Abstract
We have previously reported on the unusual human 5-hydroxytryptamine7 (h5-HT7) receptor-inactivating properties of risperidone, 9-OH-risperidone, bromocriptine, methiothepin, metergoline, and lisuride. Inactivation was defined as the inability of 10 μM 5-HT to stimulate cAMP accumulation after brief exposure and thorough removal of the drugs from HEK293 cells expressing h5-HT7 receptors. Herein we report that brief exposure of the h5-HT7 receptor-expressing cells to inactivating drugs, followed by removal of the drugs, results in potent and efficacious irreversible inhibition of forskolin-stimulated adenylate cyclase activity. Pretreatment, followed by removal of the inactivating drugs inhibited 10 μM forskolin-stimulated adenylate cyclase activity with potencies similar to the drugs' affinities for the h5-HT7 receptor. The actions of the inactivating drugs were pertussis toxin-insensitive, indicating the lack of Gi in their mechanism(s) of action. Methiothepin and bromocriptine maximally inhibited 10 μM forskolin-stimulated adenylate cyclase, whereas the other drugs produced partial inhibition, indicating the drugs are inducing slightly different inactive conformations of the h5-HT7 receptor. Maximal effects of these inactivating drugs occurred within 15 to 30 min of exposure of the cells to the drugs. A Gs-mediated inhibition of forskolin-stimulated activity has never been reported. The inactivating antagonists seem to induce a stable conformation of the h5-HT7 receptor, which induces an altered state of Gs, which, in turn, inhibits forskolin-mediated stimulation of adenylate cyclase. These and previous observations indicate that the inactivating antagonists represent a unique class of drugs and may reveal GPCR regulatory mechanisms previously unknown. These drugs may produce innovative approaches to the development of therapeutic drugs.
The 5-HT7 receptor is one of 14 5-HT receptors expressed in mammalian tissues (Teitler and Herrick-Davis, 1994; Gerhardt and van Heerikhuizen, 1997; Hoyer and Martin, 1997; Raymond et al., 2001; Hoyer et al., 2002; Kroeze et al., 2002). It was discovered through homology cloning and is expressed in various areas of the human brain and in peripheral tissues, including important blood vessels in the cerebral vasculature (Bard et al., 1993; Lovenberg et al., 1993; Shen et al., 1993; Teitler and Herrick-Davis, 1994; Hedlund and Sutcliffe, 2004). 5-HT7 receptor antagonists are being developed for possible use in various clinical conditions, including migraine (Terrón, 1997), sleep (Lovenberg et al., 1993), psychosis (Bard et al., 1993; Lovenberg et al., 1993; Shen et al., 1993), and depression (Bard et al., 1993; Lovenberg et al., 1993; Shen et al., 1993; Hedlund and Sutcliffe, 2004).
Risperidone is a highly prescribed atypical antipsychotic drug (Bhana and Spencer, 2000; Green, 2000; Love and Nelson, 2000; Schneider et al., 2006). It is one of a group of drugs believed to initiate their effects through interactions with the D2 dopamine and 5-HT2A serotonin receptors (Meltzer et al., 1989; Roth et al., 1994). These interactions have been shown to be classic competitive antagonist interactions (Roth et al., 1994; Smith et al., 2006). In previous publications, using h5-HT7 receptor-expressing HEK293 cells, we reported the rapid, potent inactivation of h5-HT7 receptor stimulation of cAMP production by six antagonists: risperidone, 9-OH-risperidone, methiothepin, bromocriptine, metergoline, and lisuride (Smith et al., 2006; Knight et al., 2009). The mechanism seems to involve the pseudo-irreversible interaction of the drugs with the h5-HT7 receptor, thus occluding the orthosteric binding site and preventing stimulation by 5-HT.
However, several observations indicated that a simple pseudo-irreversible blockade might not fully explain the effect of the inactivating antagonists. Risperidone and 9-OH-risperidone irreversibly inhibited only 50% of the h5-HT7 orthosteric binding sites, whereas the other four inactivators irreversibly inhibited all the h5-HT7 receptor binding sites (Knight et al., 2009). In addition, metergoline's potency as an inactivator was significantly lower than predicted from its affinity for the h5-HT7 receptor, whereas the other five inactivators' potencies matched their affinities for the h5-HT7 receptor.
The effects of forskolin on adenylate cyclase activity have been studied extensively (Darfler et al., 1982; Stengel et al., 1982; Alousi et al., 1991; Tang and Gilman, 1995; Dessauer et al., 1997; Insel and Ostrom, 2003). Although the major effect of forskolin is to directly stimulate adenylate cyclase activity, this stimulation can be regulated by GPCRs through G-proteins (Neer, 1978, 1986; Bender et al., 1984; Taussig et al., 1993; Tesmer et al., 2002). The predominant regulation of forskolin-stimulated adenylate cyclase activity is mediated by activation of Gi/o-coupled GPCRs, which partially inhibit forskolin-stimulated adenylate cyclase activity (Neer, 1978, 1986; Bender et al., 1984; Taussig et al., 1993; Tesmer et al., 2002). Agonist-mediated modulation of forskolin-stimulated adenylate cyclase has been reported to be produced through Gs-coupled receptors (Alousi et al. 1991). This effect is relatively minor and usually manifests as a potentiation of forskolin-stimulated activity. It should be noted that the GPCR-mediated regulation of forskolin-stimulated adenylate cyclase activity occurs through activity of agonists on GPCR. There seem to be no reports of acute effects of antagonists on forskolin-stimulated adenylate cyclase activity. During the previously reported investigation of h5-HT7 receptor inactivation by risperidone and other inactivating antagonists (Knight et al., 2009), forskolin-stimulated adenylate cyclase activity was monitored as a control for cellular capacity to produce cAMP. It was anticipated that forskolin-stimulated adenylate cyclase activity would not be affected by the inactivating drugs. As described below, the inactivating antagonists produced unique effects on h5-HT7 receptor activity, demonstrated by the persistent inhibition of forskolin-stimulated cAMP in cells exposed to this novel group of drugs. These effects provide significant information concerning the mechanism by which the inactivating antagonists produce their effects on h5-HT7 receptor-mediated cAMP production.
Materials and Methods
cAMP Assay. Total cAMP accumulation was measured using the LANCE cAMP Detection kit (PerkinElmer Life and Analytical Sciences, Waltham, MA). Cells were cultured for 18 h in serum-free media, with and without 100 ng/ml pertussis toxin (see Results, Fig. 1). Cells were lifted using 1 ml/dish diluted tetrasodium EDTA (Versene; 1:3 in phosphate-buffered saline), followed by the addition of 11 ml/dish HEPES buffer (20 mM HEPES, 2.5 mM MgSO4, and 134 mM NaCl, pH 7.5 at 23°C). Cells were centrifuged for 3 min at 330g, supernatant was aspirated, and the cells were resuspended in HEPES buffer. Cells were pretreated with drug, incubated for 30 min at 37°C (or 15, 30, 60, and 90 min for time-course experiments), and washed three times for 10 min each in HEPES buffer. After the third wash, cells were resuspended in stimulation buffer (prepared according to the PerkinElmer LANCE cAMP instruction manual). Cells were counted with a hemacytometer and added to 96-well white opaque plates. The pretreated cells were then exposed to 10 or 35 μM forskolin for 30 min at 23°C. Detection buffer was then added (prepared according to the LANCE instruction manual). Control experiments demonstrated that this procedure produced no effect on the cells' responsiveness to forskolin (see Results). Control experiments also demonstrated that performing the forskolin stimulation at 37°C has no effect on the properties of the inactivating antagonists (see Results). Time-resolved fluorescence resonance energy transfer was detected by the Victor3 1420 plate-reader (PerkinElmer Life and Analytical Sciences).
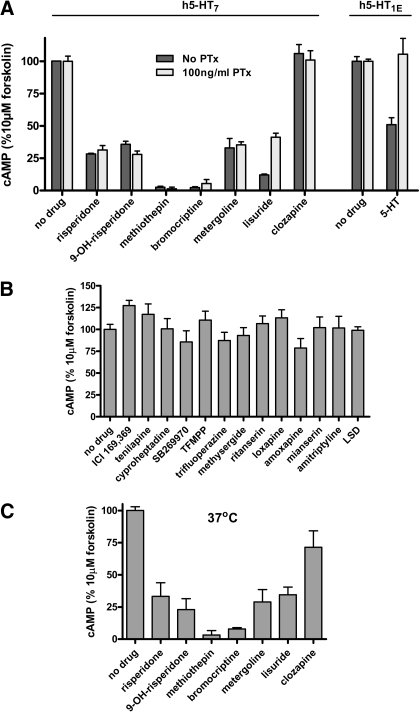
Fig. 1.
A, effect of drug pretreatment on 10 μM forskolin-stimulated cAMP production by HEK 293 cells stably expressing h5-HT7 receptors, and the lack of effect of pertussis toxin on the drug effects. Cells were cultured for 18 h in serum-free media in the absence and presence of 100 ng/ml pertussis toxin. Cells were suspended in HEPES buffer and exposed to a 10 μM concentration of drugs for 30 min. Cells were gently pelleted and resuspended in HEPES buffer and incubated at 37°C for 10 min. This drug washout procedure was repeated three times. Cells were resuspended and assayed for response to 10 μM forskolin using the LANCE cAMP Detection kit (PerkinElmer; see Materials and Methods). The results are the means ± S.E.M. of three independent experiments performed in triplicate. Risperidone, 9-OH-risperidone, bromocriptine, methiothepin, lisuride, and metergoline were significantly different from no drug treatment (p < 0.0001, one-way ANOVA). No significant main effect for pertussis toxin treatment was observed in h5-HT7 expressing cells (p = 0.104, two-way ANOVA). 5-HT1E receptor-expressing cells were tested for 5-HT-mediated inhibition of 10 μM forskolin-stimulated cAMP production in the absence and presence of pertussis toxin (positive control). PTx, pertussin toxin. B, drugs displaying no effect on forskolin-stimulated adenylate cyclase activity after thorough washout. The results are the means ± S.E.M. of three independent experiments performed in triplicate. There was no significant effect observed with these drugs (p = 0.12, one-way ANOVA). C, lack of effect of temperature on drug-induced inhibition of forskolin-stimulated adenylate cyclase activity. Cells were treated as in Fig. 1A except that forskolin stimulation was performed at 37°C rather than 23°C. There was no significant main effect on inhibition of forskolin-stimulated adenylate cyclase activity (p = 0.45, two-way ANOVA).
Risperidone and Metergoline Pretreatment Experiments. Cells were lifted and centrifuged as above. Cells were incubated for 30 min with 10 μM metergoline or 10 μM risperidone at 37°C. The cells were then washed three times for 10 min each with HEPES buffer. After the third wash, cells were resuspended in HEPES buffer and treated with bromocriptine or methiothepin for 30 min at 37°C. Cells were then washed three times for 10 min each with HEPES buffer. After the third wash, cells were resuspended in stimulation buffer (prepared according to the LANCE instruction manual). Cells were then counted using a hematocytometer and added to 96-well opaque plates. The pretreated cells were then exposed to 10 μM forskolin for 30 min at room temperature. Plates were read on a Victor3 plate-reader (PerkinElmer Life and Analytical Sciences).
Adenylate Cyclase and Gαs Immunoblots. Rabbit polyclonal A cyclase V/VI (H-130), Gαs (K-20), ERK2, and donkey anti-rabbit horseradish peroxidase were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Suspended cells were treated with the specified drugs for 30 min at 37°C then washed (as described above) three times for 10 min each with HEPES buffer. The cells were rinsed once with 1× phosphate-buffered saline and spun for 3 min at 330g. The cells were lysed for 30 min on ice with radioimmunoprecipitation assay cell lysis buffer with protease inhibitors. The pellets were then further disrupted by shearing the DNA with a 26-gauge needle and another 30-min incubation on ice. The cell lysates were spun for 20 min at 14,000g at 4°C. The supernatants containing the total cell lysate were flash frozen and stored at -80°C until needed. BCA assay was done on the cell treatments to determine the concentration of protein in each sample. Twenty micrograms of protein/treatment were loaded on to 10% Tris-HCl polyacrylamide gradient gels (Bio-Rad Laboratories, Hercules, CA) to be separated using electrophoresis and then transferred to nitrocellulose membranes. Membranes were then blocked for 1 h with 5% nonfat dry milk before overnight incubation with the primary antibody at 4°C. The membrane was washed and incubated with the secondary antibody and the bound antibodies were visualized using Pierce ECL Western blotting Substrate (Pierce, Rockford IL). The membranes were then washed, stripped, re-blotted with the ERK-2 antibody, and visualized as above.
Results
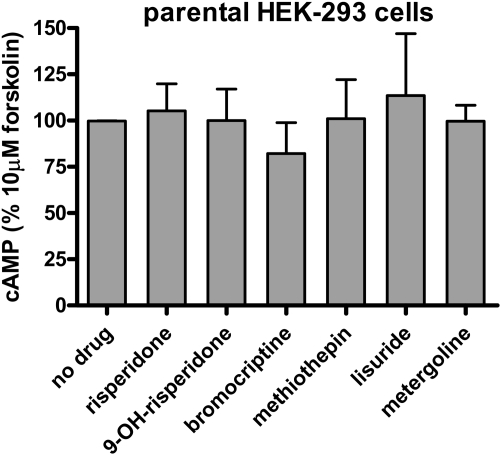
Fig. 1, A and B, displays the results of screening 20 drugs for forskolin-inactivating properties. These drugs were selected based on preliminary radioligand binding studies indicating they had high-to-moderate affinities for the h5-HT7 receptor. The h5-HT7 receptor expressing HEK293 cells were first exposed to 1 μM drug for 30 min, followed by three washouts. The cells were then exposed to 10 μM forskolin for 30 min. Inactivation was defined as the inability, or a reduced ability, of the cells to produce cAMP in response to forskolin stimulation after the thorough washout of drug. Six of the drugs tested displayed this property: risperidone, 9-OH-risperidone, methiothepin, bromocriptine, metergoline, and lisuride. These six drugs had been previously characterized as h5-HT7 receptor inactivators (Smith et al., 2006; Knight et al., 2009). The six drugs tested that had inactivating ability exhibited h5-HT7 receptor affinities that ranged from 0.4 to 143 nM. Lisuride (0.4 nM), risperidone (2 nM), methiothepin (3 nM), 9-OH-risperidone (10 nM), metergoline (16 nM), and bromocriptine (143 nM) displayed inactivating properties (Fig. 1A). Other drugs tested that did not display inactivating properties are listed with their h5-HT7 receptor affinities: amoxapine (69 nM), amitriptyline (96 nM), cyproheptadine (24 nM), loxapine (258 nM), mianserin (64 nM), ritanserin (468 nM), the selective 5-HT7 receptor antagonist SB269970 (2 nM), tenilapine (153 nM), 1-(3-trifluoromethylphenyl)piperazine (1624 nM), trifluoperazine (497 nM), the high-affinity 5-HT2 receptor antagonist ICI169369 (393 nM), clozapine (30 nM), methysergide (32 nM), and d-lysergic acid diethylamide (3 nM). Of the six inactivators, methiothepin and bromocriptine seemed to produce the most efficacious inhibition of 10 μM forskolin-stimulation (Fig. 1). To determine whether the h5-HT7 receptor might be stimulating a Gi, thereby inhibiting forskolin-stimulated adenylate cyclase activity, we pretreated the h5-HT7-receptor expressing cells with pertussis toxin, which inactivates Gi (Kaslow et al., 1987). Although the h5-HT7 receptor is a well characterized Gs-coupled receptor, it is possible that the “inactivating antagonists” are inducing a persistent state of the receptor that stimulates Gi, thereby inhibiting forskolin-stimulated adenylate cyclase activity. As shown in Fig. 1A, pretreatment of the h5-HT7-receptor expressing cells with pertussis toxin produced no main effect on the inactivating antagonists' activity (p = 0.104, two-way ANOVA). The lack of effect of pertussis toxin indicates Gi is not involved in the inhibition of forskolin stimulated adenylate cyclase activity. Figure 1A displays the positive control for the pertussis toxin: 5-HT1E-mediated inhibition of forskolin-stimulated cAMP accumulation is blocked, indicating that the pertussis toxin is active. Thus the lack of effect of pertussis toxin on the inhibition of forskolin-stimulated cAMP accumulation in the h5-HT7 receptor-expressing cells indicates no involvement of Gi. Figure 1B displays the lack of effect of 13 antagonists (i.e., “noninactivating antagonists”) on forskolin-stimulated adenylate cyclase. Figure 1C displays the lack of effect of performing the assay at 37°, rather than 23°, on the inactivating antagonist drugs' activity. Figure 2 displays the lack of effect of the inactivating antagonists on forskolin-stimulated adenylate cyclase activity in HEK-293 cells not expressing h5-HT7 receptors. Taken together, the data in Figs. 1 and 2 indicate that six of 20 drugs tested produced the inactivating effect on forskolin-stimulated adenylate cyclase activity, this effect is mediated through the h5-HT7 receptor rather than through a nonspecific mechanism, and the effect is not due to some temperature-induced alteration in the assay conditions.
Fig. 2.
Effect of drug pretreatment on 10 μM forskolin-stimulated cAMP production by parental HEK 293 cells. Cells were cultured for 18 h in serum-free media. Cells were suspended in HEPES buffer and exposed to a10 μM concentration of drugs for 30 min. Cells were gently pelleted, and resuspended in HEPES buffer and incubated at 37°C for 10 min. This drug washout procedure was repeated three times. Cells were resuspended and assayed for response to 10 μM forskolin using the LANCE cAMP detection kit (PerkinElmer; see Materials and Methods). The results are the means ± S.E.M. of three independent experiments performed in triplicate.
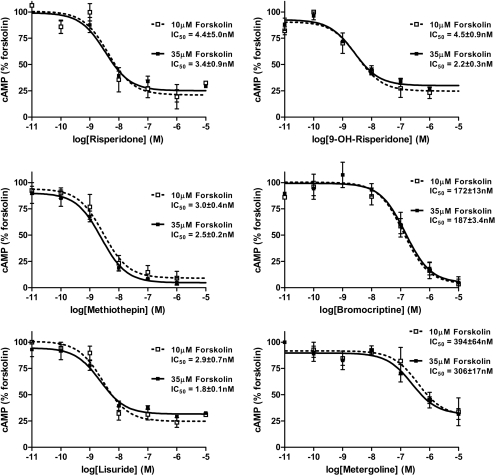
To obtain more information on this novel ability to irreversibly inhibit forskolin-stimulated adenylate cyclase activity, concentration-response curves for the inactivating effect were produced (Fig. 3; Table 1). The h5-HT7 receptor expressing HEK293 cells were first exposed to increasing concentrations of drug for 30 min, followed by three washouts. The cells were then exposed to 10 or 35 μM forskolin for 30 min. Risperidone, 9-OH-risperidone, bromocriptine, methiothepin, metergoline, and lisuride displayed high potencies for producing the inactivation effect, with similar IC50 values for 10 or 35 μM forskolin stimulation. The similar potencies of the inactivating drugs on 10 or 35 μM forskolin-stimulated activity (Fig. 3, Table 1) indicate the mechanism-of-action is not a competitive one. An important observation from the data in Fig. 3 is that the inactivators seem to have different maximal levels of inhibition (Table 2). Methiothepin and bromocriptine irreversibly inhibit forskolin-stimulated activity more effectively than risperidone, 9-OH-risperidone, metergoline, or lisuride. These results indicate the possibility of either one mechanism with multiple efficacies among the inactivating drugs (full and partial inactivators) or multiple mechanisms of inhibition among the inactivating drugs. The major observation was that methiothepin and bromocriptine are fully efficacious in irreversibly inhibiting forskolin-stimulated adenylate cyclase activity, whereas the other four inactivating drugs display less efficacy.
Fig. 3.
Concentration-response curves for inactivation of 10 and 35 μM forskolin-stimulated cAMP production in HEK-293 cells expressing h5-HT7 receptors. Cells were suspended in HEPES buffer and exposed to buffer (control) or varying concentrations of the six drugs displaying inactivating properties (see Fig. 1). After the drug washout procedure (see Materials and Methods) the cells were exposed to 10 or 35 μM forskolin for 30 min. cAMP levels were determined using the LANCE cAMP Detection kit (Perkin-Elmer). The results are the means ± S.E.M. of three independent experiments performed in triplicate. Dotted lines denote 10 μM forskolin; solid lines denote 35 μM forskolin.
TABLE 1.
Potencies of the six h5-HT7 inactivating drugs as inhibitors of forskolin-stimulated adenylate cyclase activity
After exposure of the h5-HT7-receptor-expressing HEK293 cells to varying concentrations of drugs for 30 min, the drugs were removed by repeated washing. Activity was determined by exposing the cells to 10 or 35 μM forskolin for 30 min. cAMP was detected using the LANCE cAMP detection kit (see Materials and Methods). Results are the means ± S.E.M. of three independent experiments performed in triplicate. Also included are the Ki values determined from homogenate binding studies (Knight et al., 2009). Increasing forskolin concentration had no effect on drug potencies (p = 0.492, two-way ANOVA).
|
Drug
|
Kia
|
IC50
|
|
|---|---|---|---|
| 10 μM Forskolin | 35 μM Forskolin | ||
| nM | nM | ||
| Risperidone | 1.8 ± 0.3 | 4.4 ± 2.1 | 3 ± 0.9 |
| 9-OH Risperidone | 10 ± 1.7 | 4.5 ± 0.9 | 2.2 ± 0.3 |
| Bromocriptine | 143 ± 56 | 172 ± 13 | 187 ± 3.4 |
| Lisuride | 0.4 ± 0.2 | 2.9 ± 0.7 | 1.8 ± 0.1 |
| Metergoline | 16 ± 2 | 394 ± 64 | 306 ± 17 |
| Methiothepin | 3.0 ± 0.5 | 3.0 ± 0.4 | 2.5 ± 0.2 |
Membrane homogenate binding (Knight et al., 2009).
TABLE 2.
Maximal effects of the six inactivating drugs on 10 and 35 μM forskolin-stimulated activity
h5-HT7-receptor expressing cells were exposed to 10 μM drugs for 30 min, thoroughly washed, then exposed to 10 or 35 μM forskolin for 30 min (see Fig. 3). The values listed are the percentage of forskolin-stimulated activity observed in cells not exposed to inactivators. cAMP was detected using the LANCE kit (see Materials and Methods). Results are the mean ± S.E.M. of three independent experiments.
| Inactivator (10 μM) | 10 μM Forskolin | 35 μM Forskolin |
|---|---|---|
| % control | ||
| Risperidone | 21 ± 3 | 25 ± 2 |
| 9-OH-Risperidone | 25 ± 2 | 30 ± 6 |
| Methiothepin | 9 ± 4 | 4 ± 3 |
| Bromocriptine | 4 ± 2 | 5 ± 2 |
| Metergoline | 30 ± 3.5 | 31 ± 12 |
| Lisuride | 25 ± 4 | 32 ± 8 |
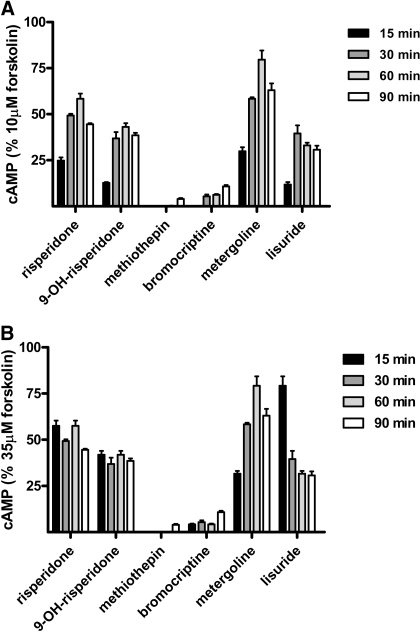
To determine whether the difference in efficacies could be due to kinetic differences between the drugs in producing the inactivating effect, time-course experiments were performed (Fig. 4). Cells were pretreated with each of the inactivators for 15, 30, 60, and 90 min and then subjected to the usual washout and assay procedures (see Materials and Methods). Although several interesting observations were made in these experiments (see Discussion), it is clear that the lower efficacy of risperidone, 9-OH-risperidone, metergoline, and lisuride relative to methiothepin and bromocriptine cannot be due to slower kinetics. The lesser effect observed for several of the drugs at the 90-min time point is contrary to this possibility.
Fig. 4.
Time course of maximal inhibition of forskolin-stimulated adenylate cyclase activity by h5-HT7 inactivators. Cells were exposed to 10 μM inactivators for varying incubation times. After the drug washout procedure (see Materials and Methods), the cells were exposed to 10 μM (A) or 35 μM (B) forskolin, and cAMP was detected as described above. Results are the means ± S.E.M. of three independent experiments. Although there was a significant effect of time on the drugs (p < 0.001, two-way ANOVA), this effect does not account for the difference between the maximal effects of the different drugs (see Discussion).
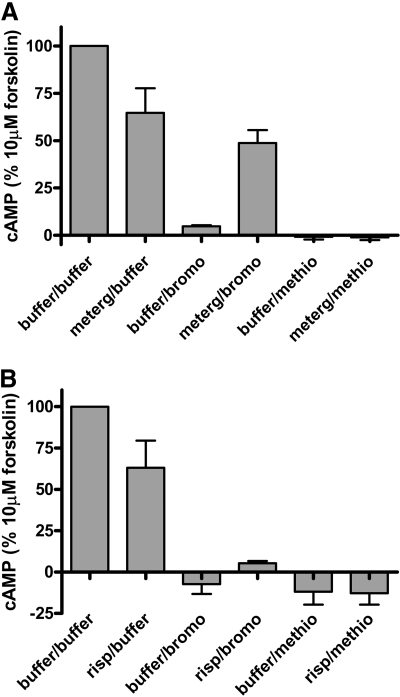
Another possible rationale for the difference in efficacies could be different mechanisms involving distinct sites on the h5-HT7 receptor mediating the effects of the inactivators. To obtain information on this possibility, h5-HT7 receptor-expressing cells were exposed to 10 μM metergoline or risperidone (partial inactivators) for 30 min, washed, and then exposed to 10 μM methiothepin or bromocriptine (full inactivators). Metergoline and risperidone were chosen based on previous observations, indicating that they display somewhat different properties as inactivators (Knight et al., 2009). As shown in Fig. 5A, pretreatment with metergoline resulted in a blunting of the effect of bromocriptine but had no effect on methiothepin's inactivating activity. Risperidone pretreatment had no effect on either methiothepin or bromocriptine (Fig. 5B). Taken together, these results indicate that methiothepin, bromocriptine, risperidone, and metergoline produce inactivation by somewhat different mechanisms (see Discussion).
Fig. 5.
Effect of metergoline or risperidone pretreatment on inactivator inhibition of forskolin-stimulated adenylate cyclase. Cells were exposed to 10 μM metergoline or risperidone for 30 min, thoroughly washed (see Materials and Methods), exposed to 10 μM methiothepin or bromocriptine for 30 min, and thoroughly washed. After the drug washout procedure (see Materials and Methods) the cells were exposed to 10 μM forskolin, and cAMP was detected as described above. Results are the means ± S.E.M. of three independent experiments. Metergoline blunted the effects of bromocriptine (p < 0.001) but had no effect on methiothepin's activity. Risperidone had no significant effect on methiothepin or bromocriptine (see Discussion).
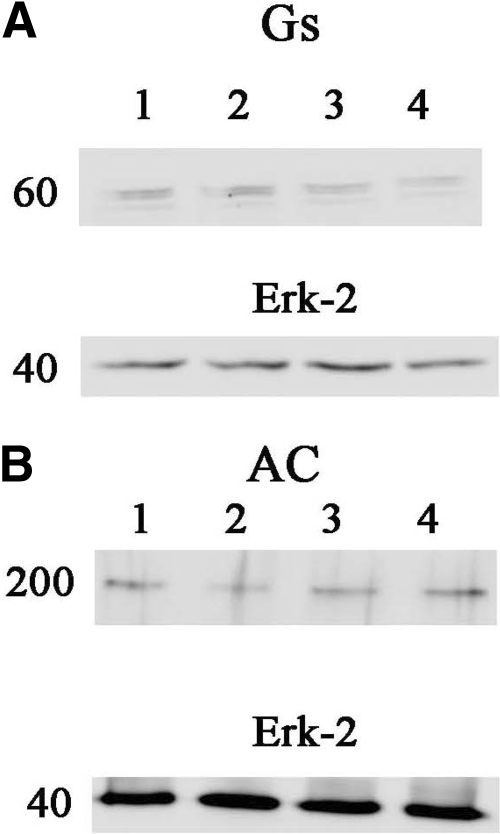
Figure 6 displays the results of Western blots for adenylate cyclase (isoforms V and VI) and Gs (α subunit) subsequent to exposure of the h5-HT7 receptor-expressing cells to no drug, 10 μM clozapine, methiothepin, or risperidone. None of the drugs produced any effect on the levels of adenylate cyclase or Gs, indicating that the loss of activity subsequent to inactivating drug treatment is not due to some dramatic effect on the cellular levels of these signal transduction components.
Fig. 6.
Levels of Gs and adenylate cyclase are not altered by inactivating drug exposure: Western blots using primary antibodies specific for Gs (α subunit) (A) and adenylate cyclase (subtypes V and VI) (B) were performed. There were no detectable differences between cells exposed to no drug (lane 1), 10 μM clozapine (lane 2), 10 μM methiothepin (lane 3), or 10 μM risperidone (lane 4). Cells were thoroughly washed before lysing and preparation. The blots were stripped after probing for either Gs or adenylate cyclase and reprobed for total ERK-2 as a loading control. Shown are representative blots that were performed twice.
Discussion
The results presented herein continue a series of unusual observations involving the h5-HT7 receptor (Smith et al., 2006; Knight et al., 2009). Six of a total of 20 drugs that have been tested produce an irreversible inactivation of the h5-HT7 receptor, as judged by inhibition of 10 μM 5-HT stimulation of cAMP production. A pseudo-irreversible interaction between the “inactivating drugs” and the h5-HT7 receptor seems to be the cause of this unusual effect (Smith et al., 2006). Five of the drugs seem to produce a complete inhibition of 5-HT-stimulated receptor activity, with the possible exception of metergoline (Knight et al., 2009). The pseudo-irreversible block of the orthosteric binding site on the h5-HT7 receptor seems to explain the inactivation of the receptor. However, it was also noted that risperidone and 9-OH-risperidone, which potently and fully inactivate 5-HT-stimulated h5-HT7 receptor, irreversibly block only 50% of the binding sites (Knight et al., 2009). This observation indicates that receptor occupancy is not sufficient to fully predict the effect of the inactivators on the h5-HT7 receptor activity. These results indicated that risperidone and 9-OH-risperidone, although producing a pseudo-irreversible complex with the h5-HT7 receptor, might be, in addition, interacting with the h5-HT7 receptor in a somewhat different manner than the other four inactivators.
In the present study another unusual observation is described. Forskolin-stimulated adenylate cyclase activity is due to the direct interaction of forskolin with all the isoforms of adenylate cyclase, except for the ram sperm form of the enzyme (Stengel et al. 1982). The interaction of activated Gs seems to have a secondary influence on the ability of forskolin to stimulate adenylate cyclase (Neer, 1978, 1986; Bender et al., 1984; Taussig et al., 1993; Tesmer et al., 2002). A more pronounced inhibitory influence of Gi-coupled receptors on forskolin-stimulated adenylate cyclase is a well documented cellular mechanism (Mons and Cooper, 1995). However the involvement of Gi in the current study has been eliminated, in that pertussis toxin, an irreversible inhibitor of Gi, has no effect on the inactivating properties of drugs presented (Fig. 1). Agonist stimulation of receptors, acting through GTP-binding proteins, has been shown to slightly increase forskolin-stimulated adenylate cyclase activity (Darfler et al., 1982; Alousi et al., 1991; Insel and Ostrom, 2003). Antagonist-induced inhibition of forskolin-stimulated adenylate cyclase is a novel observation. The irreversible effects of the six inactivating drugs on forskolin-stimulated adenylate cyclase activity, particularly that of methiothepin and bromocriptine, are unprecedented. Furthermore, these results indicate that the inactivating drugs, particularly methiothepin and bromocriptine, must be doing more than simply irreversibly occluding the orthosteric binding site on the h5-HT7 receptor. The effects on forskolin-stimulated adenylate cyclase suggest that the inactivators are inducing a stable, persistent, inactive state of the h5-HT7 receptor that, in turn, is inducing an inactive state of Gs. The inactive Gs must be inducing a state of adenylate cyclase with less than maximal forskolin-stimulated potential. In other words, the effects on forskolin-stimulated activity observed reveal several things about the receptor-inactivating mechanism of these drugs. First, the inactivators irreversibly induce a state of the receptor that inactivates Gs, eliminating the receptor-mediated cAMP stimulation, as reported previously (Smith et al., 2006; Knight et al., 2009). Second, the Gs/adenylate cyclase interaction may be altered, inhibiting forskolin's ability to stimulate adenylate cyclase. The similar potencies of the inactivating drugs on 10 or 35 μM forskolin-stimulated activity (Fig. 3, Table 1) indicates that the mechanism of action is not a competitive one. The maximal degree of irreversible inhibition of forskolin-stimulation varies among the different inactivators and is not predicted by maximal receptor occupancy (Knight et al., 2009).
To determine the possibility that the lower maximal effect of metergoline was due to a slower onset of effect, time-course experiments were performed (Fig. 4). Overall, the effect of prolonged exposure of cells to the inactivating drugs does not seem to have a major effect. However, it was noted that metergoline did become less effective with prolonged exposure, especially in the 35 μM forskolin experiments. This result is the opposite of what one would expect if the lower maximal effect of metergoline observed in Fig. 3 was due to a slower rate of onset for metergoline compared with the other inactivating drugs. The observed loss of effect of metergoline may indicate that this drug slowly dissociates, and after dissociation the receptor can become reactivated. This possibility is currently being investigated. There is a dramatic difference in the effect of 15-min lisuride exposure depending on whether 10 or 35 μM forskolin is used as the stimulant (Fig. 4). This suggests that the effects of lisuride at early time points may be reversed by increasing forskolin concentrations. This possibility is also currently being investigated.
The results in Fig. 5A, which show that metergoline pretreatment blunts the effects of bromocriptine but does not blunt the effects of methiothepin, are especially notable. These results are consistent with the complete blockade of the 5-HT7 receptor by metergoline, preventing subsequent effects at the orthosteric site by bromocriptine. However, metergoline does not blunt the inactivating effects of methiothepin. These results imply that bromocriptine and metergoline share the same mechanism, probably a pseudo-irreversible interaction with the h5-HT7 receptor. Methiothepin seems to have properties that allow it to overcome the presence of metergoline at the h5-HT7 receptor; i.e., methiothepin and bromocriptine induce different states of the h5-HT7 receptor that result in the complete inactivation of the h5-HT7 receptor.
The results in Fig. 5B, demonstrating that risperidone pretreatment is ineffective in blunting the effects of either bromocriptine or methiothepin, are also notable. These results are consistent with previous results indicating that risperidone's mechanism of action is different from metergoline's (Knight et al., 2009). Risperidone and 9-OH-risperidone have been shown to completely inactivate the h5-HT7 receptor and irreversibly block 50% of the receptors, whereas metergoline irreversibly blocks all the h5-HT7 receptors and produces a profound inactivation of the h5-HT7 receptor. The results in Fig. 4 reinforce the possibility of a different mechanism of action between risperidone and metergoline. Thus Fig. 4 indicates methiothepin, bromocriptine, risperidone, and metergoline differ in their interactions with the h5-HT7 receptor, as judged by forskolin-stimulated adenylate cyclase activity. The details of these differences are under investigation.
The results presented herein and in the two previous papers (Smith et al., 2006; Knight et al., 2009) are highly unusual and unprecedented. However, a published article (Krobert et al., 2006) has reported similar effects with four drugs used in our studies. In this report, the authors found that whereas mesulergine, SB269970, and clozapine produced little or no effect on the levels of [3H]5-carboxamidotryptamine-labeled h5-HT7 receptors after removal of the drugs, methiothepin reduced binding levels by 79%, with no change in affinity for the radioligand. These results correspond with the results we have reported previously: methiothepin inactivates the h5-HT7 receptor and inhibits [3H]5-HT labeling of the h5-HT7 receptor after removal; mesulergine, SB269970, and clozapine do not produce these effects (Smith et al., 2006; Knight et al., 2009). We found no other studies that examined the 5-HT7 receptor after removal of drugs.
The findings displayed in these studies, and in the previous articles (Smith et al., 2006; Knight et al., 2009), indicate that the h5-HT7 receptor operates in a unique manner. Although many antagonists behave in a characteristically competitive fashion, 6 of the 20 antagonists tested seem to induce a stable state of the receptor that 1) involves pseudo-irreversible binding, 2) induces a stable inactivated state of Gs, which in turn 3) induces a stable inactivated state of adenylate cyclase that includes complete or partial occlusion of the forskolin binding site. The inhibition of the forskolin-stimulated adenylate cyclase activity may not involve an occlusion of the binding site but rather an altered, inactive conformation of adenylate cyclase that is resistant to forskolin stimulation. Studies are under way to investigate the predicted consequences of this model of h5-HT7 function (i.e., a stable complex between the inactivating drug, h5-HT7 receptor, Gs, and adenylate cyclase).
In summary, the results presented add another novel observation concerning the effects of inactivating drugs on the h5-HT7 receptor, through what appears to be a pseudo-irreversible complex (Smith et al., 2006; Knight et al., 2009). The irreversible inhibition of forskolin-stimulated activity provides strong evidence of the production of an inactivated state of Gs by the inactivators, acting through the h5-HT7 receptor. The different ability of the inactivators to inhibit forskolin-stimulated adenylate cyclase indicates that the inactivators produce different states of the h5-HT7 receptor. This situation is highly analogous to that of the classic case of full and partial receptor agonists adapted to this novel class of inactivating drugs. It is becoming increasingly clear that the inactivators are inducing a novel state of the h5-HT7 receptor, revealing properties previously unobserved. As the effects of the inactivating drugs are examined in more detail, variations in the mechanism of action of these drugs are becoming clear. The inactivating drugs seem to possess properties that produce effects on GPCRs that are distinct from competitive antagonists and thus may lead to the discovery of novel GPCR regulatory mechanisms. These novel regulatory mechanisms may be targets for the development of novel therapeutic drugs.
This work was supported by National Institutes of Health National Institute of Mental Health [Grant MH56650].
ABBREVIATIONS: 5-HT, 5-hydroxytryptamine; h, human; HEK, human embryonic kidney; GPCR, G protein-coupled receptor; SB269970, (2R)-1-[(3-hydroxyphenyl)sulfonyl]-2-[2-(4-methyl-1-pipe ridinyl)ethyl]pyrrolidine hydrochloride; ICI169369, 2-(2-dimethylamino ethylthio)-3-phenyl quinoline; ANOVA, analysis of variance.
References
- Alousi AA, Jasper JR, Insel PA, and Motulsky HJ (1991) Stoichiometry of receptor-Gs-adenylate cyclase interactions. FASEB J 5 2300-2303. [DOI] [PubMed] [Google Scholar]
- Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, and Weinshank RL (1993) Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J Biol Chem 268 23422-23426. [PubMed] [Google Scholar]
- Bender JL, Wolf LG, and Neer EJ (1984) Interaction of forskolin with resolved adenylate cyclase components. Adv Cyclic Nucleotide Protein Phosphorylation Res 17 101-109. [PubMed] [Google Scholar]
- Bhana N and Spencer CM (2000) Risperidone: a review of its use in the management of the behavioural and psychological symptoms of dementia. Drugs Aging 16 451-471. [DOI] [PubMed] [Google Scholar]
- Darfler FJ, Mahan LC, Koachman AM, and Insel PA (1982) Stimulation of forskolin of intact S49 lymphoma cells involves the nucleotide regulatory protein of adenylate cyclase. J Biol Chem 257 11901-11907. [PubMed] [Google Scholar]
- Dessauer CW, Scully TT, and Gilman AG (1997) Interactions of forskolin and ATP with the cytosolic domains of mammalian adenylyl cyclase. J Biol Chem 272 22272-22277. [DOI] [PubMed] [Google Scholar]
- Gerhardt CC and van Heerikhuizen H (1997) Functional characteristics of heterologously expressed 5-HT receptors. Eur J Pharmacol 334 1-23. [DOI] [PubMed] [Google Scholar]
- Green B (2000) Focus on risperidone. Curr Med Res Opin 16 57-65. [PubMed] [Google Scholar]
- Hedlund PB and Sutcliffe JG (2004) Functional, molecular and pharmacological advances in 5-HT7 receptor research. Trends Pharmacol Sci 25 481-486. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, and Martin GR (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71 533-554. [DOI] [PubMed] [Google Scholar]
- Hoyer D and Martin G (1997) 5-HT receptor classification and nomenclature: towards a harmonization with the human genome. Neuropharmacology 36 419-428. [DOI] [PubMed] [Google Scholar]
- Insel PA and Ostrom RS (2003) Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell Mol Neurobiol 23 305-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslow HR, Lim LK, Moss J, and Lesikar DD (1987) Structure-activity analysis of the activation of pertussis toxin. Biochemistry 26 123-127. [DOI] [PubMed] [Google Scholar]
- Knight JA, Smith C, Toohey N, Klein MT, and Teitler M (2009) Pharmacological analysis of the novel, rapid, and potent inactivation of the human 5-hydroxytryptamine7 receptor by risperidone, 9-OH-risperidone, and other inactivating antagonists. Mol Pharmacol 75 374-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krobert KA, Andressen KW, and Levy FO (2006) Heterologous desensitization is evoked by both agonist and antagonist stimulation of the human 5-HT(7) serotonin receptor. Eur J Pharmacol 532 1-10. [DOI] [PubMed] [Google Scholar]
- Kroeze WK, Kristiansen K, and Roth BL (2002) Molecular biology of serotonin receptors—structure and function at the molecular level. Curr Top Med Chem 2 507-528. [DOI] [PubMed] [Google Scholar]
- Love RC and Nelson MW (2000) Pharmacology and clinical experience with risperidone. Expert Opin Pharmacother 1 1441-1453. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, and Siegel BW. (1993) A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 11 449-458. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, and Lee JC (1989) Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 PKi values. J Pharmacol Exp Ther 251 238-246. [PubMed] [Google Scholar]
- Mons N and Cooper DM (1995) Adenylate cyclases: critical foci in neuronal signaling. Trends Neurosci 18 536-542. [DOI] [PubMed] [Google Scholar]
- Neer EJ (1978) Multiple forms of adenylate cyclase. Adv Cyclic Nucleotide Res 9 69-83. [PubMed] [Google Scholar]
- Neer EJ (1986) Guanine nucleotide-binding proteins involved in transmembrane signaling. Symp Fundam Cancer Res 39 123-136. [PubMed] [Google Scholar]
- Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, and Garnovskaya MN (2001) Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther 92 179-212. [DOI] [PubMed] [Google Scholar]
- Roth BL, Craigo SC, Choudhary MS, Uluer A, Monsma FJ, Jr., Shen Y, Meltzer HY, and Sibley DR (1994) Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J Pharmacol Exp Ther 268 1403-1410. [PubMed] [Google Scholar]
- Schneider LS, Dagerman K, and Insel PS (2006) Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 14 191-210. [DOI] [PubMed] [Google Scholar]
- Shen Y, Monsma FJ, Jr., Metcalf MA, Jose PA, Hamblin MW, and Sibley DR (1993) Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J Biol Chem 268 18200-18204. [PubMed] [Google Scholar]
- Smith C, Rahman T, Toohey N, Mazurkiewicz J, Herrick-Davis K, and Teitler M (2006) Risperidone irreversibly binds to and inactivates the h5-HT7 serotonin receptor. Mol Pharmacol 70 1264-1270. [DOI] [PubMed] [Google Scholar]
- Stengel D, Guenet L, Desmier M, Insel P, and Hanoune J (1982) Forskolin requires more than the catalytic unit to activate adenylate cyclase. Mol Cell Endocrinol 28 681-690. [DOI] [PubMed] [Google Scholar]
- Tang WJ and Gilman AG (1995) Construction of a soluble adenylyl cyclase activated by Gs alpha and forskolin. Science 268 1769-1772. [DOI] [PubMed] [Google Scholar]
- Taussig R, Iñiguez-Lluhi JA, and Gilman AG (1993) Inhibition of adenylyl cyclase by Gi alpha. Science 261 218-221. [DOI] [PubMed] [Google Scholar]
- Teitler M and Herrick-Davis K (1994) Multiple serotonin receptor subtypes: molecular cloning and functional expression. Crit Rev Neurobiol 8 175-188. [PubMed] [Google Scholar]
- Terrón JA (1997) Role of 5-ht7 receptors in the long-lasting hypotensive response induced by 5-hydroxytryptamine in the rat. Br J Pharmacol 121 563-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesmer JJ, Sunahara RK, Fancy DA, Gilman AG, and Sprang SR (2002) Crystallization of complex between soluble domains of adenylyl cyclase and activated Gs alpha. Methods Enzymol 345 198-206. [DOI] [PubMed] [Google Scholar]