Abstract
One of the goals of pharmacogenomics is the use of genetic variants to predict an individual's response to treatment. Although numerous candidate and genome-wide associations have been made for cardiovascular response-outcomes, little is known about how a given polymorphism imposes the phenotype. Such mechanisms are important, because they tie the observed human response to specific signaling alterations and thus provide cause-and-effect relationships, aid in the design of hypothesis-based clinical studies, can help to devise workaround drugs, and can reveal new aspects of the pathophysiology of the disease. Here we discuss polymorphisms within the adrenergic receptor network in the context of heart failure and β-adrenergic receptor blocker therapy, where multiple approaches to understand the mechanism have been undertaken. We propose a comprehensive series of studies, ranging from transfected cells, transgenic mice, and ex vivo and in vitro human studies as a model approach to explore mechanisms of action of pharmacogenomic effects and extend the field beyond observational associations.
Chronic heart failure represents a significant treatment challenge, and although various pharmacologic therapies have been introduced over the past 2 decades, mortality remains high, ∼40 to 50% of individuals dying within 5 years of diagnosis and ∼25% dying within the first year. Well recognized within the field is the high degree of interindividual variability in the response to drugs in the treatment of heart failure that is not readily attributed to clinical, demographic, or environmental factors (van Campen et al., 1998). This variability has led to investigation of potential genetic factors that influence drug responsiveness in heart failure, a field termed pharmacogenomics (or pharmacogenetics). In this article, we review the population genomics, molecular properties, and the results of clinical studies of common polymorphisms within the adrenergic receptor signaling network in heart failure, with an emphasis on linking potential molecular mechanisms to the human phenotypes. Here we refer to a polymorphism as a variation in a sequence, compared with a common reference sequence, that is found to occur with an allele frequency of 1% or greater in any population.
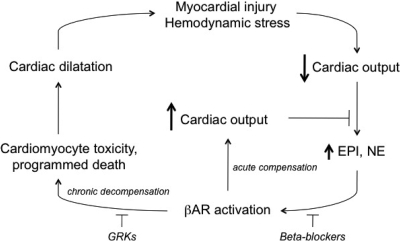
In acute loss of organ perfusion from virtually any cause, or when an increase in perfusion is required, such as during exercise or stress, the body responds by activation of the sympathetic nervous system. Increases in cardiac output are due to activation of cardiomyocyte β-adrenergic receptors (βAR). This system is well adapted for acute (short-term) needs for enhanced cardiac performance, consistent with the “fight-or-flight” nature of the sympathetic nervous system. However, chronic stimulation represents a less-than-optimal response to this physiologic need, and can ultimately contribute to pathogenic effects. In chronic heart failure, a persistently activated sympathetic nervous system is primarily manifested by increased plasma norepinephrine, a result of enhanced release of the neurotransmitter from presynaptic nerve terminals such as those that innervate the heart. Such persistent βAR activation in the failing heart, which has limited metabolic and physiologic reserves, leads to worsening heart failure. β-Adrenergic receptor blockers (β-blockers) appear to exert their therapeutic effect in heart failure by breaking this cycle, via antagonizing the effects of norepinephrine at cardiomyocyte βAR (Fig. 1). With proper titration, cardiac energetics improve, the cardiotoxic effects of the β1AR subtype are diminished, and “reverse remodeling” of heart failure associated gene-expression patterns occurs, leading to enhanced cardiac output and survival (Bristow, 2003; Mann and Bristow, 2005). With improved cardiac function and systemic perfusion, sympathetic activation is reduced as reflected in lower plasma norepinephrine levels. Cardiac βAR function, which is reduced in the failing heart, also improves with β-blocker treatment (Brodde, 2007). Indeed, the expression and function of βAR in chronic heart failure is in constant flux, based on hemodynamic status and treatment effects. β1- and β2AR are expressed on human cardiomyocytes, with β1AR being the primary mediator of catecholamine-mediated increases in inotropy and chronotropy. In chronic heart failure, β1AR expression is decreased, whereas β2AR expression is relatively stable; both β1- and β2AR functional coupling is desensitized (Bristow et al., 1990). The down-regulation and desensitization of β1AR is thought to be due to chronically elevated catecholamines and may be enhanced due to increased expression of G-protein coupled receptor kinases (GRKs), which is also observed in human heart failure (Hata et al., 2004). GRKs phosphorylate β1- and β2AR, resulting in the binding of β-arrestin to the receptor, which sterically interdicts between receptor and Gs and promotes uncoupling. The decreased βAR function in heart failure is not sufficient, however, to protect the heart from the aforementioned vicious cycle. An intriguing conundrum surrounds how best to break the cycle. Several studies have shown that inhibiting the increase in GRK expression has a salutary effect in animal models of heart failure (Hata et al., 2004). This is accompanied by restored βAR function. Because β-arrestin also acts as a signaling molecule (DeWire et al., 2007), it is not entirely clear whether decreasing these other effects of altered GRK expression improves cardiac function, which results in restoration of βAR signaling, or whether improved βAR signaling is the primary event that results in long-term restoration of cardiac function.
Fig. 1.
The vicious cycle of chronic catecholamine stimulation in heart failure. With an acute event and a healthy heart, βAR activation by catecholamines increases cardiac output without deleterious effects (within limits). With prolonged stimulation of a failing heart, which has less capacity to respond, continued forced contractility has extensive maladaptive consequences leading to worsening cardiac output. The cycle can be attenuated by β-blockers or GRK activity. See Introduction for discussion.
Presynaptic cardiac nerve terminals express two adrenergic receptors that act in a negative feedback manner to limit NE release. These receptors, the α2A- and α2CAR subtypes, seem to control release of NE from high- and low-frequency stimuli, respectively (Hein et al., 1999). Double-knockout α2A/α2C mice develop heart failure, presumably due to unregulated NE release (Hein et al., 1999). Even heterozygous [α2C(+/-)] knockout mice, under the conditions of pressure overload from transaortic constriction, develop heart failure, suggesting that one or both of these α2AR subtypes may be potential drug targets for heart failure (Gilsbach et al., 2007).
A Mechanistic Approach for Pharmacogenomic Studies
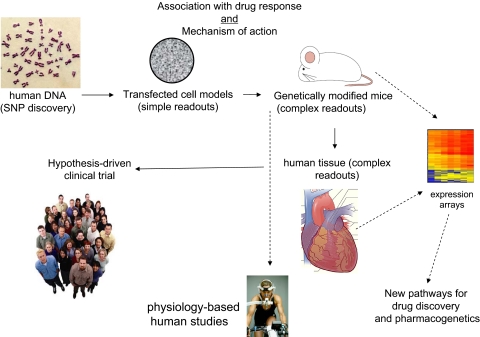
Our initial investigation of potential pharmacogenomic loci for β-blocker treatment in heart failure was focused on the primary therapeutic target, the β1AR. This candidate gene approach (as opposed to unbiased genomic-wide scans) is appropriate, in our opinion, for pharmacogenomic studies where the drug target and the downstream signaling events, are well established. This approach is outlined in Fig. 2. Initial polymorphism discovery is carried out using a reference set of genomic DNA from a collection of ethnically diverse individuals, such as the Human Variation Panel of the Coriell Institute (http://ccr.coriell.org/nigms/cells/humdiv.html). Typically, samples from 40 European Americans (whites), 40 African Americans, and 40 Asians are used. This provides for a 95% probability of detecting (at least once) polymorphisms with allele frequencies of ∼0.03 (3%) if the polymorphism is completely confined to one racial group, and ∼0.01 if found in all three racial groups. Although it might be desirable to use samples derived from patients with the disease of interest, the prevailing notion is that common polymorphisms will be present in the “normal” population, perhaps at a different allele frequency than the diseased population, but nevertheless present, and thus a reference group such as that indicated above is acceptable for polymorphism discovery. In the approach discussed here (Fig. 2), any nonsynonymous polymorphisms, as well as those in the promoter, or other untranslated regions, are studied in vitro in transfected cell systems. From these results, the generation of transgenic mice is considered, which provides an opportunity to explore the variants in the cell type of interest, in the organ of interest, under the relevant physiologic conditions. And of course, physiologic measures can be obtained that can be correlated with signaling events. In tandem with mouse studies, human tissue studies can be used for physiologic measurements if freshly obtained, or signaling and expression studies if any banked tissue is available. In addition, small cohorts of genotyped patients can be studied for short-term physiologic phenotypes, such as the response exercise or to infusions of agonists or antagonists. At this juncture in these studies, a phenotype may well be established at the molecular and physiologic levels using these multiple approaches. Based on this information a hypothesis-based clinical trial can be designed to ascertain potential effects in humans of the variant(s) in question on drug response or a suitable previously conducted trial with archived DNA can be used. In this article, we will focus on two genes, the β1AR and GRK5, because the multiple investigative strategies shown in Fig. 2 have been completed and represent examples of the approach.
Fig. 2.
An approach to exploring relevance of candidate-gene polymorphisms. Dashed lines represent ancillary studies that use reagents or cohorts collected within the main sequence of events (solid lines).
Variations in the β1AR Gene
Cell-Based Studies. Using this targeted approach to defining the genetic determinants of β-blocker efficacy, we began by examining the coding region of the intronless β1AR gene. Two common nonsynonymous SNPs were found (Table 1) at nucleotides 145 (A/G), which results in a Ser (major allele) or Gly at amino acid 49 of the extracellular amino terminus of the receptor, and at nucleotide 1165 (G/C), which results in an Arg (major allele) or Gly at amino acid 389. It is noteworthy that Gly at position 389 was found when the human β1AR was first cloned, and has been termed the “wild-type” receptor for the majority of structure-function studies, yet it is the less common allele in most populations. However, in African Americans, Gly has approximately the same prevalence as Arg. We have thus refrained from using the term wild type for either variant but simply refer to them by allele, such as β1Arg389 or β1Gly389.
TABLE 1.
Coding region variations of the β1AR and GRK5 genes. MAF, minor allele frequency
|
Gene Name
|
Common Name
|
Nucleotide
Variabilitya,b
|
Amino Acid
Variabilitya
|
MAF
|
|
|---|---|---|---|---|---|
| White | Black | ||||
| % | |||||
| ADRB1 | β1AR | 145 (A/G) | 49 (Ser/Gly) | 15 | 13 |
| ADRB1 | β1AR | 1165 (C/G) | 389 (Arg/Gly) | 27 | 42 |
| ADRB1 | β1AR | 1166 (G/T) | 389 (Arg/Leu) | <0.1 | 0.9 |
| GRK5 | GRK5 | 122 (A/T) | 41 (Gln/Leu) | 1.3 | 23 |
| GRK5 | GRK5 | 840 (G/A) | 304 (Arg/His) | <0.01 | 0 |
| GRK5 | GRK5 | 1274 (C/T) | 425 (Thr/Met) | 0 | 0.02 |
| GRK5 | GRK5 | 1624 (C/G) | 542 (Pro/Ala) | <0.01 | 0 |
The most common allele in a general U.S. population (composed of 12% African Americans) is the first provided.
Nucleotide position is relative to the AUG initiator codon.
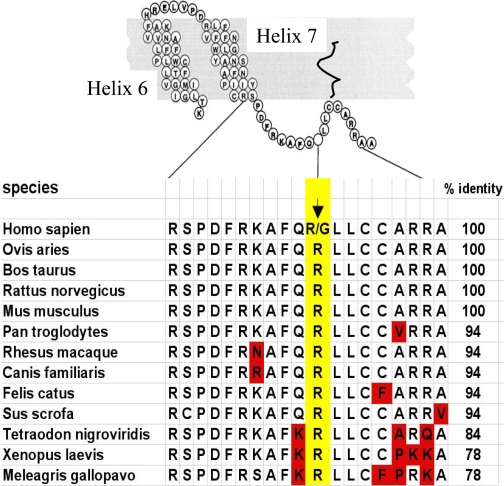
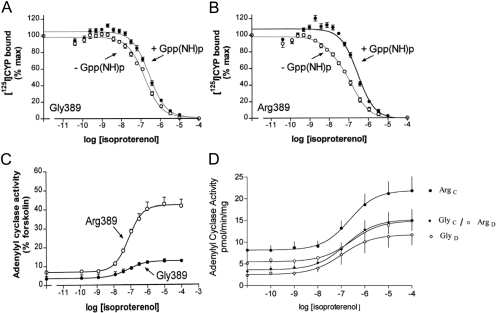
β1Arg/Gly389. The variation at amino acid 389 lies within a predicted fourth intracellular loop, formed by a stretch of ∼12 amino acids from the distal seventh transmembrane spanning-domain to the membrane-anchoring palmitoylated cysteine(s). By analogy with other G protein-coupled receptors, this fourth loop is an α-helix. As shown in Fig. 3, this region of the β1AR is highly conserved among many diverse species. In addition, Arg is found in the analogous position in every species for which sequence data are available, except for humans, where Arg or Gly is found. Given the nature of these two amino acids, this homology analysis suggested that the variation at position 389 might have functional relevance. Competition binding studies (Mason et al., 1999) in partially purified cell membranes from stably transfected Chinese hamster fibroblasts (CHW cells) expressing both receptors at equivalent levels revealed high-affinity agonist binding that was readily detected with Arg389 but rarely found with Gly389 (Fig. 4, A and B). In addition, the Arg389 curves shifted to the right and became monophasic with the addition of guanosine-5′-(β,γ-imino)triphosphate, whereas the Gly389 curves were unaffected by guanine nucleotide. This indicated a larger proportion of receptors that could attain the high-affinity active state (%RH) for Arg389 compared with Gly389 and, consequently, a greater change in free energy upon agonist binding and subsequent coupling. In the presence of guanosine-5′-(β,γ-imino)triphosphate, the low-affinity binding constant (KL) was the same for both receptors. Consistent with these results, guanosine 5′-O-(3-[35S]thio)triphosphate binding with transfected COS-7 cell membranes was greater with the Arg389 variant than with the Gly389 variant. Adenylyl cyclase activities in membranes revealed slightly greater basal activities with the Arg389 receptor and ∼3-fold greater isoproterenol-stimulated activation for Arg389 than for Gly389 (Fig. 4C). Similar results were found with the agonists epinephrine and norepinephrine. Another group (Joseph et al., 2004) performed similar studies in transfected Chinese hamster ovary cells at low expression levels and also found that Arg389 had greater Gs coupling, confirming our studies in CHW cells. In their studies, the isoproterenol-stimulated cAMP increase with β1Arg389 was ∼30-fold greater than β1Gly389. These investigators also determined binding affinities for a number of antagonists and partial agonists, including metoprolol, carvedilol, bisoprolol, and propranolol, and found no differences in affinity between the Arg and Gly receptors. Intramolecular fluorescence resonant energy transfer has been used to assess the inverse agonist activities of bisoprolol, metoprolol, and carvedilol (Rochais et al., 2007). In these studies, all three antagonists evoked a fluorescence resonant energy transfer change (consistent with a conformational change in the receptor) for both the Arg and Gly forms of the receptor, but carvedilol acting at β1Arg389 displayed the greatest change. The locations of the energy donor and energy acceptor moieties in the intramolecular domains of G protein-coupled receptors markedly affects resonant energy transfer ratios (Swift et al., 2007), and thus it is difficult to fully interpret these studies in relation to a physiologic outcome. Nevertheless, they do suggest that there may be compound-specific phenotypes for the position 389 variants.
Fig. 3.
Conservation of amino acid sequences within the fourth intracellular loop of the β1AR for various species.
Fig. 4.
Properties of the β1AR Arg389 and Gly389 variants in transfected cell membranes. A and B, results from agonist competition studies. C, results from agonist-promoted adenylyl cyclase studies. D, results from short-term agonist-promoted desensitization studies. C, control; D, desensitized by pre-exposure to isoproterenol.
The more favorable conformation for agonist-promoted Gs-coupling of the Arg389 receptor versus the Gly389 receptor suggested that GRK-promoted desensitization might also be greater for Arg389, because phosphorylation is conformation-dependent. The aforementioned transfected CHW cells were treated with vehicle or vehicle with 10 μM isoproterenol for 20 min and washed, membranes were prepared, and agonist-stimulated adenylyl cyclase activation was determined. The results revealed ∼50% greater desensitization for Arg389 compared with Gly389 (Rathz et al., 2003). It is noteworthy that when one examines the absolute values of the activities, the impact on signaling of genetic variation is of the same magnitude as homologous desensitization. As can be seen in Fig. 4D, the desensitized Arg389 receptor signals equivalently to the control (not desensitized) Gly389 receptor.
β1Ser/Gly49. The Ser49 and Gly49 β1ARs were stably expressed in CHW as well as human embryonic kidney 293 cells, and in studies by our laboratory, we found no differences in agonist-promoted coupling to adenylyl cyclase in either cell line (Rathz et al., 2002). Another group, however, reported an increased basal and agonist-stimulated adenylyl cyclase activities with the Gly49 receptor in transfected human embryonic kidney 293 cells (Levin et al., 2002). However, both groups found that these recombinantly expressed β1ARs underwent little agonist-promoted down-regulation (loss of net receptor expression) after 24 h of exposure to high concentrations of isoproterenol under cell culture conditions. Indeed, in some instances, expression increased. Both groups used incubations with cyclohexamide to block new receptor synthesis, and under these conditions, agonist-promoted down-regulation of both Ser49 and Gly49 forms of the receptor was noted. In addition, both groups reported an increase in agonist-promoted down-regulation of the Gly49 receptor compared with the Ser49 receptor (55 versus 36%, respectively, in our studies). This polymorphism is localized to the extracellular amino terminus of the β1AR, ∼33 bases 3′ to a glycosylation site. On SDS-polyacrylamide gel electrophoresis, the Ser49 receptor migrated at two molecular weights, suggesting a homodimer or altered glycosylation compared with Gly49. Although a homodimer cannot be excluded, the high-molecular-weight species of Ser49 was sensitive in vivo, and in vitro, to inhibitors of N-glycosylation (Rathz et al., 2002). Because glycosylation can affect receptor trafficking, these observations may represent the mechanism of altered down-regulation, although it is unclear based on its position how altered glycosylation is evoked by the polymorphism. Taken together, although we recognize that these studies are dependent on highly reductionist model systems, we have considered that the β1Gly49 receptor may alter cardiac phenotypes in heart failure, potentially because of enhanced down-regulation.
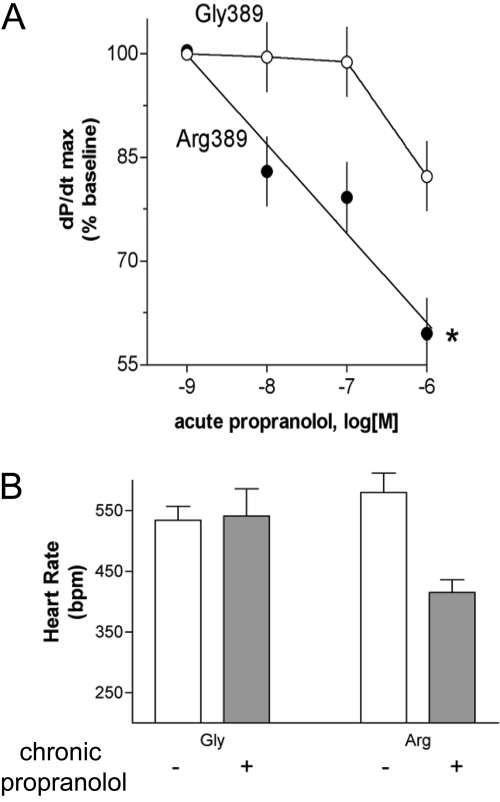
Transgenic Mouse Studies. To assess the relevance of the β1Arg389 and β1Gly389 receptors to cardiac function, transgenic mice (FVB/N strain) overexpressing the two human receptors on myocytes were generated using the α-myosin heavy chain promoter (Mialet Perez et al., 2003). We had previously generated β2AR-overexpressing cardiac mice and had delineated the appropriate expression levels over background βAR expression that provide for differentiation between two β2ARs with known differences in Gs coupling (Turki et al., 1996). We thus chose two β1AR-overexpressing mouse lines expressing either the Arg389 or Gly389 β1AR at matched levels of ∼1000 fmol/mg for most of the longitudinal studies. The salient features of their physiologic function are shown in Fig. 5. Both baseline and maximal dobutamine-stimulated contractility (the first derivative of pressure by time) were greater for Arg389 hearts compared with Gly389 at 3 months of age (Fig. 5A). These results were consistent with the transfected cell studies and revealed the relevance of the polymorphisms in the cell type of interest and at the level of intact organ function. It is noteworthy that at 6 months of age, Arg389 mice continued to have enhanced baseline contractility but were not responsive to dobutamine (Fig. 5B). We found that this desensitization was due to a decreased affinity for receptor-Gs coupling and a decrease in protein expression of Gαs and AC5/6, which paralleled a loss of ventricular function and failure by 9 months of age. This suggested a change in the coupling of the Arg389 receptor that occurred during the development of β1AR-mediated cardiomyopathy, a term that we (unfortunately) termed a “phenotypic switch.” However, subsequent studies in human hearts (see Human Studies) showed only a modest change in the magnitude of the contractile differences between Arg and Gly hearts in end-stage heart failure compared with normal hearts, so in humans, there does not appear to be a reversal of the phenotypes. Additional experiments with the transgenic mice revealed the first evidence that there may be a differential response to β-blockers based on genotype (Mialet Perez et al., 2003). Acute infusion of propranolol into hearts in the ex vivo work-performing model (Fig. 6A) revealed that Arg389 hearts had a dose-dependent decrease in contractility, whereas Gly389 hearts had a smaller decrease that was only found at the highest concentration. In vivo studies were carried out by administering propranolol in the drinking water of 4-month-old mice for 1 month and monitoring heart rate by echocardiography. As shown in Fig. 6B, only Arg389 mice displayed a decrease in heart rate.
Fig. 5.
Physiologic effects of human Arg389 and Gly389 β1ARs expressed on myocytes of transgenic mice. Mice were studied in the ex vivo, work-performing model. The first derivative of pressure per unit time (+dP/dtmax) is a measure of contraction. A, 3-month-old mice; B, 6-month-old mice.
Fig. 6.
Differential cardiac responses to β-blockade in Arg389 and Gly389 β1AR transgenic hearts. A, results from acute infusion of propranolol in the ex vivo work-performing model. B, results from a 1-month oral administration of propranolol with heart rates determined in intact mice by echocardiography.
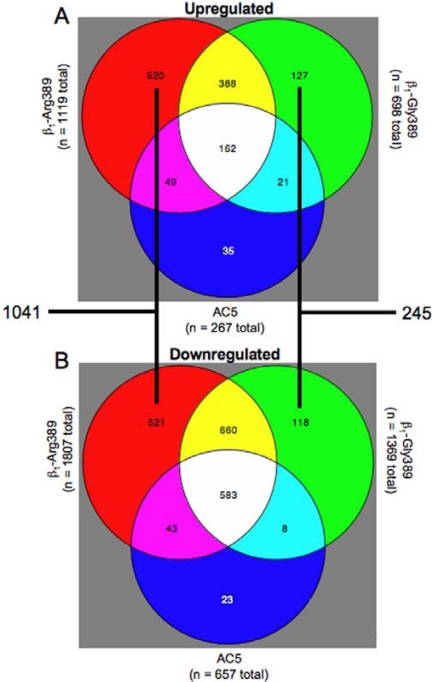
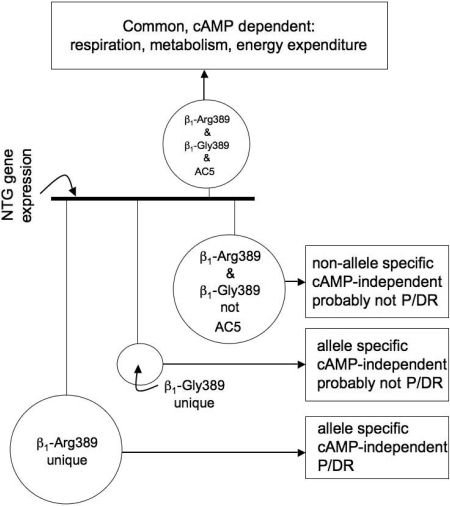
As in Fig. 2, ancillary studies have been carried out in young transgenic mouse hearts examining gene transcript expression to begin to understand potential unique signaling properties of the Arg389 and Gly389 receptors. Given that these mice had overexpressed receptors and that the Arg389 mice develop heart failure by 9 months of age, we used 3-month-old mice whose hearts showed no anatomic, histologic, biochemical, or physiologic evidence of heart failure or other pathologic features. Nevertheless, the limitations of the model call for careful interpretation. We were particularly concerned that the enhanced inotropy/chronotropy in the resting state of the Arg389 versus Gly389 hearts might simply result in regulation of energy-related genes proportional to the physiologic enhancement. To provide a “filter” for such events, we used the type V adenylyl cyclase (ACV) cardiomyocyte-specific overexpressing mouse that we had previously generated (Tepe et al., 1999). We had shown that the hearts from these mice had persistently elevated resting and agonist-stimulated contractility throughout their lives, with no evidence of pathologic consequences. The extent of the enhanced inotropy and chronotropy (using nontransgenic mice as the reference) was the same as what we observed for the hyperfunctional Arg389 mice. Thus we could separate AC/protein kinase A (PKA)-dependent events from Arg (or Gly)-specific events by comparing these with the events found with the ACV hearts. Gene expression was ascertained from six hearts in each of the four groups (nontransgenic, Arg389, Gly389, and ACV) using a complete mouse genome array representing 39,000 transcripts. These results are depicted in the Venn diagrams of Fig. 7. As can be seen, 1041 genes were uniquely regulated by the Arg389 transgene (i.e., not found with Gly389 or ACV). In contrast, 245 genes were uniquely regulated in the Gly389 hearts. Moreover, as expected, there were relatively large overlap groups of genes that were coregulated by the two β1ARs or ACV. Pathway analysis algorithms (such as Ingenuity and ToppGene) revealed a number of networks that were uniquely regulated by Arg389. The most statistically significant network involved regulation of extracellular matrix-associated genes known to be activated directly or indirectly by TGFβ (Swift et al., 2008). These data could benefit from pathway-building exercises by other groups, and the raw data are provided in the supplement to the aforementioned article. In general, we have considered the scheme depicted in Fig. 8 as a way to identify new pharmacogenomic or disease-risk genes within the context of the Arg389 polymorphism. The expression of genes in the nontransgenic mice is set as the “reference.” And the perturbations imposed by the transgenes were considered as four possibilities, initially stratified by cAMP-dependent and -independent events. Within the cAMP-dependent mechanism, a number of genes are altered in the β1-Arg and β1-Gly hearts, and by definition, this group includes all transcripts altered in AC5 hearts. Not surprisingly, the genes in this group are dominated by those associated with respiration and energy metabolism, given that cAMP/PKA activation is a major mechanism by which cardiac inotropy and chronotropy are increased. In the non-cAMP-dependent set of genes, some are common to both β1-Arg389 and β1-Gly389. These represent, then, pathways that are activated by these receptors in a non-allele-specific manner that does not involve cAMP signaling and are unlikely to be allele-specific pathogenic or pharmacogenomic loci. The genes whose transcripts were altered in a non-cAMP/PKA-dependent, allele-specific manner represent unique signaling that is apparently dependent on the single amino acid difference in the β1AR at position 389 and may represent genes or pathways that might provide insight into heart failure pathogenesis or novel therapies directed toward heart failure in those with the Arg389 genotype.
Fig. 7.
Distribution of transcripts that are regulated in a unique, or common, manner in the hearts from the three indicated transgenic mice. The numbers in each region represent the number of genes significantly up- or down-regulated compared with nontransgenic littermates. The partition sizes are not to scale.
Fig. 8.
Potential mechanistic implications of filtered gene expressions from β1-Arg389, β1-Gly389, and ACV transgenic mouse hearts. The sizes of the circles are proportional to the number of genes in each pool. P, pathogenic; DR, drug response.
Human Studies
Physiological Outcomes. To further assess the roles of the position 389 alleles in the context of human heart failure, a number of studies with physiologic endpoints have been carried out with relatively small cohorts. In one such study, graded exercise testing in 263 patients with class III/IV heart failure was performed with peak oxygen consumption (VO2) as the major outcome (Wagoner et al., 2002). There was a readily apparent difference in VO2 between β1Arg389 homozygotes and Gly389 homozygotes (17.7 ± 0.4 versus 14.5 ± 0.6 ml/kg/min, P = 0.006). Heterozygotes had a VO2 that was between the homozygous subjects at 16.9 ± 0.6. There was no confounding by etiology of heart failure, baseline left ventricular ejection fraction (LVEF), or β-blocker use. When stratified by the position 49 polymorphism, there were few homozygous β1Gly49 subjects, but homozygous Ser49 patients had higher VO2 than Gly49 carriers (homozygotes and heterozygotes). However, this appears to be driven by Arg389, because of high linkage disequilibrium between the 49 and 389 alleles. This study, then, confirmed the hyperdynamic nature of the β1Arg389 heart in humans. These results of exercise responsiveness have been replicated recently by another group using patients with heart failure (Sandilands et al., 2005), but the association does not appear to hold in healthy subjects (Leineweber et al., 2006). It is noteworthy, however, that direct assessment of cardiac β1AR function by dobutamine infusion in normal subjects has shown differential responses by the β1Arg389 genotype (Bruck et al., 2005). These investigators showed not only enhanced heart rate and contractility in Arg389 versus Gly389 homozygous subjects, but also found that dobutamine-stimulated plasma renin activity was markedly greater in the Arg389 individuals, indicating that renal β1AR function is also affected by this polymorphism. Finally, they report that the β-blocker bisoprolol decreased dobutamine-promoted cardiac and renin responses more potently in those subjects with Arg389 versus Gly389. These studies, then, are consistent with our findings in the transgenic mouse (Mialet Perez et al., 2003) and human exercise studies (Wagoner et al., 2002) as well as isolated human hearts (see below). In another study, the effects on LVEF of β-blockade with carvedilol in 224 patients with heart failure was assessed after >6 months of treatment (Mialet Perez et al., 2003). After titration, the dose of carvedilol was the same in β1Arg389 versus Gly389 patients. Arg389 homozygotes showed a greater improvement in LVEF than Gly389 homozygotes (8.7 ± 1.1 versus 0.93 ± 1.7%; P < 0.02). Heterozygotes seemed to show improvement similar to the Arg homozygotes (7.02 ± 1.5%). Similar results with the β-blocker metoprolol were reported in another study of 61 heart failure patients (Terra et al., 2005). These studies have recently been replicated in 135 patients treated with carvedilol (Molenaar et al., 2007). Here, the respective improvements for the two homozygous states were 18 versus 6%, heterozygotes being 11%. In our view, these types of studies, which are highly focused and hypothesis-driven with an emphasis on human physiology, provide an important link between cell- or animal-based studies and longitudinal studies of patients with heart failure. In addition, they provide for a refinement of the hypothesis, or the study design, for such large patient studies.
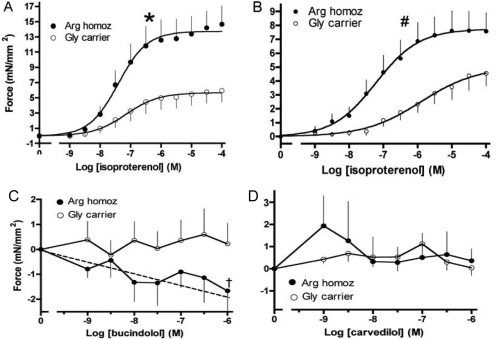
Ex Vivo Cardiac Mechanics. The effects of β1AR genotype on human responses have been assessed using right ventricular trabeculae from human nonfailing and failing hearts, studied in organ-bath preparations (Liggett et al., 2006). Nonfailing (normal) hearts were donor hearts that were not ultimately used for transplant due to ABO incompatibility or other noncardiac issues. Failing hearts were obtained at the time of cardiac transplantation for end-stage heart failure. These results are shown stratified by genotype in Fig. 9. In nonfailing hearts from persons homozygous for Arg389, the maximal contractile force was greater than that of hearts from Gly389 carriers (Fig. 9A). The absolute difference in force between Arg389 and Gly389 responses in these nonfailing trabeculae amounted to ∼8 mN/mm2 greater for Arg389. In failing hearts, this phenotype was maintained, but the absolute differences were not as great (Fig. 9B; note scale change), amounting to 3.5 mN/mm2. Therefore, although there is no “switch” or “reversal” in the phenotypes, as was suggested in the mice, there is an attenuation of the phenotypic differences by genotype between nonfailing and failing hearts. This may be due to the Arg389 receptor's undergoing greater signal desensitization during the catecholamine excess that accompanies end-stage heart failure, which is consistent with the previously discussed cell-based studies (Rathz et al., 2003). It is noteworthy that by radioligand binding, there were no differences in β1- or β2AR expression levels between Arg389 and Gly389 hearts. Additional experiments in failing hearts have also been carried out with carvedilol, and the atypical β-blocker bucindolol (Fig. 9, C and D). Here, trabeculae were prestimulated with forskolin, which provides conditions for detecting weak partial agonist or inverse agonist effects. Bucindolol caused a dose-dependent decrease in force generation in trabeculae from Arg389 homozygous hearts but not from Gly389 carrier hearts. In contrast, carvedilol acted as a neutral antagonist in trabeculae with either genotype.
Fig. 9.
Contractile responses to ligands in right ventricular trabeculae from human failing and nonfailing hearts. Data are shown stratified by Arg389 homozygous and Gly389 carrier genotypes.
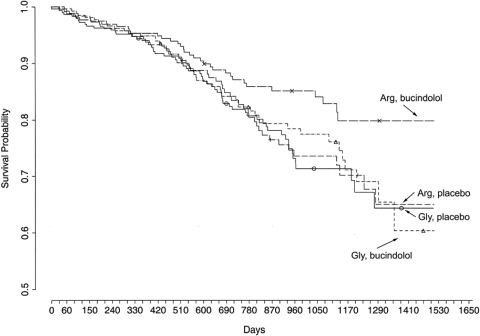
Heart Failure Survival. Taken together, the cell, transgenic mouse, ex vivo human heart, and human physiological studies all indicated that the Arg389 form of the β1AR achieves a greater signaling potential than the Gly389 form and that those with the Arg389 form have greater short-term responses to β-blockers. These differences could have physiological or pharmacologic relevance in heart failure. To ascertain the consequences of this polymorphic variation for heart failure survival, we genotyped archived DNA from a prospective, double-blinded, placebo-controlled trial of the β-blocker bucindolol in class III-IV heart failure. The trial, the β-Blocker Evaluation of Survival Trial (BEST), was terminated before achieving its enrollment goals because of an interim analysis indicating little efficacy regarding the pre-defined endpoints and a lack of investigator equipoise (BEST Trial Investigators, 2001).
One thousand forty patients from BEST consented to the substudy. The clinical characteristics of the placebo and bucindolol groups were well matched, as were these groups when further stratified by homozygous β1Arg389 or -Gly389 carriers (see Supporting Information Table 4 in Liggett et al., 2006). Of particular note, there were no differences in age, sex, race, LVEF, etiology of heart failure, New York Heart Association class, or baseline heart rates and blood pressures, among the groups. The primary outcome was all-cause mortality, adjudicated heart failure hospitalizations, and the combined endpoint. The statistical comparisons were with these outcomes comparing the placebo and bucindolol groups in Arg389 and Gly389 patients. Because the study had the placebo arms, the potential effects of the polymorphism on heart failure progression in the absence of β-blocker could be assessed, and because comparisons were between bucindolol and placebo by genotype, any subtle differences evoked by a polymorphism on an endpoint could be detected and attributed to a drug-specific effect. Cumulative survival curves were constructed by Kaplan-Meier methods and the Cox proportional-hazards regression model was used to examine the effects of treatment stratified by the indicated genotype. Results were adjusted for age, sex, and race. Because of the limited number of comparisons and a hypothesis that was based on the results we found in the cell-based, mouse-based, and human ventricle studies, we considered P values <0.05 as significant, without adjustments for multiple comparisons. The main results of this study are summarized in Fig. 10. As can readily be observed, one group had improved survival, which was the one with patients having the β1Arg389 homozygous genotype who were receiving the active drug (bucindolol). In contrast, Gly389 carriers had identical survival, whether they were on placebo or bucindolol. It is noteworthy that by looking at the two placebo groups, we can conclude that the polymorphism does not seem to significantly alter survival in the absence of β-blocker. For Arg389 patients treated with bucindolol compared with placebo, the hazard ratio (HR) = 0.62, 95% confidence interval (CI) = 0.40 to 0.96, P = 0.03, indicating an improvement in survival with bucindolol in those with this genotype. This same comparison in Gly carriers revealed no difference in survival (HR = 0.90, 95% CI = 0.62 to 1.30, P = 0.57), indicative of no treatment response to bucindolol. There was also an apparent influence of β1AR genotype on heart failure exacerbations during bucindolol treatment, as measured by hospitalization as a result of heart failure. With this outcome, patients with Arg389 compared with placebo had HR = 0.64, 95% CI = 0.46 to 0.88, P = 0.006. In contrast, Gly389 carriers showed no benefit of the drug compared with placebo in terms of hospitalizations (HR = 0.86, 95% CI = 0.64 to 1.15, P = 0.30). For the combined outcome of time to first heart failure hospitalization or death, a bucindolol-associated favorable treatment effect was evident for Arg patients compared with placebo (HR = 0.66, 95% CI = 0.50 to 0.88, P = 0.004), but it was not apparent in bucindolol-treated Gly389 carriers versus placebo (HR = 0.87, 95% CI = 0.67 to 1.11, P = 0.250). The β149 polymorphisms provided no additional predictive value. An issue for this study was the known small, but significant, difference in the frequency of the Gly allele between blacks and nonblacks (Table 1) and the fact that, in the entire BEST cohort (BEST Trial Investigators, 2001), bucindolol's mortality effect in blacks seemed to be less favorable than in nonblacks. We considered whether our findings were based simply on being able to proportionately identify blacks that would place them into the Gly group (the nonresponders). If the effect was due to some other African gene(s), then it is conceivable that the 389 allele was being used only as an ancestral marker. However, based on the number of blacks in the study (≈20%), the allele frequency difference would have needed to be >10-fold in order for Gly to be a meaningful surrogate for “blacks only” identification and to affect overall outcome. Furthermore, the HRs were adjusted for race and, nevertheless, an advantage was observed for Arg but not Gly patients. We also carried out this same analysis excluding the black subjects, thus removing any potential for confounding by race. For mortality, Arg patients treated with bucindolol had an HR = 0.56, 95% CI = 0.34 to 0.90, P = 0.017, versus placebo. The bucindolol treatment HR for Gly = 0.81, 95% CI = 0.53 to 1.24, P = 0.34, versus placebo.
Fig. 10.
Kaplan-Meyer survival curves for patients in the BEST trial, stratified by β1AR genotype and drug/placebo. Those with the Arg389 homozygous genotype had a significantly decreased mortality compared with those with the same genotype receiving placebo. In contrast, Gly389 carriers showed no apparent response to bucindolol compared with placebo (see Heart Failure Survival for hazard ratios).
There are several unique components of the BEST Substudy that are noteworthy when considering comparisons with other studies of β-blocker efficacy and β1AR polymorphisms. BEST used the β-blocker bucindolol, which has some distinct pharmacologic properties. Of all β-blockers that have been studied in the treatment of heart failure, bucindolol shows the greatest sympatholytic effect (i.e., norepinephrine lowering), which are similar in magnitude to the effects of the central imidazoline receptor agonist moxonidine (Cohn et al., 2003). In addition, bucindolol acts as an inverse agonist in human heart preparations at the β1Arg389, but not the β1Gly389 (Liggett et al., 2006). On the other hand, metoprolol and carvedilol act as neutral antagonists without inverse activity in these isolated human hearts. Finally, bucindolol has similar binding affinities for both β1AR and β2AR, and is thus a nonselective β-blocker. Concerning trial design, BEST was a multicenter, prospective, randomized, and placebo-controlled trial, with stringent entry criteria and extensive patient phenotyping. Given the above, and the ∼38% improvement in outcomes observed in Arg389 patients taking bucindolol versus placebo, it is important to consider whether these findings are also true for the β-blockers currently available and in common use for heart failure treatment in the United States, metoprolol and carvedilol. To our knowledge, there is only one β1AR polymorphism study in heart failure (White et al., 2003) with a placebo arm, which is a substudy of MERIT-HF. It is noteworthy that ∼45% of these patients had mild heart failure (class II), mean follow-up period was only 12 months, and there were few deaths, so the combined outcome of hospitalization and death was used. The analysis compared outcome by genotype in the combined cohort of placebo- and metoprolol-treated patients, and the major conclusion was that Gly389 patients did not have improved outcomes. It is not possible from this study to assess whether there was a pharmacogenetic effect because comparisons within the two treatment arms, by genotype, were not performed. Shin et al. (2007) reported the results of a longitudinal observational study of 227 heart-failure patients with survival as the endpoint, where 81% were receiving an unspecified β-blocker. No association with β1AR polymorphisms was noted, but a two-locus haplotype of the β2AR subtype was associated with poor survival. de Groote et al. (2005), in a study of 444 white patients with heart failure, all of whom were treated with either bisoprolol or carvedilol, found a different β2AR 2-locus haplotype associated with survival (univariant analysis only) and no association with a β1AR polymorphism. Finally, a retrospective two-center catheterization-laboratory registry study of 637 heart failure patients who were being treated with either carvedilol or metoprolol, has been recently reported examining the coding β1AR polymorphism, those of the β2AR, and a surrogate for an α2CAR polymorphism (Sehnert et al., 2008). No associations between any of these polymorphisms and survival were found. In contrast to the BEST Substudy, this study was retrospective, patient enrollment was based on having a cardiac catheterization (a potential selection bias), a formal β-blocker titration protocol was not in place, and compliance was not monitored past 6 months. So, any differences between this study (or the aforementioned other studies) and the BEST Substudy may be due to the “noise” from their designs or to the effects of the β1AR polymorphism being specific for bucindolol.
A βAR-Desensitizing GRK5 Polymorphism Is Protective in Experimental and Clinical Heart Failure and Mimics β-Blockers
Whereas minute-by-minute cardiac function is critically regulated by βAR signaling stimulated by systemically circulating or locally released sympathetic catecholamines, these responses are contextually modulated by G-protein receptor kinases or GRKs. As described above, under normal conditions, this acute stress response transiently activates downstream βAR signaling pathways that increase ventricular ejection performance and heart rate. After the exertion is over or stress is relieved, cardiac output and catecholamine levels normalize. However, chronically depressed cardiac output in systolic heart failure persistently activates these same catecholaminergic-βAR signaling pathways, resulting in cardiomyocyte toxicity that creates a downward functional spiral of worsening heart failure that stimulates more catecholamines, which further injure the heart, and so forth (Fig. 1). As discussed earlier, an important mechanism that could potentially protect myocardium from the pathological consequences of uninterrupted βAR signaling is GRK-mediated phosphorylation of myocardial βAR (Ferguson, 2001; Pitcher et al., 1998), which recruits β-arrestins that displace bound G-proteins, thus partially uncoupling βARs from signaling to Gs. β-Arrestins also target βARs to clathrin-coated pits for endocytic receptor internalization, resulting in βAR down-regulation, and serve as “signals” themselves, by way of their capacity to chaperone other molecules (DeWire et al., 2007).
The prototypical cardiovascular GRK is GRK2, originally designated β-adrenergic receptor kinase (Benovic et al., 1986; Benovic et al., 1987). Of seven mammalian GRKs (Benovic et al., 1989), the most highly abundant in myocardium are GRK2 and GRK5 (Kunapuli and Benovic, 1993; Premont et al., 1994), both of which can desensitize agonist-occupied βAR. Despite these similarities, GRK2 and GRK5 are structurally distinct and are members of separate GRK subfamilies (Premont et al., 1995), which are GRKs 1 and 7 (retinal opsin kinases), GRKs 2 and 3 (i.e., β-adrenergic receptor kinases 1 and 2), and GRKs 4, 5, and 6.
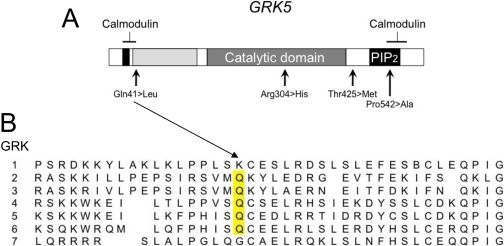
In clinical heart failure, the cycle of sympathetic stimulation leading to βAR dysfunction can be interrupted with pharmacological βAR blockade, which prolongs group mean survival in heart failure (see Fig. 1) (Packer et al., 1996; MERIT-HF Study Group, 1999). An experiment of nature suggests that increased activity of GRK5 can provide some of the benefits associated with pharmacological β-blockers (“genetic-β-blockade”) and thus act as a pharmacogenomic locus by indicating which individuals would not benefit from “exogenous” β-blockade. A comprehensive screening for coding polymorphisms of GRK2 and GRK5 found no common nonsynonymous polymorphisms in GRK2 but identified four allelic variants of GRK5: cDNA nucleotide position 122 A/T changes glutamine (Gln) to leucine (Leu) at amino acid 41 in the amino terminus, adjacent to a calmodulin binding domain; nucleotide 840 G/A changes arginine to histidine at amino acid 304 within the catalytic domain; nucleotide 1274 C/T changes threonine to methionine at amino acid 425 in the carboxyl terminus, not corresponding to any known functional domain; and nucleotide 1624 C/G changes proline to alanine at amino acid 542 within the carboxyl terminus calmodulin-binding domain (Fig. 11A) (Liggett et al., 2008). The amino acids encoded by three of the four major GRK alleles are completely conserved within members of the same GRK subfamily, and glutamine at amino acid 41 is conserved in all nonretinal opsin human GRKs (including each of the splice variants of GRK4 and GRK6) (Fig. 11B), and across mammalian species in GRK5 (Liggett et al., 2008). GRK5 variation at amino acids 304, 425, and 542 was infrequent (<2% allele frequency) in a diverse human cohort. In contrast, the GRK5 Leu41 allele, whereas rare in whites (allele frequency 0.01-0.02), was common among African Americans (allele frequency, 0.20; prevalence of heterozygous carriers, 0.35; and homozygous, 0.05).
Fig. 11.
Sequence comparison of human GRKs. The nonretinal GRKs (2, 3, 4, 5, 6) all have Gln in position 41 analogous to GRK5 (yellow bar). The most common polymorphism of GRK5 is this Gln→Leu variation.
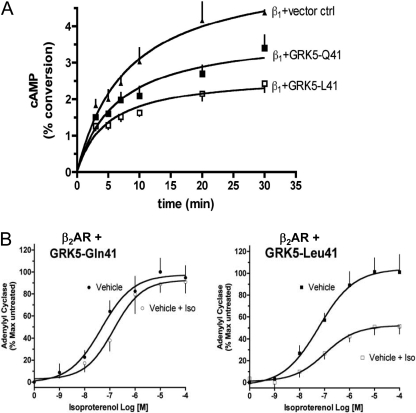
As might be expected for a polymorphism within a putative regulatory domain, rather than within the catalytic domain, the Leu41 substitution did not affect intrinsic in vitro GRK kinase activity measured by in vitro rhodopsin phosphorylation. However, when the pharmacological properties of the GRK5 Leu41 minor allele were compared with those of “wild-type” GRK5 Gln41 in the intact cell setting, a differential effect on βAR signaling was found. Each variant was coexpressed at equivalent levels with either human β1AR (Liggett et al., 2008) or β2AR (Wang et al., 2008) in cultured Chinese hamster ovary cells. Cells expressing GRK5 Leu41 showed enhanced agonist-promoted desensitization of βAR-stimulated adenylyl cyclase activity for both βAR subtypes (Fig. 12). In the case of β2AR, studies of intact cells also revealed increased agonist-mediated receptor phosphorylation and internalization. Improved GRK5 Leu41-mediated desensitization of a PKA phosphorylation site β2AR mutant further demonstrated that PKA was not involved, implicating GRK phosphorylation sites in enhanced βAR receptor uncoupling. Together, these studies show that βAR phosphorylation, desensitization, and internalization from GRK5 Leu41 encoded by the variant allele accelerates uncoupling from adenylyl cyclase and more efficiently attenuates βAR signaling. In other words, the gain of GRK5 desensitization function in the variant manifests as a more rapid loss of βAR signaling in cell-based systems.
Fig. 12.
Enhanced βAR desensitization evoked by the GRK5-Leu41 polymorphism. A, β1AR were coexpressed with GRK5-Gln41 or GRK5-Leu41, and the kinetics of isoproterenol promoted cAMP accumulation were determined. The Leu41 responses are quenched to a greater extent, and more rapidly, than those from cells expressing Gln41. B, in a different model of desensitization, β2AR was expressed with the two GRKs and exposed to vehicle or isoproterenol for 30 min and washed, and membranes were prepared. Adenylyl cyclase activities were then performed with the indicated concentrations. The β2AR with Leu41 coexpressed underwent a greater degree of desensitization compared with Gln41.
In considering the functional impact of the GRK5 Glu-to-Leu substitution at amino acid 41, one must consider the important calmodulin binding domain spanning amino acids 20 to 39 (Pronin et al., 1997). Binding of calcium-bound calmodulin inhibits GRK5 catalytic activity, alters its membrane-binding properties, thereby increasing its ability to phosphorylate soluble (nonreceptor) substrates, and alters the pattern of GRK5 autophosphorylation (Freeman et al., 1998; Sallese et al., 2000). GRK5 has a much greater affinity for calcium-bound calmodulin than GRK2 (IC50 of ∼40 nM versus 2 μM), suggesting that increases in intracellular calcium may specifically inhibit GRK5. Currently, these interactions must be considered speculative as the specific impact of the variant allele on GRK5-calmodulin interactions has not yet been examined.
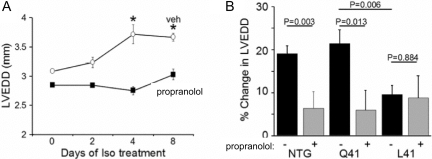
Both GRK5 and GRK2 desensitize myocardial βAR. Because previous studies in mice had demonstrated that genetic ablation of GRK2 in the heart exacerbated catecholamine cardiomyopathy produced by long-term isoproterenol infusion (Matkovich et al., 2006), we predicted that improved βAR desensitization and more rapid attenuation of toxic βAR signaling by GRK5 Leu41would have the reciprocal effects (i.e., would protect against heart failure in the same model). To test this hypothesis, cardiac-specific transgenic mice expressing equivalent levels of either human GRK5 Gln41 or GRK5 Leu41 were created and subjected to comparative detailed analyses of cardiac phenotypes at baseline and after long-term isoproterenol infusion (Liggett et al., 2008). Overexpression of human GRK5 at low levels (∼5-fold endogenous levels) in these studies produced no baseline phenotype, which contrasts with a previous report of diminished basal and catecholamine-stimulated cardiac function in transgenic mice expressing comparatively high (30-fold endogenous levels) expression of bovine GRK5 (Chen et al., 2001). Both wild-type GRK5 and the Leu41 variant shifted the concentration-response curve for isoproterenol-stimulation of contractility in isolated perfused hearts significantly and equally to the right, consistent with decreased sensitivity of cardiac βAR to agonist. Most importantly, subacute desensitization of isoproterenol (βAR)-stimulated cardiac contractility was significantly accelerated in the GRK5 Leu41 transgenics, compared with wild-type GRK5 overexpressors, in perfused mouse hearts (Fig. 13A). Moreover, when left ventricular dilation and contractile function were examined at progressive time intervals after isoproterenol mini-pump implantation, GRK5 Leu41-expressing mice were protected from the adverse consequences of chronic βAR stimulation, similar to treatment with the nonselective βAR antagonist, propranolol (Fig. 13B). In contrast, mice overexpressing wild-type GRK5 Gln41 developed the characteristic dilated catecholamine cardiomyopathy in response to isoproterenol, although they could still be protected by pharmacological βAR blockade (Liggett et al., 2008).
Fig. 13.
GRK5-Leu41 protects against catecholamine-mediated left-ventricular dilatation in mice. A, isoproterenol administered by mini-pump evokes an increase in the left ventricular end diastolic dimension (LVEDD) in nontransgenic (NTG) mice that is blocked by propranolol. B, expression of GRK5-Leu41 results in no increase in LVEDD during isoproterenol infusion and propranolol has no effect. In contrast, GRK5-Gln41 mice displayed equivalent levels of increased LVEDD, and the same response to propranolol, as do NTG mice.
The results of cell-based and transgenic mouse studies suggested that both the pharmacological and physiological consequences of expressing GRK5 Leu41 are similar to being treated with β-blockers (i.e., to decrease βAR signaling under conditions of chronic agonist stimulation). Because heart failure is the medical condition in which treatment with pharmacological β-blockers most dramatically affects the disease [β-blockade improves left ventricular function in failing hearts and decreases mortality rates by approximately half in those with heart failure (Fauchier et al., 2007)], we explored the effects of GRK5 genotype on clinical outcome in human heart failure. Because the minor allele shows significant prevalence in African Americans (>40% carry one or two alleles), but not whites (<2% carry any allele), we genotyped the GRK5 Gln/Leu41 locus in 375 African Americans who had been recruited into a longitudinal National Institutes of Health/National Heart, Lung, and Blood Institute-funded study of heart failure. Because GRK5 Leu41 mimics β-blocker treatment in experimental heart failure, we measured how disease outcome (time from heart failure onset to death or cardiac transplantation) was modified by the interaction of GRK5 genotype and β-blocker treatment status. There was no difference in GRK5 Leu41 allele frequency in nonaffected African Americans versus those with dilated or ischemic cardiomyopathy, showing that the GRK5 Leu41 polymorphism does not modify the risk for developing heart failure. However, functional allelic variants that are not risk factors for a disease may nevertheless change disease outcome or response to specific therapies if the pathological pathway they modify is activated only after onset of the disease, such as hyperactivation of cardiac catecholaminergic signaling in heart failure. (For this reason, β-blockers are used to treat heart failure rather than to prevent it.) Indeed, we observed that GRK5 Leu41 carriers not treated with β-blockers had longer transplant-free survival times than their wild-type GRK5 counterparts (Fig. 14). Cox proportional hazards modeling with adjustment for age and sex described a protective effect of GRK5 Leu41 in subjects not treated with β-blockers (Hazard ratio, 0.28; 95% confidence interval, 0.12-0.66; P = 0.004) that was comparable with protection afforded by pharmacological β-blockade in wild-type GRK5 subjects (Hazard ratio, 0.19; 95% confidence interval, 0.10-0.34; P < 0.001).
Fig. 14.
Association of GRK5 polymorphisms, β-blocker usage, and survival in human heart failure. Shown are Kaplan-Meyer curves with the proportion of patients surviving as the y-axis. In homozygous GRK5-Gln41 patients, β-blockers (BB) have a characteristic effect, with an improvement in survival. In GRK5-Leu41 carrier patients, survival in the absence of β-blocker treatment is similar to that of GRK5-Gln41 patients taking β-blockers (green line). In the GRK5-Leu41 patients, there was no major improvement in survival in those receiving β-blockers over those who were not.
As a clinical study, the GRK5 heart failure study has some noteworthy limitations: it was performed in a relatively small number of subjects recruited from a single referral center and with a limited number of endpoints. The decision to treat or not with β-blockers was not randomized (nor could it ethically have been), and the results are therefore subject to unknown factors possibly related to intolerance of the drug on one hand (e.g., asthma or hemodynamic instability) or for indications for β-blocker therapy other than heart failure (e.g., ischemic heart disease) on the other. Thus, these human findings need to be replicated in larger, multicenter trials, and randomized prospective trials will be required before this gene-drug interaction should be considered to justify changing in the current class 1 indication for β-blockers in heart failure for even a subgroup of heart failure patients. In the meantime, the strong concordance between GRK5 Leu41 effects in recombinant cell culture systems, in transgenic mice, and in the initial study of human heart failure is sufficiently striking to suggest that gene-drug interactions such as this may have more impact on interindividual variability in treatment response than is now recognized.
Beyond Individual Polymorphisms—The Challenge of Haplotypes
The β1AR (Small et al., 2008), β2AR (Drysdale et al., 2000), α2AAR (Small et al., 2006), and α2CAR (Small et al., 2004) genes have polymorphisms in their promoter, 5′UTR, and 3′UTR regions, as well as their coding regions. Although individual analysis of a promoter SNP, for example, could reveal a phenotype using reporter assays, it is difficult to know the relevance of this individual SNP within the context of all the other variations in these intronless genes. This situation is somewhat different from a coding SNP that has a marked phenotype in terms of ligand binding, G-protein coupling, or desensitization, where such functions are clearly relevant to drug response. We have therefore proposed that promoter, 5′UTR, and 3′UTR SNPs should be studied within the context of the other SNPs as they occur in nature (i.e., in the context of the haplotype). The number of theoretically possible haplotypes is 2N, where N is the number of SNPs. However, the human population is fairly young, and thus there has not been enough time for so many recombination events to have the number of haplotypes approach this theoretical number. It is noteworthy that we (Small et al., 2003) and others (Stephens et al., 2001) have found that the number of haplotypes for any gene is approximately equal to the number of SNPs × 1.1 (SNP and haplotype frequency 0.01 or greater). Hence, for the α2CAR, we found 20 SNPs (and thus a theoretical 1.04 × 106 haplotypes), but only 24 haplotypes (Small et al., 2004). The α2CAR haplotypes represent an informative example of the complexity that this next step in genetic variation-phenotype association studies presents. Table 2 shows the SNPs and the haplotypes of the α2CAR in African Americans, whites, and Asians. It is readily appreciated that certain haplotypes are cosmopolitan (i.e., 1, 2), whereas others are overrepresented in certain racial groups (i.e., 8, 14). Second, it is clear that only seven haplotypes occur at an allele frequency ≥0.05 in at least one racial group. However, genotyping to identify only these seven leaves ∼10 to 15% of subjects with incorrect haplotypes (if a limited haplotype-tagged SNP approach is used for identification) or grouped as “other.” In addition, in the 3′UTR (position r), there is a 21-base pair deletion. In the insertion form, there is a SNP within this stretch of nucleotides (position s). Depending on the genotyping technique, the s polymorphism might be called in the presence of the r deletion, because the wild-type allele would not be found. Another issue revolves around the 12-nucleotide in-frame deletion polymorphism (position q) that occurs in the third intracellular loop of the receptor, deleting amino acids 322 to 325. The α2CDel322-325 has been studied in transfected cells and found to be markedly dysfunctional (Small et al., 2000); it has been associated with several heart failure phenotypes (Gerson et al., 2003; Lobmeyer et al., 2007; Small et al., 2002). However, Del322-325 is found in nine different α2CAR haplotypes. What if promoter polymorphisms within a haplotype increased expression? Would that move the cellular or clinical phenotype to an intermediate phenotype? Or, conversely, SNPs that lower expression could accentuate the Del322-325 effect, again adding heterogeneity to the phenotypes. Indeed, using whole-gene transfections, we have shown that haplotype does affect receptor expression (Small et al., 2004). Thus in a clinical study, genotyping only at Del322-325 does not give one all the pertinent information that may affect drug response or other traits. The need to genotype more SNPs, and to impute haplotypes, would seem to require greater numbers of subjects for clinical trials because there are more genotypic bins. And, some would argue, additional power considerations must be made because of multiple comparisons. However, in candidate gene studies, it seems inappropriate to genotype at one SNP position when there are multiple SNPs, particularly when molecular studies have shown haplotype effects. It is noteworthy that reliance on linkage disequilibrium between an SNP within a gene and several other SNPs in the gene must be shown to be valid before studies. For the α2CAR, even over a small number of bases (∼4300), low levels of linkage disequilibrium were found between some SNPs, and the degree of linkage disequilibrium varied by race (Small et al., 2004). This represents a major weakness of unbiased genome-wide association studies. Multiple SNPs of the α2CAR could be on a chip for such study, and due to low linkage disequilibrium, none would have identified (tracked with) the Del322-325 polymorphism.
TABLE 2.
Haplotypes of the α2C -adrenergic receptor gene
Shown are the allele frequencies of the haplotypes for each ethnic group. See Small et al. (2004) for the position in the gene relative to the location code.
|
HAP #
|
Promoter
|
5'UTR
|
Coding
|
3'UTR
|
AA
|
W
|
As
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | g | h | i | j | k | l | m | n | o | p | q | r | s | t | ||||
| % | |||||||||||||||||||||||
| 1 | T | C | C | G | C | C | G | T | T | C | G | G | C | C | C | T | Ins | Ins | T | G | 23.8 | 58.8 | 39.6 |
| 2 | T | C | C | G | C | C | G | T | T | C | G | G | C | C | C | T | Ins | Ins | C | C | 13.8 | 13.8 | 12.5 |
| 3 | T | C | C | G | C | C | G | G | T | C | G | G | C | C | C | T | Del | Ins | C | C | 21.3 | 0 | 8.3 |
| 4 | T | C | C | G | C | C | G | G | T | C | G | G | C | C | G | T | Del | Ins | C | C | 7.5 | 0 | 0 |
| 5 | T | G | T | G | C | C | G | T | T | A | C | G | C | C | C | T | Ins | Ins | C | C | 7.5 | 0 | 0 |
| 6 | T | C | C | G | C | C | G | T | T | C | G | G | C | C | C | T | Del | Ins | C | C | 5 | 0 | 0 |
| 7 | T | C | C | T | C | C | G | G | T | C | G | G | C | C | C | T | Del | Ins | C | C | 5 | 0 | 0 |
| 8 | C | C | C | G | T | C | G | T | T | C | G | A | C | C | C | T | Ins | Del | a | G | 2.5 | 7.5 | 27.1 |
| 9 | T | C | C | G | C | C | G | G | T | C | G | G | C | C | C | C | Del | Ins | C | C | 2.5 | 3.75 | 0 |
| 10 | T | C | C | G | C | C | G | T | T | C | G | G | C | C | C | T | Ins | Del | a | G | 1.25 | 0 | 2.1 |
| 11 | T | C | C | G | C | C | G | G | T | C | G | G | C | C | C | T | Ins | Ins | T | G | 2.5 | 0 | 0 |
| 12 | T | G | T | G | C | C | G | T | T | C | C | G | C | C | C | T | Ins | Ins | C | C | 2.5 | 0 | 0 |
| 13 | T | C | C | G | C | C | G | T | G | C | G | G | C | C | C | T | Ins | Ins | T | G | 0 | 2.5 | 0 |
| 14 | T | C | C | G | C | C | G | T | T | C | G | G | C | G | C | T | Ins | Ins | C | C | 0 | 11.3 | 0 |
| 15 | C | C | C | G | C | C | G | G | T | C | G | G | C | C | C | T | Del | Ins | C | C | 1.25 | 0 | 0 |
| 16 | C | C | C | G | C | C | G | T | T | C | G | G | C | C | C | T | Ins | Ins | C | C | 1.25 | 0 | 0 |
| 17 | C | C | C | G | C | C | G | T | T | C | G | G | C | C | C | T | Ins | Del | a | G | 1.25 | 0 | 0 |
| 18 | T | C | C | G | C | C | A | T | T | C | G | G | A | C | C | T | Del | Ins | T | G | 1.25 | 0 | 0 |
| 19 | C | C | C | G | C | C | G | G | T | C | G | G | C | C | C | C | Del | Del | a | C | 0 | 1.25 | 0 |
| 20 | T | C | C | G | C | C | A | G | T | C | G | G | A | C | C | T | Del | Ins | C | C | 0 | 1.25 | 0 |
| 21 | C | C | C | G | T | C | G | T | T | C | G | A | C | C | C | C | Ins | Del | a | G | 0 | 0 | 4.2 |
| 22 | C | C | C | G | T | C | A | T | T | C | G | A | A | C | C | T | Ins | Del | a | G | 0 | 0 | 2.1 |
| 23 | C | C | C | G | T | T | G | T | T | C | G | A | C | C | C | T | Ins | Del | a | G | 0 | 0 | 2.1 |
| 24 | T | C | C | G | C | C | G | T | T | C | G | G | C | C | C | C | Ins | Ins | T | G | 0 | 0 | 2.1 |
AA, African American; W, white; As, Asian;
Not applicable due to deletion at r.
Conclusions
The β1AR and GRK5 polymorphisms discussed seem to play a role in the response to β-blocker therapy, and a mechanistic basis for the effects has arisen from studies using multiple approaches. Each of these types of studies, using transfected cells, transgenic mouse, ex vivo human hearts, and human physiological outcomes, has limitations. But taken together, they build a case for cause-and-effect for a polymorphism and a drug-response phenotype. They also provide new insights into alternative therapies and the pathophysiology of heart failure. Nevertheless, questions remain as to their roles in individualizing heart failure therapy, and there may be other polymorphisms that have an even greater impact that are yet to be discovered. Nevertheless, we propose that effort be expended in both clinical studies as well as mechanistic studies so that pharmacogenomics can include well conducted clinical trials and a linking of these results to mechanism of action.
Acknowledgments
We thank Esther Moses for manuscript preparation.
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL045967, HL077101, HL052318].
ABBREVIATIONS: βAR, β-adrenergic receptor; β-blockers, β-adrenergic receptor blockers; GRK, G-protein coupled receptor kinase; NE, norepinephrine; AR, adrenergic receptor; CHW, Chinese hamster fibroblast; PKA, protein kinase A; VO2, oxygen consumption; ACV, adenylyl cyclase type V; LVEF, left ventricular ejection fraction; UTR, untranslated region; SNP, single nucleotide polymorphism.
References
- Benovic JL, DeBlasi A, Stone WC, Caron MG, and Lefkowitz RJ (1989) Βeta-adrenergic receptor kinase: primary structure delineates a multigene family. Science 246 235-240. [DOI] [PubMed] [Google Scholar]
- Benovic JL, Mayor F Jr, Staniszewski C, Lefkowitz RJ, and Caron MG (1987) Purification and characterization of beta-adrenergic receptor kinase. J Biol Chem 262 9026-9032. [PubMed] [Google Scholar]
- Benovic JL, Strasser RH, Caron MG, and Lefkowitz RJ (1986) Beta-adrenergic receptor kinase: identification of a novel protein kinase phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci U S A 83 2797-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEST Trial Investigators (2001) A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 344 1659-1667. [DOI] [PubMed] [Google Scholar]
- Bristow M (2003) Antiadrenergic therapy of chronic heart failure: surprises and new opportunities. Circulation 107 1100-1102. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Hershberger RE, Port JD, Gilbert EM, Sandoval A, Rasmussen R, Cates AE, and Feldman AM (1990) Beta-adrenergic pathways in nonfailing and failing human ventricular myocardium. Circulation 82 I12-I25. [PubMed] [Google Scholar]
- Brodde OE (2007) Beta-adrenoceptor blocker treatment and the cardiac beta-adrenoceptor-G-protein(s)-adenylyl cyclase system in chronic heart failure. Naunyn Schmiedebergs Arch Pharmacol 374 361-372. [DOI] [PubMed] [Google Scholar]
- Bruck H, Leineweber K, Temme T, Weber M, Heusch G, Philipp T, and Brodde OE (2005) The Arg389Gly beta1-adrenoceptor polymorphism and catecholamine effects on plasma-renin activity. J Am Coll Cardiol 46 2111-2115. [DOI] [PubMed] [Google Scholar]
- Chen EP, Bittner HB, Akhter SA, Koch WJ, and Davis RD (2001) Myocardial function in hearts with transgenic overexpression of the G protein-coupled receptor kinase 5. Ann Thorac Surg 71 1320-1324. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Pfeffer MA, Rouleau J, Sharpe N, Swedberg K, Straub M, Wiltse C, and Wright TJ (2003) Adverse mortality effect of central sympathetic inhibition with sustained-release moxonidine in patients with heart failure (MOXCON). Eur J Heart Fail 5 659-667. [DOI] [PubMed] [Google Scholar]
- de Groote P, Lamblin N, Helbecque N, Mouquet F, Mc Fadden E, Hermant X, Amouyel P, Dallongeville J, and Bauters C (2005) The impact of beta-adrenoreceptor gene polymorphisms on survival in patients with congestive heart failure. Eur J Heart Fail 7 966-973. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, and Shenoy SK (2007) Beta-arrestins and cell signaling. Annu Rev Physiol 69 483-510. [DOI] [PubMed] [Google Scholar]
- Drysdale CM, McGraw DW, Stack CB, Stephens JC, Judson RS, Nandabalan K, Arnold K, Ruano G, and Liggett SB (2000) Complex promoter and coding region beta 2-adrenergic receptor haplotypes alter receptor expression and predict in vivo responsiveness. Proc Natl Acad Sci U S A 97 10483-10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauchier L, Pierre B, de Labriolle A, and Babuty D (2007) Comparison of the beneficial effect of beta-blockers on mortality in patients with ischaemic or non-ischaemic systolic heart failure: a meta-analysis of randomised controlled trials. Eur J Heart Fail 9 1136-1139. [DOI] [PubMed] [Google Scholar]
- Ferguson SS (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53 1-24. [PubMed] [Google Scholar]
- Freeman JL, De La Cruz EM, Pollard TD, Lefkowitz RJ, and Pitcher JA (1998) Regulation of G protein-coupled receptor kinase 5 (GRK5) by actin. J Biol Chem 273 20653-20657. [DOI] [PubMed] [Google Scholar]
- Gerson MC, Wagoner LE, McGuire N, and Liggett SB (2003) Activity of the uptake-1 norepinephrine transporter as measured by I-123 MIBG in heart failure patients with a loss-of-function polymorphism of the presynaptic alpha2C-adrenergic receptor. J Nucl Cardiol 10 583-589. [DOI] [PubMed] [Google Scholar]
- Gilsbach R, Brede M, Beetz N, Moura E, Muthig V, Gerstner C, Barreto F, Neubauer S, Vieira-Coelho MA, and Hein L (2007) Heterozygous alpha 2C-adrenoceptor-deficient mice develop heart failure after transverse aortic constriction. Cardiovasc Res 75 728-737. [DOI] [PubMed] [Google Scholar]
- Hata JA, Williams ML, and Koch WJ (2004) Genetic manipulation of myocardial beta-adrenergic receptor activation and desensitization. J Mol Cell Cardiol 37 11-21. [DOI] [PubMed] [Google Scholar]
- Hein L, Altman JD, and Kobilka BK (1999) Two functionally distinct alpha2-adrenergic receptors regulate sympathetic neurotransmission. Nature 402 181-184. [DOI] [PubMed] [Google Scholar]
- Joseph SS, Lynham JA, Grace AA, Colledge WH, and Kaumann AJ (2004) Markedly reduced effects of (-)-isoprenaline but not of (-)-CGP12177 and unchanged affinity of beta-blockers at Gly389-beta1-adrenoceptors compared to Arg389-beta1-adrenoceptors. Br J Pharmacol 142 51-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunapuli P and Benovic JL (1993) Cloning and expression of GRK5: a member of the G protein-coupled receptor kinase family. Proc Natl Acad Sci U S A 90 5588-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leineweber K, Bruck H, Temme T, Heusch G, Philipp T, and Brodde OE (2006) The Arg389Gly beta1-adrenoceptor polymorphism does not affect cardiac effects of exercise after parasympathetic inhibition by atropine. Pharmacogenet Genomics 16 9-13. [DOI] [PubMed] [Google Scholar]
- Levin MC, Marullo S, Muntaner O, Andersson B, and Magnusson Y (2002) The myocardium-protective Gly-49 variant of the beta 1-adrenergic receptor exhibits constitutive activity and increased desensitization and down-regulation. J Biol Chem 277 30429-30435. [DOI] [PubMed] [Google Scholar]
- Liggett SB, Cresci S, Kelly RJ, Syed FM, Matkovich SJ, Hahn HS, Diwan A, Martini JS, Sparks L, Parekh RR, et al. (2008) A GRK5 polymorphism that inhibits beta-adrenergic receptor signaling is protective in heart failure. Nat Med 14 510-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggett SB, Mialet-Perez J, Thaneemit-Chen S, Weber SA, Greene SM, Hodne D, Nelson B, Morrison J, Domanski MJ, Wagoner LE, et al. (2006) A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure. Proc Natl Acad Sci U S A 103 11288-11293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobmeyer MT, Gong Y, Terra SG, Beitelshees AL, Langaee TY, Pauly DF, Schofield RS, Hamilton KK, Herbert Patterson J, Adams KF Jr., et al. (2007) Synergistic polymorphisms of beta1 and alpha2c-adrenergic receptors and the influence on left ventricular ejection fraction response to beta-blocker therapy in heart failure. Pharmacogenet Genomics 17 277-282. [DOI] [PubMed] [Google Scholar]
- Mann DL and Bristow MR (2005) Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation 111 2837-2849. [DOI] [PubMed] [Google Scholar]
- Mason DA, Moore JD, Green SA, and Liggett SB (1999) A gain-of-function polymorphism in a G-protein coupling domain of the human beta1-adrenergic receptor. J Biol Chem 274 12670-12674. [DOI] [PubMed] [Google Scholar]
- Matkovich SJ, Diwan A, Klanke JL, Hammer DJ, Marreez Y, Odley AM, Brunskill EW, Koch WJ, Schwartz RJ, and Dorn GW 2nd (2006) Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. Circ Res 99 996-1003. [DOI] [PubMed] [Google Scholar]
- MERIT-HF Study Group (1999) Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 353 2001-2007. [PubMed] [Google Scholar]
- Mialet Perez J, Rathz DA, Petrashevskaya NN, Hahn HS, Wagoner LE, Schwartz A, Dorn GW, and Liggett SB (2003) Β1-adrenergic receptor polymorphisms confer differential function and predisposition to heart failure. Nat Med 9 1300-1305. [DOI] [PubMed] [Google Scholar]
- Molenaar P, Chen L, Semmler AB, Parsonage WA, and Kaumann AJ (2007) Human heart beta-adrenoceptors: beta1-adrenoceptor diversification through `affinity states' and polymorphism. Clin Exp Pharmacol Physiol 34 1020-1028. [DOI] [PubMed] [Google Scholar]
- Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, and Shusterman NH (1996) The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 334 1349-1355. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, and Lefkowitz RJ (1998) G protein-coupled receptor kinases. Annu Rev Biochem 67 653-692. [DOI] [PubMed] [Google Scholar]
- Premont RT, Inglese J, and Lefkowitz RJ (1995) Protein kinases that phosphorylate activated G protein-coupled receptors. FASEB J 9 175-182. [DOI] [PubMed] [Google Scholar]
- Premont RT, Koch WJ, Inglese J, and Lefkowitz RJ (1994) Identification, purification, and characterization of GRK5, a member of the family of G protein-coupled receptor kinases. J Biol Chem 269 6832-6841. [PubMed] [Google Scholar]
- Pronin AN, Satpaev DK, Slepak VZ, and Benovic JL (1997) Regulation of G protein-coupled receptor kinases by calmodulin and localization of the calmodulin binding domain. J Biol Chem 272 18273-18280. [DOI] [PubMed] [Google Scholar]
- Rathz DA, Brown KM, Kramer LA, and Liggett SB (2002) Amino acid 49 polymorphisms of the human beta1-adrenergic receptor affect agonist-promoted trafficking. J Cardiovasc Pharmacol 39 155-160. [DOI] [PubMed] [Google Scholar]
- Rathz DA, Gregory KN, Fang Y, Brown KM, and Liggett SB (2003) Hierarchy of polymorphic variation and desensitization permutations relative to beta 1- and beta 2-adrenergic receptor signaling. J Biol Chem 278 10784-10789. [DOI] [PubMed] [Google Scholar]
- Rochais F, Vilardaga JP, Nikolaev VO, Bünemann M, Lohse MJ, and Engelhardt S (2007) Real-time optical recording of beta1-adrenergic receptor activation reveals supersensitivity of the Arg389 variant to carvedilol. J Clin Invest 117 229-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallese M, Iacovelli L, Cumashi A, Capobianco L, Cuomo L, and De Blasi A (2000) Regulation of G protein-coupled receptor kinase subtypes by calcium sensor proteins. Biochim Biophys Acta 1498 112-121. [DOI] [PubMed] [Google Scholar]
- Sandilands AJ, Parameshwar J, Large S, Brown MJ, and O'Shaughnessy KM (2005) Confirmation of a role for the 389R>G beta-1 adrenoceptor polymorphism on exercise capacity in heart failure. Heart 91 1613-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehnert AJ, Daniels SE, Elashoff M, Wingrove JA, Burrow CR, Horne B, Muhlestein JB, Donahue M, Liggett SB, Anderson JL, et al. (2008) Lack of association between adrenergic receptor genotypes and survival in heart failure patients treated with carvedilol and metoprolol. J Am Coll Cardiol 52 644-651. [DOI] [PubMed] [Google Scholar]
- Shin J, Lobmeyer MT, Gong Y, Zineh I, Langaee TY, Yarandi H, Schofield RS, Aranda JM Jr, Hill JA, Pauly DF, et al. (2007) Relation of beta(2)-adrenoceptor haplotype to risk of death and heart transplantation in patients with heart failure. Am J Cardiol 99 250-255. [DOI] [PubMed] [Google Scholar]
- Small KM, Brown KM, Seman CA, Theiss CT, and Liggett SB (2006) Complex haplotypes derived from noncoding polymorphisms of the intronless alpha2A-adrenergic gene diversify receptor expression. Proc Natl Acad Sci U S A 103 5472-5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small KM, Forbes SL, Rahman FF, Bridges KM, and Liggett SB (2000) A four amino acid deletion polymorphism in the third intracellular loop of the human alpha 2C-adrenergic receptor confers impaired coupling to multiple effectors. J Biol Chem 275 23059-23064. [DOI] [PubMed] [Google Scholar]
- Small KM, Mialet-Perez J, and Liggett SB (2008) Genetic variation within the beta1-adrenergic receptor gene results in haplotype-specific expression phenotypes. J Cardiovasc Pharmacol 51 106-110. [DOI] [PubMed] [Google Scholar]
- Small KM, Mialet-Perez J, Seman CA, Theiss CT, Brown KM, and Liggett SB (2004) Polymorphisms of the cardiac presynaptic alpha2C adrenergic receptors: diverse intragenic variability with haplotype-specific functional effects. Proc Natl Acad Sci U S A 101 13020-13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small KM, Tanguay DA, Nandabalan K, Zhan P, Stephens JC, and Liggett SB (2003) Gene and protein domain-specific patterns of genetic variability within the G-protein coupled receptor superfamily. Am J Pharmacogenomics 3 65-71. [DOI] [PubMed] [Google Scholar]
- Small KM, Wagoner LE, Levin AM, Kardia SL, and Liggett SB (2002) Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med 347 1135-1142. [DOI] [PubMed] [Google Scholar]
- Stephens JC, Schneider JA, Tanguay DA, Choi J, Acharya T, Stanley SE, Jiang R, Messer CJ, Chew A, Han JH, et al. (2001) Haplotype variation and linkage disequilibrium in 313 human genes. Science 293 489-493. [DOI] [PubMed] [Google Scholar]
- Swift SM, Gaume BR, Small KM, Aronow BJ, and Liggett SB (2008) Differential coupling of Arg- and Gly389 polymorphic forms of the beta1-adrenergic receptor leads to pathogenic cardiac gene regulatory programs. Physiol Genomics 35 123-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift SM, Schwarb MR, Mihlbachler KA, and Liggett SB (2007) Pleiotropic beta-agonist-promoted receptor conformations and signals independent of intrinsic activity. Am J Respir Cell Mol Biol 36 236-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepe NM, Lorenz JN, Yatani A, Dash R, Kranias EG, Dorn GW2 and Liggett SB (1999) Altering the receptor-effector ratio by transgenic overexpression of type V adenylyl cyclase: enhanced basal catalytic activity and function without increased cardiomyocyte Β-adrenergic signalling. Biochem 38 16706-16713. [DOI] [PubMed] [Google Scholar]
- Terra SG, Pauly DF, Lee CR, Patterson JH, Adams KF, Schofield RS, Belgado BS, Hamilton KK, Aranda JM, Hill JA, et al. (2005) beta-Adrenergic receptor polymorphisms and responses during titration of metoprolol controlled release/extended release in heart failure. Clin Pharmacol Ther 77 127-137. [DOI] [PubMed] [Google Scholar]
- Turki J, Lorenz JN, Green SA, Donnelly ET, Jacinto M, and Liggett SB (1996) Myocardial signalling defects and impaired cardiac function of a human beta 2-adrenergic receptor polymorphism expressed in transgenic mice. Proc Natl Acad Sci U S A 93 10483-10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Campen LC, Visser FC, and Visser CA (1998) Ejection fraction improvement by beta-blocker treatment in patients with heart failure: an analysis of studies published in the literature. J Cardiovasc Pharmacol 32 (Suppl 1): S31-S35. [DOI] [PubMed] [Google Scholar]
- Wagoner LE, Craft LL, Zengel P, McGuire N, Rathz DA, Dorn GW 2nd, and Liggett SB (2002) Polymorphisms of the beta1-adrenergic receptor predict exercise capacity in heart failure. Am Heart J 144 840-846. [DOI] [PubMed] [Google Scholar]
- Wang WC, Mihlbachler KA, Bleecker ER, Weiss ST, and Liggett SB (2008) A polymorphism of G-protein coupled receptor kinase5 alters agonist-promoted desensitization of beta2-adrenergic receptors. Pharmacogenet Genomics 18 729-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HL, de Boer RA, Maqbool A, Greenwood D, van Veldhuisen DJ, Cuthbert R, Ball SG, Hall AS, and Balmforth AJ (2003) An evaluation of the beta-1-adrenergic receptor Arg389Gly polymorphism in individuals with heart failure: a MERIT-HF sub-study. Eur J Heart Fail 5 463-468. [DOI] [PubMed] [Google Scholar]