). However, presence of 1-heptanol during IVM impaired (P < 0.05) the ability of DOs co-cultured with COCs during IVM or IVM+IVF to develop into blastocysts. Otherwise and in the absence of the gap junction disruptor during IVM, similar proportions (P > 0.05) of COCs cultured separately or co-cultured with DOs developed to the different embryo stages (Table 3). The proportions of embryos at different stages at the end of the experiment were similar (P > 0.05) for DOs cultured separately, DOs co-cultured with COCs during IVF only, and DOs co-cultured in the presence of 1-heptanol; none of these embryos reached the blastocyst stage (Table 3). For all treatment groups producing blastocysts, there were no differences (P > 0.05) in number of blastomeres/embryo (range, 78 to 95 blastomeres).
DISCUSSION
This study revealed that domestic cat cumulus cells and their communication with the oocyte were involved in the spontaneous meiotic resumption, the nuclear maturation induced by FSH/LH, the cytoplasmic maturation, and the eventual successful fertilization/early embryonic development. Spontaneous meiotic resumption was inhibited in the absence of FSH/LH during IVM by cumulus cell removal or disruption of cumulus-oocyte communications. Although gonadotropins could stimulate the DOs or the disrupted COCs to resume meiosis, maturation beyond the MI stage could not be fully achieved. Perhaps most intriguing was what appeared to be diffusible/paracrine factors from cumulus-enclosed oocytes that could positively influence meiotic progression and cytoplasmic maturation of DOs co-cultured in the same microdrop. As a result, it was possible to achieve normal nuclear maturation and developmental competence in DOs that were co-cultured with intact COCs during IVM. In addition, presence of COCs with expanded cumulus cells during IVF improved the poor fertilizability of DOs.
Intact cat COCs had the capacity of spontaneously resuming their nuclear maturation in the absence of FSH/LH as previously reported in the same species (15) and other animal models (mouse, 22; bovine, 36; and porcine, 37). From our study, however, it was clear that meiosis did not re-initiate in DOs or in COCs with disrupted cumulus-oocyte communications, which is different from that observed in the mouse (18), bovine (19) and porcine (17) models where meiosis spontaneously resumes. Upon removal of cumulus cells or disruption of cumulus-oocyte communications, cAMP level probably did not drop and suppress inhibition of meiotic resumption as in intact COCs (15). As a result, the oocyte spontaneous meiotic resumption was prevented as if intracellular level of cAMP was maintained artificially high (5).
Incomplete meiotic resumption in DOs or disrupted COCs supplemented with FSH/LH in culture also has been observed in the mouse (18) and bovine (38) model. It is important to note that compared to LH, the role of FSH probably was more direct and critical during IVM because this gonadotropin is rather known to up-regulate LH receptor formation in cumulus cells (39, 40, 41). Furthermore, an FSH effect on cat DOs only could be explained by the presence of FSH receptors on the oocyte membrane that, in turn, triggered meiotic resumption, as it has been demonstrated in human and pig oocytes (42). If existing in the cat, then this alternative signaling pathway could lead to the MI stage with DOs or COCs with disrupted communication failing to fully advance to MII. Thus, more studies of the MI/MII transition in these types of oocytes are warranted, including examining kinase activity, which has been found to be insufficient in poor quality or prepubertal oocytes to advance to MII (13, 27).
The ability of the DOs to proceed through MI and reach the MII was dictated by the presence of COCs in the same culture drop. The benefits of ‘group culture’ is not new for the cat as Spindler et al. (43) demonstrated enhanced development in vitro for marginal quality embryos co-incubated with better quality conspecific or even heterospecific counterparts. The present study supported the idea that there were paracrine factors from the cumulus-enclosed oocyte that appeared to positively affect meiotic progression of DOs maintained in the same microenvironment. Although the specifics are unknown, this favorable effect could be exerted by antioxidant properties conferred by the COCs, especially since it is now recognized that exogenous antioxidants improve maturation in vitro in the cat (35) and support specifically the MI to MII transition in pig oocytes (37). In addition, the intracellular pH of the growing mouse oocyte is controlled through pH-regulatory mechanisms residing in the granulosa cells, at least until the oocyte becomes self-sufficient in homeostasis (3). Interestingly, cat DOs were apparently able to take advantage of the pH-regulation activity of the cumulus cells from co-cultured COCs and not be compromised by intracellular pH variations.
Like in the bovine model (21), co-culture with intact COCs during IVM and IVF was optimal for cat DOs. As for bovine oocytes (20, 21, 44), it is apparent that intact cumulus-oocyte interaction is critical for ensuring complete nuclear and cytoplasmic maturation as well as promoting gamete interaction and embryogenesis. However, we revealed for the first time that paracrine factors produced by the cumulus-enclosed oocyte were beneficial to co-cultured DOs to reach comparable levels of nuclear and cytoplasmic maturation. In addition, beneficial effect of co-culture did not require any direct contact between DOs and COCs during IVM and IVF as already shown with bovine or mouse oocytes (21, 24). Interestingly, there seemed to be no advantage to any of the cat COCs in being co-cultured with DOs, which was not in agreement with a previous study in cows (45) that revealed that oocytes void of cumulus cells appear to be secreting beneficial factors. This likely was due to a difference in ratio between DOs and COCs (1:1 in our study) in comparison with the ratio (5:1) used in the latter report.
Disruption of cumulus-oocyte communications using 1-heptanol mainly compromised the ability of COCs to reach MII which resulted in fewer fertilized oocytes and compromised the beneficial effect on DOs. However, the presence of 1-heptanol did not decrease the proportion of COCs with expanded cumulus cells as previously observed in cow oocytes (4, 38). Interestingly, expanded cumulus cells benefited the sperm penetration in the COC itself as well as in DOs present in the same microdrop. It is known that microenvironment created by expanded cumulus cells in cow oocytes includes increased amounts of hyaluronic acid that enhance the spermatozoon ability to achieve capacitation and fertilization (8). Besides this, it is likely that expanded cumulus cells from co-cultured COCs also released other unknown beneficial factors during IVF that could support sperm capacitation and then penetration of DOs.
It was important to note that percentages of cleavage and fertilization were comparable among treatments suggesting that once fertilized, oocytes cleaved and likely did not experience developmental arrest at the pronuclear stage. Low proportions of 4-cell stage embryos at 48 hpi among the cleaved embryos (in comparison with control conditions) revealed slower kinetics of first cleavages which subsequently translated in poor blastocyst formation as seen in the bovine model (46). This was the case when groups of DOs were cultured separately or co-cultured with COCs only during IVF. The reason could be that no beneficial cytoplasmic factors (for COCs and DOs cultured in the same microdrop) were delivered by cumulus cells during IVM (through open cumulus-oocyte communications) as demonstrated in the bovine (21). Indeed, studies have demonstrated the positive role of glutathione (GSH) accumulation during IVM on the ability of cow and cat oocytes to reach the blastocyst stage after fertilization (15, 21, 47). For the present study, DOs separately may well have accrued less intracellular GSH. As a result, we now are examining the impact of supplementing thiol compounds during IVM of DOs cultured alone to promote their developmental potential.
Denuded bovine oocytes cultured alone have the capacity to advance to blastocysts in vitro after IVF (21, 25, 26, 33). However, in our system, cat oocytes void of cumulus cells failed to become blastocysts, largely because of poor nuclear and cytoplasmic maturation followed by a poor fertilization success. Our observations also confirmed that FSH/LH could not affect the developmental competence of DOs when cultured separately (11). Specifically, the few DOs cultured separately that fertilized and cleaved, failed to develop beyond the 8-cell stage. Hoffert et al. (48) has demonstrated that the maternal-to-zygotic control of development (MZT) occurs in the cat embryo at about the 8-cell division in the IVM/IVF process. Since maternal developmental factors are cytoplasmic in origin, it seems plausible that insufficient cytoplasmic maturation may have precipitated MZT failure. This poor function also could compromise genetic manifestations, similar to the aberrant Oct-4 expression reported in the mouse DOs that results in abnormal blastomere numbers (49). Interestingly, co-culture with COCs seemed to restore DOs cumulus-mediating effects involved in oocyte nuclear and cytoplasmic maturation, such as leptin differentially regulated gene expression recently described in the bovine model (50). Fertilization success and blastocyst formation in the co-cultured DOs in the present cat study were comparable to results routinely obtained in our laboratory with COCs (35). However, because DOs might have spindle abnormalities (51), ongoing studies are examining the incidence of mosaicism in blastocysts and embryo viability after transfer into conspecific recipient females.
Our study strongly endorses the concept of cross-talk among DOs and COCs cultured together. Biological benefits conferred by the presence of COCs at different steps are summarized in Figure 2. Although DOs can re-initiate their meiosis (step 1), paracrine factors A and B from cumulus-enclosed oocytes benefit to the progression of DOs to the MII (step 2) as well as their cytoplasmic maturation (step 3), respectively. During IVF, presence of expanded cumulus cells positively impact the fertilizability of DOs (Factors C; step 4). These new finding expand the original work of Spindler et al. (43) who found that developmental success of cat embryos in vitro was enhanced in intermediate quality IVM/IVF zygotes that were co-mingled with equal or high quality embryos from cats or even the cow. What is intriguing, of course, is that the cross-talk extends to oocyte-to-oocyte communication, with the co-cultured COCs being the source of the unknown advantageous factor(s) (Fig. 2). Benefits derived from group culture of COCs from the same follicular size and stage has not been demonstrated clearly for any other species studied to date, including the cow (52, 53). Furthermore, to-date, there has been no evidence for similar type of interactions among oocytes within the ovary (54). Clearly a priority now is identifying the paracrine factor(s) while determining if this phenomenon and mechanism is species-specific by using intact cow oocytes to support denuded cat oocytes.
Fig. 2.

A hypothetical cat model for mechanisms involved during in vitro maturation (IVM) and fertilization (IVF) of denuded oocytes (DOs) in co-culture with cumulus-oocyte complexes (COCs) that release paracrine factors at different steps (2, 3, and 4). Germinal vesicle stage (GV), metaphase I stage (MI), metaphase II stage (MII), glutathione (GSH).
We discovered a phenomenon that rather is a peculiarity of gamete in vitro culture. Overcoming the absence of cumulus cells is possible and also appears as a new way to control in vitro cellular events during oocyte IVM. Since there is no spontaneous meiotic resumption in cat DOs, enhancing oocyte developmental competence by attenuating the spontaneous meiotic resumption of oocytes in vitro appears easier than in other species (6). Thus, we could potentially better direct cellular activities in DOs and mimic optimal in vivo processes in terms of timing, molecular mechanisms, as well as metabolic activities (13, 15, 55) to improve IVM success of oocytes. We therefore currently are developing a synthetic IVM medium for DOs. This simple culture system could be a solution to improve IVM and IVF success of oocytes with compromised cumulus-oocyte communications (prepubertal oocytes, 27; poor quality oocytes, 14, 35). It also will provide technical solutions for the in vitro culture of immature oocytes denuded for micromanipulation or cryopreservation (33, 34, 56). Such novel approaches using the cat model could help develop better strategies to circumvent infertility problems in human.
Acknowledgments
This study was supported by Grant Number K01 RR020045 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Funding also was received from the American University. Authors thank Drs. Michael Cranfield and Brent Whitaker (Maryland Line Animal Rescue) and Dr. Darby Thornburgh (Petworth Animal Hospital) for providing domestic cat ovaries.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eppig JJ. The relationship between cumulus cell-oocyte coupling, oocyte meiotic maturation, and cumulus expansion. Develop Biol. 1982;89:268–72. doi: 10.1016/0012-1606(82)90314-1. [DOI] [PubMed] [Google Scholar]
- 2.Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121:647–53. doi: 10.1530/rep.0.1210647. [DOI] [PubMed] [Google Scholar]
- 3.Fitzharris G, Baltz JM. Granulosa cells regulate intracellular pH of the murine growing oocyte via gap junctions: development of independent homeostasis during oocyte growth. Development. 2006;133:591–9. doi: 10.1242/dev.02246. [DOI] [PubMed] [Google Scholar]
- 4.Vozzi C, Formenton A, Chanson A, Senn A, Sahli R, Shaw P, et al. Involvement of connexin 43 in meiotic maturation of bovine oocytes. Reproduction. 2001;122:619–28. [PubMed] [Google Scholar]
- 5.Thomas RE, Armstrong DT, Gilchrist RB. Bovine cumulus cell-oocyte gap junctional communication during in vitro maturation in response to manipulation of cell-specific cyclic adenosine 3′,5′-monophosophate levels. Biol Reprod. 2004;70:548–56. doi: 10.1095/biolreprod.103.021204. [DOI] [PubMed] [Google Scholar]
- 6.Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008 Jan 5; doi: 10.1093/humupd/dmm040. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Wu C, Rui R, Dai J, Zhang C, Ju S, Xie B, et al. Effects of cryopreservation on the developmental competence, ultrastructure and cytoskeletal structure of porcine oocytes. Mol Reprod Dev. 2006;73:1454–62. doi: 10.1002/mrd.20579. [DOI] [PubMed] [Google Scholar]
- 8.Tanghe S, Van Soom A, Mehrzad J, Maes D, Duchateau L, de Kruif A. Cumulus contributions during bovine fertilization in vitro. Theriogenology. 2003;60:135–49. doi: 10.1016/s0093-691x(02)01360-2. [DOI] [PubMed] [Google Scholar]
- 9.de Matos DG, Miller K, Scott R, Tran CA, Kagan D, Nataraja SG, et al. Leukemia inhibitory factor induces cumulus expansion in immature human and mouse oocytes and improves mouse two-cell rate and delivery rates when it is present during mouse in vitro oocyte maturation. Fertil Steril. 2008 Jan 25; doi: 10.1016/j.fertnstert.2007.10.061. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Luciano AM, Modina S, Vassena R, Milanesi E, Lauria A, Gandolfi F. Role of intracellular cyclic adenosine 3′,5′-monophosphate concentration and oocyte-cumulus cells communications on the acquisition of the developmental competence during in vitro maturation of bovine oocyte. Biol Reprod. 2004;70:465–72. doi: 10.1095/biolreprod.103.020644. [DOI] [PubMed] [Google Scholar]
- 11.Sirard MA, Desrosier S, Assidi M. In vivo and in vitro effects of FSH on oocyte maturation and developmental competence. Theriogenology. 2007;68 (Suppl 1):71–6. doi: 10.1016/j.theriogenology.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 12.Nogueira D, Ron-El R, Friedler S, Schachter M, Raziel A, Cortvrindt R, et al. Meiotic arrest in vitro by phosphodiesterase 3-inhibitor enhances maturation capacity of human oocytes and allows subsequent embryonic development. Biol Reprod. 2006;74:177–84. doi: 10.1095/biolreprod.105.040485. [DOI] [PubMed] [Google Scholar]
- 13.Bogliolo L, Leoni G, Ledda S, Zedda MT, Bonelli P, Madau L, et al. M-phase promoting factor (MPF) and mitogen activated protein kinases (MAPK) activities of domestic cat oocytes matured in vitro and in vivo. Cloning Stem Cells. 2004;6:15–23. doi: 10.1089/15362300460743790. [DOI] [PubMed] [Google Scholar]
- 14.Wood TC, Wildt DE. Effect of the quality of the cumulus-oocyte complex in the domestic cat on the ability of oocytes to mature, fertilize and develop into blastocysts in vitro. J Reprod Fertil. 1997;110:355–60. doi: 10.1530/jrf.0.1100355. [DOI] [PubMed] [Google Scholar]
- 15.Luvoni GC, Chigioni S, Perego L, Lodde V, Modina S, Luciano AM. Effect of gonadotropins during in vitro maturation of feline oocytes on oocyte-cumulus cells functional coupling and intracellular concentration of glutathione. Anim Reprod Sci. 2006;96:66–78. doi: 10.1016/j.anireprosci.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Comizzoli P, Wildt DE, Pukazhenthi BS. Impact of anisosmotic conditions on structural and functional integrity of cumulus-oocyte complexes at the germinal vesicle stage in the domestic cat. Mol Reprod Dev. 2008;75:345–54. doi: 10.1002/mrd.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyano T, Ebihara M, Goto Y, Hirao Y, Nagai T, Kato S. Inhibitory action of hypoxanthine on meiotic resumption of denuded pig follicular oocytes in vitro. J Exp Zool. 1995;273:70–5. doi: 10.1002/jez.1402730109. [DOI] [PubMed] [Google Scholar]
- 18.Faerge I, Terry B, Kalous J, Wahl P, Lessl M, Ottesen JL, et al. Resumption of meiosis induced by meiosis-activating sterol has a different signal transduction pathway than spontaneous resumption of meiosis in denuded mouse oocytes cultured in vitro. Biol Reprod. 2001;65:1751–58. doi: 10.1095/biolreprod65.6.1751. [DOI] [PubMed] [Google Scholar]
- 19.Mayes MA, Sirard MA. Effect of type 3 and type 4 phosphodiesterase inhibitors on the maintenance of bovine oocytes in meiotic arrest. Biol Reprod. 2002;66:180–4. doi: 10.1095/biolreprod66.1.180. [DOI] [PubMed] [Google Scholar]
- 20.Fatehi AN, Zeinstra EC, Kooij RV, Colenbrander B, Bevers MM. Effect of cumulus cell removal of in vitro matured bovine oocytes prior to in vitro fertilization on subsequent cleavage rate. Theriogenology. 2002;57:1347–55. doi: 10.1016/s0093-691x(01)00717-8. [DOI] [PubMed] [Google Scholar]
- 21.Luciano AM, Lodde V, Beretta MS, Colleoni S, Lauria A, Modina S. Developmental capability of denuded bovine oocyte in a co-culture system with intact cumulus-oocyte complexes: role of cumulus cells, cyclic adenosine 3′,5′-monophosphate, and glutathione. Mol Reprod Dev. 2005;71:389–97. doi: 10.1002/mrd.20304. [DOI] [PubMed] [Google Scholar]
- 22.Downs SM, Gilles R, Vanderhoef C, Humpherson PG, Leese HJ. Differential response of cumulus cell-enclosed and denuded mouse oocytes in a meiotic induction model system. Mol Reprod Dev. 2006;73:379–89. doi: 10.1002/mrd.20416. [DOI] [PubMed] [Google Scholar]
- 23.Torre ML, Munari E, Albani E, Levi-Setti PE, Villani S, Faustini M, et al. In vitro maturation of human oocytes in a follicle-mimicking three-dimensional coculture. Fertil Steril. 2006;86:572–76. doi: 10.1016/j.fertnstert.2006.02.090. [DOI] [PubMed] [Google Scholar]
- 24.Ge L, Han D, Lan GC, Zhou P, Liu Y, Zhang X, et al. Factors affecting the in vitro action of cumulus cells on the maturing mouse oocytes. Mol Reprod Dev. 2008;75:136–42. doi: 10.1002/mrd.20753. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto S, Saeki K, Nagao Y, Minami N, Yamada M, Utsumi K. Effects of cumulus cell density during in vitro maturation of the developmental competence of bovine oocytes. Theriogenology. 1998;49:1451–63. doi: 10.1016/s0093-691x(98)00091-0. [DOI] [PubMed] [Google Scholar]
- 26.Geshi M, Takenouchi N, Yamauchi N, Nagai T. Effects of sodium pyruvate in nonserum maturation medium on maturation, fertilization, and subsequent development of bovine oocytes with or without cumulus cells. Biol Reprod. 2000;63:1730–4. doi: 10.1095/biolreprod63.6.1730. [DOI] [PubMed] [Google Scholar]
- 27.Cecconi S, Mauro A, Capacchietti G, Berardinelli P, Bernabò N, Di Vincenzo AR, et al. Meiotic maturation of incompetent prepubertal sheep oocytes is induced by paracrine factor(s) released by gonadotropin-stimulated oocyte-cumulus cell complexes and involves MAPK activation. Endocrinology. 2008;149:100–7. doi: 10.1210/en.2007-0874. [DOI] [PubMed] [Google Scholar]
- 28.Pukazhenthi BS, Neubauer K, Jewgenow K, Howard J, Wildt DE. The impact and potential etiology of teratospermia in the domestic cat and its wild relatives. Theriogenology. 2006;66:112–21. doi: 10.1016/j.theriogenology.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Comizzoli P, Widlt DE, Pukazhenthi BS. Oocyte quality and in vitro culture in the domestic cat. ART & Science. 2004;4:11–3. [Google Scholar]
- 30.Pukazhenthi B, Comizzoli P, Travis AJ, Wildt DE. Applications of emerging technologies to the study and conservation of threatened and endangered species. Reprod Fertil Dev. 2006;18:77–90. doi: 10.1071/rd05117. [DOI] [PubMed] [Google Scholar]
- 31.Qiao TW, Liu N, Miao DQ, Zhang X, Han D, Ge L, et al. Cumulus cells accelerate aging of mouse oocytes by secreting a soluble factor(s) Mol Reprod Dev. 2008;75:521–28. doi: 10.1002/mrd.20779. [DOI] [PubMed] [Google Scholar]
- 32.Ruppert-Lingham CJ, Paynter SJ, Godfrey J, Fuller BJ, Shaw RW. Developmental potential of murine germinal vesicle stage cumulus-oocyte complexes following exposure to dimethylsulphoxide or cryopreservation: loss of membrane integrity of cumulus cells after thawing. Hum Reprod. 2003;18:392–98. doi: 10.1093/humrep/deg071. [DOI] [PubMed] [Google Scholar]
- 33.Modina S, Beretta MS, Lodde V, Lauria A, Luciano AM. Cytoplasmic changes and developmental competence of bovine oocyte cryopreserved without cumulus cells. Eur J Histochem. 2004;48:337–46. [PubMed] [Google Scholar]
- 34.Bogliolo L, Ariu F, Fois S, Rosati I, Zedda MT, Leoni G, et al. Morphological and biochemical analysis of immature ovine oocytes vitrified with or without cumulus cells. Theriogenology. 2007;68:1138–49. doi: 10.1016/j.theriogenology.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Comizzoli P, Wildt DE, Pukazhenthi BS. Overcoming poor in vitro nuclear maturation and developmental competence of domestic cat oocytes during the non-breeding season. Reproduction. 2003;126:809–16. [PubMed] [Google Scholar]
- 36.Byskov AG, Yding Andersen C, Hossaini A, Guoliang X. Cumulus cells of oocyte-cumulus complexes secrete a meiosis-activating substance when stimulated with FSH. Mol Reprod Dev. 1997;46:296–305. doi: 10.1002/(SICI)1098-2795(199703)46:3<296::AID-MRD8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 37.Tao Y, Zhou B, Xia G, Wang F, Wu Z, Fu M. Exposure to L-ascorbic acid or alpha-tocopherol facilitates the development of porcine denuded oocytes from metaphase I to metaphase II and prevents cumulus cells from fragmentation. Reprod Domest Anim. 2004;39:52–7. doi: 10.1046/j.1439-0531.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- 38.Atef A, Francois P, Christian V, Marc-Andre S. The potential role of gap junction communication between cumulus cells and bovine oocytes during in vitro maturation. Mol Reprod Dev. 2005;71:358–67. doi: 10.1002/mrd.20281. [DOI] [PubMed] [Google Scholar]
- 39.Okazaki T, Nishibori M, Yamashita Y, Shimada M. LH reduces proliferative activity of cumulus cells and accelerates GVBD of porcine oocytes. Mol Cell Endocrinol. 2003;209:43–50. doi: 10.1016/j.mce.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Calder MD, Caveney AN, Smith LC, Watson AJ. Responsiveness of bovine cumulus-oocyte-complexes (COC) to porcine and recombinant human FSH, and the effect of COC quality on gonadotropin receptor and Cx43 marker gene mRNAs during maturation in vitro. Reprod Biol Endocrinol. 2003;1:1–14. doi: 10.1186/1477-7827-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu M, Chen X, Yan J, Lei L, Jin S, Yang J, et al. Luteinizing hormone receptors expression in cumulus cells closely related to mouse oocyte meiotic maturation. Front Biosci. 2007;12:1804–13. doi: 10.2741/2189. [DOI] [PubMed] [Google Scholar]
- 42.Méduri G, Charnaux N, Driancourt MA, Combettes L, Granet P, Vannier B, et al. Follicle-stimulating hormone receptors in oocytes? J Clin Endocrinol Metab. 2002;87:2266–76. doi: 10.1210/jcem.87.5.8502. [DOI] [PubMed] [Google Scholar]
- 43.Spindler RE, Crichton EG, Agca Y, Loskutoff N, Critser J, Gardner DK, et al. Improved felid embryo development by group culture is maintained with heterospecific companions. Theriogenology. 2006;66:82–92. doi: 10.1016/j.theriogenology.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, Jiang S, Wozniak PJ, Yang X, Godke RA. Cumulus cell function during bovine oocyte maturation, fertilization, and embryo development in vitro. Mol Reprod Dev. 1995;40:338–44. doi: 10.1002/mrd.1080400310. [DOI] [PubMed] [Google Scholar]
- 45.Hussein TS, Thompson JG, Gilchrist RB. Oocyte-secreted factors enhance oocyte developmental competence. Dev Biol. 2006;296:514–21. doi: 10.1016/j.ydbio.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 46.Lequarre AS, Marchandise J, Moreau B, Massip A, Donnay I. Cell cycle duration at the time of maternal zygotic transition for in vitro produced bovine embryos: effect of oxygen tension and transcription inhibition. Biol Reprod. 2003;69:1707–13. doi: 10.1095/biolreprod.103.017178. [DOI] [PubMed] [Google Scholar]
- 47.de Matos DG, Furnus CC, Moses DF, Baldassarre H. Effect of cysteamine on glutathione level and developmental capacity of bovine oocyte matured in vitro. Mol Reprod Dev. 1995;42:432–6. doi: 10.1002/mrd.1080420409. [DOI] [PubMed] [Google Scholar]
- 48.Hoffert KA, Anderson GB, Wildt DE, Roth TL. Transition from maternal to embryonic control of development in IVM/IVF domestic cat embryos. Mol Reprod Dev. 1997;48:208–15. doi: 10.1002/(SICI)1098-2795(199710)48:2<208::AID-MRD8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 49.Chang HC, Liu H, Zhang J, Grifo J, Krey LC. Developmental incompetency of denuded mouse oocytes undergoing maturation in vitro is ooplasmic in nature and is associated with aberrant Oct-4 expression. Hum Reprod. 2005;7:1958–68. doi: 10.1093/humrep/dei003. [DOI] [PubMed] [Google Scholar]
- 50.Paula-Lopes FF, Boelhauve M, Habermann FA, Sinowatz F, Wolf E. Leptin promotes meiotic progression and developmental capacity of bovine oocytes via cumulus cell-independent and -dependent mechanisms. Biol Reprod. 2007;76:532–41. doi: 10.1095/biolreprod.106.054551. [DOI] [PubMed] [Google Scholar]
- 51.Li GP, Bunch TD, White KL, Rickords L, Liu Y, Sessions BR. Denuding and centrifugation of maturing bovine oocytes alters oocyte spindle integrity and the ability of cytoplasm to support parthenogenetic and nuclear transfer embryo development. Mol Reprod Dev. 2006;73:446–51. doi: 10.1002/mrd.20436. [DOI] [PubMed] [Google Scholar]
- 52.Carolan C, Lonergan P, Khatir H, Mermillod P. In vitro production of bovine embryos using individual oocytes. Mol Reprod Dev. 1996;45:145–50. doi: 10.1002/(SICI)1098-2795(199610)45:2<145::AID-MRD6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 53.Hagemann LJ, Weilert LL, Beaumont SE, Tervit HR. Development of bovine embryos in single in vitro production (sIVP) systems. Mol Reprod Dev. 1998;51:143–7. doi: 10.1002/(SICI)1098-2795(199810)51:2<143::AID-MRD3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 54.Takagi M, Choi YH, Kamishita H, Acosta TJ, Wijayagunawardane MP, Miyamoto A, et al. Oocyte quality of small antral follicles coexisting with cystic follicles in the ovaries of the cow. Anim Reprod Sci. 1998;51:195–203. doi: 10.1016/s0378-4320(98)00070-0. [DOI] [PubMed] [Google Scholar]
- 55.Spindler RE, Pukazhenthi BS, Wildt DE. Oocyte metabolism predicts the development of cat embryos to blastocyst in vitro. Mol Reprod Dev. 2000;56:163–71. doi: 10.1002/(SICI)1098-2795(200006)56:2<163::AID-MRD7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 56.Liu H, Zhang J, Krey LC, Grifo JA. In-vitro development of mouse zygotes following reconstruction by sequential transfer of germinal vesicles and haploid pronuclei. Hum Reprod. 2000;15:1997–2002. doi: 10.1093/humrep/15.9.1997. [DOI] [PubMed] [Google Scholar]


