Abstract
Bone marrow is a major target of benzene toxicity, and NAD- (P)H:quinone oxidoreductase (NQO1), an enzyme protective against benzene toxicity, is present in human bone marrow endothelial cells, which form the hematopoietic stem cell vascular niche. In this study, we have employed a transformed human bone marrow endothelial cell (TrHBMEC) line to study the adverse effects induced by the benzene metabolite hydroquinone. Hydroquinone inhibited TrHBMEC tube formation at concentrations that were not overtly toxic, as demonstrated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide or sulforhodamine B analysis. Hydroquinone was found to up-regulate chondromodulin-I (ChM-I), a protein that promotes chondrocyte growth and inhibits endothelial cell growth and tube formation. Recombinant human ChM-I protein inhibited tube formation in TrHBMECs, suggesting that up-regulation of ChM-I may explain the ability of hydroquinone to inhibit TrHB-MEC tube formation. To explore this possibility further, anti-ChM-I small interfering RNA (siRNA) was used to deplete ChM-I mRNA and protein. Pretreatment with anti-ChM-I siRNA markedly abrogated hydroquinone-induced inhibition of tube formation in TrHBMECs. Overexpression of the protective enzyme NQO1 in TrHBMECs inhibited the up-regulation of ChM-I and abrogated the inhibition of tube formation induced by hydroquinone. In summary, hydroquinone treatment up-regulated ChM-I and inhibited tube formation in TrHBMECs; NQO1 inhibited hydroquinone-induced up-regulation of ChM-I in TrHB-MECs and protected cells from hydroquinone-induced inhibition of tube formation. This study demonstrates that ChM-I up-regulation is one of the underlying mechanisms of inhibition of tube formation and provides a mechanism that may contribute to benzene-induced toxicity at the level of bone marrow endothelium.
Benzene is an occupational and environmental pollutant. Long-term exposure to benzene can induce aplastic anemia, myelodysplastic syndrome, and leukemia (Travis et al., 1994). Although questions remain regarding its mechanism of action, it is known that benzene requires metabolism to induce its adverse effects, and hydroquinone is considered to be one of the major toxic metabolites derived from benzene (Ross, 1996). Benzene is metabolized to phenolic derivates mainly in the liver (Ross, 1996), and both catechol and hydroquinone have been shown to persist in bone marrow after benzene exposure (Rickert et al., 1979). Through autooxidation or peroxidase-mediated oxidation, hydroquinone can be converted to reactive 1,4-benzoquinone capable of reacting with proteins, DNA, and lipids (Ross, 2000). NADPH:quinone oxidoreductase 1 (NQO1; DT-diaphorase), a flavin-containing quinone reductase, can protect cells from toxic effects via reduction of quinone substrates to less reactive hydroquinone forms, which are more water soluble and easily conjugated and excreted (Ross, 1997).
The major target of benzene toxicity is bone marrow, where the main hematopoietic cell generating system is in close contact with the stromal microenvironment. Bone marrow stroma is composed of a variety of different cell types providing structural and functional support for hematopoiesis. Among these cell types, endothelial cells have a specific biological relevance. Recent advances in stem cell studies have revealed two hematopoietic stem cell (HSC) niches that play an important role in the homeostatic regulation of HSCs: the osteoblastic niche (osteoblasts and hematopoietic cells) and the vascular niche (hematopoietic cells and endothelial cells) (Friedenstein et al., 1974; Dexter et al., 1977; Caplan and Dennis, 2006; Li and Li, 2006). The vascular niche offers an alternative niche for mobilized stem cells that promotes proliferation and further differentiation or maturation and release into the circulatory system (Avecilla et al., 2004; Kopp et al., 2005). Damage to the vascular niche could result in abnormal hematopoiesis. Diseases of myelosuppression, aplastic anemia, and leukemia can all be characterized by abnormal proliferation of blood cells resulting from abnormal and poorly regulated hematopoiesis. Therefore, in this study, hydroquinone-induced toxicity in the bone marrow vascular microenvironment was examined by using transformed human bone marrow endothelial cells (TrHBMECs) as a model system.
Among the endothelial cell functions, capillary tube formation is a prerequisite for the establishment of a continuous vessel lumen and further angiogenesis (Matsumura et al., 1997). Recent advances in hypovascular mesenchymal tissues have unveiled some endothelial cell tube formation inhibitors, including ChM-I and tenomodulin (Hiraki and Shukunami, 2005). It is noteworthy that ChM-I was identified as one of the genes up-regulated by treatment of TrHB-MECs with hydroquinone in an Affymetrix microarray study in our lab (Zhou et al., 2008). ChM-I was originally identified as a chondrocyte growth promoting factor and then purified as an angiogenesis inhibitor that inhibits DNA synthesis, cell growth, and tube formation in endothelial cells (Hiraki et al., 1991). ChM-I has also been reported to promote apoptosis in human coronary artery endothelial cells (HCAECs) (Yoshioka et al., 2006). ChM-I is initially synthesized in a type II transmembrane glycoprotein precursor form with total of 334 amino acids in human. After C-terminal cleavage by the furin endoprotease, it is secreted from the cell immediately as a mature but shorter 120-amino acid protein (Hiraki et al., 1999; Azizan et al., 2001). ChM-I is expressed at high levels in avascular tissues such as cartilage and cornea, but under normal conditions, ChM-I expression in endothelium is very low (Hiraki and Shukunami, 2000; Hiraki and Shukunami, 2005). In this work, a tube-formation assay was first performed to assess the ability of hydroquinone to inhibit TrHBMEC tube formation. Further studies were then performed to investigate whether ChM-I was a mediator of the effects of hydroquinone on tube formation in TrHBMEC. In addition, based on the documented protective role of NQO1 against benzene toxicity, we overexpressed NQO1 in TrHBMEC to investigate whether NQO1 could protect cells from hydroquinone-induced up-regulation of ChM-I and inhibition of tube formation.
Materials and Methods
Materials
Hydroquinone, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and β-mercaptoethanol were obtained from Sigma Chemical (St. Louis MO). Human ChM-I siRNA sequences were obtained from Dharmacon RNA Technologies (Lafayette, CO). RNeasy kit was obtained from QIAGEN (Valencia, CA). Recombinant human ChM-I and rabbit polyclonal antisera against human ChM-I were generated in the Hiraki lab (Hiraki et al., 1999). Mouse anti-NQO1 monoclonal antibody was generated in our lab. Mouse anti-β-Actin antibody was obtained from Sigma. Lipofectamine 2000 transfection reagent was obtained from Invitrogen (Carlsbad, CA). Matrigel was obtained from BD Biosciences (San Jose, CA). Butyl Sepharose 4 Fast Flow was obtained from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK). Amicon Ultra-4 3000 nominal molecular weight limit centrifugal filter devices were obtained from Millipore (Billerica, MA).
Cell Culture
TrHBMEC is a transformed microvascular endothelial cell line established from primary bone marrow endothelial cells by Dr. Babette B. Weksler (Weill Medical College, Cornell University, New York, NY) (Schweitzer et al., 1997). Cells were cultured on 0.2% (w/v) gelatin-coated cell culture plates in Dulbecco's modified Eagle's medium (low glucose) supplemented with 5% (v/v) fetal bovine serum, penicillin (100 units/ml), streptomycin (100 units/ml), 3 mM l-glutamine, 10 mM HEPES, and 1% (v/v) BME vitamins (Invitrogen, Carlsbad, CA). This medium was used for all experiments. Cells were maintained at 37°C in 5% CO2. Cells with passage numbers ranging from 19 to 25 were used in this study.
MTT Cell Proliferation Assay
TrHBMEC proliferation after treatment with hydroquinone was measured using the MTT colorimetric assay. In these studies, TrHBMEC were seeded at 2 to 3 × 103 cells/well in 96-well plates and allowed to attach for 16 h. Medium was removed by aspiration and the TrHBMEC were treated with the hydroquinone (1-1000 μM) in complete medium for 24 h, after which the medium was removed and replaced with 200 μl of fresh medium. After 72 h, the medium was removed and an additional PBS wash was performed to avoid the possibility of a colorimetric reaction between MTT and hydroquinone. MTT (50 μg) in complete medium (50 μl) was added to each well for a further 4-h incubation. Cell viability was determined by measuring the cellular reduction of MTT (Mosmann, 1983) to the crystalline formazan product, dissolved by the addition of dimethyl sulfoxide (100 μl). Optical density was determined at 550 nm using a microplate reader (Thermomax; Molecular Devices, Sunnyvale, CA). The IC50 values were defined as the concentration of hydroquinone that resulted in 50% reduction in cell number compared with the control. These values were determined from percentage of control versus hydroquinone concentration.
Quantitative Real Time RT-PCR of the ChM-I Gene
The relative amount of ChM-I mRNA was assessed using TaqMan probe real-time PCR with the ABI 7500 system (Applied Biosystems, Foster City, CA). TaqMan probes for ChM-I (assay ID Hs00170877_m1) and β-actin assay were purchased from Applied Biosystems (Foster City, CA), β-Actin was used as the internal control. All of the reactions were run in triplicate or quadruplicate. The -fold increase of ChM-I mRNA in TrHBMECs after hydroquinone treatment, normalized to an endogenous β-Actin reference and relative to untreated control, is given by 2-ΔΔCT (where ΔΔCT = ΔCT hydroquinone treatment - ΔCT untreated control, and ΔCT = threshold cycles for target ChM-I - threshold cycles for reference β-Actin).
Human ChM-I siRNA Transfection
Lipofectamine 2000 transfection reagents were obtained from Invitrogen. Cells (5 × 105) were seeded into 100-mm plates the day before transfection with 15 ml of medium containing serum but without antibiotics. On the day of transfection, 600 pmol of siRNA was diluted in 1.5 ml of culture medium without serum and antibiotics, which resulted in a final working siRNA concentration of 33 nM after adding complexes to cells. Lipofectamine 2000 transfection reagent (30 μl) was added to another 1.5 ml of culture medium without serum and antibiotics with gentle mix and incubated for 5 min at room temperature. Then the diluted oligomer and lipofectamine 2000 were mixed gently and incubated at room temperature for another 20 min to allow for the formation of transfection complexes. The complexes were added onto the cells and mixed gently by rocking the plate back and forth. Cells containing transfection complexes were then incubated under their normal growth conditions for various periods until harvest.
Purification of Recombinant Human ChM-I
Recombinant human ChM-I was prepared as described previously (Hiraki et al., 1999).
Recombinant Human NQO1 Transfection
Fugene HD transfection reagent (Roche Applied Bioscience, Indianapolis, IN) and the cytomegalovirus-driven mammalian expression vector pcDNA3.0 containing human wild-type NQO1 cDNA (Winski et al., 1998) were used in transfections. Cells were allowed to grow to 80% confluence. Fugene HD transfection reagent, DNA, and diluent mix was made according to the Fugene HD manufacturer's instructions. Cells transfected for at least 18 h were subjected for further hydroquinone treatment or tube-formation assay.
Immunoblot Analysis
Mature ChM-I in Medium. TrHBMEC culture media (10 ml for each sample) were collected after treatment. The medium was first loaded to butyl Sepharose column according to the manufacturer's instructions and was then eluted with 20 mM Na2HPO4 with 40% acetonitrile; collected fractions were concentrated using an Amicon Ultra-4 3000 nominal molecular weight limit centrifugal filter devices to a similar volume. Samples were then diluted in 5× Laemmli sample buffer to a total volume of 120 μl and heated to 90°C for 5 min. The same volumes of samples (25 μl) were then separated by 12% SDS-PAGE.
Cell Lysate Proteins. TrHBMECs were scraped into PBS and collected by centrifugation. Cell pellets were resuspended in 100 μl of radioimmunoprecipitation assay buffer with protease inhibitors added and sonicated on ice for 15 s. Sonicates were centrifuged at 13,000 rpm for 5 min to remove cellular debris. After centrifugation, the supernatant was assayed for protein concentrations using the method of Lowry (1951). Protein samples (15 μg to 50 μg) were diluted in 5× Laemmli sample buffer and heated to 90°C for 5 min, then separated by 12% SDS-PAGE. After SDS-PAGE separation, samples were transferred to polyvinylidene difluoride membranes in 25 mM Tris and 192 mM glycine containing 20% (v/v) methanol at 200 mA for 1 h. After transfer, membranes were placed overnight in blocking buffer (10 mM Tris-HCl, pH 8, containing 125 mM NaCl, 0.2% (v/v) Tween 20, and 5% (w/v) nonfat dry milk). Antibodies diluted in blocking buffer were used at the following dilutions and times: polyclonal antisera against ChM-I 1:5000, 1 h at room temperature or 4°C overnight; anti-β-actin, 1:10,000 for 0.5 h at room temperature or 4°C overnight. For all analysis, horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG were diluted at 1:5000 in blocking buffer for 0.5 to 1 h at room temperature. Protein bands were visualized using luminol-based enhanced chemiluminescence as described by the manufacturer (PerkinElmer Life and Analytical Sciences, Waltham, MA). Densitometry analysis software was Photoshop 7.0 (Adobe Systems, Mountain View, CA).
Generation and Validation of ChM-I Antibody
The polyclonal antibody against human ChM-I precursor and mature protein was generated and validated in the Hiraki lab as described previously (Hiraki et al., 1999). Validation was also extended in the Ross lab. First, the recombinant human ChM-I protein was verified using matrix-assisted laser desorption ionization/time-of-flight mass spectrometry, and then the reactivity of the antibody against the recombinant human ChM-I protein was confirmed. The antibody was also validated using ChM-I protein generated from TnT Quick-Coupled Transcription/Translation Systems (cell free protein expression; Promega, Madison, WI) using the full-length coding region. The antibody was further validated by the detection of decreased formation of ChM-I resulted from anti-ChM-I siRNA treatment.
Tube-Formation Assay
Six- or 24-well plates were coated with 150 or 30 μl of growth factor-reduced matrigel (BD Biosciences) per well and incubated at 37°C for 30 min. TrHBMECs (105 or 2 × 104; siRNA-pretreated cells were cells transfected with anti-ChM-I siRNA for 48 h) were seeded onto each well in 5 ml or 1 ml media, respectively (with or without hydroquinone/ChM-I treatment). Cells were allowed to grow for 16 h for tube formation observations (10× objective lens, 10× ocular lens, inverted phase contrast microscope; TMS-F; Nikon, Tokyo, Japan). Pictures were taken directly from the ocular lens using a Nikon Coolpix 990 digital camera. Total lengths of tube-like structures per field were measured using image processing and analysis software (NIH Image J ver. 1.41; http://rsb.info.nih.gov/nih-image/). Each experiment was repeated at least three times.
Statistical Analysis
Values are presented as the mean ± S.D. Statistical significance was evaluated using the student's t test for comparisons between two mean values. One-way ANOVA with Tukey post hoc test was used to assess significance between experimental groups. A value of P < 0.05 was considered significant.
Results
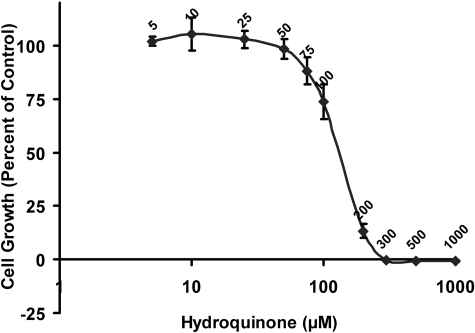
Determination of Nonovertly Toxic Concentrations of Hydroquinone in TrHBMECs. To assess hydroquinone-induced cytotoxicity in TrHBMECs and to generate a dose that did not demonstrate overt toxicity, MTT growth inhibition assays were performed. To avoid the reaction between the residual hydroquinone and MTT, an extra wash with PBS was performed before the addition of MTT. The calculated IC50 of hydroquinone in TrHBMECs was approximately 139 μM (Fig. 1), which is in agreement with data obtained using trypan blue exclusion as a marker of toxicity (Bironaite et al., 2004). Using an SRB assay to measure cellular protein content, the IC50 value for hydroquinone in TrHBMECs was calculated to be 167 μM, which correlates well with the MTT assay results, because IC50 values obtained using the SRB method are usually higher than those obtained using the MTT method (Vichai and Kirtikara, 2006). Hydroquinone at a dose of 10 μM was chosen for further studies.
Fig. 1.
Assessment of hydroquinone-induced toxicity in TrHBMECs with MTT assay. TrHBMEC proliferation after hydroquinone treatment was assessed using MTT assay. TrHBMECs were exposed to hydroquinone (5-1000 μM) for 3 days. Results are expressed as the mean ± S.D. of three independent determinations. The calculated IC50 is 139 μM.
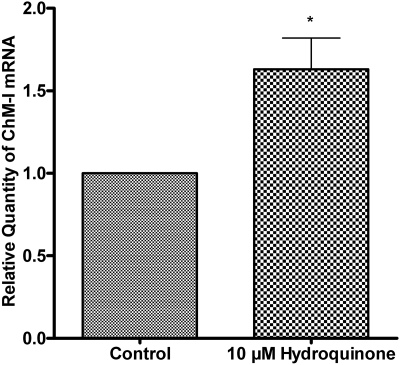
Hydroquinone Up-Regulated ChM-I mRNAs in TrHBMEC. To understand how hydroquinone alters TrHBMEC function, we use 10 μM hydroquinone to treat TrHBMECs. ChM-I was identified as one of the genes up-regulated by hydroquinone treatment, from an absent call to a present call in an Affymetrix microarray study (Zhou et al., 2008). To verify ChM-I mRNA up-regulation by hydroquinone treatment, TaqMan probe quantitative RT-PCR was performed to evaluate ChM-I mRNA levels after hydroquinone treatment in TrHBMECs. As shown in Fig. 2, hydroquinone treatment successfully up-regulated ChM-I mRNA by approximately 1.6-fold in TrHBMECs compared with untreated control cells. Pretreatment with actinomycin D to inhibit the de novo mRNA synthesis in TrHBMECs blocked the up-regulation of ChM-I precursor protein (data not shown), confirming that hydroquinone up-regulated ChM-I at the level of mRNA in TrHBMECs.
Fig. 2.
Hydroquinone up-regulated ChM-I mRNA in TrHBMECs. TrHBMECs were treated with 10 μM hydroquinone for 4 h. The relative amount of ChM-I mRNA was assessed by TaqMan probe real-time PCR. The -fold increase of ChM-I mRNA is given by 2-ΔΔCT. Values are normalized against endogenous β-actin. Data represent means ± S.D. from four experiments. *, p < 0.05 is considered significant compared with untreated control.
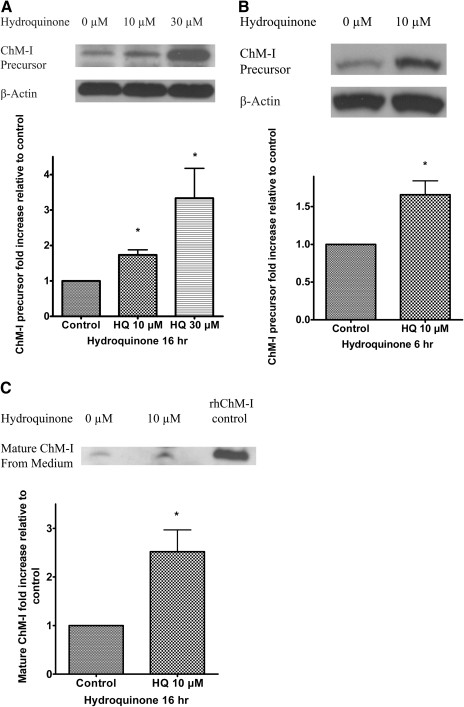
Hydroquinone Up-Regulated ChM-I Protein in TrHBMECs. ChM-I is initially synthesized in precursor form, a type II transmembrane glycoprotein (334 amino acids in human), which requires cleavage by the furin endoprotease to secret the mature 120-amino acid protein (Hiraki et al., 1999; Azizan et al., 2001). The increased expression of ChM-I mRNA in TrHBMECs suggested a potential up-regulation of ChM-I protein. Immuno-blot assays were performed to detect the ChM-I precursor protein in TrHBMEC lysates. As shown in Fig. 3A, hydroquinone treatment for 16 h up-regulated ChM-I precursor in a dose-dependent manner and up-regulation of ChM-I precursor could be observed as early as 6 h after treatment with 10 μM hydroquinone treatment (Fig. 3B). To ensure that hydroquinone did not block the maturation of ChM-I, the up-regulation of mature ChM-I protein levels in cell culture medium was examined. As shown in Fig. 3C, the immunoblot results clearly demonstrated that mature ChM-I in medium was also increased by hydroquinone treatment. The increase of mature ChM-I in medium confirmed the secretion of ChM-I from TrHBMECs.
Fig. 3.
Hydroquinone up-regulated ChM-I precursor and mature ChM-I protein in TrHBMECs. TrHBMECs were treated with hydroquinone at indicated concentrations and hours. ChM-I precursor protein levels were examined in TrHBMEC sonicates by immunoblot analysis. β-Actin was included as a loading control (A and B). Mature ChM-I protein in culture media were also examined by immunoblot analysis after partial removal of serum proteins and centrifugal concentration. Recombinant human ChM-I (rhChM-I) was included as a positive control (C). Blots represent similar results from three independent experiments. The density of the bands was determined and plotted as the relative -fold change compared with the untreated control. *, p < 0.05 is considered significant compared with untreated control.
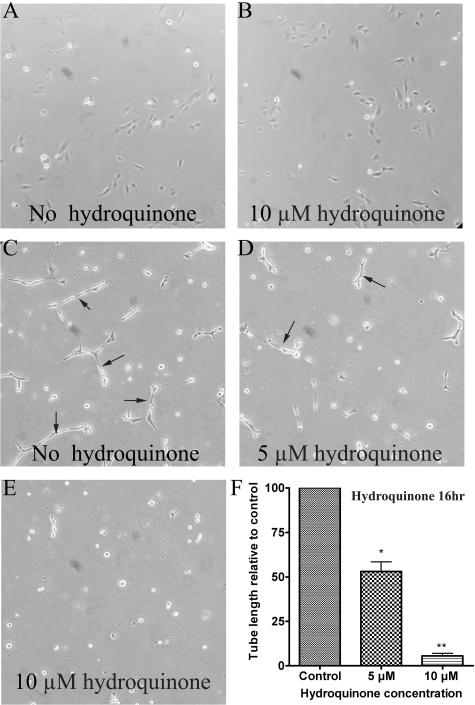
Hydroquinone Inhibited TrHBMEC Tube Formation in Matrigel. One important function of ChM-I is to inhibit endothelial cell tube formation (Shukunami and Hiraki, 2007). Because hydroquinone-induced up-regulation of ChM-I mRNA and protein were both confirmed, we next determined whether hydroquinone could inhibit TrHBMEC tube formation. A tube-formation assay was performed in matrigel-coated plates as shown in Fig. 4, C to E. A parallel control study using gelatin-coated plate was also performed as shown in Fig. 4, A and B. Hydroquinone treatment did not induce obvious morphological change or cell death in cells grown in gelatin-coated plates (Fig. 4, A and B). However, hydroquinone treatment inhibited TrHBMEC tube formation in a dose-dependent manner on cells grown in matrigel (Fig. 4, C to E), it almost completely inhibited TrHBMEC tube formation at 10 μM, a concentration that was not overtly toxic, as demonstrated by trypan blue exclusion (Bironaite et al., 2004) or MTT (Fig. 1) or SRB analysis (data not shown).
Fig. 4.
Hydroquinone inhibited TrHBMEC tube formation in matrigel. Hydroquinone did not induce obvious morphological change and cell death when TrHBMECs were seeded on gelatin-coated plates (A and B). However, hydroquinone inhibited TrHBMEC tube formation in a dose-dependent manner when cells were seeded on matrigel-coated plates; 10 μM hydroquinone almost completely inhibited TrHBMEC tube formation (C-E). Arrows indicate the tube structures. Data represent similar results from three independent experiments. Total tube length per field was measured by image processing and analysis software. *, p < 0.05 is considered significant compared with untreated control. **, p < 0.05 is considered significant compared with untreated control and 5 μM hydroquinone treatment (F).
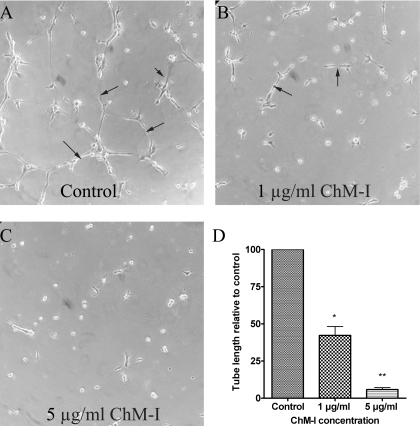
Recombinant Human ChM-I Inhibited TrHBMEC Tube Formation in Matrigel. To support the hypothesis that hydroquinone up-regulated ChM-I and inhibited tube formation in TrHBMECs, we examined the effect of direct addition of purified mature human ChM-I protein on the formation of tubes in TrHBMECs. Although ChM-I has been demonstrated to be able to inhibit tube formation in different endothelial cell lines, such as human umbilical vein endothelial cells (Oshima et al., 2004), bovine carotid artery endothelial cells (Hiraki et al., 1999), and HCAECs (Yoshioka et al., 2006), it has not been tested on TrHBMECs. As shown in Fig. 5, recombinant human mature ChM-I inhibited TrHBMEC tube formation in matrigel in a dose-dependent manner.
Fig. 5.
Recombinant human ChM-I inhibited TrHBMEC tube formation in matrigel. Recombinant human ChM-I mature protein inhibited TrHBMEC tube formation in a dose-dependent manner (A-C). Arrows indicate the tube structures. Data represent similar results from three independent experiments. Total tube length per field was measured by image processing and analysis software. *, p < 0.05 is considered significant compared with untreated control. **, p < 0.05 is considered significant compared with untreated control and 1 μg/ml ChM-I treatment (D).
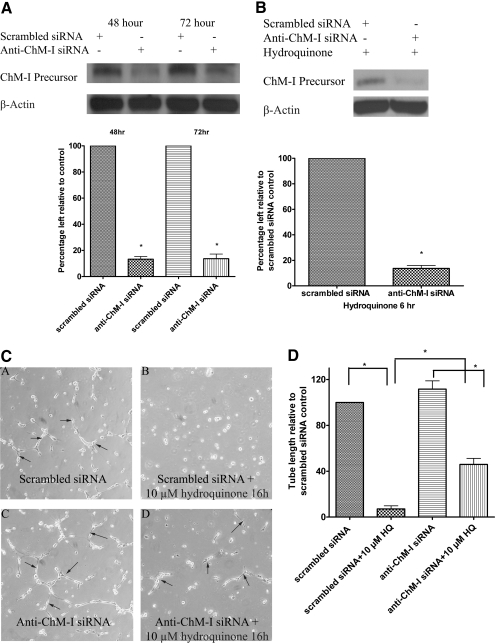
Pretreatment with Anti-ChM-I siRNA in TrHBMEC Knocked Down ChM-I Protein and Abrogated the Inhibition of Tube Formation Induced by Hydroquinone Treatment. To define whether the elevation of ChM-I by hydroquinone contributes to hydroquinone-induced tube formation inhibition in TrHBMECs, cells were first pretreated with anti-ChM-I siRNA to deplete ChM-I mRNA and ChM-I protein. As shown in Fig. 6A, ChM-I precursor protein was successfully knocked down more than 80% by ChM-I siRNA between 48 and 72 h after siRNA transfection. It is noteworthy that pretreatment with anti-ChM-I siRNA inhibited hydroquinone-induced up-regulation of ChM-I (Fig. 6B). After 48 h of anti-ChM-I siRNA treatment, cells were lifted and seeded onto matrigel-coated plates to perform the tube-formation assay. Cells treated with either nontargeting control siRNA or anti-ChM-I siRNA maintained tube formation ability when there was no hydroquinone present. However, tube formation was inhibited in TrHBMECs pretreated with nontargeting siRNA in the presence of hydroquinone (10 μM), whereas inhibition of tube formation was markedly abrogated in TrHBMECs pretreated with anti-ChM-I siRNA and subsequently challenged with hydroquinone (Fig. 6, C and D). These data strongly suggest that ChM-I elevated by hydroquinone treatment may be a novel mechanism contributing to hydroquinone-induced inhibition of tube formation in TrHBMEC.
Fig. 6.
Pretreatment with anti-ChM-I siRNA in TrHBMECs knocked down ChM-I protein and abrogated the inhibition of tube formation induced by hydroquinone treatment. TrHBMECs were treated with either scramble siRNA or anti-ChM-I siRNA for the indicated times. ChM-I precursor levels were examined in TrHBMEC sonicates by immunoblot analysis. β-Actin was included as a loading control. Blots demonstrated a successful knockdown of ChM-I proteins by anti-ChM-I siRNA treatment (A). Anti-ChM-I siRNA treatment also efficiently inhibited hydroquinone-induced up-regulation of ChM-I (B). Blots are representative of three independent experiments. The density of the bands was determined and plotted as the relative -fold change compared with the scrambled siRNA control. *, p < 0.05 is considered significant compared with scrambled siRNA control (A and B). TrHBMECs pretreated with either scrambled or anti-ChM-I siRNA for 48 h were lifted to perform tube-formation assay in the presence or absence of hydroquinone (10 μM). All cells maintained tube formation abilities in the absence of hydroquinone (C, subpanels A and C). Hydroquinone treatment completely inhibited tube formation in TrHBMECs with scrambled siRNA pretreatment (C, subpanel B) but only partially inhibited tube formation in cells pretreated with anti-ChM-I siRNA (C, subpanel D). Arrows indicate the tube structures. Data represent similar results from four independent experiments. Total tube length per field was measured by image processing and analysis software. *, p < 0.05 (D).
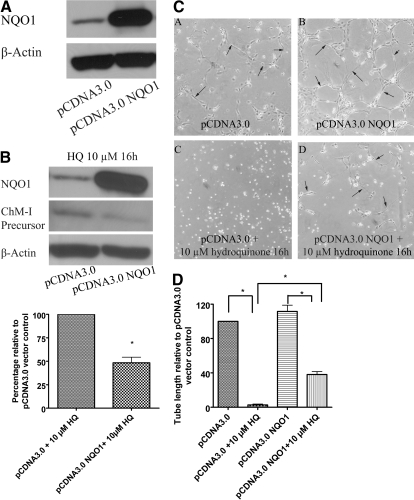
Overexpression of NQO1 in TrHBMECs Protects Cells from Hydroquinone-Induced Up-Regulation of ChM-I and Inhibition of Tube Formation. NQO1 is known to be a protective enzyme against benzene toxicity. We hypothesized that overexpression of NQO1 could protect TrHBMECs from hydroquinone-induced up-regulation of ChM-I and inhibition of tube formation. TrHBMECs were treated with either a control plasmid or plasmid encoding human NQO1. Overexpression of NQO1 was confirmed in TrHBMECs by immunoblot analysis (Fig. 7A). Immunoblot analysis also demonstrated that cells overexpressing NQO1 inhibited hydroquinone-induced up-regulation of ChM-I precursor protein (Fig. 7B). Cells transfected with pcDNA3.0 harboring NQO1 (the NQO1 overexpression vector) or control vector were then lifted to perform the tube-formation assay in the presence of hydroquinone. Hydroquinone inhibited tube formation in cells transfected with control vector, but the inhibition was abrogated in cells transfected with NQO1 overexpression vector, as shown in Fig. 7, C and D.
Fig. 7.
Overexpression of NQO1 in TrHBMECs protects cells from hydroquinone-induced up-regulation of ChM-I and inhibition of tube formation. TrHBMECs were first transfected with either control vector or NQO1 overexpression vector for 18 to 24 h; blot (A) demonstrated a successful overexpression of NQO1 in TrHBMECs. Cells were then subjected to hydroquinone treatment or the tube-formation assay in the presence or absence of 10 μM hydroquinone. ChM-I precursor protein levels after hydroquinone challenge were examined in TrHBMEC sonicates by immunoblot analysis. Overexpression of NQO1 inhibited hydroquinone-induced up-regulation of ChM-I (B). β-Actin was included as a loading control. Hydroquinone (10 μM) inhibited tube formation in TrHBMECs transfected with control vector (C, subpanels A and C) but the inhibition was abrogated in TrHBMEC transfected with NQO1 overexpression vector (C, subpanels B and D). Arrows indicate the tube structures. Data represent similar results from three independent experiments. Total tube length per field was measured by image processing and analysis software. *, p < 0.05 (D).
Discussion
Long-term exposure to benzene can result in bone marrow toxicity and is associated with the development of a variety of blood dyscrasias, including lymphocytopenia, thrombocytopenia, pancytopenia, and aplastic anemia. Some people develop myelodysplastic syndrome, which can ultimately progress into acute myelogenous leukemia (Stillman et al., 1997). A recent study showed that workers exposed to low levels of benzene exhibited a significant decrease in white blood cells and platelets (Lan et al., 2004). These data suggest that a characteristic of the early stages of chronic benzene toxicity could be ineffective hematopoiesis, a process that requires structural and functional support from the bone marrow stroma, including the vascular microenvironment formed by bone marrow endothelial cells. Hydroquinone, a major benzene metabolite, was demonstrated to induce adverse effects in TrHBMEC (Figs. 1 and 4) suggesting that benzene could induce ineffective hematopoiesis through damaging the HSC vascular microenvironment.
ChM-I is mainly expressed in avascular tissues (Hiraki and Shukunami, 2000; Hiraki and Shukunami, 2005). ChM-I mRNA was reported as an absent call in nontreated TrHBMECs in our microarray study (Zhou et al., 2008), and low expression of ChM-I was confirmed by RT-PCR. With hydroquinone treatment, both ChM-I mRNA and protein were up-regulated in TrHBMECs. A similar up-regulation was observed using human umbilical vein endothelial cells (data not shown). The increased expression of ChM-I in TrHBMECs suggests a novel pathway through which hydroquinone could induce toxicity in TrHBMECs. These findings led us to identify ChM-I as a novel mediator in hydroquinone-induced tube formation in TrHBMEC and provide a potential mechanism of how hydroquinone induces adverse effects in a critical bone marrow stromal compartment. The effect of recombinant human ChM-I protein on tube formation inhibition and the abrogation of hydroquinone-induced inhibition of tube formation by siRNA knockdown provided strong support for the hypothesis. Mature ChM-I is a multifunctional glycoprotein secreted from cells. It is known to promote chondrocyte growth and to inhibit endothelial cell growth, DNA synthesis, and tube formation. It has also been reported to be able to suppress T-cell responses and synovial cell proliferation (Setoguchi et al., 2004). The eventual destiny of endothelial cells treated with ChM-I could be apoptosis, as observed in HCAECs treated with ChM-I (Yoshioka et al., 2006). The accumulation of ChM-I elevated by hydroquinone could result in adverse effects in the bone marrow endothelial microenvironment critical for the development and differentiation of HSCs.
Recent studies have shown that endothelial cell tube formation requires the activation of the Rho GTPases Cdc42 and Rac1. Cell surface proteolysis mediated through membrane type 1 matrix metalloproteinase is also necessary to create vascular guidance tunnels within the three-dimensional matrix environment (Davis et al., 2007). Whether the above-mentioned molecules are involved in ChM-I mediated inhibition of tube formation requires further study.
Hydroquinone has been demonstrated to up-regulate both message and protein levels of ChM-I, but its exact transcriptional mechanisms of hydroquinone up-regulation of ChM-I expression remains unknown. The ChM-I core promoter region contains an AP-1 binding site (Yanagihara et al., 2000), and hydroquinone has been reported to enhance AP-1 activation in TF-1 erythroleukemia cells (Zheng et al., 2004). Whether hydroquinone can up-regulate ChM-I via AP-1 activation needs further investigation. Aoyama et al. (2004) have reported that methylation status in ChM-I core promoter region determines cell-specific ChM-I expression, and Bollati et al. (2007) have reported their observations of changes in DNA methylation pattern in subjects exposed to low-dose benzene. It is also possible that hydroquinone up-regulates ChM-I expression via altering methylation status in the ChM-I promoter region.
NQO1's protective role against benzene toxicity has been documented (Ross, 1997; Rothman et al., 1997; Bauer et al., 2003). The NQO1*2 polymorphism is a single-nucleotide polymorphism, defined as a C-to-T change at position 609 of the human NQO1 cDNA, corresponding to a proline-to-serine change at position 187 of the protein (Ross et al., 1996). The mutant NQO1*2 protein is rapidly degraded by the ubiquitin proteasomal system, resulting in an absence of NQO1 protein in persons carrying the NQO1*2/*2 genotype (Moran et al., 1999). Epidemiological studies have associated the NQO1*2 genotype with an increased risk of benzene-induced myeloid toxicity and a variety of de novo and therapy induced leukemias (Ross, 2005). Overexpression of NQO1 in TrHBMECs decreased the expression of ChM-I and abrogated the inhibition of tube formation induced by hydroquinone, further confirming its protective role against benzene induced toxicity. These studies demonstrate the potential role of NQO1 against hydroquinone-induced inhibition of endothelial cell tube formation in bone marrow and suggest that persons carrying the NQO1*2 genotype may be more susceptible to benzene metabolite-induced stromal compartment damage.
In summary, hydroquinone up-regulated ChM-I and inhibited tube formation in TrHBMECs, suggesting that elevation of ChM-I by hydroquinone is a novel mechanism underlying hydroquinone-induced inhibition of tube formation. In addition, this study provides a potential mechanism by which hydroquinone induces adverse effects in the bone marrow endothelial microenvironment critical to the development and differentiation of HSC.
This work was supported by National Institutes of Health National Institute of Environmental Health Sciences [Grant ES09554]
ABBREVIATIONS: NQO1, NADPH:quinone oxidoreductase 1; HSC, hematopoietic stem cell; TrHBMEC, transformed human bone marrow endothelial cell; ChM-I, chondromodulin-I; HCAEC, human coronary artery endothelial cell; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; siRNA, small interfering RNA; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; RT, reverse transcription; PAGE, polyacrylamide gel electrophoresis; SRB, sulforhodamine B; AP-1, activator protein 1.
References
- Aoyama T, Okamoto T, Nagayama S, Nishijo K, Ishibe T, Yasura K, Nakayama T, Nakamura T, and Toguchida J (2004) Methylation in the core-promoter region of the chondromodulin-I gene determines the cell-specific expression by regulating the binding of transcriptional activator Sp3. J Biol Chem 279 28789-28797. [DOI] [PubMed] [Google Scholar]
- Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, et al. (2004) Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med 10 64-71. [DOI] [PubMed] [Google Scholar]
- Azizan A, Holaday N, and Neame PJ (2001) Post-translational processing of bovine chondromodulin-I. J Biol Chem 276 23632-23638. [DOI] [PubMed] [Google Scholar]
- Bauer AK, Faiola B, Abernethy DJ, Marchan R, Pluta LJ, Wong VA, Roberts K, Jaiswal AK, Gonzalez FJ, Butterworth BE, et al. (2003) Genetic susceptibility to benzene-induced toxicity: role of NADPH:quinone oxidoreductase-1. Cancer Res 63 929-935. [PubMed] [Google Scholar]
- Bironaite D, Siegel D, Moran JL, Weksler BB, and Ross D (2004) Stimulation of endothelial IL-8 (eIL-8) production and apoptosis by phenolic metabolites of benzene in HL-60 cells and human bone marrow endothelial cells. Chem Biol Interact 149 177-188. [DOI] [PubMed] [Google Scholar]
- Bollati V, Baccarelli A, Hou L, Bonzini M, Fustinoni S, Cavallo D, Byun HM, Jiang J, Marinelli B, Pesatori AC, et al. (2007) Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res 67 876-880. [DOI] [PubMed] [Google Scholar]
- Caplan AI and Dennis JE (2006) Mesenchymal stem cells as trophic mediators. J Cell Biochem 98 1076-1084. [DOI] [PubMed] [Google Scholar]
- Davis GE, Koh W, and Stratman AN (2007) Mechanisms controlling human endothelial lumen formation and tube assembly in three-dimensional extracellular matrices. Birth Defects Res C Embryo Today 81 270-285. [DOI] [PubMed] [Google Scholar]
- Dexter TM, Allen TD, and Lajtha LG (1977) Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol 91 335-344. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, and Keiliss-Borok IV (1974) Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 17 331-340. [DOI] [PubMed] [Google Scholar]
- Hiraki Y, Mitsui K, Endo N, Takahashi K, Hayami T, Inoue H, Shukunami C, Tokunaga K, Kono T, Yamada M, et al. (1999) Molecular cloning of human chondromodulin-I, a cartilage-derived growth modulating factor, and its expression in Chinese hamster ovary cells. Eur J Biochem 260 869-878. [DOI] [PubMed] [Google Scholar]
- Hiraki Y and Shukunami C (2000) Chondromodulin-I as a novel cartilage-specific growth-modulating factor. Pediatr Nephrol 14 602-605. [DOI] [PubMed] [Google Scholar]
- Hiraki Y and Shukunami C (2005) Angiogenesis inhibitors localized in hypovascular mesenchymal tissues: chondromodulin-I and tenomodulin. Connect Tissue Res 46 3-11. [DOI] [PubMed] [Google Scholar]
- Hiraki Y, Tanaka H, Inoue H, Kondo J, Kamizono A, and Suzuki F (1991) Molecular cloning of a new class of cartilage-specific matrix, chondromodulin-I, which stimulates growth of cultured chondrocytes. Biochem Biophys Res Commun 175 971-977. [DOI] [PubMed] [Google Scholar]
- Kopp HG, Avecilla ST, Hooper AT, and Rafii S (2005) The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 20 349-356. [DOI] [PubMed] [Google Scholar]
- Lan Q, Zhang L, Li G, Vermeulen R, Weinberg RS, Dosemeci M, Rappaport SM, Shen M, Alter BP, Wu Y, et al. (2004) Hematotoxicity in workers exposed to low levels of benzene. Science 306 1774-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z and Li L (2006) Understanding hematopoietic stem-cell microenvironments. Trends Biochem Sci 31 589-595. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, and Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193 265-267. [PubMed] [Google Scholar]
- Matsumura T, Wolff K, and Petzelbauer P (1997) Endothelial cell tube formation depends on cadherin 5 and CD31 interactions with filamentous actin. J Immunol 158 3408-3416. [PubMed] [Google Scholar]
- Moran JL, Siegel D, and Ross D (1999) A potential mechanism underlying the increased susceptibility of individuals with a polymorphism in NAD(P)H:quinone oxidoreductase 1 (NQO1) to benzene toxicity. Proc Natl Acad Sci U S A 96 8150-8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65 55-63. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Sato K, Tashiro F, Miyazaki J, Nishida K, Hiraki Y, Tano Y, and Shukunami C (2004) Anti-angiogenic action of the C-terminal domain of tenomodulin that shares homology with chondromodulin-I. J Cell Sci 117 2731-2744. [DOI] [PubMed] [Google Scholar]
- Rickert DE, Baker TS, Bus JS, Barrow CS, and Irons RD (1979) Benzene disposition in the rat after exposure by inhalation. Toxicol Appl Pharmacol 49 417-423. [DOI] [PubMed] [Google Scholar]
- Ross D (1996) Metabolic basis of benzene toxicity. Eur J Haematol Suppl 60 111-118. [DOI] [PubMed] [Google Scholar]
- Ross D (1997) Quinone reductases, in Comprehensive Toxicology, Volume 3: Biotransformation (Guengerich FP ed) pp 179-198, Pergamon, New York.
- Ross D (2000) The role of metabolism and specific metabolites in benzene-induced toxicity: evidence and issues. J Toxicol Environ Health A 61 357-372. [DOI] [PubMed] [Google Scholar]
- Ross D (2005) Functions and distribution of NQO1 in human bone marrow: potential clues to benzene toxicity. Chem Biol Interact 153-154 137-146. [DOI] [PubMed] [Google Scholar]
- Ross D, Traver RD, Siegel D, Kuehl BL, Misra V, and Rauth AM (1996) A polymorphism in NAD(P)H:quinone oxidoreductase (NQO1): relationship of a homozygous mutation at position 609 of the NQO1 cDNA to NQO1 activity. Br J Cancer 74 995-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman N, Smith MT, Hayes RB, Traver RD, Hoener B, Campleman S, Li GL, Dosemeci M, Linet M, Zhang L, et al. (1997) Benzene poisoning, a risk factor for hematological malignancy, is associated with the NQO1 609C→T mutation and rapid fractional excretion of chlorzoxazone. Cancer Res 57 2839-2842. [PubMed] [Google Scholar]
- Schweitzer KM, Vicart P, Delouis C, Paulin D, Dräger AM, Langenhuijsen MM, and Weksler BB (1997) Characterization of a newly established human bone marrow endothelial cell line: distinct adhesive properties for hematopoietic progenitors compared with human umbilical vein endothelial cells. Lab Invest 76 25-36. [PubMed] [Google Scholar]
- Setoguchi K, Misaki Y, Kawahata K, Shimada K, Juji T, Tanaka S, Oda H, Shukunami C, Nishizaki Y, Hiraki Y, et al. (2004) Suppression of T cell responses by chondromodulin I, a cartilage-derived angiogenesis inhibitory factor: therapeutic potential in rheumatoid arthritis. Arthritis Rheum 50 828-839. [DOI] [PubMed] [Google Scholar]
- Shukunami C and Hiraki Y (2007) Chondromodulin-I and tenomodulin: the negative control of angiogenesis in connective tissue. Curr Pharm Des 13 2101-2112. [DOI] [PubMed] [Google Scholar]
- Stillman WS, Varella-Garcia M, Gruntmeir JJ, and Irons RD (1997) The benzene metabolite, hydroquinone, induces dose-dependent hypoploidy in a human cell line. Leukemia 11 1540-1545. [DOI] [PubMed] [Google Scholar]
- Travis LB, Li CY, Zhang ZN, Li DG, Yin SN, Chow WH, Li GL, Dosemeci M, Blot W, and Fraumeni JF Jr. (1994) Hematopoietic malignancies and related disorders among benzene-exposed workers in China. Leuk Lymphoma 14 91-102. [DOI] [PubMed] [Google Scholar]
- Vichai V and Kirtikara K (2006) Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 1 1112-1116. [DOI] [PubMed] [Google Scholar]
- Winski SL, Hargreaves RH, Butler J, and Ross D (1998) A new screening system for NAD(P)H:quinone oxidoreductase (NQO1)-directed antitumor quinones: identification of a new aziridinylbenzoquinone, RH1, as a NQO1-directed antitumor agent. Clin Cancer Res 4 3083-3088. [PubMed] [Google Scholar]
- Yanagihara I, Yamagata M, Sakai N, Shukunami C, Kurahashi H, Yamazaki M, Michigami T, Hiraki Y, and Ozono K (2000) Genomic organization of the human chondromodulin-1 gene containing a promoter region that confers the expression of reporter gene in chondrogenic ATDC5 cells. J Bone Miner Res 15 421-429. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Yuasa S, Matsumura K, Kimura K, Shiomi T, Kimura N, Shukunami C, Okada Y, Mukai M, Shin H, et al. (2006) Chondromodulin-I maintains cardiac valvular function by preventing angiogenesis. Nat Med 12 1151-1159. [DOI] [PubMed] [Google Scholar]
- Zheng JH, Pyatt DW, Gross SA, Le AT, Kerzic PJ, and Irons RD (2004) Hydroquinone modulates the GM-CSF signaling pathway in TF-1 cells. Leukemia 18 1296-1304. [DOI] [PubMed] [Google Scholar]
- Zhou H, Kepa J, Siegel D, Hiraki Y, and Ross D (2008) Benzene metabolite hydroquinone up-regulates chondromodulin I and inhibits tube formation in human bone marrow endothelial cells (Abstract), in Proceedings of the 99th Annual Meeting of the American Association for Cancer Research; 2008 April 12-16; San Diego, CA. p. 608. American Association for Cancer Research, Philadelphia.